Abstract
There have been several reports that convulsions, although rare, occur in patients who receive fluoroquinolones. In this study, the proconvulsant effects exhibited by a novel series of 6-desfluoroquinolones and some classic quinolones on pentylenetetrazole (PTZ)-induced seizures in mice were evaluated and compared. Animals were intraperitoneally injected with vehicle or quinolone derivatives (5 to 100 μg/g of body weight) 30 min before the subcutaneous (s.c.) administration of PTZ (40 μg/g). In each experiment, mice were then observed for 1 h to monitor for the incidence and onset of clonic seizures. The order of proconvulsant activity in our epileptic model was MF5184 > MF5187 > pefloxacin > MF5189 > ofloxacin > ciprofloxacin > MF5140 > MF5181 > MF5137 > rufloxacin > MF5143 > MF5158 > MF5191 > MF5128 > MF5138 > cinoxacin > MF5142 > norfloxacin > nalidixic acid. The relationship between the chemical structure and the proconvulsant activity of 6-desfluoroquinolone derivatives was studied. We observed that, in terms of toxicity to the central nervous system (CNS), besides the heterocyclic side chain (moiety) at the C-7 position, the C-6 substituent also appears to play an important role. In particular, a hydrogen at the C-6 position seemed to be responsible for major neurotoxic activity in comparison to an amino group located in the same position. The relationship between lipophilicity and proconvulsant activity was also investigated. We did not find any clear relationship between a higher level of lipophilicity and major proconvulsant properties. Although the principal mechanism by which quinolones induce potentiation of the proconvulsant effects of PTZ cannot be easily determined, it is possible that the convulsions are caused by drug interactions, because both PTZ and quinolones are believed to increase excitation of the CNS by inhibition of γ-aminobutyric acid binding to receptors.
Because of their excellent activities, quinolone antibacterial agents have been widely adopted for use in clinical practice. Progress in the development of new quinolones came with the introduction of a fluorine atom at the C-6 position on the parent nuclei. This modification, with the concomitant presence of an appropriate base at C-7, yielded compounds with enhanced antibacterial activity in comparison with the activities of the predecessors (8). Although the precise role of the fluorine atom at C-6 has never been clearly defined, it continues to be a fundamental structural feature of all synthesized analogues (fluoroquinolones) which have been marketed recently or are currently undergoing clinical development. In the continuing search for new, potent quinolone derivatives, recently a new series of quinolones (6-desfluoroquinolones), in which the usual fluorine atom at the C-6 position was excluded or replaced by an amino group, were synthesized (4–6). We now want to determine if this modification, besides providing a higher level of antibacterial activity, is able to reduce the side effects on the central nervous system (CNS) that characterize the fluoroquinolone class. In fact, several clinical observations indicated the possible incidence of undesirable adverse reactions and drug interactions following quinolone use (3, 7, 21, 24, 31) and many attempts to make quinolones with minimized side effects have been made, but without proved results up to now. Among various adverse effects on the CNS, convulsions remain a serious problem. In fact, convulsions have been reported more frequently for individuals predisposed to epileptic seizures and for patients who have received both fluoroquinolones and either theophylline or certain nonsteroidal anti-inflammatory drugs (3, 7, 21, 24, 31). In previous studies, members of our team described a convulsant behavioral pattern following the concomitant administration of quinolones and theophylline in a particular strain of rats, namely, genetically epilepsy-prone rats (11, 13). In addition, members of our team demonstrated that quinolones potentiate the convulsant effects elicited by cefazolin or imipenem in mice (12, 14). It is well-known that quinolones competitively inhibit in vitro the binding of [3H]γ-aminobutyric acid ([3H]GABA), [3H]muscimol, and [3H]diazepam to benzodiazepine (BDZ)-GABA receptors in postsynaptic membranes in a concentration-dependent manner (2, 30, 35, 36). However, as GABA antagonists, quinolones could be expected to be proconvulsant when given to animals in combination with an agent that blocks GABA-mediated inhibition, such as pentylenetetrazole (PTZ) (28, 33). In a recent study members of our team also demonstrated that drugs both enhancing GABAergic transmission and inhibiting excitatory amino acid transmission are able to antagonize seizures induced by pefloxacin (PFX) in mice (15). In an attempt to clarify the relationship between chemical structure and epileptogenic activity, in the present study we examined the proconvulsant properties of a selected set of desfluoroquinolone derivatives (MF) and compared their effects with those of classic quinolones, such as PFX, ciprofloxacin (CIP), ofloxacin (OFX), norfloxacin (NOR), rufloxacin (RFX), cinoxacin (CIN), and nalidixic acid (NAL) on a subconvulsant dose of PTZ in mice. While toxicity to the CNS appears to be primarily influenced by the nature of the C-7 substituent (2, 19), nothing is known about the possible role played by the C-6 substituent, because almost all of the clinically useful quinolones are 6-fluoroquinolones.
MATERIALS AND METHODS
Animals.
Male ICR mice (20 to 24 g, 42 to 48 days old) were purchased from Nossan (Correzzana, Milano, Italy) and, during a quarantine period lasting at least 10 days, housed in wire-mesh cages and kept in an air-conditioned room (temperature, 23 ± 2°C; humidity, 55% ± 15%; light cycle, 12 h/day). They were allowed free access to commercial feed (Nossan) and tap water and were acclimated to laboratory conditions until the experiments were carried out. After the quarantine period, mice were randomly assigned to experimental groups (10 mice for each dose).
Procedures involving animals and their care were carried out in conformity with institutional guidelines and the policy and legal directives of the European Communities Council.
General epileptic behavior.
A group of mice (control) was intraperitoneally (i.p.) injected with vehicle (see “drugs” below), and 30 min later the animals were subcutaneously (s.c.) injected with different doses (10 to 90 μg/g of body weight) of PTZ. Different groups of mice were i.p. injected with different doses (5 to 100 μg/g) of PFX, CIP, OFX, NOR, RFX, CIN, NAL, and MF derivatives (MF5137, MF5138, MF5158, MF5191, MF5142, MF5128, MF5143, MF5140, MF5181, MF5187, MF5189, or MF5184), and 30 min later the animals were again injected with PTZ (40 μg/g, s.c.). Animals were placed in a Plexiglas box (0.04 by 0.04 by 0.03 m) and observed over the following 1 or 2 h for the incidence and onset of clonic convulsions. Ten mice were used for each dose level studied. The intensity of seizure response was scored on the following scale: 0, no response; 1, wild running; 2, clonus; 3, tonus; and 4, respiratory arrest (12). At the end of experiments, animals which showed seizures were euthanatized with diethyl ether anesthesia.
ECoG analysis.
Electrocortical (ECoG) activity was recorded (8-channel ECoG machine; OTE Biomedica, Florence, Italy) through four permanently implanted steel screw electrodes, inserted bilaterally into the frontoparietal area. At least three mice treated with the largest dose of vehicle plus PTZ and some treated with a quinolone derivative (PFX, MF5184, or MF5137) plus PTZ were studied for changes in ECoG activity.
Lipophilicity measurements.
The partition coefficient values, log P (octanol/water), were calculated by MedChem software release 3.5 (10) and are listed as clog P values in Tables 1 and 2.
TABLE 1.
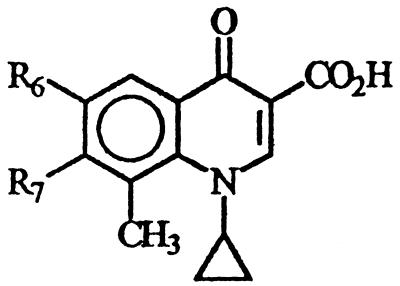
Chemical structure, convulsant doses, molecular weights, and partition coefficient values of 6-desfluoroquinolone derivatives tested in ICR mice
| Compound | R6 | R7 | CD50a (95% confidence interval)
|
Mol wt | clog Pb | |
|---|---|---|---|---|---|---|
| μg/g | nmol/g | |||||
| MF-5128 | NH2 | 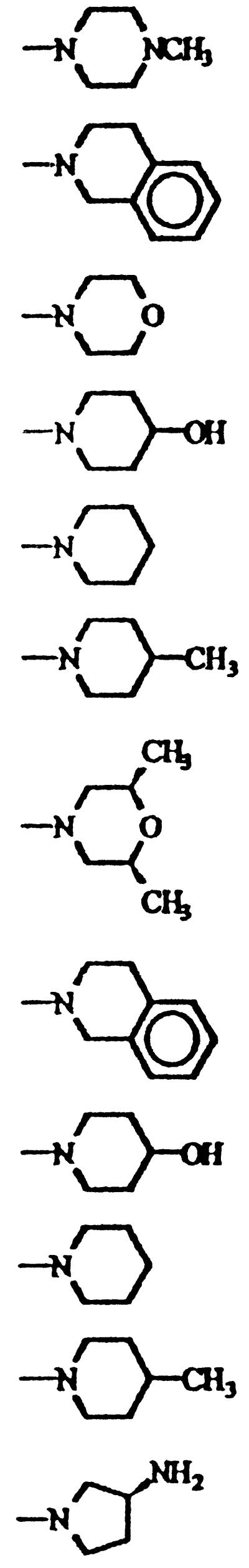 |
55.3 (29.9–102.1) | 128.8 | 429.35 | −0.695 |
| MF-5137 | NH2 | 37.2 (25.3–49.3) | 95.5 | 389.45 | 3.305 | |
| MF-5138 | NH2 | 50.8 (33.7–76.6) | 133.7 | 379.84 | 0.951 | |
| MF-5140 | NH2 | 35.1 (18.7–65.9) | 89.1 | 393.87 | 0.508 | |
| MF-5143 | NH2 | 40.2 (12.5–129.3) | 106.4 | 377.87 | 2.595 | |
| MF-5142 | NH2 | 52.8 (33.2–84.0) | 134.7 | 391.90 | 3.114 | |
| MF-5158 | NH2 | 46.7 (30.6–71.2) | 125.7 | 371.44 | 1.989 | |
| MF-5184 | H | 10.1 (5.3–19.2) | 27.0 | 377.44 | 4.373 | |
| MF-5187 | H | 16.3 (8.6–30.7) | 47.6 | 342.39 | 1.341 | |
| MF-5181 | H | 30.5 (15.7–59.2) | 93.4 | 326.40 | 3.663 | |
| MF-5191 | H | 43.3 (30.4–61.7) | 127.2 | 340.42 | 3.947 | |
| MF-5189 | H | 28.5 (21.6–37.6) | 73.6 | 387.18 | −0.820 | |
TABLE 2.
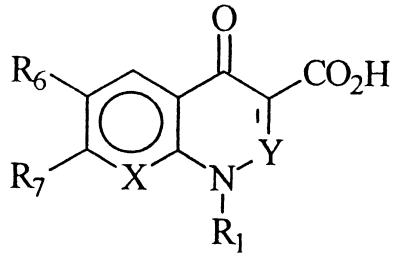
Chemical structure, convulsant doses, molecular weights, and partition coefficient values of classic quinolone derivatives tested in ICR mice
| Compound | R1 | X | Y | R6 | R7 | CD50a (95% confidence interval)
|
Mol wt | clog Pb | |
|---|---|---|---|---|---|---|---|---|---|
| μg/g | nmol/g | ||||||||
| CIN | C2H5 | CH | N | OCH2O | 35.1 (16.6–74.2) | 133.8 | 262.22 | 0.259 | |
| CIP | c-C3H5 | CH | CH | F | 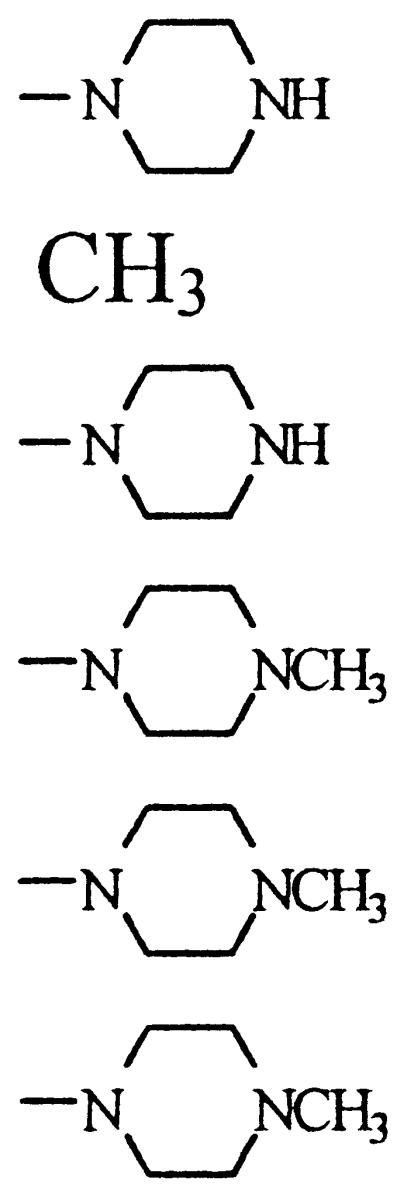 |
33.0 (19.8–54.9) | 85.5 | 385.82 | −0.805 |
| NAL | C2H5 | N | CH | H | 55.8 (40.6–76.7) | 240.3 | 232.24 | 0.909 | |
| NOR | C2H5 | CH | CH | F | 52.8 (33.7–76.6) | 165.3 | 319.34 | −0.630 | |
| OFX | OCHCH(CH3)CH2OCH | CH | F | 28.9 (15.6–55.6) | 80.0 | 361.37 | −0.097 | ||
| PFX | C2H5 | CH | CH | F | 22.5 (13.8–36.5) | 48.3 | 465.49 | 0.225 | |
| RFX | OCHCH2CH2SCH | CH | F | 39.2 (25.4–60.5) | 98.03 | 399.87 | −0.062 | ||
Statistical analyses.
Statistical comparisons among groups of control and drug-treated animals were made by using Fisher’s exact probability test (incidence of the seizure phases) or the Mann-Whitney U test (median seizure score ± interquartile range). The incidence (as a percentage) of each seizure phase was determined for each dose of quinolone derivative administered. The values were plotted against the corresponding doses by a computer construction of the dose-response curves to calculate the convulsant dose for 50% of the animals (CD50). CD50s (with 95% confidence limits) for each compound and each phase of seizure response were estimated according to the method of Litchfield and Wilcoxon (23) and were determined in both nanomoles per gram and micrograms per gram. The relative convulsant activities were determined by comparison of respective CD50s. At least 40 animals were used to calculate each CD50. For some compounds statistical differences in seizure latencies were studied by Bonferroni’s corrected Student’s t test. The obtained CD50s were plotted as a function of the clog P values by linear regression analysis performed by means of the Microcal Origin program (25).
Drugs.
PFX mesylate dihydrate was purchased from Rhone-Poulenc Pharma S.p.A. (Milano, Italy), CIN was purchased from Eli Lilly & Co (Indianapolis, Ind.), CIP hydrochloride monohydrate was purchased from Bayer (Leverkusen, Germany), OFX was purchased from Sigma Tau Laboratories (Pomezia, Italy), NOR was purchased from I.S.F. Laboratories (Trezzano S/N Milano, Italy), RFX hydrochloride was purchased from Mediolanum Farmaceutici (Milano, Italy), and NAL and PTZ were purchased from Sigma (Milano, Italy). PTZ, PFX mesylate, RFX hydrochloride, and CIP hydrochloride were dissolved in sterile saline, while CIN, NAL, OFX, and NOR were dissolved in 0.1 N NaOH and sterile saline. MF5128 dihydrochloride, MF5137, MF5138 hydrochloride, MF5140 hydrochloride, MF5143 hydrochloride, MF5142 hydrochloride, MF5158, MF5184, MF5187, MF5181, MF5191, and MF5189 acetate were synthesized at the Institute of Chemistry and Technology of Drug (University of Perugia, Perugia, Italy) and purchased from Mediolanum Farmaceutici. They were dissolved in a 50:50 solution of dimethylsulfoxide and 5% carboxymethylcellulose. All drug solutions were prepared immediately before injection (volume, 0.1 ml per 10 g of body weight).
RESULTS
During a search for new antibacterial agents, new 6-desfluoroquinolones derivatives were selected for study. All compounds are characterized by a cyclopropyl group at the N-1 position, a methyl group at the C-8 position, and different heterocyclic bases at the C-7 position. In addition, MF5128, MF5137, MF5138, MF5140, MF5143, MF5142, and MF5158 bear an amino group at the C-6 position (and so are collectively called 6-amino derivatives), while MF5184, MF5187, MF5181, MF5191, and MF5189 have a hydrogen in the same position (and thus are collectively called 6-hydrogen derivatives). Their chemical structures are shown in Table 1, while those of classic quinolone derivatives tested in the present study are shown in Table 2.
General epileptic behavior.
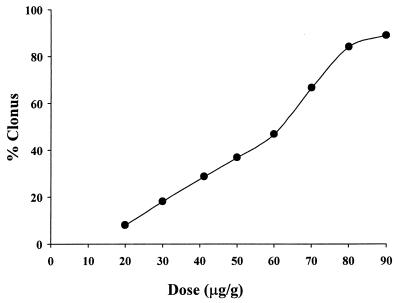
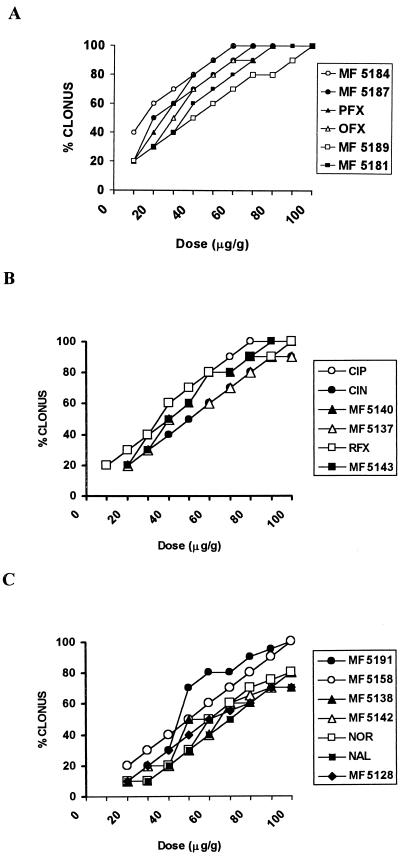
The intensity of seizures after single injections of different doses of PTZ (10 to 90 μg/g, s.c.) in ICR mice is shown in Fig. 1, while the effects of a combined treatment with several doses of quinolone derivatives (5 to 100 μg/g) and a subconvulsant dose of PTZ (40 μg/g, s.c.) are shown in Fig. 2 (10 mice were used for each dose studied). The relative CD50s (with 95% confidence limits) of vehicle plus PTZ and different quinolones plus PTZ are reported in Tables 1 and 2. The rank order for the proconvulsant potency of the compounds tested was MF5184 > MF5187 > PFX > MF5189 > OFX > CIP > MF5140 > MF5181 > MF5137 > RFX > MF5143 > MF5158 > MF5191 > MF5128 > MF5138 > CIN > MF5142 > NOR > NAL.
FIG. 1.
Incidence of clonic convulsions induced by different doses of PTZ (10 to 90 μg/g, s.c.) in ICR mice.
FIG. 2.
Dose-response curves illustrating the effects of the administration of different doses (10 to 100 μg/g, i.p.) of quinolone derivatives followed 30 min later by a dose of PTZ (40 μg/g, s.c.) in mice (10 animals for each group).
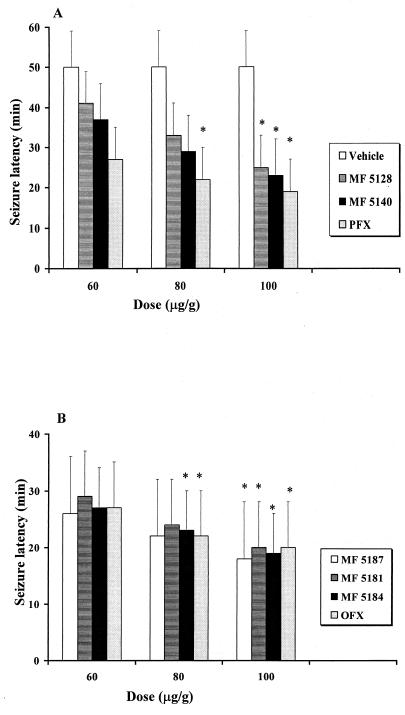
Tables 1 and 2 show that the most of the quinolones studied increased the incidence of clonic phases induced by a subconvulsant dose of PTZ (40 μg/g, s.c.). Significant differences in proconvulsant effects among the quinolones tested were also observed. The percentage of mice showing seizures when pretreated with the 6-hydrogen derivative MF5184, MF5187 or PFX 30 min before receiving PTZ (40 μg/g, s.c.) was significantly increased (P < 0.01) in comparison to the percentage of mice that showed seizures after receiving vehicle plus PTZ (40 μg/g, s.c.) (Fig. 1). Latency times decreased and the incidence of convulsions and death increased gradually as the doses of the compounds were increased. All quinolones at doses of 100 μg/g were able to significantly reduce the latency of seizures; however, no significant difference has been observed among groups receiving various quinolones (Fig. 3). In particular, MF5184 and MF5187 produced a high incidence of seizures at lower concentrations compared to other compounds tested. Similar and less evident effects were observed in mice pretreated with the same doses of MF5189 and OFX, while with CIP and MF5140 the incidence of convulsant effects was still less evident. MF5181, MF5137, RFX, and MF5143 were increasingly less likely to boost the convulsant properties of PTZ. In particular, MF5158, MF5191, MF5128, MF5138, CIN, and MF5142 and to a greater extent NOR and NAL failed to significantly modify the occurrence of PTZ-induced seizures, when compared to the vehicle plus PTZ group by both Fisher’s exact probability test and the Mann-Whitney U test. These quinolone derivatives did not exhibit any convulsant activity when administered alone in the same doses (data not shown).
FIG. 3.
Seizure latency of different quinolones i.p. injected 30 min before PTZ (40 μg/g, s.c.) in mice (10 animals for each group) Open columns in panel A indicate control group (vehicle plus PTZ); other columns indicate quinolone derivatives plus PTZ. ∗, significantly different (P < 0.05) from the value for the group receiving vehicle plus PTZ.
The present data clearly display a dose-dependent effect for several of 6-desfluoroquinolone compounds tested. The dose-response curves reflect the onset of clonic phase but not the seizure duration.
ECoG activity.
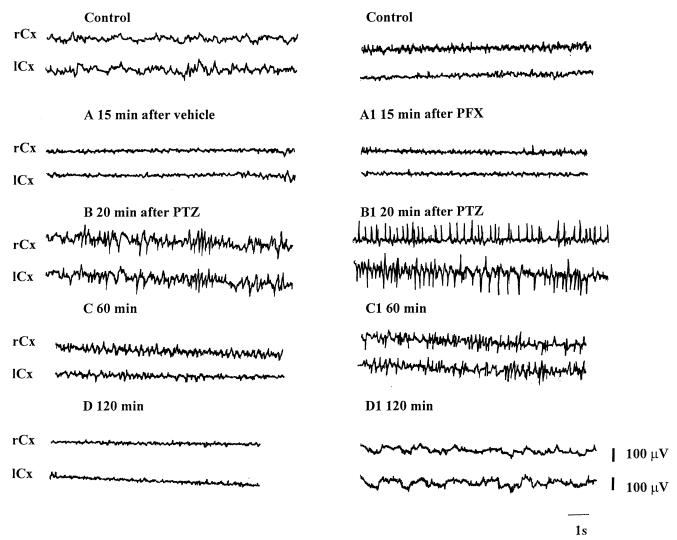
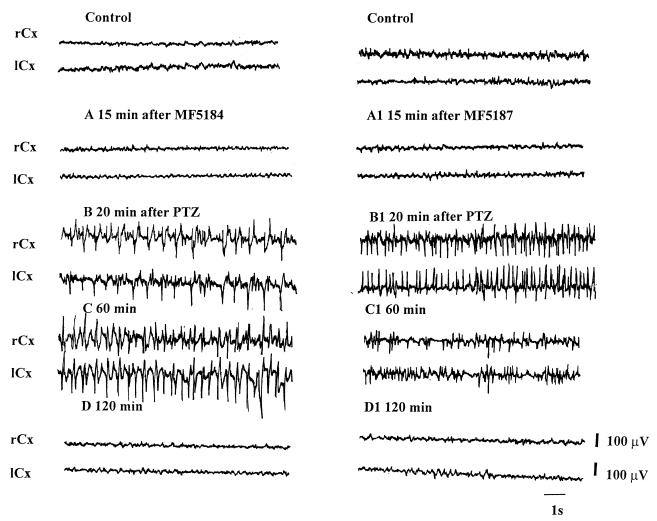
In animals used to study ECoG activity, we observed that the electrocorticographic epileptic discharges appeared more rapidly in mice pretreated with quinolones than in those that received vehicle plus PTZ. In particular, these epileptic discharges appeared more rapidly in animals pretreated with MF5184, MF5187, and PFX than in those pretreated with NAL. Animals pretreated with the other quinolones showed electrocorticographic epileptic discharges with intermediate latency (data not shown). The electrocorticographic epileptic pattern was similar for mice that received vehicle plus PTZ and those that received the quinolone derivative plus PTZ (Fig. 4 and 5).
FIG. 4.
Electrocortical patterns from the right and left frontoparietal cortex (rCx and lCx) after administration of vehicle (i.p.) plus PTZ (40 μg/g, s.c.) (A, B, C, and D) or PFX (80 μg/g, i.p.) plus PTZ (40 μg/g, s.c.) (A1, B1, C1, and D1) in mice. Electrocorticographic recordings were made 15 min after the injection of vehicle or PFX (A and A1), and 20 min (B and B1), 60 min (C and C1), and 120 min (D and D1) after the injection of PTZ.
FIG. 5.
Electrocortical patterns from the right and left frontoparietal cortex (rCx and lCx) after administration of MF5184 (80 μg/g, i.p.) plus PTZ (40 μg/g, s.c.) (A, B, C, and D) or MF5187 (80 μg/g, i.p.) plus PTZ (40 μg/g, s.c.) (A1, B1, C1, and D1) in mice. Electrocorticographic recordings were made 15 min after the injection of MF5184 and MF5187 (A and A1) and 20 min (B and B1), 60 min (C and C1), and 120 min (D and D1) after the injection of PTZ.
Lipophilicity data.
In order to determine if there is some correlation between lipophilicity and the proconvulsant effects of the tested quinolones, the relative lipophilicity (clog P) values of the 6-desfluoroquinolones and classic quinolones were calculated and are shown in Tables 1 and 2, respectively. The clog P for the 6-desfluoroquinolone derivatives decreases in the order MF5184 > MF5191 > MF5181 > MF5137 > MF5142 > MF5143 > MF5158 > MF5187 > MF5138 > MF5140 > MF5128 > MF5189 (Table 1), while for conventional quinolones the decreasing order is NAL > CIN > PFX > RFX > OFX > NOR > CIP (Table 2). In general, the 6-desfluoroquinolone derivatives are less lipophilic than classic quinolones. In addition, for each quinolone series as well as for the whole set of the tested quinolones, the correlation between lipophilicity and proconvulsant potency was evaluated by linear regression analysis. In every case, a very low R2 value was obtained (6-amino derivatives, R2 = 0.020; 6-hydrogen derivatives, R2 = 0.016; classic fluoroquinolones, R2 = 0.206; whole quinolone set, R2 = 0.016), indicating no direct correlation between clog P and CD50s.
DISCUSSION
Although a variety of studies regarding the CNS effects of quinolones have already been conducted, the mechanism of epileptogenic activity of quinolones is largely unresolved (2, 19, 30, 35). In the present study, the convulsant properties of a series of 6-desfluoroquinolones were investigated and compared with the properties of conventional quinolones. Our results demonstrated that compounds have different potentials for potentiating the convulsant effects of PTZ, a well-known convulsant drug which is believed to block the actions of GABA through its effects at the chloride ionophore coupled to the BDZ-GABAA receptor complex (28, 33). These results are in agreement with those observed by Enginar and Eroglu (20), who demonstrated that OFX reduced the threshold of convulsion induced by PTZ. It was suggested by different authors that quinolones are able to increase the excitation of the CNS by inhibition of GABA binding to receptors (2, 30, 35, 36). In particular, since the epileptogenic activities of some quinolones were suppressed by compounds that enhance GABAergic neurotransmission, i.e., muscimol and diazepam, which act selectively on the BDZ-GABAA receptor complex, and were influenced at neurotoxic doses by baclofen, a GABAB receptor agonist, the epileptogenic activity of quinolones most likely involves the BDZ-GABAA receptor complex (2, 15, 26, 36). In particular, Akahane et al. (2) demonstrated that the active site in the quinolone molecule responsible for the inhibition of GABA receptor binding was, at least in part, unsubstituted piperazine or aminopyrrolidine moieties at the C-7 position of the parent molecule, which have structures similar to those of certain GABA receptor agonists. Thus, the enhanced convulsive activity caused by the concomitant administration of quinolones and PTZ in mice may be mediated through additional inhibitory effects on GABA transmission.
However, the concentration needed for an interaction of a quinolone and the GABA receptor is rather high, and it varied among the different quinolones tested by a factor of ∼100. Thus, it appears questionable that a specific interaction of quinolones with the GABA receptor can alone explain the proconvulsant activity of these compounds (17). Indeed, in contrast to the GABAergic mechanism, some authors suggested that other receptors, such as opioid and excitatory amino acid receptors, may also be involved in CNS effects of quinolones (1, 13, 16, 18, 30, 37). Therefore, a GABAergic mechanism is thought to be an essential part of but not the sole component of the mechanism by which quinolones induce seizures; glutamate is also suspected of being involved (1, 13, 37). Furthermore, recent studies have clearly shown that compounds which antagonize ionotropic glutamate receptors inhibit quinolone-induced seizures in mice; therefore, it was suggested that it is most likely that excessive activation of glutamate receptors occurs secondarily to or concomitantly with the impairment of the inhibitory GABAergic neurotransmission caused by quinolones and that it is essential for the propagation of seizures (1, 15, 37).
In the present experiments, we observed the different proconvulsant activities of the several compounds tested, and a relationship between the chemical structure and the epileptogenic properties of the 6-desfluoroquinolones can be outlined. In fact, from a head-to-head comparison of the 6-amino derivatives and their 6-hydrogen counterparts, it is possible to observe that having an amino group in the C-6 position makes compounds less toxic than having a hydrogen in the same position (compare MF5137 and MF5184, MF5143 and MF5181, MF5140 and MF5187, and MF5142 and MF5191).
Looking at the C-7 substituent, we observed that in general the 4-methyl-1-piperidinyl group yields compounds with weaker proconvulsant effects when either an amino group (MF5142) or a hydrogen (MF5191) is present at the C-6 position. In addition, in the 6-hydrogen series, the same weak proconvulsant effect was also observed with the cis-3,5-dimethylmorpholinyl group (MF5158). On the other hand, in both 6-desfluoroquinolone series, the substituents that were highly proconvulsant were the tetrahydro-isoquinolinyl (MF5137 and MF5184) and 4-hydroxypiperidinyl (MF5140 and MF5187) groups.
Another point that should be taken into consideration is lipophilicity, because the various degrees of proconvulsant activity exhibited by the compounds might be partially related to their lipophilicity. It has been suggested, in fact, that there is a connection between the lipophilic character of quinolones and the occurrence of CNS reactions (24). Generally, quinolones possess a very low lipophilicity, but it is possible that some quinolones, such as PFX, which is one of the most lipophilic of the fluoroquinolones, cross the blood brain barrier more readily than others (9, 22). However, our study did not demonstrate any clear correlation between the lipophilicity and proconvulsant potency of the several quinolones tested. In fact, among the classic quinolones, PFX shows high lipophilicity coupled with the highest proconvulsant activity (potency), but NAL, which is the most lipophilic compound, is also the least toxic. In the 6-desfluoroquinolone series, it can be observed that the 6-amino derivatives are less lipophilic and less toxic than their 6-hydrogen counterparts, indicating a direct correlation between lipophilic character and occurrence of CNS effects. However, looking at the piperidinyl derivatives in both series (MF5140, MF5143, MF5142, MF5187, MF5181, and MF5191), the lipophilicity decreases in the order of 4-methyl-1-piperidinyl > peridinyl > 4-hydroxy-1-piperidinyl derivatives, while the proconvulsant effect increases in the same order, showing a reverse correlation. Additionally, the lack of correlation between the clog P values and the CD50 was clearly confirmed by the very low R2 values resulting from linear regression analyses. Although further research is necessary, it now appears that factors other than lipophilicity may be responsible for proconvulsant potency.
This possibility compels us to consider the involvement of pharmacokinetic mechanisms. In fact, it is possible that the higher proconvulsant activity of some compounds, such as PFX, may be related to the concentrations reached by the drug in the brain or to a slow clearance of the compound from the cerebral area. In fact, Sato et al. (29) reported that the transport mechanism for two quinolones, OFX and lomefloxacin, across the blood-cerebrospinal fluid (CSF) barrier might involve a process of sequestration from CSF into blood. Moreover, unidirectional efflux (sequestration) from CSF into blood by saturable active transport has been proposed for many drugs, e.g., some β-lactam antibiotics (32, 34). Recently, Ooie and coworkers (27) have demonstrated that among several conventional quinolones, marked differences exist in the steady-state concentration ratio between CSF and brain tissue. This phenomenon may contribute to the differences in neurotoxicity of the present compounds.
In conclusion, we suggest that the nature of the substituents at different positions on the quinolone nucleus may play an important role in the CNS effects of these compounds. Therefore, the design and development of new quinolone derivatives with broader antibacterial activity and better pharmacokinetics but no CNS effects are attractive therapeutic goals.
In addition, although additional studies are needed to investigate the mechanism that causes the CNS side effects of quinolones, physicians should consider the possible epileptogenic activity of these compounds when treating patients with predisposing epileptic factors or when the penetration of quinolones into the brain via a damaged blood-brain barrier is enhanced.
ACKNOWLEDGMENTS
Financial support from the Italian Ministry of the University and Scientific and Technological Research (MURST) and the Italian Council for Research (CNR, Rome, Italy) is gratefully acknowledged.
We thank Antonino Giacopello and Fabio Giuffrè for their skillful technical assistance.
REFERENCES
- 1.Akahane K, Kato M, Takayama S. Involvement of inhibitory and excitatory neurotransmitters in levofloxacin- and ciprofloxacin-induced convulsions in mice. Antimicrob Agents Chemother. 1993;37:1764–1770. doi: 10.1128/aac.37.9.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akahane K, Sekiguchi M, Une T, Osada Y. Structure-epileptogenicity relationship of quinolones with special reference to their interaction with γ-aminobutyric acid receptor sites. Antimicrob Agents Chemother. 1989;33:1704–1708. doi: 10.1128/aac.33.10.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anastasio G D, Menscer D, Little J M., Jr Norfloxacin and seizures. Ann Intern Med. 1988;109:169–170. doi: 10.7326/0003-4819-109-2-169. [DOI] [PubMed] [Google Scholar]
- 4.Cecchetti V, Clementi S, Cruciani G, Fravolini A, Pagella P G, Savino A, Tabarrini O. 6-Aminoquinolones: a new class of quinolone antibacterials? J Med Chem. 1995;38:973–982. doi: 10.1021/jm00006a017. [DOI] [PubMed] [Google Scholar]
- 5.Cecchetti V, Fravolini A, Lorenzini M C, Tabarrini O, Terni P, Xin T. Studies on 6-aminoquinolones: synthesis and antibacterial evaluation of 6-amino-8-methylquinolones. J Med Chem. 1996;39:436–445. doi: 10.1021/jm950558v. [DOI] [PubMed] [Google Scholar]
- 6.Cecchetti V, Fravolini A, Palumbo M, Sissi C, Tabarrini O, Terni P, Xin T. Potent 6-desfluoro-8-methylquinolones as new lead compounds in antibacterial chemotherapy. J Med Chem. 1996;39:4952–4957. doi: 10.1021/jm960414w. [DOI] [PubMed] [Google Scholar]
- 7.Christ W. Central nervous system toxicity of quinolones: human and animal findings. J Antimicrob Chemother. 1990;26(Suppl. B):219–225. doi: 10.1093/jac/26.suppl_b.219. [DOI] [PubMed] [Google Scholar]
- 8.Chu D T W, Fernandes P B. Structure-activity relationships of the fluoroquinolones. Antimicrob Agents Chemother. 1989;33:131–135. doi: 10.1128/aac.33.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalhoff A. A review of quinolone tissue pharmacokinetics. In: Fernandes P B, editor. Quinolones. J. R. Barcelona, Spain: Prous Science Publishers, S.A.; 1989. pp. 277–313. [Google Scholar]
- 10.Daylight Chemical Information Systems, Inc. CLOGP3 user guide: MedChem software release 3.5. Irvine, Calif: Daylight Chemical Information Systems, Inc.; 1988. [Google Scholar]
- 11.De Sarro A, De Sarro G B. Responsiveness of genetically epilepsy-prone rats to aminophylline-induced seizures and interactions with quinolones. Neuropharmacology. 1991;30:169–176. doi: 10.1016/0028-3908(91)90200-u. [DOI] [PubMed] [Google Scholar]
- 12.De Sarro A, Ammendola D, De Sarro G B. Effects of some quinolones on imipenem-induced seizures in DBA/2 mice. Gen Pharmacol. 1994;25:369–379. doi: 10.1016/0306-3623(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 13.De Sarro A, Trimarchi G R, Ammendola D, De Sarro G. Repeated treatment with quinolones potentiates the seizures induced by aminophylline in genetically epilepsy-prone rats. Gen Pharmacol. 1992;23:853–859. doi: 10.1016/0306-3623(92)90237-e. [DOI] [PubMed] [Google Scholar]
- 14.De Sarro A, Zappalá M, Chimirri A, Grasso S, De Sarro G B. Quinolones potentiate cefazolin-induced seizures in DBA/2 mice. Antimicrob Agents Chemother. 1993;37:1497–1503. doi: 10.1128/aac.37.7.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Sarro G, Nava F, Calapai G, De Sarro A. Effects of some excitatory amino acid antagonists and drugs enhancing γ-aminobutyric acid neurotransmission on pefloxacin-induced seizures in DBA/2 mice. Antimicrob Agents Chemother. 1997;41:427–434. doi: 10.1128/aac.41.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimpfel W, Dalhoff A, von Keutz E. In vitro modulation of hippocampal pyramidal cell response by quinolones: effects of HA 966 and γ-hydroxybutyric acid. Antimicrob Agents Chemother. 1996;40:2573–2576. doi: 10.1128/aac.40.11.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimpfel W, Spüler M, Dalhoff A, Hofmann W, Schlüter G. Hippocampal activity in the presence of quinolones and fenbufen in vitro. Antimicrob Agents Chemother. 1991;35:1142–1146. doi: 10.1128/aac.35.6.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodd P R, Davies L P, Warson W E J, Nielsen B, Dyer J A, Wong L S, Johnston G A R. Neurochemical studies on quinolone antibiotics: effects on glutamate, GABA and adenosine systems in mammalian CNS. Pharmacol Toxicol. 1989;64:404–411. doi: 10.1111/j.1600-0773.1989.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 19.Domagala J M. Structure-activity and structure-side-effect relationships for quinolone antibacterials. J Antimicrob Chemother. 1994;33:685–706. doi: 10.1093/jac/33.4.685. [DOI] [PubMed] [Google Scholar]
- 20.Enginar N, Eroglu L. The effect of ofloxacin and ciprofloxacin on pentylenetetrazole-induced convulsions in mice. Pharmacol Biochem Behav. 1991;39:587–589. doi: 10.1016/0091-3057(91)90132-l. [DOI] [PubMed] [Google Scholar]
- 21.Fass R J. Adverse reactions associated with quinolones. Quinolones Bull. 1987;3:5–6. [Google Scholar]
- 22.Gonzalez I P, Henwood I M. Pefloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1989;37:712–721. doi: 10.2165/00003495-198937050-00003. [DOI] [PubMed] [Google Scholar]
- 23.Litchfield J T, Wilcoxon F. A simplified method of evaluating dose-effects experiments. J Pharmacol Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 24.Lucet I C, Tilly H, Lerebours G, Gres I L, Piguet H. Neurological toxicity related to pefloxacin. J Antimicrob Chemother. 1988;21:811–812. doi: 10.1093/jac/21.6.811. [DOI] [PubMed] [Google Scholar]
- 25.Microcal Software Inc. Microcal Origin program, version 3.5. Northampton, Mass: Microcal Software Inc.; 1991. [Google Scholar]
- 26.Mohler H. GABAergic synaptic transmission. Regulation by drugs. Arzneim-Forsch. 1992;42:211–214. [PubMed] [Google Scholar]
- 27.Ooie T, Suzuki H, Terasaki T, Sugiyama Y. Comparative distribution of quinolone antibiotics in cerebrospinal fluid and brain in rats and dogs. J Pharmacol Exp Ther. 1996;278:590–596. [PubMed] [Google Scholar]
- 28.Ramanjaneyulu R, Ticku M K. Interaction of pentamethylenetetrazole and tetrazole analogues with the picrotoxinin site of the benzodiazepine-GABA receptor-ionophore complex. Eur J Pharmacol. 1984;98:337–345. doi: 10.1016/0014-2999(84)90282-6. [DOI] [PubMed] [Google Scholar]
- 29.Sato H, Okezaki E, Yamamoto S, Nagata O, Kato H, Tsuji A. Entry of the new quinolone antibacterial agents ofloxacin and NY-198 into the central nervous system in rats. J Pharmacobio-Dyn. 1988;11:386–394. doi: 10.1248/bpb1978.11.386. [DOI] [PubMed] [Google Scholar]
- 30.Segev S, Rehavi M, Rubinstein E. Quinolones, theophylline, and diclofenac interactions with the γ-aminobutyric acid receptor. Antimicrob Agents Chemother. 1988;32:1624–1626. doi: 10.1128/aac.32.11.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semel J D, Allen N. Seizures in patients simultaneously receiving theophylline and pefloxacin or ciprofloxacin or metronidazole. South Med J. 1991;84:465–468. doi: 10.1097/00007611-199104000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Spector R. Ceftriaxone pharmacokinetics in the central nervous system. J Pharmacol Exp Ther. 1986;236:380–383. [PubMed] [Google Scholar]
- 33.Squires R F, Saederup E, Grawley J N, Skolnick P, Paul S M. Convulsant potencies of tetrazole are highly correlated with action on GABA/picrotoxin receptor complexes in brain. Life Sci. 1984;35:1439–1444. doi: 10.1016/0024-3205(84)90159-0. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki H, Sawada Y, Sugiyama Y, Iga T, Hanano M, Spector R. Transport of imipenem, a novel carbapenem antibiotic, in the rat central nervous system. J Pharmacol Exp Ther. 1989;250:979–984. [PubMed] [Google Scholar]
- 35.Tsuji A, Sato H, Kume Y, Tamai I, Okezaki E, Nagata O, Kato H. Inhibitory effects of quinolone antibacterial agents on γ-aminobutyric acid binding to receptor sites in rat brain membranes. Antimicrob Agents Chemother. 1988;32:190–194. doi: 10.1128/aac.32.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unseld E, Ziegler G, Gemeinhardt A, Janssen U, Klotz U. Possible interaction of fluoroquinolones with the benzodiazepine-GABAA-receptor complex. Br J Clin Pharmacol. 1990;30:63–70. doi: 10.1111/j.1365-2125.1990.tb03744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams P D, Helton D R. The proconvulsive activity of quinolone antibiotics in an animal model. Toxicol Lett. 1991;58:23–28. doi: 10.1016/0378-4274(91)90186-a. [DOI] [PubMed] [Google Scholar]