Abstract
Although SARS-CoV-2 is primarily an airborne risk, the COVID-19 pandemic also highlighted the need for self-disinfection surfaces that could withstand the demand of high occupant densities characteristic of public transportation systems. The aim of this study was to evaluate the durability and antiviral activity of a copper film deployed for 90 days in two high touch locations within an active metropolitan bus and railcar. The antiviral efficacy of this copper film after being deployed in transit vehicles for 90 days (deployed copper film) was then compared to new (unused) copper film to determine if frequent touches and cleaning protocols could decrease the efficacy of the copper films. Deployed copper film, new copper film, and aluminium foil (positive control) coupons were inoculated with ~1 × 106 MS2 virus particles, allowed a contact time of either 5- or 10-min, and analysed for residual viral infectiousness. On both new and deployed copper films, MS2 was completely inactivated (≥5 log reduction) at both time points. These results suggest that the copper film may provide the durability demanded by high touch public spaces while maintaining the antiviral activity necessary to reduce exposure risk and viral transmission via surfaces in public transportation settings.
Keywords: antimicrobial film, bacteriophage, copper, disinfection, efficacy, MS2
Introduction
The early stages of the COVID-19 pandemic raised concerns for millions of commuters utilizing public transportation systems throughout the United States. For example, in New York City it has been estimated that 46–51% of residents use the subway as their primary source of transportation (Sy et al. 2021). Where public transportation is a primary infrastructure, it is characterized by high occupant density in confined spaces, which may increase the risk of disease transmission. Additionally, mass transportation environments contain a significant amount of frequently touched surfaces that may act as fomites for disease transmission by constantly facilitating a flux of microbes among humans and their surroundings (Hsu et al. 2016; Gohli et al. 2019). Although surface contamination is likely a minor contributor to the transmission and exposure risk for SARS-CoV-2 (the virus that causes COVID-19) (Moreno et al. 2021), contaminated surfaces can play a key role in the spread of viral infections (Boone and Gerba 2007). This recognition led to exploratory measures that could reduce viral transmission in mass transportation environments by utilizing self-disinfecting surfaces, such as copper films, placed in high touch areas where routine cleaning may be inconsistent or easily outpaced by the usage demand or frequency of repeated touches.
The antimicrobial properties of copper are well known and have demonstrated efficacy against many bacterial and viral agents (Grass et al. 2011; Poggio et al. 2020). The most widespread public health applications for copper and copper alloys have been to combat the prevalence of hospital-associated infections (HAIs), with copper products often being utilized in water filtration systems or as a self-disinfecting surface (Grass et al. 2011; Schmidt et al. 2016; Vincent et al. 2016). In 2008, the Environmental Protection Agency recognized copper surfaces for their antimicrobial properties (Grass et al. 2011); and as of this writing, copper is the first and only metal to be awarded this status. Approximately 500 copper and copper alloy surfaces have been registered under the U.S. Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) as antimicrobial public health materials that have proven to be effective and pose no risk to public health (Copper Alliance Development Inc 2021). Furthermore, copper and copper alloys have been used to demonstrate antimicrobial effects against five strains of bacteria (Grass et al. 2011; Vincent et al. 2016; Copper Alliance Development Inc 2021).
The current study aimed to investigate the efficacy of a copper film placed in high touch areas in a mass transportation environment for 90 days. MS2, a non-enveloped bacteriophage, was selected for this study because it has been commonly used to assess the efficacy of virucidal decontaminants (Bae and Schwab 2008; D’Souza 2010).
Results and discussion
Copper films placed in two high touch locations (as shown in Fig. 1) were evaluated for residual antiviral activity using MS2 as the test virus. Both post-deployed and new films were attached to aluminium foil and cut into 2- by 4-cm coupons for analysis. Aluminium foil coupons of the same size were used as positive controls. Copper test and aluminium positive control coupons were each inoculated with ~1 × 106 plaque forming unit (PFU) MS2 and initially allowed a 30-min contact time (railcar samples only), but upon observing full MS2 inactivation (data not shown), the copper films were challenged with additional tests using 5- and 10-min contact times. Viricidal efficacy was calculated as the difference between the positive control and test sample Log10 recoveries (De Vries and Hamilton 1999). Virus recoveries from positive control coupons ranged from 6·6 to 6·8 Log10 PFU, while recoveries from both types of copper films were at or below the detection limit (i.e., 1 Log10 PFU), providing an overall log reduction ≥5. This result is significant considering EPA guidelines stating disinfectants with viricidal claims must demonstrate at least a 3-log reduction of the virus on tested surfaces (USEPA 2018) as part of the registration requirements. The analysis method used in this study had a detection limit of 10 PFU per sample, therefore a 1 Log10 recovery (instead of 0 Log10) was reported and utilized in efficacy calculations for samples with no observable plaques to avoid inflating viricidal efficacies.
Figure 1.

Photographs of representative copper film deployment locations on hand railing in a bus (left) and a railcar (right).
Recoveries (expressed as Log10 virus PFU recovered) and antiviral efficacy data (expressed as Log10 Reduction) for MS2 for both the new and post-deployed copper films are shown in Fig. 2. Railcar and bus deployed copper films were tested on separate days, and recovery results indicate that the post-deployed copper film maintained antiviral activity similar to new, unused copper film.
Figure 2.
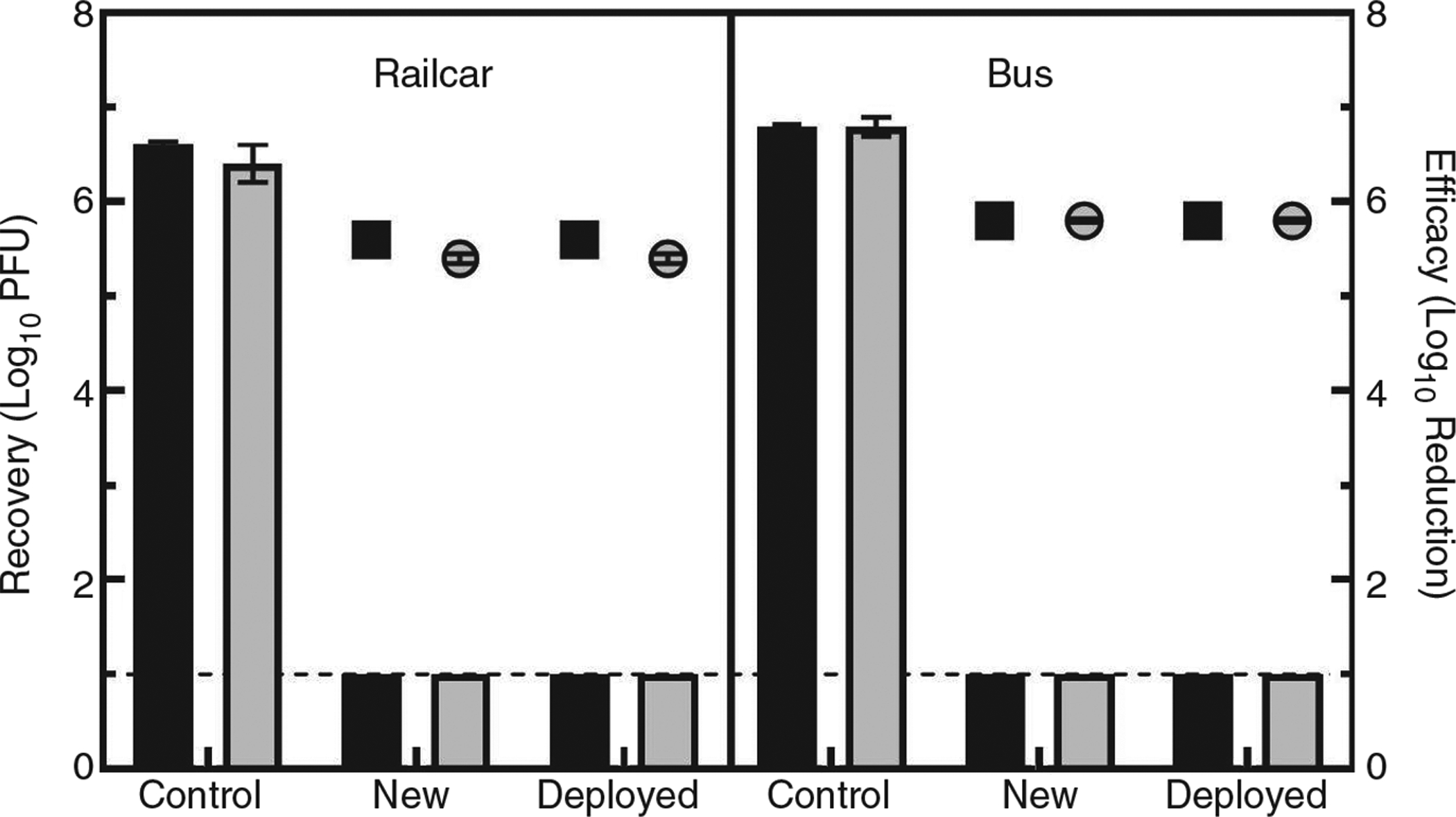
Efficacy of new and post-deployed copper film against MS2. Recovery (bars) and efficacy (symbols) of new and post-deployed (deployed) copper film, from the railcar location and bus location are reported as mean Log10 PFU (±standard deviation) for recoveries (left y-axis), and mean Log10 reduction (±pooled standard error) for efficacy (right y-axis). Black bars represent recovery following a 5-min contact time, gray bars represent recovery following a 10-min contact time, black squares represent efficacy following a 5-min contact time, and gray circles represent efficacy following a 10-min contact time. The horizontal line at 1·0 indicates the analytical limit of detection for the assay (i.e., 1 Log10 PFU recovered).
Mass transit systems are critical to everyday life for millions of people, and the potential role in the transmission of infectious disease, as well as bioterrorism risk, is significant (Zhang et al. 2016; Gohli et al. 2019). As a result, there is a widespread interest in tools for disease and bioterrorism surveillance in major transportation systems. A recent metagenomic analysis of surfaces was used to characterize the microbial diversity and live bacterial communities existing across a city-wide mass transit environment (Afshinnekoo et al. 2015). Results showed that most of the recovered DNA sequences matched organisms from taxa commonly associated with human skin, but nearly half (48%) of the DNA detected did not match any known organism. Further, swab samples were used to recover viable organisms which could be cultured on standard media, and of those recoveries, ~28% were resistant to common antibiotics (kanamycin, chloramphenicol, and ampicillin). Such baseline data demonstrate the important role of surfaces in capturing, harbouring, and potentially spreading microorganisms, including pathogens or select biological agents. Therefore, consideration of pre-emptive mitigation measures such as placement of self-sanitizing surfaces in public spaces has received a renewed interest. For example, a 2012 ten-week study measured bacterial levels on brass (copper alloy), glass, and wood surfaces (e.g. railings and door pulls) located in Grand Central Terminal (New York City, NY) (Michels and Michels 2016). Significant reductions (84–97%) in bacterial loads were reported for the copper alloy surfaces when compared to the non-copper materials. In this case, long-standing terminal infrastructure containing copper alloys appeared to maintain antimicrobial characteristics even after decades of deployment in high touch areas. Similarly, the data reported here illustrate that the antiviral activity of copper films used in high touch surface areas can be sustained over relatively short periods of time (up to 90 days) despite the potentially heavy microbial burden and frequent surface cleaning.
Other environments that may benefit from surfaces equipped with copper films include hospitals and health care facilities. In 2002, hospitalized patients in the United States were estimated to have a 4·5% risk of developing a hospital acquired infection (HAI), resulting in nearly 99 000 estimated deaths (Klevens 2007) and adding an estimated $28–45 billion (2007 dollars) to annual health-care costs (Scott 2009; Stone 2009). Although the idea that environmental contaminants are responsible for HAIs is debated, the possibility that touching of contaminated surfaces may be a source of transfer to patients must be considered (Casey et al. 2010). As a result, the CDC has guidelines and recommendations for surface cleaning and disinfection in hospitals and other health care settings (Rutala and Weber 2008), and surfaces that incorporate copper or copper alloys may provide added antimicrobial benefit between routine cleanings. Some studies have shown that the use of copper surfaces in health care settings may significantly reduce the microbial burden over sustained periods (Casey et al. 2010; Schmidt et al. 2012; Michels and Michels 2016). And at least one study demonstrated that placing copper alloy surfaces onto six high touch objects in ICU rooms reduced the risk of HAI by more than 50% for all study sites (Salgado et al. 2013). These studies and the data presented herein suggest that copper film could be an effective option for controlling or reducing microbial transmission from surfaces in areas that experience high occupant density and/or frequent touches. Additional studies should be performed to evaluate the antiviral efficacy of these surface coatings over a longer deployment period, with shorter contact times that may represent intervening time between separate passenger touches, under varied environmental conditions, against wet- and dry-deposited contaminants, and in public locations which may be subjected to more frequent surface cleanings that would further explore the promising findings of this initial investigation.
Materials and methods
Bacteriophage propagation
The non-enveloped bacteriophage MS2 (ATCC 15597-B1) and its host, Escherichia coli (ATCC-15597), were obtained from the American Type Culture Collection. Viral stocks were propagated in the log phase bacterial host (OD600 = 0·6–0·9), Escherichia coli using Luria-Bertani Broth (Difco, 244610, Sparks, MD) and a conventional soft agar overlay method (Kropinski et al. 2009). Following overnight incubation at 35°C, sterile cell spreaders were used to gently scrape soft agar overlays from three 100 mm diameter plates into a sterile 50-ml conical tube containing 15 ml of SM buffer (Teknova, S0249, Hollister, CA). Tubes were vortexed for 1–2 min to break up agar clumps then centrifuged at 7000 g for 15 min. Supernatant was removed and filtered through a 0·2-micron syringe filter (Corning PES syringe filters, 431229, Corning, NY). Aliquots of 1 ml were stored in cryovials (Thermo Fisher Scientific, AY509X33, Waltham, MA) at −80°C until use.
Field test conditions
Commercially available, self-adhesive copper films (CuVerro® V, 91·3% Copper, EPA registration #853535, East Alton, IL) were placed on the hand railing and stanchions of both buses and railcars operating within the Los Angeles Metropolitan Transportation Authority system, as shown in Fig. 1. The films were left in place for 90 days (February through April 2021) and were subject to cleaning with a direct spray and wipe using either Turbo Kill and/or Monofoil M when the vehicles were out of service, usually at night. While ridership during the copper film deployment period was down an average of approximately 55% across the overall system, the estimated weekday ridership still averaged over 12 500 000 per month (Los Angeles Metro 2021). After 90 days, a subset of the deployed copper film was removed and shipped to the EPA RTP Microbiology Laboratory in Durham, NC for analysis. Copper films were adhered to aluminium foil for transport, then cut into individual coupons (2- by 4-cm) prior to inoculation and analysis.
Sample inoculation and analysis
New and unused CuVerro® V copper film (stored at room temperature in the laboratory) was adhered to aluminium foil, then cut into 2- by 4-cm coupons to remain consistent with the handling of the deployed copper film. Copper film test coupons and positive control aluminium foil coupons were inoculated in triplicate with 10 μl of an MS2 virus preparation (concentration 1 × 108 MS2 virus particles per ml) stabilized in 5% fetal bovine serum (FBS) (10082139, Gibco, certified heat inactivated). The 10 μl inoculum was delivered by pipetting the inoculum in a line lengthwise along the centre of the coupon, then using the pipette tip to spread the inoculum over the surface of the coupon. The inoculated coupons were stored in covered petri-dishes at ambient conditions within a Type 2 Biological Safety Cabinet (~23°C, 54–57% RH) for the entirety of the contact time. After the prescribed contact time (5- or 10-min), virus particles were recovered from both test and control coupons by continuously vortexing each coupon for 2 min in a 50-ml conical tube containing 10 ml of extraction buffer composed of 10 mmol l−1 ethylenediaminetetraacetic acid (EDTA) in 10% Dey-Engley (DE) Broth (BD 281910, Difco, Fisher Scientific, Waltham, MA) prepared in 1X phosphate buffered saline (PBS) (P0196, 10X solution, Teknova, Hollister, CA). Inoculation controls were prepared by directly inoculating 10 ml of extraction buffer. Negative controls were prepared by placing a non-inoculated 2- by 4-cm coupon for each material type (new copper, deployed copper, and aluminium foil) in extraction buffer and processing them in same manner as the test coupons.
Sample plating and analysis
Optical density measurements of the E. coli cultures were taken before plating as indicators of bacterial host log phase growth (OD600 = 0·6–0·9). A tenfold dilution series was prepared as appropriate in 1X PBS for each sample. Undiluted and diluted aliquots of either 100 μl or 1 ml were plated with 100 μl of log phase E. coli in triplicate using a conventional soft agar overlay method (Kropinski et al. 2009). Plates were incubated overnight at 35°C and manually enumerated the following day. The inoculum titer was determined each test day by performing a tenfold dilution series directly from the inoculum, plating 100 μl aliquots of each dilution in triplicate, and enumerating plaques the following day.
Post-test neutralization assays
Post-test neutralization assays were performed to verify that the extraction buffer was effectively chelating the copper and quenching residual antimicrobial activity. These assays were performed by removing 5 ml aliquots from non-inoculated samples (i.e. negative controls) and directly inoculating the aliquots with 10 μl of the virus preparation (concentration 1 × 108 MS2 virus particles per ml). Inoculation controls were prepared by directly inoculating extraction buffer. Following inoculation, samples were vortexed to mix, incubated at room temperature for ≥10-min, then plated. For effective neutralization, test sample recoveries should approximate control recoveries. Recoveries from these assays demonstrated effective neutralization and were as follows: Log10 6·3, 6·4, 6·4 and 6·3 for the inoculum titer, inoculation controls, deployed copper film, and new copper film, respectively. These data verify the effectiveness of the neutralizer.
Significance and Impact of the Study:
The use of commercially available copper films placed in high touch areas could be an effective supplemental measure for reducing the exposure risk and transmission of infectious diseases in mass transportation environments.
Acknowledgements
This research was initiated following the identification of knowledge gaps by the Los Angeles Metro Transit Authority. This effort was directed by a principal investigator from the US EPA Office of Research and Development’s (ORD) Center for Environmental Solutions and Emergency Response (CESER), using the support of a project team that consisted of US EPA and HSMMD. Additionally, the authors would like to thank EPA contractors Brian Ford, Rachael Baartmans, and Lesley Mendez Sandoval.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The EPA, through its Office of Research and Development, directed the research described herein conducted through contract 68HERC20D0018 with Jacobs Technology, Inc. It has been subjected to the Agency’s review and has been approved for publication. Mention of trade names, products or services does not convey official EPA approval, endorsement, or recommendation.
Conflict of Interest
No conflict of interest was declared.
Data Availability Statement
All data supporting this study and manuscript are available on data.gov.
References
- Afshinnekoo E, Meydan C, Chowdhury S, Jaroudi D, Boyer C, Bernstein N, Maritz JM, Reeves D et al. (2015) Geospatial resolution of human and bacterial diversity with city-scale metagenomics. Cell Syst 1, 72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J and Schwab KJ (2008) Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Appl Environ Microbiol 74, 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone SA and Gerba CP (2007) Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol 73, 1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey AL, Adams D, Karpanen TJ, Lambert PA, Cookson BD, Nightingale P, Miruszenko L, Shillam R et al. (2010) Role of copper in reducing hospital environment contamination. J Hosp Infect 74, 72–77. [DOI] [PubMed] [Google Scholar]
- Copper Alliance Development Inc (2021) EPA registration copper stewardship site. Viewed 24 August 2021. https://www.copperalloystewardship.com/
- De Vries TA and Hamilton MA (1999) Estimating the antimicrobial log reduction: Part 1. Quantitative assays. Quant Microbiol 1, 29–45. [Google Scholar]
- D’Souza DH and Xiaowei SU (2010) Efficacy of chemical treatments against murine norovirus, feline calicivirus, and MS2 bacteriophage. Foodborne Pathog Dis 7, 319–326. [DOI] [PubMed] [Google Scholar]
- Gohli J, Boifot KO, Moen LV, Pastuszek P, Skogan G, Udekwu KI and Dybwad M (2019) The subway microbiome: seasonal dynamics and direct comparison of air and surface bacterial communities. Microbiome 7, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass G, Rensing C and Solioz M (2011) Metallic copper as an antimicrobial surface. Appl Environ Microbiol 77, 1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T, Joice R, Vallarino J, Abu-Ali G, Hartmann EM, Shafquat A, DuLong C, Baranowski C et al. (2016) Urban transit system microbial communities differ by surface type and interaction with humans and the environment. mSystems 1, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens RM, Edwards JR, Richards CL Jr, Horan TC, Gaynes RP, Pollock DA and Cardo DM (2007) Estimating health care-associated infections and deaths in U.S. Hospitals, 2002. Public Health Rep (Washington, DC: 1974) 122, 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski AM, Mazzocco A, Waddell TE, Lingohr E and Johnson RP (2009) Enumeration of bacteriophages by double agar overlay plaque assay. In Bacteriophages ed. Walker JM pp. 69–76. Totowa, NJ: Humana Press. [DOI] [PubMed] [Google Scholar]
- Los Angeles Metro (2021) Interactive Estimated Ridership Stats. Viewed 18 August 2021. https://isotp.metro.net/MetroRidership/IndexSys.aspx
- Michels H and Michels C (2016) Copper alloys-the new old weapon in the fight against infectious disease. Curr Trends Microbiol 10, 23–45. [Google Scholar]
- Moreno T, Pinto RM, Bosch A, Moreno N, Alastuey A, Minguillon MC, Anfruns-Estrada E, Guix S et al. (2021) Tracing surface and airborne SARS-CoV-2 RNA inside public buses and subway trains. Environ Int 147, 106326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggio C, Colombo M, Arciola CR, Greggi T, Scribante A and Dagna A (2020) Copper-alloy surfaces and cleaning regimens against the spread of SARS-CoV-2 in dentistry and orthopedics. From fomites to anti-infective nanocoatings. Materials (Basel) 13, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutala WA, Weber DJ and the Healthcare Infection Control Practices Advisory Committee (2008) Guideline for Disinfection and Sterilization in Healthcare Facilities. Viewed 24 August 2021. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/.
- Salgado CD, Sepkowitz KA, John JF, Cantey JR, Attaway HH, Freeman KD, Sharpe PA, Michels HT et al. (2013) Copper surfaces reduce the rate of healthcare-acquired infections in the intensive care unit. Infect Control Hosp Epidemiol 34, 479–486. [DOI] [PubMed] [Google Scholar]
- Schmidt MG, Attaway HH, Sharpe PA, John J Jr, Sepkowitz KA, Morgan A, Fairey SE, Singh S et al. (2012) Sustained reduction of microbial burden on common hospital surfaces through introduction of copper. J Clin Microbiol 50, 2217–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MG, von Dessauer B, Benavente C, Benadof D, Cifuentes P, Elgueta A, Duran C and Navarrete MS (2016) Copper surfaces are associated with significantly lower concentrations of bacteria on selected surfaces within a pediatric intensive care unit. Am J Infect Control 44, 203–209. [DOI] [PubMed] [Google Scholar]
- Scott RD (2009) The direct medical cost of healthcare-associated infections in U.S. Hospitals and the benefits of prevention. GA: Centers for Disease Control and Prevention (No. CS200891-A). [Google Scholar]
- Stone PW (2009) Economic burden of healthcare-associated infections: an american perspective. Expert Rev Pharmacoecon Outcomes Res 9, 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy KTL, Martinez ME, Rader B and White LF (2021) Socioeconomic disparities in subway use and COVID-19 outcomes in New York city. Am J Epidemiol 190, 1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA (ed.) (2018) Product performance test guideline, ocspp 810.2200, disinfectants for use on environmental surfaces, guidance for efficacy testing, [epa 712-c-17–004]. In US Environmental Protection Agency pp. 19. Washington, DC: U.S. Environmental Protection Agency (US EPA). [Google Scholar]
- Vincent M, Hartemann P and Engels-Deutsch M (2016) Antimicrobial applications of copper. Int J Hyg Environ Health 219, 585–591. [DOI] [PubMed] [Google Scholar]
- Zhang N, Huang H, Duarte M and Zhang JJ (2016) Dynamic population flow based risk analysis of infectious disease propagation in a metropolis. Environ Int 94, 369–379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting this study and manuscript are available on data.gov.


