Abstract
Stroke is the second leading cause of mortality globally with higher burden and younger age in low‐middle income countries (LMICs) than high‐income countries (HICs). However, it is unclear to what extent differences in healthcare access and quality (HAQ) and prevalence of risk factors between LMICs and HICs contribute to younger age of stroke in LMICs. In this systematic review, we conducted meta‐analysis of 67 articles and compared the mean age of stroke between LMICs and HICs, before and after adjusting for HAQ index. We also compared the prevalence of main stroke risk factors between HICs and LMICs. The unadjusted mean age of stroke in LMICs was significantly lower than HICs (63.1 vs. 68.6), regardless of gender (63.9 vs. 66.6 among men, and 65.6 vs. 70.7 among women) and whether data were collected in population‐ (64.7 vs. 69.5) or hospital‐based (62.6 vs. 65.9) studies (all p < 0.01). However, after adjusting for HAQ index, the difference in the mean age of stroke between LMICs and HICs was not significant (p ≥ 0.10), except among women (p = 0.048). In addition, while the median prevalence of hypertension in LMICs was 23.4% higher than HICs, the prevalence of all other risk factors was lower in LMICs than HICs. Our findings suggest a much larger contribution of HAQ to the younger mean age of stroke in LMICs, as compared with other potential factors. Additional studies on stroke care quality and accessibility are needed in LMICs.
Introduction
Stroke is the second leading cause of morbidity and mortality, 1 , 2 and the third leading cause of disability 3 , 4 globally. The burden of stroke in low‐middle income countries (LMICs) is higher than in high‐income countries (HICs) and this gap is increasing. 4 , 5 Recent global burden of disease (GBD) studies reported that although stroke incidence, prevalence, and mortality rates decreased in HICs in the recent decades, there have been no significant changes in the incidence of stroke in LMICs. 6 One of the reasons for this difference is limited access to diagnostic techniques for identifying stroke subtypes in LMICs, as CT imaging or MRI facilities are not readily available or not affordable by some LMICs. 2 However, it is not clear how much of these differences could be attributed to differences in access to diagnostic techniques. Also, evidence shows that the age‐adjusted incidence of stroke in LMICs exceeded that in HICs by 20% for the first time during 2000–2008. 7 Despite a global decline in the age‐standardized stroke death rate, the decline has been slower in LMICs than HICs. 8 Specifically, the mortality rate of stroke has been reduced to half in HICs but only reduced by 15% in LMICs. 9 Furthermore, the global absolute number of people who developed new stroke, died, survived, or remained disabled from stroke has almost doubled from 1990 to 2017 with a major bulk of stroke burden in LMICs in 2017 (80% all incident strokes, 77% all stroke survivors, and 87% of all deaths from stroke). 6 , 10 , 11
Although aging is postulated as an explanation for the increased rates of stroke over time, about one‐fourth of ischemic strokes occur in working‐aged individuals in the HICs, and new strokes strike an estimated 3.6 million young people each year. 12 There is also evidence suggesting that stroke may occur at a younger age in LMICs as compared to HICs. 13 , 14 , 15 , 16 However, despite over two‐thirds of stroke deaths worldwide occurring in LMICs, high quality and reliable data from population‐based prospective studies are sparse, especially in LMICs. 17 For example, the data on stroke epidemiology and mortality may be hospital‐based in many LMICs, potentially ignoring non‐hospitalized cases and lacking generalizability to the whole population. 18 In addition, limited number of studies from LMICs provide reliable data on the age of stroke and its differences by sex, stroke etiology, and subtypes.
The increasing prevalence of known risk factors such as high blood pressure, dyslipidemia, diabetes, obesity, and atrial fibrillation (AF) are possible reasons driving stroke rates and stroke burden. 19 , 20 , 21 Although global data show that 90.5% of the stroke burden are attributable to such modifiable risk factors, 22 the role of differences in their epidemiology along with other stroke risk factors between HICs and LMICs and their possible contribution to the increasing burden and younger age of stroke in LMICs is unclear. Therefore, in addition to comparing epidemiology of the aforementioned risk factors, we also sought to determine if other factors contribute to the younger age of stroke in LMICs. For example, studies have shown differences in access to healthcare, health technologies, and relative rates of stroke risk factors between HICs and LMICs. 22 A systematic analysis suggested that healthcare access and quality (HAQ) may have direct impacts on health outcomes, including stroke mortality. 11 , 23 , 24 , 25 However, to our knowledge, the possible contribution of HAQ to the younger age of stroke in LMICs is unclear.
We systematically review the existing literature on the mean age of stroke in different countries and compare it between HICs and LMICs by gender, stroke subtypes, and whether the data were collected in a population‐ or hospital‐based setting, before and after adjusting for HAQ index. In addition, using publicly available global data, we compared the prevalence of main stroke risk factors including hypertension, diabetes, high cholesterol, obesity, and AF between HICs and LMICs.
Methods
Economic classification
We used publicly available data from the World Bank's 2020 fiscal year (2020–2021) classification of countries' income levels based on gross national income (GNI) per capita (current US$) of the previous calendar year (2019): low‐income (≤$1035 GNI), lower‐middle income ($1036 to $4045 GNI), upper‐middle income ($4046 to $12,535 GNI), and high‐income (≥$12,536 GNI). 26 In this study, we have classified countries with low‐, lower‐middle‐, and upper‐middle income economies as LMICs.
Mean age of stroke in LMICs and HICs
Study design, literature search strategy, and data extraction
This is a systematic review of peer‐reviewed articles to assess the differences in the mean age of stroke in HICs and LMICs. Following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines, 27 (PRISMA Checklist is in Table S6) we initially searched PubMed database using MeSH terms through a concept‐based search strategy with keywords “stroke” AND “mean age” to identify articles. Then, we restricted the searches to articles that were published in English from January 2000 to July 2021 with human adult patients. Duplicates were removed and the titles of the remaining articles were reviewed to exclude articles with irrelevant titles. We further reviewed the abstracts, and full texts of the remaining articles to identify the list of articles that met eligibility criteria to be included in meta‐analysis. We extracted data from each article for the mean age of stroke by gender, stroke subtypes, and whether the data were collected in a hospital‐ or population‐based setting and organized the information into a Microsoft Excel database. The database was organized according to the author, publication year, country name, region, population data, and economic classification as LMICs or HICs.
HAQ index
We used publicly available GBD 28 data on HAQ index, which was constructed using GBD 2016 estimates for 32 causes of death that may be treatable with effective care to approximate personal HAQ for countries and territories, as well as selected subnational locations, over time. 23 In brief, GBD 2016 Healthcare Access and Quality Collaborators used estimates for age‐ and risk‐standardized death rates from 24 non‐cancer causes and age‐standardized mortality‐to‐incidence ratios for eight cancers that are considered amenable to healthcare, to construct the HAQ Index providing an overall score of 0–100 (0 and 100 indicate worst and best, respectively). 23 For our systematic review, we only analyzed the country‐level HAQ index, which accounted for 195 countries.
Meta‐analysis
As variances for the variables in each country were not available, we excluded the estimation of variance for each country and focused on estimation of mean age for these variables. Based on mean estimation from each country, we have estimated the weighted sum and variance, using on the population size in 2019 by World Bank as weights. We used univariable weighted generalized linear models (GLMs) to compare the mean age of stroke (i.e., dependent variable) between LMICs and HICs, as well as for subgroup analyses comparing the mean age of stroke between LMICs and HICs by gender, stroke subtypes, and whether the data sources were population‐based or hospital‐based. In addition, we used multivariable GLMs that account for population weights to compare the mean age of stroke between LMICs and HICs, as well as subgroup analyses adjusted for HAQ index. As variance data were not available within the country level, the test for homogeneity of variance was not performed.
Prevalence of stroke risk factors
Data source identification and definition of risk factors
We focused on five stroke risk factors including hypertension, diabetes, high cholesterol, obesity, and AF with the most recent version of publicly available global country‐level data observatories from either World Health Organization (WHO), 29 GBD, 28 or International Diabetes Foundation (IDF). 30 Specifically, we used 2015 WHO data 31 for prevalence of hypertension (systolic blood pressure ≥140 or diastolic blood pressure ≥90), 2008 WHO data 32 for prevalence of high cholesterol (total cholesterol ≥6.2 mmol/L), 2016 WHO data 33 for prevalence of overweight and obesity (body mass index [BMI] ≥25 and BMI ≥30, respectively), and 2019 GBD data 28 for prevalence of AF. For prevalence of diabetes (fasting blood glucose ≥7.0 mmol/L or on medication), we identified data from 2019 GBD, 28 2014 WHO, 34 to 2019 IDF. 30 For all the aforementioned risk factors, we only included country‐level data.
Data extraction and analysis
Data on the prevalence of stroke risk factors from different resources were standardized to percentage (%). Available datasets for each stroke risk factors were all downloaded in the form of Microsoft Excel files, and were merged by data source, publication year, country name, region, population data, economic classification, LMICs, and HICs binary data based on the economic classification, age range (if available), gender (if available), prevalence percentage, and its range (minimum, maximum). Since the country‐level variance data for the prevalence of risk factors were not available, we excluded estimation of variance for each country.
All statistical procedures were conducted using SAS 9.4 software. 35 All hypotheses were tested at a 5% level of significance.
Results
We identified 30,489 peer‐reviewed articles in our initial PubMed search. We only included articles in English, with adult human subjects that were published after 2000 (21,167 articles). We also removed 3042 duplicate articles before conducting other eligibility assessments. The remaining 18,125 articles were further narrowed down to 1077 by reviewing the titles. After reviewing the abstracts and full text of the 1077 articles, 67 articles that reported age of stroke in adult populations met the inclusion criteria (Fig. 1). 21 , 29 , 31 , 32 , 33 , 34 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95
Figure 1.
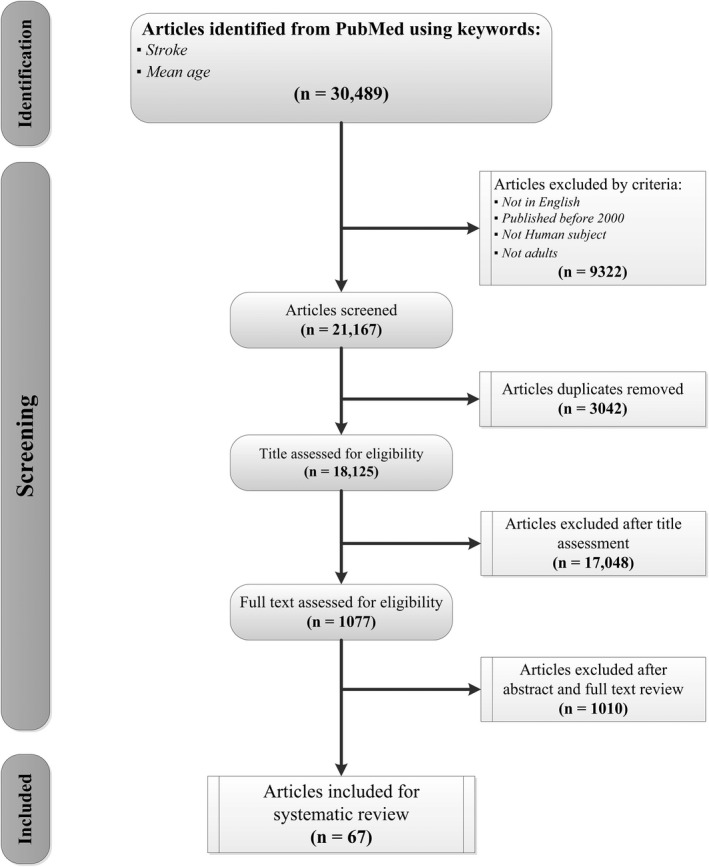
Systematic review flow chart following PRISMA guideline.
As shown in Table 1, the mean age of stroke in all countries was 64.4 years (95% CI: [62.9, 65.8]), and patients with stroke in LMICs were significantly younger than those in HICs (63.1 years vs. 68.6 years, p < 0.01). However, after adjusting for HAQ index, mean age difference of stroke between LMICs and HICs was not statistically significant (adjusted mean age difference [LMICs mean−HICs mean] [95% CI]: −0.9 [−4.2, 2.5], p = 0.61). The subgroup analysis with gender‐specific data revealed that the overall mean age of stroke was 64.1 years among men and 66.1 years among women. In the unadjusted model, the mean age of stroke patients in LMICs were significantly lower than that in HICs among both men and women, with larger difference in women (unadjusted mean age difference [95% CI]: females −5.1 years [−9.5, −0.8] vs. men −2.8 years [−7.1, 1.6]). However, only the mean age difference of female patients remained significant after adjusting for HAQ index (adjusted mean age difference [95% CI]: women −6.8 years [−13.5, −0.2], p = 0.048 vs. men −1.5 years [−6.1, 3.0], p = 0.49). Based on stroke subtypes, mean age of stroke was 67.5 years for ischemic stroke and 61.6 years for intracerebral hemorrhage. While the unadjusted mean age of ischemic stroke in LMICs was significantly lower than that in HICs (66.6 years vs. 69.9 years, p = 0.03), the difference was not statistically significant after accounting for HAQ index (66.6 years vs. 66.5 years, p = 0.96). Although the unadjusted mean age of patients with intracerebral hemorrhage in LMICs were younger than that in HICs, the difference was not statistically significant (60.9 years vs. 73.2 years, p = 0.46). However, mean age difference of patients with intracerebral hemorrhage between LMICs and HICs became marginally significant after adjusting for HAQ index (adjusted mean age difference [95% CI]: −9.5 years [−21.7, 2.6], p = 0.10). Similarly, overall mean age of patients with stroke was 66.3 years based on population‐based studies, and 62.9 years based on hospital‐based studies. Patients with stroke in LMICs were significantly younger than those in HICs, regardless of whether the data were from population‐ or hospital‐based studies, though with a larger difference observed in population‐based studies (unadjusted mean age difference between LMICs and HICs [95% CI]: population‐based studies −4.8 years [−8.8, −0.8] vs. hospital‐based studies −3.3 years [−6.7, 0.0]). However, such differences were not significant after adjusting for HAQ index (adjusted mean age of patients in LMICs vs. HICs: hospital‐based studies 63.9 years vs. 62.3 years, p = 0.51, population‐based studies 67.1 years vs. 65.8 years, p = 0.56). Details on the comparison of mean age of patients with stroke among LMICs and HICs are displayed in Table 1 and Figure 2.
Table 1.
Meta‐analysis on mean age of stroke in LMICs and HICs, before and after adjusting for HAQ index.
| Mean age of stroke (years) | Unadjusted | Adjusted 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | LMICs | HICs | Mean difference 2 | p value 3 | LMICs | HICs | Mean difference 2 | p value 3 | |
| Overall (N = 52) 4 | 64.4 (62.9, 65.8) | 63.1 (61.1, 65.1) | 68.6 (66.9, 70.2) | −5.4 (−7.9, −2.9) | < 0.01 | 65.1 (63.6, 66.6) | 65.9 (63.3, 68.5) | −0.9 (−4.2, 2.5) | 0.61 |
| By gender 5 | |||||||||
| Male (N = 19) | 64.1 (62.8, 65.4) | 63.9 (62.0, 65.7) | 66.6 (63.6, 69.7) | −2.8 (−7.1, 1.6) | < 0.01 | 64.5 (62.9, 66.0) | 66.0 (61.9, 70.1) | −1.5 (−6.1, 3.0) | 0.49 |
| Female (N = 19) | 66.1 (64.2, 68.0) | 65.6 (62.8, 68.3) | 70.7 (67.0, 74.5) | −5.1 (−9.5, −0.8) | < 0.01 | 64.8 (62.6, 67.0) | 71.6 (65.6, 77.6) | −6.8 (−13.5, −0.2) | 0.048 |
| By stroke sub‐type | |||||||||
| Ischemic (N = 16) 6 | 67.5 (66.1, 69.0) | 66.6 (65.2, 68.1) | 69.9 (67.1, 72.8) | −3.3 (−6.1, −0.6) | 0.03 | 66.6 (65.6, 67.5) | 66.5 (64.4, 68.6) | 0.1 (−2.3, 2.4) | 0.96 |
| Intracerebral hemorrhage (N = 7) 7 | 61.6 (57.9, 65.3) | 60.9 (58.5, 63.4) | 73.2 (45.2, 101.3) | −12.3 (−25.1, 0.4) | 0.46 | 59.9 (56.8, 62.9) | 69.4 (56.8, 81.9) | −9.5 (−21.7, 2.6) | 0.10 |
| By method of data collection | |||||||||
| Hospital‐based (N = 26) 8 | 62.9 (61.3, 64.5) | 62.6 (60.5, 64.7) | 65.9 (63.0, 68.8) | −3.3 (−6.7, 0.0) | < 0.01 | 63.9 (62.4, 65.3) | 62.3 (58.1, 66.6) | 1.5 (−3.2, 6.3) | 0.51 |
| Population‐based (N = 28) 9 | 66.3 (64.1, 68.4) | 64.7 (61.0, 68.4) | 69.5 (67.6, 71.4) | −4.8 (−8.8, −0.8) | < 0.01 | 67.1 (64.8, 69.3) | 65.8 (62.5, 69.1) | 1.3 (−3.2, 5.9) | 0.56 |
Numbers are displayed as mean (95% CI), unless otherwise noted. LMICs, low‐middle income countries; HICs, high‐income countries; HAQ, health access and quality.
Adjusted for HAQ index.
Mean difference = pooled mean age difference (LMICs−HICs).
Pooled/Satterthwaite for variance estimation for t‐test.
LMICs: N = 26, HICs N = 26.
LMICs: N = 9, HICs N = 10.
LMICs: N = 9, HICs N = 7.
LMICs: N = 4, HICs N = 3.
LMICs: N = 16, HICs N = 10.
LMICs: N = 12, HICs N = 16.
Figure 2.
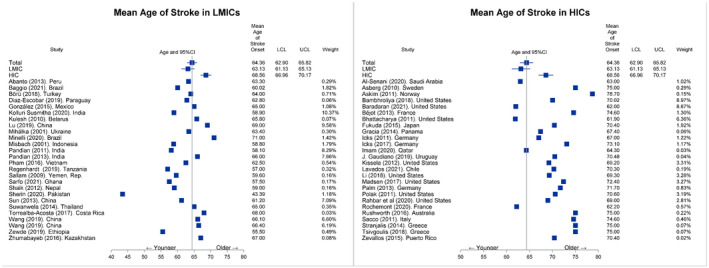
Forest plot on mean age of stroke in LMICs and HICs. LMICs, low‐middle income countries; HICs, high‐income countries. [Colour figure can be viewed at wileyonlinelibrary.com]
In Table 2, we compared median and range of prevalence of the five main known risk factors for stroke including hypertension, diabetes, high cholesterol, obesity, and AF, between LMICs and HICs using publicly available data. Our findings show that median prevalence of hypertension was 23.4% higher in LMICs than HICs (25.8% vs. 20.9%, difference ratio [DR] = 23.4%). However, median prevalence of all other risk factors was lower in LMICs as compared to HICs (Table 2). For diabetes, the median prevalence of both type I and II diabetes in LMICs was 3.4% higher than that in HICs (median prevalence: 7.2% vs. 7.0%, DR = 3.4%). However, specific median prevalence of type I and type II diabetes was 48.9% and 10.4% lower in LMICs than HICs (DR = −18.6%), respectively (median prevalence in LMICs vs. HICs: type I diabetes 0.2% vs. 0.4%, and type II diabetes 5.5% vs. 6.1%). High cholesterol was 57.0% less prevalent in LMICs compared to HICs, which showed the largest relative difference among the five risk factors (7.3% vs. 16.9%, DR = −57.0%).
Table 2.
Comparison of the prevalence of stroke risk factors between LMICs and HICs.
| Median estimated prevalence (%) | Worldwide | LMICs | HICs | Difference ratio (%) 1 |
|---|---|---|---|---|
| Hypertension 2 | 24.6 (11, 33.4) | 25.8 (13.7, 33.4) | 20.9 (11.0, 32.4) | 23.4 |
| Diabetes 3 | ||||
| All | 7.2 (1.0, 30.5) | 7.2 (1.0, 30.5) | 7.0 (2.1, 29.2) | 3.4 |
| Type I | 0.2 (0.1, 0.9) | 0.2 (0.1, 0.5) | 0.4 (0.1, 0.9) | −48.9 |
| Type II | 5.7 (2.3, 20.9) | 5.5 (2.3, 20.9) | 6.1 (2.7, 17.4) | −10.4 |
| High cholesterol 4 | 9.1 (2.5, 29.1) | 7.3 (2.5, 15.7) | 16.9 (8.7, 29.1) | −57.0 |
| Obesity 5 | ||||
| BMI ≥25 (overweight) | 54.9 (18.3, 88.5) | 48.1 (18.3, 83.5) | 59.1 (27.2, 88.5) | −18.6 |
| BMI ≥30 (obese) | 20.6 (2.1, 61) | 17.1 (2.1, 52.9) | 23.1 (4.3, 61.0) | −26.0 |
| Atrial fibrillation 6 | 0.55 (0.16, 1.44) | 0.48 (0.16, 1.18) | 0.87 (0.33, 1.44) | −45.0 |
Numbers are displayed as median (minimum, maximum), unless otherwise noted. LMICs, low‐middle income countries; HICs, high‐income countries.
Difference ratio (%) = (LMICs median−HICs median)/HICs median × 100.
2015 World Health Organization (WHO) 31 data from 133 LMICs and 60 HICs.
2019 Global Burden of Disease (GBD), 28 2014 WHO, 86 and 2019 International Diabetes Foundation 30 data from 134 LMICs and 66 HICs.
2008 WHO 32 data from 131 LMICs and 60 HICs.
2016 WHO 33 data from 133 LMICs and 60 HICs.
2019 GBD 28 data from 134 LMICs and 66 HICs.
Similarly, the median prevalence of obesity and AF were 26.0% and 45.0% lower in LMICs than HICs, respectively (DR = −45.0%). Additional detailed data on comparisons of age‐ and gender‐specific prevalence of each risk factor between LMICs and HICs are provided in Tables S1–S1.
Discussion
In this systematic review, we summarized the relevant published literature on the mean age of stroke in LMICs and HICs. While the overall mean age of stroke among all countries was 64.4 years, patients with stroke in LMICs were about 5.5 years younger than those in HICs. Moreover, the mean age of stroke patients in LMICs was significantly younger than in HICs regardless of the patients' gender, stroke sub‐types, and whether the data were collected at population or hospital level. However, after adjusting for HAQ index, the difference in the mean age of stroke between LMICs and HICs did not remain significant in any main and subgroup analyses, except among women, where adjusted mean age of stroke patients remained significantly younger in LMICs than HICs. In addition, we found that hypertension was the most prevalent risk factor in LMICs, with a median prevalence that was 23.4% higher than that in HICs. However, median prevalence of all other risk factors was lower in LMICs compared to HICs.
Our findings on the younger age of stroke in LMICs than HICs are consistent with findings from a review of 23 population‐based studies indicating a moderate positive correlation (r = 0.60) between country's SES measures including lower per capita gross domestic product (GDP) and total health expenditures per capita, and occurrence of stroke at a younger age. 96 However, they did not compare mean age at stroke between countries with low and high GDP or conduct a meta‐analysis to report a pooled estimate. Our findings are also consistent with the GBD data showing that patients with incident and fatal ischemic and hemorrhagic stroke in LMICs were 3–5 years younger than those in HICs. Their findings suggested that while the worldwide mean age of people with incident and fatal ischemic and hemorrhagic stroke has increased from 1990 to 2017, this increase was most dramatically noted in HICs. 6 , 16 However, they did not report the mean age of stroke by gender subgroups or adjust for any potential confounders. A recent study, “Global Outcome Assessment Lifelong after stroke in young adults” (GOAL) initiative, combined data from individual young patients with stroke from 32 cohorts in 29 countries and reported that LMICs had less vascular risk factors, but a higher 3‐month mortality than those from HICs (OR = 2.49; 95% CI 1.42–4.36). 5
To our knowledge, our study is the first to report that the significant difference in the mean age of stroke between LMICs and HICs diminished after accounting for health care access and quality as measured by country‐level HAQ index. This finding suggests that the younger age of stroke in LMICs is not necessarily related to the overall country income level, and that the HAQ differences between LMICs and HICs could explain, at least in part, the higher burden due to younger age of stroke in LMICs.
Despite the decreasing trends in the incidence and mortality of new and recurrent stroke especially in HICs, a substantial portion of strokes in both HICs and LMICs are further preventable by proper management of major modifiable risk factors for stroke such as hypertension, diabetes, hyperlipidemia, obesity, and antithrombotic therapy. 97 For example, despite the fact that hypertension is the most common modifiable risk factor for stroke, affecting about one‐third of US adults over 20 years of age, blood pressure was controlled in only about half of adults in the US with hypertension. 98 Findings of the cross‐sectional phase of the Prospective Urban Rural Epidemiology (PURE) study also showed a large gap between detection and control of hypertension across all countries. Specifically, they have shown that awareness, treatment, and control of hypertension were lower in LMICs than HICs, as well as in rural than urban settings within LMICs. 99 For example, a survey of prevention strategies in Pakistan revealed that only 63% of physicians prescribed antihypertensive therapy if blood pressure was 140/90 mmHg or greater, and 75% did not routinely check the lipid profile. 4 This lack of awareness, under‐diagnosis and suboptimal management of modifiable risk factors can also contribute to the high burden and younger age of stroke in LMICs despite the overall lower prevalence of other stroke risk factors that we observed in our analyses.
Another possible explanation for the younger age of stroke in LMICs may be the limited availability of accurate data on the prevalence of risk factors and stroke, mainly due to lack of infrastructure for high‐quality prospective, population‐based, and clinical studies in LMICs. The World Stroke Organization‐WHO‐Lancet Neurology Commission on Stroke has recently published the results of a survey assessing the status of stroke services (surveillance, prevention, acute stroke, and rehabilitation) in LMICs compared to HICs. Their findings showed that only a small proportion of LMICs has stroke surveillance activities that is stroke registries, active or recently completed risk factors surveys, participation in either population‐based or clinical research for stroke, etc. 89 This lack of prospective studies and surveillance result in data that significantly underestimate the actual prevalence of stroke and cardiovascular risk factors in the LMICs as compared to HICs. For example, Miranda et al 100 have shown that data on the distribution of cardiovascular risk factors in Latin American and Caribbean (LAC) region are not only very limited, but also the available data have significant variation in the levels of prevalence. For instance, the CARMELA study 101 that was conducted in seven major urban cities in LAC, reported markedly different hypertension prevalence ranging from 24% to 29% in Buenos Aires (Argentina), Santiago (Chile), and Barquisimeto (Venezuela), whereas from 9% to 13% in Quito (Ecuador), Mexico City (Mexico), Lima (Peru), and Bogota (Colombia). However, the prevalence of diabetes in these cities were similar to global estimates that was about 7%. 101 Therefore, developing capacity for high quality, population‐based, and clinical research in LMICs is warranted.
Along with the aforementioned key contributors to the younger age of stroke in LMICs, there are other potential risks, such as household air pollution, urbanization, poor diet, and social vulnerability that vary by country's economic level. 102 , 103 For example, there is evidence suggesting 21% of all stroke deaths are attributable to air pollution globally, 104 and 87% of this burden occurs in LMICs, particularly concentrated in sub‐Saharan Africa and South and East Asia. 105 However, reliable global data about these factors are not available, especially from LMICs. Therefore, burden of these risk factors and their possible associations with stroke, especially in LMICs, require further investigation.
Limitations
We acknowledge certain limitations of our study. First, although we made every attempt to include as much data as possible from LMICs, such data particularly from population‐based studies with high methodological quality are scarce and publication bias is also possible. Second, LMICs might have had lower rates of neuroimaging investigations than HICs, which can limit the ability to distinguish between stroke types particularly in LMICs. Furthermore, for the stroke risk factors, we only considered main stroke risk factors with available data covering most of the HICs and LMICs. There are additional risk factors for stroke such as smoking, level of physical activity, diet/nutrition, and air pollution that are not included in this analysis. Since the country‐level variance data were not available for the age of stroke and the prevalence of risk factors, homogeneity of variance test was not performed.
Conclusions
In our systematic review, we found that although strokes occur at younger ages in LMICs as compared to HICs regardless of gender, subtype of stroke and whether the data are collected at population or hospital level, the younger age of stroke in LMICs than in HICs is not necessarily related to the overall country income level. Specifically, our analyses suggest health care access and quality as a main contributor to higher burden and younger age of stroke in all countries. In addition, women in LMICs may be more susceptible to stroke at younger ages than those in HICs even after accounting for HAQ. Therefore, improving the quality and accessibility of care, especially for vulnerable populations such as women can reduce the gap in mean age and burden of stroke between LMICs and HICs. In addition, developing capacity for high quality population‐based and clinical research in LMICs is crucial.
Conflict of Interest
The authors declare that they have no conflict of interest.
Supporting information
Table S1. Comparison of the prevalence of hypertension (systolic blood pressure ≥140 or diastolic blood pressure ≥90) among adults from age group of ≥25 years between LMICs and HICs.
Table S2. Comparison of the prevalence of diabetes (fasting blood glucose ≥7.0 mmol/L or on medication) between LMICs and HICs.
Table S3. Comparison of the prevalence of high cholesterol (total cholesterol ≥6.2 mmol/L) among adults from age group of ≥25 years between LMICs and HICs.
Table S4. Comparison of the prevalence of obesity (BMI ≥30) and overweight (BMI ≥25) between LMICs and HICs.
Table S5. Comparison of the prevalence of atrial fibrillation between LMICs and HICs.
Table S6. PRISMA 2020 Checklist.
Acknowledgments
The Biostatistics/Epidemiology/Research Design (BERD) component of the Center for Clinical and Translational Sciences (CCTS) provided support for this project. CCTS‐BERD is mainly funded by the NIH Centers for Translational Science Award (NIH CTSA), awarded to the University of Texas Health Science Center at Houston in 2006 by the National Center for Research Resources (NCRR) and its renewal by National Center for Advancing Translational Sciences (NCATS) in 2012 and a new grant in 2019. The content is solely the responsibility of the authors and does not necessarily represent the official views of NCRR or NCATS.
Funding Information Grant No. (UL1 RR024148), funded by NCRR in 2006. Grant No. (UL1 TR000371), funded by NCATS in 2012. Grant No. (UL1 TR003167), funded by NCATS in 2019.
Funding Statement
This work was funded by National Center for Advancing Translational Sciences (NCATS) grants UL1 TR000371 and UL1 TR003167; National Center for Research Resources (NCRR) grant UL1 RR024148.
References
- 1. Hsieh FI, Lien LM, Chen ST, et al. Get With the Guidelines‐stroke performance indicators: surveillance of stroke care in the Taiwan Stroke Registry: Get With the Guidelines‐Stroke in Taiwan. Circulation. 2010;122:1116‐1123. [DOI] [PubMed] [Google Scholar]
- 2. Donkor ES. Stroke in the 21(st) century: a snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat. 2018;2018:3238165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson W, Onuma O, Owolabi M, Sachdev S. Stroke: a global response is needed. Bull World Health Organ. 2016;94:634‐a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pandian JD, Kalkonde Y, Sebastian IA, Felix C, Urimubenshi G, Bosch J. Stroke systems of care in low‐income and middle‐income countries: challenges and opportunities. Lancet. 2020;396:1443‐1451. [DOI] [PubMed] [Google Scholar]
- 5. Jacob MA, Ekker MS, Allach Y, et al. Global differences in risk factors, etiology, and outcome of ischemic stroke in young adults: a worldwide meta‐analysis: the goal‐initiative. Neurology. 2021. doi: 10.1212/wnl.0000000000013195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krishnamurthi RV, Ikeda T, Feigin VL. Global, regional and country‐specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the Global Burden of Disease Study 2017. Neuroepidemiology. 2020;54:171‐179. [DOI] [PubMed] [Google Scholar]
- 7. Lee H, Nam YS, Lee KM. Development‐assistance strategies for stroke in low‐ and middle‐income countries. J Korean Med Sci. 2015;30 suppl 2(suppl 2):S139‐S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Global Burden of Disease (GBD) 2016 Stroke Collaborators . Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:439‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Habibi‐Koolaee M, Shahmoradi L, Niakan Kalhori SR, Ghannadan H, Younesi E. Prevalence of stroke risk factors and their distribution based on stroke subtypes in Gorgan: a retrospective hospital‐based study‐2015–2016. Neurol Res Int. 2018;2018:2709654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langhorne P, O'Donnell MJ, Chin SL, et al. Practice patterns and outcomes after stroke across countries at different economic levels (INTERSTROKE): an international observational study. Lancet. 2018;391:2019‐2027. [DOI] [PubMed] [Google Scholar]
- 12. Krishnamurthi RV, Moran AE, Feigin VL, et al. Stroke prevalence, mortality and disability‐adjusted life years in adults aged 20–64 years in 1990–2013: data from the Global Burden of Disease 2013 study. Neuroepidemiology. 2015;45:190‐202. [DOI] [PubMed] [Google Scholar]
- 13. Prince MJ, Wu F, Guo Y, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385:549‐562. [DOI] [PubMed] [Google Scholar]
- 14. Gezmu T, Schneider D, Demissie K, Lin Y, Gizzi MS. Risk factors for acute stroke among South Asians compared to other racial/ethnic groups. PLoS One. 2014;9:e108901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feigin VL, Krishnamurthi RV, Parmar P, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology. 2015;45:161‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first‐ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1, e259‐e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feigin VL. Stroke epidemiology in the developing world. Lancet. 2005;365:2160‐2161. [DOI] [PubMed] [Google Scholar]
- 18. Kolominsky‐Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long‐term survival in ischemic stroke subtypes: a population‐based study. Stroke. 2001;32:2735‐2740. [DOI] [PubMed] [Google Scholar]
- 19. Khaku AS & Tadi P Cerebrovascular disease (stroke). StatPearls [Internet]. 2020. January 31, 2021.
- 20. Owolabi MO, Johnson W, Khan T, Feigin V. Effectively combating stroke in low‐ and middle‐income countries: placing proof in pragmatism—the Lancet Neurology Commission. J Stroke Med. 2018;1:65‐67. doi: 10.1177/2516608518776706 [DOI] [Google Scholar]
- 21. Lip GYH, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142:1489‐1498. [DOI] [PubMed] [Google Scholar]
- 22. Feigin VL, Roth GA, Naghavi M, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913‐924. [DOI] [PubMed] [Google Scholar]
- 23. Collaborators GHAaQ . Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. Lancet. 2018;391:2236‐2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kruk ME, Gage AD, Arsenault C, et al. High‐quality health systems in the Sustainable Development Goals era: time for a revolution. Lancet Glob Health. 2018;6:e1196‐e1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Brien EC, Wu J, Zhao X, et al. Healthcare resource availability, quality of care, and acute ischemic stroke outcomes. J Am Heart Assoc. 2017;6:e003813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The World Bank New World Bank country classifications by income level: 2020–2021. 2021. Accessed August 10, 2021. https://blogs.worldbank.org/opendata/new‐world‐bank‐country‐classifications‐income‐level‐2020‐2021
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Global Health Data Exchange . Global Burden of Disease (GBD) Results Tool 2021. 2021. Accessed July 21, 2021. http://ghdx.healthdata.org/gbd‐results‐tool
- 29.World Health Organization. The global health observatory. 2021.
- 30.International Diabetes Federation. Diabetes data portal. 2019. Accessed July 19, 2021. https://www.diabetesatlas.org/data/en/
- 31.World Health Organization. The global health observatory: raised blood pressure (SBP>=140 OR DBP>=90) (age‐standardized estimate). 2021. [7/21/2021].
- 32. World Health Organization . The global health observatory: cholestrol‐raised total cholestrol >=6.2 mmol/L. 2021. Accessed July 21, 2021. https://www.who.int/data/gho/data/themes/topics/indicator‐groups/indicator‐group‐details/GHO/cholesterol‐‐‐raised‐total‐cholesterol‐=6‐2‐mmol‐l
- 33.World Health Organization. The global health observatory: prevelance of obesity among adults, BMI>=30. 2021. Accessed July 21, 2021. https://www.who.int/data/gho/indicator‐metadata‐registry/imr‐details/2389
- 34. World Health Organization . The global health observatory: prevalence of raised fasting blood glucose. 2021. Accessed July 19, 2021. https://www.who.int/data/gho/indicator‐metadata‐registry/imr‐details/2379
- 35. SAS Institute . SAS 9.4 Online Documentation. SAS Institute Inc; 2013. [Google Scholar]
- 36. Abanto C, Ton TG, Tirschwell DL, et al. Predictors of functional outcome among stroke patients in Lima, Peru. J Stroke Cerebrovasc Dis. 2013;22:1156‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baggio JAO, Farias DH, Albuquerque LLG, et al. Functional outcomes among stroke patients in Alagoas, Brazil: observational study. Sao Paulo Med J. 2021;139:156‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Türk Börü Ü, Kulualp A, Tarhan ÖF, et al. Stroke prevalence among the Turkish population in a rural area of Istanbul: a community‐based study. SAGE Open Med. 2018;6:2050312118797565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Díaz‐Escobar L, Flores A, Seró‐Ballesteros L. Frequency of hypertension, age, and gender in cerebrovascular disease in Paraguay. Rev Mex Neurocienc. 2019;20:130‐135. [Google Scholar]
- 40. Góngora Rivera F. Perspective on stroke in Mexico. Med Univ. 2015;17:184‐187. [Google Scholar]
- 41. Susmitha K, Chandrasekhar V, Kiran G, Divya A, Rani GS, Sarangapani M, eds. Prevalence of Stroke Cases in Warangal, Telangana Region, India: A Hospital‐Based Case Study. Springer International Publishing; 2020. [DOI] [PubMed] [Google Scholar]
- 42. Kulesh SD, Filina NA, Frantava NM, et al. Incidence and case‐fatality of stroke on the East border of the European union: the Grodno Stroke Study. Stroke. 2010;41:2726‐2730. [DOI] [PubMed] [Google Scholar]
- 43. Lu MY, Chen CH, Yeh SJ, et al. Comparison between in‐hospital stroke and community‐onset stroke treated with endovascular thrombectomy. PLoS One. 2019;14:e0214883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mihálka L, Smolanka V, Bulecza B, Mulesa S, Bereczki D. A population study of stroke in West Ukraine: incidence, stroke services, and 30‐day case fatality. Stroke. 2001;32:2227‐2231. [DOI] [PubMed] [Google Scholar]
- 45. Minelli C, Cabral NL, Ujikawa LT, et al. Trends in the incidence and mortality of stroke in Matão, Brazil: the Matão Preventing Stroke (MAPS) study. Neuroepidemiology. 2020;54:75‐82. [DOI] [PubMed] [Google Scholar]
- 46. Misbach J, Ali W. Stroke in Indonesia: a first large prospective hospital‐based study of acute stroke in 28 hospitals in Indonesia. J Clin Neurosci. 2001;8:245‐249. [DOI] [PubMed] [Google Scholar]
- 47. Pandian JD, Liu M, Misbach J, Venketasubramanian N. Alternative therapies for stroke treatment in Asia. Int J Stroke. 2011;6:541‐543. [DOI] [PubMed] [Google Scholar]
- 48. Pandian JD, Sudhan P. Stroke epidemiology and stroke care services in India. J Stroke. 2013;15:128‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pham TL, Blizzard L, Srikanth V, et al. Case‐fatality and functional status three months after first‐ever stroke in Vietnam. J Neurol Sci. 2016;365:65‐71. [DOI] [PubMed] [Google Scholar]
- 50. Regenhardt RW, Biseko MR, Shayo AF, et al. Opportunities for intervention: stroke treatments, disability and mortality in urban Tanzania. Int J Qual Health Care. 2019;31:385‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sallam A‐R, Al‐Aghbari K, Awn H. The clinical profile of stroke: a Yemeni experience. Jordan Med J. 2009;43:115‐121. [Google Scholar]
- 52. Sarfo FS, Agyei M, Ogyefo I, Opare‐Addo PA, Ovbiagele B. Factors linked to chronic kidney disease among stroke survivors in Ghana. J Stroke Cerebrovasc Dis. 2021;30:105720. [DOI] [PubMed] [Google Scholar]
- 53. Shaik MM, Loo KW, Gan SH. Burden of stroke in Nepal. Int J Stroke. 2012;7:517‐520. [DOI] [PubMed] [Google Scholar]
- 54. Sherin A, Ul‐Haq Z, Fazid S, Shah BH, Khattak MI, Nabi F. Prevalence of stroke in Pakistan: findings from Khyber Pakhtunkhwa integrated population health survey (KP‐IPHS) 2016–17. Pak J Med Sci. 2020;36:1435‐1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun XG, Wang YL, Zhang N, et al. Incidence and trends of stroke and its subtypes in Changsha, China from 2005 to 2011. J Clin Neurosci. 2014;21:436‐440. [DOI] [PubMed] [Google Scholar]
- 56. Suwanwela NC. Stroke epidemiology in Thailand. J Stroke. 2014;16:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Torrealba‐Acosta G, Carazo‐Céspedes K, Chiou SH, O'Brien AT, Fernández‐Morales H. Epidemiology of stroke in Costa Rica: a 7‐year hospital‐based acute stroke registry of 1319 consecutive patients. J Stroke Cerebrovasc Dis. 2018;27:1143‐1152. [DOI] [PubMed] [Google Scholar]
- 58. Wang YJ, Li ZX, Gu HQ, et al. China Stroke Statistics 2019: a report from the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non‐communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc Neurol. 2020;5:211‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zewde YZ, Mengesha AT, Gebreyes YF, Naess H. The frequency and impact of admission hyperglycemia on short term outcome of acute stroke patients admitted to Tikur Anbessa Specialized hospital, Addis Ababa, Ethiopia: a cross‐sectional study. BMC Neurol. 2019;19:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhumabayev M, Maltabarova N, Kokoshko A. A cerebralstroke and the problem of inflammatory complications. Res J Med Sci. 2016;10:492‐497. [Google Scholar]
- 61. Al‐Senani F, Al‐Johani M, Salawati M, et al. An epidemiological model for first stroke in Saudi Arabia. J Stroke Cerebrovasc Dis. 2020;29:104465. [DOI] [PubMed] [Google Scholar]
- 62. Asberg S, Henriksson KM, Farahmand B, et al. Ischemic stroke and secondary prevention in clinical practice: a cohort study of 14,529 patients in the Swedish Stroke Register. Stroke. 2010;41:1338‐1342. [DOI] [PubMed] [Google Scholar]
- 63. Askim T, Bernhardt J, Løge AD, Indredavik B. Stroke patients do not need to be inactive in the first two‐weeks after stroke: results from a stroke unit focused on early rehabilitation. Int J Stroke. 2012;7:25‐31. [DOI] [PubMed] [Google Scholar]
- 64. Bambhroliya AB, Donnelly JP, Thomas EJ, et al. Estimates and temporal trend for US nationwide 30‐day hospital readmission among patients with ischemic and hemorrhagic stroke. JAMA Netw Open. 2018;1:e181190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Baradaran H, Delic A, Wong KH, et al. Using ultrasound and inflammation to improve prediction of ischemic stroke: a secondary analysis of the Multi‐Ethnic Study of Atherosclerosis. Cerebrovasc Dis Extra. 2021;11:37‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Béjot Y, Daubail B, Jacquin A, et al. Trends in the incidence of ischaemic stroke in young adults between 1985 and 2011: the Dijon Stroke Registry. J Neurol Neurosurg Psychiatry. 2014;85:509‐513. [DOI] [PubMed] [Google Scholar]
- 67. Bhattacharya P, Khanal D, Madhavan R, Chaturvedi S. Why do ischemic stroke and transient ischemic attack patients get readmitted? J Neurol Sci. 2011;307:50‐54. [DOI] [PubMed] [Google Scholar]
- 68. Fukuda K, Kai H, Kamouchi M, et al. Day‐by‐day blood pressure variability and functional outcome after acute ischemic stroke: Fukuoka Stroke Registry. Stroke. 2015;46:1832‐1839. [DOI] [PubMed] [Google Scholar]
- 69. Gracia F, Benzadon A, Gonzalez‐Castellon M, Armien B. The impact of cerebrovascular disease in Panama. Int J Stroke. 2014;9 suppl A100:28‐30. [DOI] [PubMed] [Google Scholar]
- 70. Icks A, Claessen H, Kvitkina T, et al. Incidence and relative risk of stroke in the diabetic and the non‐diabetic population between 1998 and 2014: a community‐based stroke register. PLoS One. 2017;12:e0188306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Icks A, Scheer M, Genz J, Giani G, Glaeske G, Hoffmann F. Stroke in the diabetic and non‐diabetic population in Germany: relative and attributable risks, 2005–2007. J Diabetes Complications. 2011;25:90‐96. [DOI] [PubMed] [Google Scholar]
- 72. Imam YZ, Kamran S, Saqqur M, et al. Stroke in the adult Qatari population (Q‐stroke) a hospital‐based retrospective cohort study. PLoS One. 2020;15:e0238865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gaudiano J, Graña D, Gómez A, et al. Búsqueda etiológica de ACV en salas de medicina del Hospital Pasteur, Montevideo‐Uruguay. Julio‐setiembre 2019. Rev Urug Med Interna. 2020;5:19‐27. [Google Scholar]
- 74. Kissela BM, Khoury JC, Alwell K, et al. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79:1781‐1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lavados PM, Hoffmeister L, Moraga AM, et al. Incidence, risk factors, prognosis, and health‐related quality of life after stroke in a low‐resource community in Chile (ÑANDU): a prospective population‐based study. Lancet Glob Health. 2021;9:e340‐e351. [DOI] [PubMed] [Google Scholar]
- 76. Li C, Baek J, Sanchez BN, Morgenstern LB, Lisabeth LD. Temporal trends in age at ischemic stroke onset by ethnicity. Ann Epidemiol. 2018;28:686‐90.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Madsen TE, Khoury J, Alwell K, et al. Sex‐specific stroke incidence over time in the Greater Cincinnati/Northern Kentucky Stroke Study. Neurology. 2017;89:990‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Palm F, Dos Santos M, Urbanek C, et al. Stroke seasonality associations with subtype, etiology and laboratory results in the Ludwigshafen Stroke Study (LuSSt). Eur J Epidemiol. 2013;28:373‐381. [DOI] [PubMed] [Google Scholar]
- 79. Polak JF, Pencina MJ, O'Leary DH, D'Agostino RB. Common carotid artery intima‐media thickness progression as a predictor of stroke in Multi‐Ethnic Study of Atherosclerosis. Stroke. 2011;42:3017‐3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rahbar M, Medrano M, Diaz‐Garelli F, et al., eds. Quality of Acute Ischemic Stroke Care in Dominican Republic: An Initial Report on Patients' Characteristics and Clinical Outcomes. European Stroke Organization (ESO)‐World Stroke Organization (WSO); 2020. Virtual. [Google Scholar]
- 81. Rochemont DR, Mimeau E, Misslin‐Tritsch C, et al. The epidemiology and management of stroke in French Guiana. BMC Neurol. 2020;20:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sacco S, Ornello R, Degan D, Tiseo C, Pistoia F, Carolei A. Declining incidence of intracerebral hemorrhage over two decades in a population‐based study. Eur J Neurol. 2016;23:1627‐1634. [DOI] [PubMed] [Google Scholar]
- 83. Stranjalis G, Loufardaki M, Koutsarnakis C, Kalamatianos T. Trends in the management and hospital outcome of spontaneous subarachnoid hemorrhage in the post‐international subarachnoid aneurysm trial era in Greece: analysis of 719 patients during a 13‐year period. World Neurosurg. 2016;88:327‐332. [DOI] [PubMed] [Google Scholar]
- 84. Tsivgoulis G, Patousi A, Pikilidou M, et al. Stroke incidence and outcomes in northeastern Greece: the Evros Stroke Registry. Stroke. 2018;49:288‐295. [DOI] [PubMed] [Google Scholar]
- 85. Zevallos J, Santiago F, González J, et al. Burden of stroke in Puerto Rico. Int J Stroke. 2015;10:117‐119. [DOI] [PubMed] [Google Scholar]
- 86. World Health Organization . The global health observatory: prevelance of raised fasting blood sugar 2016. Accessed July 15, 2021. https://www.who.int/data/gho/indicator‐metadata‐registry/imr‐details/2379
- 87. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095‐2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bai Y, Wang YL, Shantsila A, Lip GYH. The global burden of atrial fibrillation and stroke: a systematic review of the clinical epidemiology of atrial fibrillation in Asia. Chest. 2017;152:810‐820. [DOI] [PubMed] [Google Scholar]
- 89. Owolabi MO, Thrift AG, Martins S, et al. The state of stroke services across the globe: report of World Stroke Organization‐World Health Organization surveys. Int J Stroke. 2021;16(8):889‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Owolabi MO, Akarolo‐Anthony S, Akinyemi R, et al. The burden of stroke in Africa: a glance at the present and a glimpse into the future. Cardiovasc J Afr. 2015;26(2 suppl 1):S27‐S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pandian JD, Verma SJ, Arora D, et al. INSTRuCT: protocol, infrastructure, and governance. Stroke. 2021;52:e574‐e580. [DOI] [PubMed] [Google Scholar]
- 92. Ezzati M, Obermeyer Z, Tzoulaki I, Mayosi BM, Elliott P, Leon DA. Contributions of risk factors and medical care to cardiovascular mortality trends. Nat Rev Cardiol. 2015;12:508‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kaur P, Kwatra G, Kaur R, Pandian JD. Cost of stroke in low and middle income countries: a systematic review. Int J Stroke. 2014;9:678‐682. [DOI] [PubMed] [Google Scholar]
- 94. Bernhardt J, Urimubenshi G, Gandhi DBC, Eng JJ. Stroke rehabilitation in low‐income and middle‐income countries: a call to action. Lancet. 2020;396:1452‐1462. [DOI] [PubMed] [Google Scholar]
- 95. Rahbar M, Medrano M, Diaz‐Garelli F, et al., eds. Comparison of age at the oneset of stroke and prevalence of risk factors between high‐income and low‐middle income countries featuring dominican republic: a systematic review. 13th WORLD STROKE CONGRESS 2021 October 28–29, 2021; Virtual.
- 96. Sposato LA, Saposnik G. Gross domestic product and health expenditure associated with incidence, 30‐day fatality, and age at stroke onset: a systematic review. Stroke. 2012;43:170‐177. [DOI] [PubMed] [Google Scholar]
- 97. Guzik A, Bushnell C. Stroke epidemiology and risk factor management. Continuum (Minneap Minn). 2017;23(1, Cerebrovascular Disease):15‐39. [DOI] [PubMed] [Google Scholar]
- 98. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics‐2016 update: a report from the American Heart Association. Circulation. 2016;133:e38‐e360. [DOI] [PubMed] [Google Scholar]
- 99. Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high‐, middle‐, and low‐income countries. JAMA. 2013;310:959‐968. [DOI] [PubMed] [Google Scholar]
- 100. Miranda JJ, Barrientos‐Gutiérrez T, Corvalan C, et al. Understanding the rise of cardiometabolic diseases in low‐ and middle‐income countries. Nat Med. 2019;25:1667‐1679. [DOI] [PubMed] [Google Scholar]
- 101. Schargrodsky H, Hernández‐Hernández R, Champagne BM, et al. CARMELA: assessment of cardiovascular risk in seven Latin American cities. Am J Med. 2008;121:58‐65. [DOI] [PubMed] [Google Scholar]
- 102. Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high‐income, middle‐income, and low‐income countries (PURE): a prospective cohort study. Lancet. 2020;395:795‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yusuf S, Rangarajan S, Teo K, et al. Cardiovascular risk and events in 17 low‐, middle‐, and high‐income countries. N Engl J Med. 2014;371:818‐827. [DOI] [PubMed] [Google Scholar]
- 104. Schraufnagel DE, Balmes JR, Cowl CT, et al. Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies' Environmental Committee, Part 2: air pollution and organ systems. Chest. 2019;155:417‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. World Health Organization . Ambient air pollution: a global assessment of exposure and burden of disease. 2016. Accessed July 21, 2021. https://apps.who.int/iris/bitstream/handle/10665/250141/9789241511353‐eng.pdf?sequence=1&isAllowed=y
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of the prevalence of hypertension (systolic blood pressure ≥140 or diastolic blood pressure ≥90) among adults from age group of ≥25 years between LMICs and HICs.
Table S2. Comparison of the prevalence of diabetes (fasting blood glucose ≥7.0 mmol/L or on medication) between LMICs and HICs.
Table S3. Comparison of the prevalence of high cholesterol (total cholesterol ≥6.2 mmol/L) among adults from age group of ≥25 years between LMICs and HICs.
Table S4. Comparison of the prevalence of obesity (BMI ≥30) and overweight (BMI ≥25) between LMICs and HICs.
Table S5. Comparison of the prevalence of atrial fibrillation between LMICs and HICs.
Table S6. PRISMA 2020 Checklist.


