Abstract
Hepatic abscesses can rarely cause pericardial disease by erosion into the pericardial space and present with haemodynamic instability due cardiac tamponade. While rare, these dramatic presentations are more often due to amoebic abscesses than bacterial abscesses. Importantly, a cause must be found for any cryptogenic hepatic abscess regardless of presentation, as there is a high association with underlying malignancy. We report a previously healthy man in his 30s who presented with cardiac tamponade from perforation of a Roseomonas mucosa pyogenic hepatic abscess into the pericardium in the absence of bacteremia and biliary disease. One year later, he was found to have diffusely metastatic hepatoid carcinoma.
Keywords: pericardial disease, infection (gastroenterology), hepatitis and other GI infections, oesophageal cancer, pathology
Background
Hepatic abscesses can rarely cause pericardial disease by erosion into the pericardial space which can cause haemodynamic instability and require emergent intervention. Besides amoeba, a range of causative bacteria have been reported, with Klebsiella pneumoniae being most common. Roseomonas mucosa, a gram-negative bacterium first described in 2003, is being recognised more frequently as clinically relevant, but has yet to be reported causing hepatic abscesses.1 Due to its frequently reported antibiotic resistance and risk of nosocomial spread, knowledge of this bacteria is pertinent.1–3 Regardless of the causative organism, pyogenic hepatic abscesses, whether complicated or uncomplicated, have strong associations with underlying gastrointestinal malignancies.4 5 Therefore, all patients with cryptogenic hepatic abscess should be screened for cancers.
Case presentation
A man in his 30s with no significant medical history besides daily tobacco and marijuana smoking presented in respiratory distress. He had been ill for 6 weeks with fatigue, fever, generalised weakness and malaise and was diagnosed clinically with mononucleosis by his primary care provider. He denied unprotected sex, intravenous drug use, sick contacts, pertinent family history of disease and recent travel.
After becoming dyspneic, he presented to the emergency room where he was hypotensive (78/38 mm Hg), tachycardic in sinus rhythm (123 beats per minute) and tachypneic (49 respirations per minute), but afebrile (37.1°C). He had a high anion gap metabolic acidosis, normal troponin, leucocytosis with white cell count (WBC) 44.3 ×109/L, absolute neutrophil count of 38.1 ×109/L, thrombocytosis and anaemia. Creatine was elevated at 1.43 mg/dL on presentation but peaked at 5.65 mg/dL and liver enzymes were high with aspartate aminotransferase (AST) 10 710 units/L and alanine aminotransferase (ALT) 5 262 units/L implying ischaemic injuries.
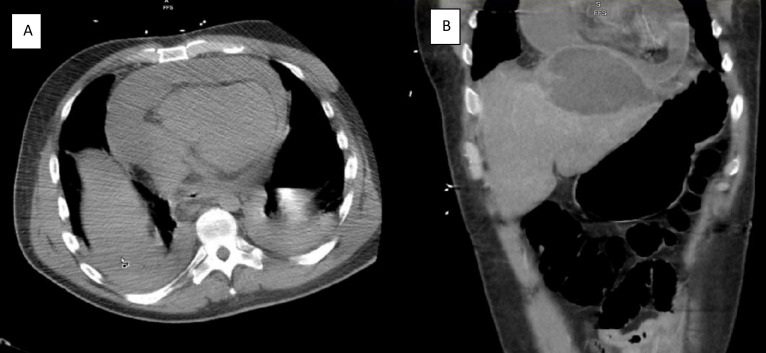
Bedside echocardiogram revealed a large pericardial effusion (video 1), but initial subxiphoid pericardiocentesis was unsuccessful due to purulent fluid return prior to accessing the pericardium. A CT scan of the chest, abdomen and pelvis (figure 1) revealed a large left hepatic lobe abscess and further described the pericardial effusion as larger anteriorly than posteriorly. He was empirically started on piperacillin/tazobactam and metronidazole and required haemodynamic support as well as continuous renal replacement therapy after developing hyperkalemia due to acute renal failure.
Video 1.
Figure 1.
(A) CT scan of the abdomen: coronal view demonstrating hepatic and pericardial abscesses. (B) CT scan of the chest: transverse view demonstrating circumferential pericardial abscess.
His hepatic abscess was drained under fluoroscopy removing 300 mL of green, viscous, extremely foul smelling fluid. Anterior pericardiocentesis resulted in the immediate return of 850 mL purulent fluid and restored cardiac function.
Investigations
Pericardial fluid studies revealed brown, turbid drainage with a pH 7.07, WBC 119.2×109/L with 95% neutrophils, red blood cells (RBC) 0.0177 x1012/L, albumin 1.1 g/dL, lactate dehydrogenase (LDH) 5438 units/L, glucose 15 mg/dL, lipase 31 units/L, protein 3.5 g/dL and no malignant cells, but an abundance of acute and chronic inflammatory cells. Culture was positive in broth only at 5 days and R. mucosa was identified by matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometry (Bruker; Billerica, Massachusetts, USA). Microscan (BD Phoenix, BD; Franklin Lakes, New Jersey, USA) revealed susceptibility to ceftriaxone and ciprofloxacin.
Prior to culture growth, investigative labwork revealed a negative HIV screen and negative autoimmune workup. Blood cultures remained negative for bacteria, fungi and acid fast bacteria. Entamoeba histolytica antibody and antigen were both negative. Magnetic resonance cholangiopancreatography did not reveal biliary pathology as the inciting cause of the abscesses.
Differential diagnosis
Due to the ruptured abscess without bacteremia or biliary pathology, the abscess was thought to be amoebic which necessitated the addition of metronidazole. Since the patient had no prior medical history or inciting events, the differential was broad and included rheumatologic disease, malignancy and tuberculosis.
Treatment
Cardiac function was restored with emergent pericardiocentesis. The hepatic and pericardial drains were left in place for 12 days. On day 5, repeat echocardiogram noted an absence of pericardial fluid. Despite this, 300 mL more fluid was removed through the pericardial drain over the next 3 days (1995 mL total) and R. mucosa was also grown from fluid drained from the hepatic abscess, indicating that the pericardial and hepatic abscesses were in fact communicating.
After culture growth and bacterial susceptibility testing, the patient was transitioned to ceftriaxone and metronidazole and haemodynamic support was weaned. He was discharged with baseline kidney function after a 20-day hospitalisation and continued 5 weeks of intravenous ceftriaxone and oral metronidazole.
Outcome and follow-up
The patient was discharged in stable condition with home nursing arranged to administer intravenous antibiotics in the outpatient setting. He successfully completed this course of antibiotics and was noted to have resolution of symptoms, normal cardiac function and normal hepatic function at 3-month follow-up. An outpatient screening colonoscopy was recommended due to the association of cryptogenic hepatic abscess with underlying malignancy, though this was not performed.
The patient presented again to the hospital 1 year later. Though he had suffered profound weight loss of 100 lbs from an initial weight of 232 lbs, his presenting complaint was dysphagia. Initial imaging suggested an oesophageal mass, so endoscopy was performed revealing a 1.5 cm fungating mass in the lower third of the oesophagus (figure 2). Follow-up MRI revealed extensive hepatic and lymph node metastases (figure 3).
Figure 2.

Endoscopic images of a 1.5 cm fungated mass in the lower third of the oesophagus.
Figure 3.

MRI of the abdomen: transverse view revealing a large hepatic metastasis.
Biopsy of the oesophageal mass revealed various morphologies including glandular differentiation with areas of solid sheets of epithelioid, highly pleomorphic neoplastic cells and dystrophic calcification (figure 4). At higher magnifications, there were supranuclear and subnuclear vacuoles and large anaplastic cells present. Staining was positive for CK20, CDX2, SATB2, glypican-3, SALL4 and alpha-fetoprotein (AFP), but negative for CK7. Due to positive staining for SALL4, initial diagnosis was extragonadal yolk sac tumour. However, after further review and considering the positive staining for AFP, consensus diagnosis was hepatoid carcinoma.
Figure 4.
(A) H&E stain at ×40 magnification revealing various morphologies with poor differentiation: glandular, solid epithelioid and highly pleomorphic neoplastic cells. (B) Weakly positive alpha-fetoprotein stain.
Discussion
Most pyogenic hepatic abscesses are polymicrobial, due to peritonitis or biliary disease in the case of gram-negative bacteria or due to gram-positive bacteremia or endocarditis.6 7 Perforation of a pyogenic liver abscess into the pericardium is rare and associated with a mortality of at least 25%, though data are limited.8 9 Pericardiocentesis for pericardial abscess is complicated, as thick fluid may drain poorly or loculate, and event-free survival is worse compared with drainage of exudative pericardial effusions.10
To date, most cases of communication between hepatic abscess and pericardium are amoebic rather than pyogenic.8 9 In a review of current literature, Cho et al. reported a case of a K. pneumoniae pyogenic liver abscess perforating into the pericardium in a 45-year-old man and identified 12 other cases with causative organisms being Staphylococcus aureus, Escherichia coli and Actinomyces sp most commonly.8 Roseomonas sp have yet to be reported as causative of hepatic abscesses.
The genus Roseomonas consists of pink pigmented, oxidative, glucose non-fermentative, gram-negative coccobacilli which were first described in 1993 and originally included six species, with R. mucosa later differentiated as a separate species from Roseomonas gilardii in 2003.1 R. mucosa is reliably identified by 16S rRNA gene sequencing and MALDI-TOF mass spectrometry, which was successfully used in our case.11
Roseomonas sp have been isolated not only from environmental samples including water, soil, air and plants but also from human skin and rarely the gastrointestinal tract.2 3 Furthermore, R. mucosa strains were isolated from a hospital environment, suggesting a human reservoir and a niche as a pathobiont.2 3 12
Most clinically relevant cases of R. mucosa infection are catheter related owing to the bacteria’s ability to form a biofilm.11 Other infections in both immunocompromised and immunocompetent hosts have been reported including: infective endocarditis, peritonitis in a peritoneal dialysis patient, spondylitis post vertebroplasty, spinal epidural abscess as well as bacteremia, cellulitis and nosocomial infections.11 13–16
While cases of R. mucosa infection are more commonly being reported, the study of this bacterium is especially pertinent due to its frequent antibiotic resistance. R. mucosa colonies are consistently resistant to cefepime, ceftazidime and ampicillin and frequently resistant to ceftriaxone and other beta-lactam antibiotics.1 Furthermore, the bacteria can form biofilms which can impair antibiotic penetration.17 Testing on multiple species of the genus Roseomonas revealed that the addition of clavulanic acid restored susceptibility to amoxicillin and ticarcillin, suggesting the production of B-lactamase.2 R. mucosa is consistently susceptible to amikacin and ciprofloxacin and frequently to imipenem.1 The causative organism in our case was confirmed susceptible to ciprofloxacin, which also would have been a viable treatment option.
Next, our patient had no risk factors for hepatic abscess or R. mucosa infection, as he had no medical history and no intravenous drug use, surgeries, biliary disease or bacteremia that could have introduced R. mucosa into the bloodstream or body cavities. Therefore, the pyogenic abscess was cryptogenic. While we had recommended screening colonoscopy after clinical stabilisation, this procedure was never performed.
In a systematic review of 12 studies, the pooled incidence of later colorectal cancer in patients with pyogenic hepatic abscesses was 8%, compared with 1.2% of controls.4 The total estimated risk of colorectal cancer post pyogenic liver abscess was 7.3 cases per 1000 person-years.5 Liver cancers have also been associated with the greatest odds of diagnosis in the first 3 months after discovery of a cryptogenic hepatic abscess.18 The most frequent cause of pyogenic hepatic abscess that precedes diagnosis of colorectal cancer was K. pneumoniae, which occurred in 93.1% of patients in pooled analysis.4 Our patient was diagnosed 12 months after discovery of hepatic abscess with hepatoid carcinoma, an extrahepatic malignancy with hepatocellular features, that was already advanced at the time of discovery.19–21
To our knowledge, roughly 40 cases have been reported involving R. mucosa as a causative organism in clinically significant infections.2 Our case is the first reported of a pyogenic hepatic abscess due to R. mucosa, representing a new causative species for hepatic abscesses, and further illustrates the rare and emergent presentation of perforation into the pericardium. Lastly, our case highlights the necessity of cancer screening in any patient diagnosed with cryptogenic liver abscess, as the risk of associated malignancy is high.
Learning points.
Perforation of a hepatic abscess into the pericardium can cause tamponade physiology and is associated with high morbidity and mortality.
Roseomonas mucosa is increasingly being recognised as clinically relevant in human disease and can cause a variety of different infections.
Cryptogenic hepatic abscesses have a high association with underlying malignancy, and any patient diagnosed with cryptogenic hepatic abscess should be screened for cancer.
Footnotes
Contributors: Case conceptualisation and design: JS and LM. Data collection and writing of draft: JS, MG and MB. Analysis and Interpretation of results and revision of manuscript: All authors. Review of literature: JS and MG. Oversight: LM. All authors have seen and approved of the final submitted manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Han XY, Pham AS, Tarrand JJ, et al. Bacteriologic characterization of 36 strains of Roseomonas species and proposal of Roseomonas mucosa sp nov and Roseomonas gilardii subsp rosea subsp nov. Am J Clin Pathol 2003;120:256–64. 10.1309/731VVGVCKK351Y4J [DOI] [PubMed] [Google Scholar]
- 2.Romano-Bertrand S, Bourdier A, Aujoulat F, et al. Skin microbiota is the main reservoir of Roseomonas mucosa, an emerging opportunistic pathogen so far assumed to be environmental. Clin Microbiol Infect 2016;22:737:737.e1–737.e7. 10.1016/j.cmi.2016.05.024 [DOI] [PubMed] [Google Scholar]
- 3.Okamoto K, Ayibieke A, Saito R, et al. A nosocomial cluster of Roseomonas mucosa bacteremia possibly linked to contaminated hospital environment. J Infect Chemother 2020;26:802–6. 10.1016/j.jiac.2020.03.007 [DOI] [PubMed] [Google Scholar]
- 4.Mohan BP, Meyyur Aravamudan V, Khan SR, et al. Prevalence of colorectal cancer in cryptogenic pyogenic liver abscess patients. do they need screening colonoscopy? A systematic review and meta-analysis. Dig Liver Dis 2019;51:1641–5. 10.1016/j.dld.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 5.Lai H-C, Lin C-C, Cheng K-S, et al. Increased incidence of gastrointestinal cancers among patients with pyogenic liver abscess: a population-based cohort study. Gastroenterology 2014;146:129–37. 10.1053/j.gastro.2013.09.058 [DOI] [PubMed] [Google Scholar]
- 6.Huang CJ, Pitt HA, Lipsett PA, et al. Pyogenic hepatic abscess. changing trends over 42 years. Ann Surg 1996;223:600–9. 10.1097/00000658-199605000-00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahimian J, Wilson T, Oram V, et al. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 2004;39:1654–9. 10.1086/425616 [DOI] [PubMed] [Google Scholar]
- 8.Cho E, Park SW, Jun CH, et al. A rare case of pericarditis and pleural empyema secondary to transdiaphragmatic extension of pyogenic liver abscess. BMC Infect Dis 2018;18:40. 10.1186/s12879-018-2953-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hara N, Kato M, Fuse K, et al. A rare case of pyogenic liver abscess complicated with cardiac tamponade. Intern Med 2008;47:563–4. 10.2169/internalmedicine.47.0854 [DOI] [PubMed] [Google Scholar]
- 10.Imazio M, Brucato A, Maestroni S, et al. Risk of constrictive pericarditis after acute pericarditis. Circulation 2011;124:1270–5. 10.1161/CIRCULATIONAHA.111.018580 [DOI] [PubMed] [Google Scholar]
- 11.Rudolph WW, Gunzer F, Trauth M, et al. Comparison of Vitek 2, MALDI-TOF MS, 16S rRNA gene sequencing, and whole-genome sequencing for identification of Roseomonas mucosa. Microb Pathog 2019;134:103576. 10.1016/j.micpath.2019.103576 [DOI] [PubMed] [Google Scholar]
- 12.Myles IA, Earland NJ, Anderson ED, et al. First-In-Human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 2018;3:e120608. 10.1172/jci.insight.120608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao S, Guo X, Guo P, et al. Roseomonas mucosa infective endocarditis in patient with systemic lupus erythematosus: case report and review of literature. BMC Infect Dis 2019;19:140. 10.1186/s12879-019-3774-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsukuma Y, Sugawara K, Shimano S, et al. A case of bacterial peritonitis caused by Roseomonas mucosa in a patient undergoing continuous ambulatory peritoneal dialysis. CEN Case Rep 2014;3:127–31. 10.1007/s13730-013-0101-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim K-Y, Hur J, Jo W, et al. Infectious spondylitis with bacteremia caused by Roseomonas mucosa in an immunocompetent patient. Infect Chemother 2015;47:194–6. 10.3947/ic.2015.47.3.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maraki S, Bantouna V, Lianoudakis E, et al. Roseomonas spinal epidural abscess complicating instrumented posterior lumbar interbody fusion. J Clin Microbiol 2013;51:2458–60. 10.1128/JCM.00512-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciofu O, Rojo-Molinero E, Macià MD, et al. Antibiotic treatment of biofilm infections. APMIS 2017;125:304–19. 10.1111/apm.12673 [DOI] [PubMed] [Google Scholar]
- 18.Koo HC, Kim YS, Kim SG, et al. Should colonoscopy be performed in patients with cryptogenic liver abscess? Clin Res Hepatol Gastroenterol 2013;37:86–92. 10.1016/j.clinre.2012.03.037 [DOI] [PubMed] [Google Scholar]
- 19.Fakhruddin N, Bahmad HF, Aridi T, et al. Hepatoid adenocarcinoma of the stomach: a challenging diagnostic and therapeutic disease through a case report and review of the literature. Front Med 2017;4:164. 10.3389/fmed.2017.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun B, Jain R, Cunningham J. Diagnosis of Hepatoid carcinoma of extrahepatic origins: cell markers and pathologic standards. BJSTR 2019;15:1–6. 10.26717/BJSTR.2019.15.002666 [DOI] [Google Scholar]
- 21.Wang L, Zhong Y, Sun L, et al. Clinical and pathological features of hepatoid carcinoma of the ovary. World J Surg Oncol 2013;11:29. 10.1186/1477-7819-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]