Abstract
Introduction
Total mesorectal excision (TME) and postoperative adjuvant chemotherapy following neoadjuvant chemoradiotherapy (CRT) is the standard treatment for locally advanced rectal cancer (LARC). However, neoadjuvant CRT has no recognised impact on reducing distant recurrence, and patients suffer from a long-lasting impairment in quality of life (QOL) associated with TME. Total neoadjuvant therapy (TNT) is an alternative approach that could reduce distant metastases and increase the proportion of patients who could safely undergo non-operative management (NOM). This study is designed to compare two TNT regimens in the context of NOM for selecting a more optimal regimen for patients with LARC.
Methods and analysis
NOMINATE trial is a prospective, multicentre, randomised phase II selection design study. Patients must have clinical stage II or III (T3-T4Nany) LARC with distal location (≤5 cm from the anal verge or for those who are candidates for abdominoperineal resection or intersphincteric resection). Patients will be randomised to either arm A consisting of CRT (50.4 Gy with capecitabine) followed by consolidation chemotherapy (six cycles of CapeOx), or arm B consisting of induction chemotherapy (three cycles of CapeOx plus bevacizumab) followed by CRT and consolidation chemotherapy (three cycles of CapeOx). In the case of clinical complete response (cCR) or near cCR, patients will progress to NOM. Response assessment involves a combination of digital rectal examination, endoscopy and MRI. The primary endpoint is the proportion of patients achieving pathological CR or cCR≥2 years, defined as the absence of local regrowth within 2 years after the start of NOM among eligible patients. Secondary endpoints include the cCR rate, near cCR rate, rate of NOM, overall survival, distant metastasis-free survival, locoregional failure-free survival, time to disease-related treatment failure, TME-free survival, permanent stoma-free survival, safety of the treatment, completion rate of the treatment and QOL. Allowing for a drop-out rate of 10%, 66 patients (33 per arm) from five institutions will be accrued.
Ethics and dissemination
The study protocol was approved by Wakayama Medical University Certified Review Board in December 2020. Trial results will be published in peer-reviewed international journals and on the jRCT website.
Trial registration number
jRCTs051200121
Keywords: chemotherapy, radiotherapy, colorectal surgery
Strength and limitations of this study.
This phase II study is the first study of total neoadjuvant therapy and non-operative management to compare the efficacy and safety of consolidation chemotherapy to sandwich chemotherapy using bevacizumab combined with capecitabine-based chemoradiotherapy for locally advanced rectal cancer.
This study includes clinical T3-T4NanyM0, mismatch repair-proficient rectal cancer with distal location.
The assessment of a clinical complete response and near clinical complete response will be performed based on predefined response criteria.
Patients treated with non-operative management will undergo intensive monitoring as per the follow-up protocol.
Confirmatory conclusions cannot be drawn from this randomised phase II study with relatively small sample size and a limited number of participating centres.
Introduction
The current standard treatment for locally advanced rectal cancer (LARC) is neoadjuvant chemoradiotherapy (CRT), total mesorectal excision (TME) and postoperative adjuvant chemotherapy. This multimodality treatment has significantly reduced local recurrence rates to <10%. However, neoadjuvant CRT has failed to reduce distant recurrence or improve disease-free survival (DFS) and overall survival (OS). Despite the adoption of adjuvant postoperative chemotherapy, distant relapse occurs in about 30% of patients at 5 years.1 The benefit of postoperative adjuvant chemotherapy following neoadjuvant CRT remains unclear, possibly due to poor compliance to chemotherapy, a longer interval between diagnosis and commencing chemotherapy and the application of suboptimal regimens.2 These limitations have led to the development of a total neoadjuvant therapy (TNT) approach, which delivers both radiotherapy and systemic chemotherapy preoperatively in an attempt to treat micrometastases earlier, increase adherence to systemic chemotherapy and improve DFS. Two recent phase III randomised controlled trials investigating TNT (RAPIDO3 and PRODIGE 23 trial)4 showed better pathological complete response (pCR) rate and fewer distant metastases in the TNT arm as compared with the standard short-course radiotherapy or CRT arm.
Numerous studies have shown that patients with a pCR have more favourable long-term oncological outcomes in terms of distant and local control,5 and this has raised the question as to whether TME can be avoided in patients with pCR. Because TME is associated with postoperative complications and late morbidity, such as bowel, sexual and urinary dysfunction,6 7 avoiding TME may provide an opportunity to reduce the morbidity and the need of a permanent stoma, and improve quality of life (QOL). In 2004, Habr-Gama et al for the first time proposed a watch-and-wait approach or non-operative management (NOM) for patients with a clinical complete response (cCR) after CRT.8 Since then, many studies—mainly retrospective observational studies—have shown NOM to be a feasible option for patients with cCR after CRT.9 10
TNT has the potential to increase the proportion of patients achieving cCR and thus being eligible for NOM;11 however, randomised trial data evaluating the efficacy of NOM in the context of TNT are lacking. OPRA was the first randomised phase II trial to address the efficacy of TNT and NOM for patients with cCR or near cCR, with the primary endpoint of 3 year DFS, as compared with standard historical controls managed with CRT and TME followed by adjuvant chemotherapy.12 In the OPRA trial, 306 patients with LARC were randomised to receive 4 months of 5-Fluorouracil (5-FU), Leucovorin and Oxaliplatin (FOLFOX) or Capecitabine and Oxaliplatin (CapeOx) either before (induction chemotherapy) or after (consolidation chemotherapy) CRT, followed by NOM for patients with cCR or near cCR. Preliminary analyses demonstrated higher 3-year organ preservation rates in the consolidation arm over the induction arm (59% vs 43%).13 Similarly, in the CAO/ARO/AIO-12 phase II trial, which randomly assigned patients to either induction or consolidation chemotherapy (three cycles of FOLFOX) before or after oxaliplatin-based CRT followed by TME, demonstrated higher pCR rates in the consolidation arm as compared with the induction arm (25% vs 17%).14 Given these results, CRT followed by consolidation chemotherapy may enable greater organ preservation, and should be preferentially considered.15
On the other hand, several studies have shown that the addition of antivascular endothelial growth factor drugs before radiotherapy can enhance the radiation response in LARC.16 17 In the GEMCAD 1402 randomised phase II trial of induction chemotherapy with 3 months of mFOLFOX6 with or without aflibercept followed by CRT and TME, patients in the aflibercept arm demonstrated a higher pCR rate than those without aflibercept (22.6% vs 13.8%).18 In a single-arm phase II trial of 3 months of mFOLFOX6 plus bevacizumab prior to CRT, we reported a pCR rate of 37% with favourable toxicity in a series of 43 patients with poor-risk LARC.19 Furthermore, a single-arm phase II study of sandwich-like neoadjuvant therapy consisting of one cycle of induction FOLFOX with bevacizumab, followed by CRT with three doses of bevacizumab, and one cycle of consolidation FOLFOX, also reported a high pCR rate (39.1%).20 Given these results, we hypothesised that sandwich-like therapy of three cycles of induction chemotherapy with bevacizumab and three cycles of consolidation chemotherapy could provide the advantages of both induction (addressing micrometastatic disease earlier and enhanced CRT response by bevacizumab) and consolidation (greater pCR or NOM rate) therapy arms. We chose long-course CRT because there was limited data on the use of short-course radiotherapy in NOM.21
To this end, we designed this randomised phase II trial (NOMINATE trial) of TNT and NOM to compare the efficacy and safety of consolidation chemotherapy (six cycles of CapeOx) to a sandwich chemotherapy regimen using bevacizumab (three cycles of CapeOx plus bevacizumab as induction chemotherapy and three cycles of CapeOx as consolidation chemotherapy) combined with capecitabine-based CRT.
Methods and analysis
Study design
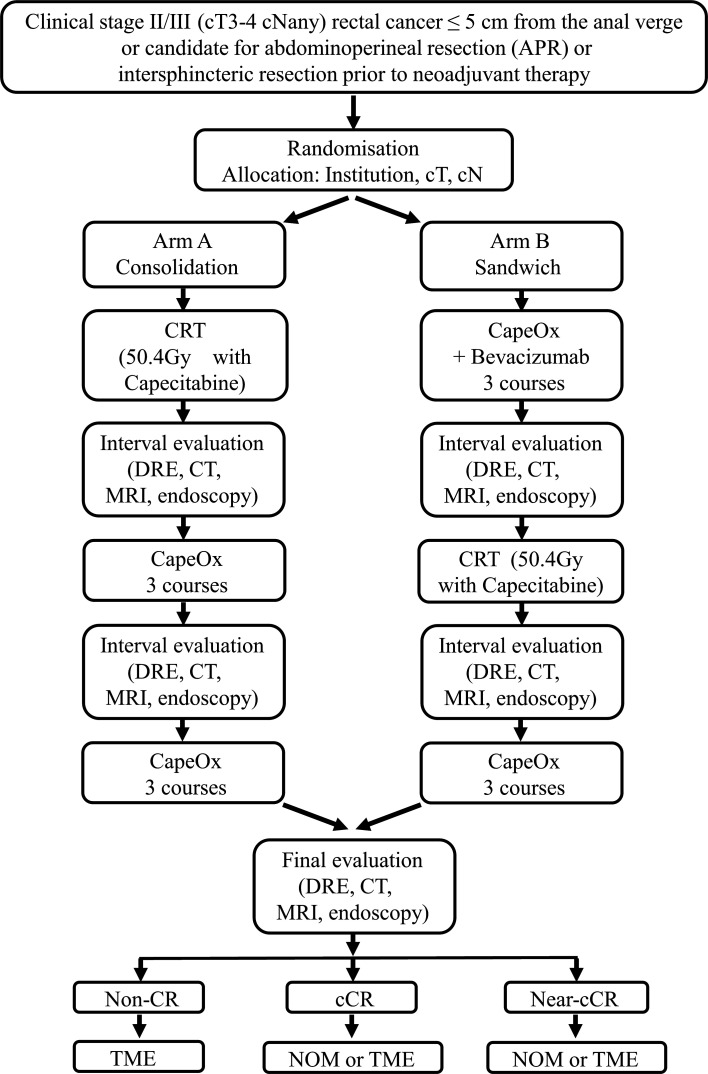
This is a prospective, multicentre, randomised phase II selection design study to compare two TNT regimens in the context of NOM for selecting a more optimal regimen for patients with LARC. The study flowchart is shown in figure 1.
Figure 1.
Study flowchart. cCR, clinical complete response; CRT, chemoradiotherapy; DRE, digital rectal examination; NOM, non-operative management; non-CR, non-complete response; TME, total mesorectal excision.
Primary endpoint
The primary endpoint is the proportion of patients achieving pCR or cCR ≥2 years among eligible patients. pCR is defined as no residual tumour cells in the surgical specimen. cCR ≥2 years is defined as the absence of local regrowth within 2 years after the start of NOM.
Secondary endpoints
Secondary endpoints include cCR rate, near cCR rate, rate of NOM, OS, distant metastasis-free survival, locoregional failure-free survival, time to disease-related treatment failure, TME-free survival, permanent stoma-free survival, safety of the treatment, completion rate of the treatment, faecal incontinence according to Wexner score22 and Low Anterior Resection Syndrome (LARS)-scale,23 and QOL according to European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)—C3024 and CR29.25 Locoregional failure includes progressive unresectable disease, local R2 resection and intrapelvic recurrence after TME. Local regrowth after NOM is not considered as locoregional failure when followed by an R0/R1 resection. Disease-related treatment failure is defined as the first occurrence of locoregional failure, distant metastasis, a new primary colorectal cancer or treatment-related death.3 In patients managed by TME, surgical morbidity, R0 resection rate, pathological stage, Dworak tumour regression grade26 will also be assessed. In patients managed by NOM, local regrowth rate, time to local regrowth, salvage surgery rate in patients with local regrowth, surgical morbidity in salvage surgery and R0 resection rate in salvage surgery will also be assessed. The grade of adverse events will be assessed according to Common Terminology Criteria for Adverse Events (CTCAE) V.5.0.
Eligibility criteria
Inclusion criteria
Histologically confirmed diagnosis of adenocarcinoma of the rectum.
The lowest part of the tumour ≤5 cm from the anal verge or patient is a candidate for abdominoperineal resection or intersphincteric resection prior to neoadjuvant therapy according to the primary surgeon.
Patients must have clinical stage II (cT3-4N0) or stage III (cT3-4N1-3) by MRI and CT.
Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0 or 1.
Age≥20 years.
Adequate organ functions within 28 days prior to entry: neutrophils≥1500/mm3, platelets≥10 × 104/mm3, haemoglobin≥9.0 g/dL, total bilirubin≤2.0 mg/dL, aspartate aminotransferase≤100 IU/L, alanine aminotransferase≤100 IU/L, serum creatinine≤1.5 mg/dL or Ccr≥60 mL/min/body, urine protein/creatinine<1.
If there is bowel obstruction or significant stricture, stoma is constructed prior to neoadjuvant therapy.
Written informed consent is obtained.
Exclusion criteria
Patients with a history of a prior malignancy within the past 5 years, except for adequately treated cancer with 5-year relative survival rate≥95%.
History of pelvic irradiation.
Administration contraindication of capecitabine, oxaliplatin, or bevacizumab.
Uncontrolled active infection.
Body temperature≥38°C at registration.
Possibly pregnant, pregnant, or nursing.
Patients with concurrent psychiatric condition or disease that would make them inappropriate candidates for entry into this study in the investigator’s judgement.
Patients with concurrent serious comorbidity (heart failure, interstitial lung disease or pulmonary fibrosis, uncontrolled diabetes, renal failure, liver failure, hypertension, thrombotic disease, gastrointestinal fistula, among other similarly serious conditions).
History of operation≤4 weeks ago or minor operation such as stoma construction≤2 weeks ago.
Deficient in mismatch repair, as determined by immunohistochemistry and/or microsatellite instability testing using pre-treatment biopsy specimens.
Other conditions not suitable for this study in the investigator’s judgement.
Sample size calculation
This study uses a ‘pick the winner’ format proposed by Simon et al.27 The expected lowest response rate of pCR or cCR≥2 years is set at 25%.28 If the difference in response rate between the treatment arms is at least 10%, 30 patients per arm (total 60 patients) is necessary to select the better treatment with a probability of≥80%. With consideration for dropouts of 10%, 33 patients per arm (total 66 patients) will be necessary. If there are no differences in response rate between treatment arms, a better treatment will be chosen in terms of secondary endpoints such as toxicity and safety of the treatment.
Registration and randomisation
Patients are registered to the study after confirming the eligibility criteria and written informed consent is obtained. Patients are requested to fill out EORTC QLQ-C30, CR29 and a questionnaire about faecal incontinence (Wexner score and LARS scale) at registration. After registration, patients are randomly assigned at a 1:1 ratio to the consolidation arm (arm A) or sandwich arm (arm B) using a minimisation method stratified by institution, cT (cT3 vs cT4) and cN (cN- vs cN+). Registration, randomisation and collection of patient information will be performed using the Viedoc electronic data capture system. Data are anonymised using a unique patient identification number.
Treatment
Arm A consists of CRT (50.4 Gy in 28 fractions and capecitabine 825 mg/m2 twice daily, days 1–5, 8–12, 15–19, 22–26, 29–33, 36–38) followed by consolidation chemotherapy (six cycles of CapeOx: capecitabine 2000 mg/m2/day, days 1–14, oxaliplatin 130 mg/m2, day 1, Q3w). Consolidation chemotherapy should start at 3–8 weeks after the last day of radiotherapy. Arm B consists of induction chemotherapy (three cycles of CapeOx plus bevacizumab: capecitabine 2000 mg/m2/day, days 1–14, oxaliplatin 130 mg/m2, day 1, bevacizumab 7.5 mg/kg, day 1, Q3w) followed by CRT (same as in arm A) followed by consolidation chemotherapy (three cycles of CapeOx). CRT should start at 3–6 weeks after the last day of induction chemotherapy, and consolidation chemotherapy should start at 3–8 weeks after the last day of radiotherapy.
Response assessment
Interval evaluation will be performed twice: after the completion of CRT and after the completion of three cycles of consolidation chemotherapy in arm A, and after the completion of induction chemotherapy and after the completion of CRT in arm B. Final response assessment will be performed at 4 (- 1/+4) weeks after the completion of all neoadjuvant treatments. In the case of cCR or near cCR, patients will progress to NOM, but TME is also permitted if patients hope to undergo radical surgery. In the case of non-CR, patients will progress to TME. Criteria for response assessment are shown in table 1.12 29–32 Final response assessment involves a combination of digital rectal examination, endoscopy and MRI, and will be discussed at online multidisciplinary-team meetings attended by the principal investigator and the local investigators.
Table 1.
Criteria for response assessment
| cCR | Near cCR | Non-CR | |
| Endoscopy | |||
| WL-C | |||
| Ulcer | Closed | Closed | Open |
| Scar | Linear and flat (white) | Irregular surface (reddish) | Incompletely closed ulcer, residual erosion or white moss |
| Protruded tumour nodule | No | No | Yes |
| Wall extension | Normal | Decreased | Poor with submucosal tumour-like deformity |
| ME | |||
| Vessel pattern (NBI) | Regular circulated/lacy | Lack of uniformity | Calibre change/irregularity |
| Surface pattern (chromoendoscopy) |
Uniformly arranged regeneration pits or hypercellular pits | Regenerated pits irregularly arranged | Residual neoplastic pit pattern |
| DRE | |||
| Normal | Smooth induration or minor mucosal abnormalities | Tumour nodules palpable | |
| MRI | |||
| T2WI | |||
| Tumour bed | Normalised rectal wall or no residual intermediate signal in the tumour bed and fibrotic hypointense signal | Residual intermediate tumour signal (regardless of the percentage of fibrotic hypointense signal) | |
| Lymph node | Downsizing of involved lymph nodes to a short-axis diameter<5 mm | Partial or no regression of involved lymph nodes with a short-axis diameter≥5 mm | |
| DWI (b800 or b1000 images) | |||
| Tumour bed | No high signal on high b-value images and no low ADC signal in the tumour bed | Presence of high signal on high b-value images and low ADC signal in the tumour bed | |
ADC, apparent diffusion coefficient; cCR, clinical complete response; DRE, digital rectal examination; DWI, diffusion-weighted images; ME, magnifying endoscopy; NBI, narrow-band imaging; near cCR, near clinical complete response; non-CR, non-complete response; T2WI, T2-weighted images; WL-C, white light conventional endoscopy.
Follow-up
Patients treated with TME will be followed with measurements of serum carcinoembryonic antigen and carbohydrate 19–9 and will be subjected to chest/abdomen/pelvis CT scan every 6 months for 5 years. Patients treated with NOM will be followed every 3 months for the first 2 years and every 6 months thereafter, as shown in table 2. In the case of near cCR, patients will be followed every 6–8 weeks for the first 6 months. Salvage TME will be recommended for patients with local regrowth after NOM; if the patient refuses TME, local resection will also be acceptable. If a patient refuses surgical resection of local regrowth, it is considered as locoregional failure.
Table 2.
Follow-up protocol for non-operative management
| Time from final response assessment | Tumour marker* | DRE | MRI† | CT‡ | Endoscopy | Adverse events | PROM§ |
| 3 months¶ | x | x | x | Rectum | x | ||
| 6 months | x | x | x | x | Rectum | x | x |
| 9 months | x | x | x | Rectum | x | ||
| 1 year | x | x | x | x | Total | x | x |
| 1 year 3 months | x | x | x | Rectum | x | ||
| 1 year 6 months | x | x | x | x | Rectum | x | |
| 1 year 9 months | x | x | x | Rectum | x | ||
| 2 years | x | x | x | x | Rectum | x | x |
| 2 years 6 months | x | x | x | x | Rectum | x | |
| 3 years | x | x | x | x | Total | x | x |
| 3 years 6 months | x | x | x | x | Rectum | x | |
| 4 years | x | x | x | x | Rectum | x | |
| 4 years 6 months | x | x | x | x | Rectum | x | |
| 5 years | x | x | x | x | Total | x |
*Tumour marker includes serum carcinoembryonic antigen and carbohydrate 19–9.
†MRI includes pelvic MRI.
‡CT includes chest/abdomen/pelvis CT.
§PROM includes EORTC QLQ—C30 and CR29, Wexner score and LARS-scale.
¶Near cCR patients will be followed every 6–8 weeks for the first 6 months.
cCR, clinical complete response; DRE, digital rectal examination; EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; LARS, Low Anterior Resection Syndrome; PROM, patient-reported outcome measure.
Statistical analysis plan
The primary analysis will be conducted when 3 years have passed since patient accrual completion. All analyses are based on descriptive data without testing because of the study design. The proportions at the primary endpoint will be estimated using an Clopper-Pearson method for binomial response. The proportions among registered patients or patients who complete the protocol treatment will also be calculated as a reference. An analysis of secondary endpoints will also be performed to complement the results at the primary endpoint, but adjustment of multiplicity will not be performed due to their exploratory nature. Kaplan–Meier method will be used to estimate OS, with 95% CIs calculated by Greenwood’s formula. Univariate Cox regression will be used to estimate HRs with 95%CIs associated with treatment arms. For endpoints with competing risk, such as distant metastasis-free survival, locoregional failure-free survival and an estimation of HRs will be performed using Fine and Grey models. The final analysis will be conducted when 6 years have passed since patient accrual completion.
Interim analysis and monitoring
Interim analysis is planned for possible early trial termination to claim futility. In Simon’s optimal two-stage design,33 when the null hypothesis is a pCR or cCR/near cCR rate of 15% versus the alternative of 30% for each arm, and power and one-sided alpha are set at 80% and 5%, respectively, 19 subjects will be accrued in the first stage. If there are three or fewer responders within these 19 subjects, enrolment in that arm will be stopped. If the number of treatment-related deaths reach two for each arm, the registration will be suspended until Data and Safety Monitoring approve the continuation of the trial. The Data Centre (Clinical Research and Medical Development Centre, Cancer Institute Hospital, Japanese Foundation for Cancer Research) will perform central monitoring every 6 months and monitoring reports will be submitted to the Data and Safety Monitoring Committee.
Translational research
Accompanying translational research about the molecular determinants of response to TNT and molecular predictors of successful organ preservation is planned. The specific study protocol for correlative translational research to the NOMINATE trial has been approved by the intuitional review boards of all participating institutions. Tumour tissue and plasma will be collected and stored at different time points after obtaining written informed consent from patients. Next-generation sequencing, such as exome sequencing, RNA sequencing, and circulating tumour DNA analysis, will be performed.
Ethics and dissemination
Wakayama Medical University Certified Review Board approved this study protocol in December 2020. The first patient was enrolled in March 2021, and the estimated study completion date is November 2030. This trial will be performed in accordance with the Declaration of Helsinki and Clinical Trials Act in Japan. Trial results of the primary and secondary endpoints will be published in peer-reviewed international journals and on the jRCT website (https://jrct.niph.go.jp/), as well as at international and national conferences.
Supplementary Material
Footnotes
Contributors: TA, ES, ST and TK developed the trial concept, wrote the protocol and drafted the manuscript. NI designed the statistical analyses for the study. AC, MH, TT, TN, ST, ShuM, ShiM, KO, TM, YH, TY, TN, KY, MU, HK and YF have made substantial contributions to the conception and design of the work and subsequent protocol revisions. TA submitted the study.
Funding: This study is funded by Japan Surgical Society Clinical Investigation Project Award.
Competing interests: KY reports honoraria from Chugai Co., Ltd.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Breugom AJ, Swets M, Bosset J-F, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 2015;16:200–7. 10.1016/S1470-2045(14)71199-4 [DOI] [PubMed] [Google Scholar]
- 2. Carvalho C, Glynne-Jones R. Challenges behind proving efficacy of adjuvant chemotherapy after preoperative chemoradiation for rectal cancer. Lancet Oncol 2017;18:e354–63. 10.1016/S1470-2045(17)30346-7 [DOI] [PubMed] [Google Scholar]
- 3. Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-Course radiotherapy followed by chemotherapy before total mesorectal excision (Tme) versus preoperative chemoradiotherapy, Tme, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:29–42. 10.1016/S1470-2045(20)30555-6 [DOI] [PubMed] [Google Scholar]
- 4. Conroy T, Bosset J-F, Etienne P-L, et al. Neoadjuvant chemotherapy with Folfirinox and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:702–15. 10.1016/S1470-2045(21)00079-6 [DOI] [PubMed] [Google Scholar]
- 5. Maas M, Nelemans PJ, Valentini V, et al. Long-Term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835–44. 10.1016/S1470-2045(10)70172-8 [DOI] [PubMed] [Google Scholar]
- 6. Matsubara N, Miyata H, Gotoh M, et al. Mortality after common rectal surgery in Japan: a study on low anterior resection from a newly established nationwide large-scale clinical database. Dis Colon Rectum 2014;57:1075–81. 10.1097/DCR.0000000000000176 [DOI] [PubMed] [Google Scholar]
- 7. Honda M, Akiyoshi T, Noma H, et al. Patient-Centered outcomes to decide treatment strategy for patients with low rectal cancer. J Surg Oncol 2016;114:630–6. 10.1002/jso.24376 [DOI] [PubMed] [Google Scholar]
- 8. Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus Nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy. Ann Surg 2004;240:711–8. 10.1097/01.sla.0000141194.27992.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 2018;391:2537–45. 10.1016/S0140-6736(18)31078-X [DOI] [PubMed] [Google Scholar]
- 10. Renehan AG, Malcomson L, Emsley R, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol 2016;17:174–83. 10.1016/S1470-2045(15)00467-2 [DOI] [PubMed] [Google Scholar]
- 11. Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol 2018;4:e180071. 10.1001/jamaoncol.2018.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith JJ, Chow OS, Gollub MJ, et al. Organ preservation in rectal adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer 2015;15:767. 10.1186/s12885-015-1632-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia-Aguilar J, Patil S, Kim JK, et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. JCO 2020;38:4008. 10.1200/JCO.2020.38.15_suppl.4008 [DOI] [Google Scholar]
- 14. Fokas E, Allgäuer M, Polat B, et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. JCO 2019;37:3212–22. 10.1200/JCO.19.00308 [DOI] [PubMed] [Google Scholar]
- 15. Shi DD, Mamon HJ. Playing with Dynamite? A cautious assessment of TnT. J Clin Oncol 2021;39:103–6. 10.1200/JCO.20.02199 [DOI] [PubMed] [Google Scholar]
- 16. Zhou Y, Guo Z, Wu Z, et al. The efficacy and safety of adding bevacizumab in neoadjuvant therapy for locally advanced rectal cancer patients: a systematic review and meta-analysis. Transl Oncol 2021;14:100964. 10.1016/j.tranon.2020.100964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhong X, Wu Z, Gao P, et al. The efficacy of adding targeted agents to neoadjuvant therapy for locally advanced rectal cancer patients: a meta-analysis. Cancer Med 2018;7:565–82. 10.1002/cam4.1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fernández-Martos C, Pericay C, Losa F, et al. Effect of aflibercept plus modified FOLFOX6 induction chemotherapy before standard chemoradiotherapy and surgery in patients with high-risk rectal adenocarcinoma: the GEMCAD 1402 randomized clinical trial. JAMA Oncol 2019;5:1566–73. 10.1001/jamaoncol.2019.2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Konishi T, Shinozaki E, Murofushi K, et al. Phase II trial of neoadjuvant chemotherapy, chemoradiotherapy, and laparoscopic surgery with selective lateral node dissection for poor-risk low rectal cancer. Ann Surg Oncol 2019;26:2507–13. 10.1245/s10434-019-07342-7 [DOI] [PubMed] [Google Scholar]
- 20. Xiao J, Chen Z, Li W, et al. Sandwich-like neoadjuvant therapy with bevacizumab for locally advanced rectal cancer: a phase II trial. Cancer Chemother Pharmacol 2015;76:21–7. 10.1007/s00280-015-2763-2 [DOI] [PubMed] [Google Scholar]
- 21. Yuval JB, Thompson HM, Garcia-Aguilar J. Organ preservation in rectal cancer. J Gastrointest Surg 2020;24:10.1007/s11605-020-04583-w:1880–8. 10.1007/s11605-020-04583-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum 1993;36:77–97. 10.1007/BF02050307 [DOI] [PubMed] [Google Scholar]
- 23. Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg 2012;255:922–8. 10.1097/SLA.0b013e31824f1c21 [DOI] [PubMed] [Google Scholar]
- 24. Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 25. Whistance RN, Conroy T, Chie W, et al. Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer 2009;45:3017–26. 10.1016/j.ejca.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 26. Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997;12:19–23. 10.1007/s003840050072 [DOI] [PubMed] [Google Scholar]
- 27. Simon R, Wittes RE, Ellenberg SS. Randomized phase II clinical trials. Cancer Treat Rep 1985;69:1375–81. [PubMed] [Google Scholar]
- 28. Petrelli F, Trevisan F, Cabiddu M, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg 2020;271:440–8. 10.1097/SLA.0000000000003471 [DOI] [PubMed] [Google Scholar]
- 29. Chino A, Konishi T, Ogura A, et al. Endoscopic criteria to evaluate tumor response of rectal cancer to neoadjuvant chemoradiotherapy using magnifying chromoendoscopy. Eur J Surg Oncol 2018;44:1247–53. 10.1016/j.ejso.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 30. Ishioka M, Chino A, Ide D, et al. Adding narrow-band imaging to Chromoendoscopy for the evaluation of tumor response to neoadjuvant therapy in rectal cancer. Dis Colon Rectum 2021;64:10.1097/DCR.0000000000001699:53–9. 10.1097/DCR.0000000000001699 [DOI] [PubMed] [Google Scholar]
- 31. Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of gastrointestinal and abdominal radiology (ESGAR) consensus meeting. Eur Radiol 2018;28:1465–75. 10.1007/s00330-017-5026-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maas M, Lambregts DMJ, Nelemans PJ, et al. Assessment of clinical complete response after chemoradiation for rectal cancer with digital rectal examination, endoscopy, and MRI: selection for Organ-Saving treatment. Ann Surg Oncol 2015;22:3873–80. 10.1245/s10434-015-4687-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10. 10.1016/0197-2456(89)90015-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.