Abstract
Objective
To investigate whether herpes zoster (HZ) was associated with subsequent increased risk of dementia diagnosis.
Methods
We conducted a historical cohort study using primary care electronic health records from the Clinical Practice Research Datalink in the United Kingdom. Individuals with incident HZ aged ≥40 years from 2000 to 2017 were matched with up to four individuals without HZ by age, sex, primary care practise and calendar time. The primary outcome was a new diagnosis of all‐cause dementia. We used the Cox proportional hazards regression adjusting for demographic, lifestyle and clinical confounders to assess any association between HZ and dementia. We investigated interactions with sex, frailty index and antiviral treatment and conducted various sensitivity analyses.
Results
The cohort comprised 177,144 individuals with HZ and 706,901 matched unexposed individuals (median age 65 years (IQR 55.1–75.0), 40% male) followed for a median duration of 4.6 years (IQR 2.0–8.1). In total, 26,585 (3%) patients had an incident dementia diagnosis recorded and 113,056 patients died (12.8%). HZ was associated with a small reduction in dementia diagnosis (adjusted HR 0.92 (95% CI 0.89–0.95)), occurring predominantly in frail individuals and females. For patients who were fit (578,115, 65%), no association was seen (adjusted HR 0.97, 95% CI 0.92–1.02). There was no association between HZ and a composite outcome of dementia or death (adjusted HR 1.00, 95% CI 0.99–1.02). Dementia risk did not vary by prescription of antiviral agents. Sensitivity analyses showed consistent results.
Interpretation
HZ was not associated with increased dementia diagnosis in a UK primary care‐based cohort.
Introduction
The global burden of dementia is rising, with the number of people living with dementia projected to reach 131.5 million in 2050 compared to 46.8 million in 2015. 1 Driven by increases in life expectancy among low‐ and middle‐income countries, this will have a major impact upon disability, dependence and quality of life, especially in settings where access to care and support services is limited. 2 Only around 40% of dementia cases are potentially preventable by action on known modifiable risk factors. 3 Combined with the persistent failure of dementia treatment trials, this has prompted renewed urgency in the search for tractable dementia risk factors.
Infections may exacerbate or accelerate progression of a range of underlying diseases. 4 , 5 , 6 , 7 In the brain, a dysregulated microglial response to injurious stimuli may disrupt usual sentinel and housekeeping pathways, leading to ongoing neuroinflammation and neuronal damage. Dysregulation of host immune pathways is implicated both in Alzheimer's disease pathogenesis, for example, through persistent amyloid beta deposition resulting from chronic interaction with microglia, and in the tauopathies, which are associated with accumulation of pro‐inflammatory microglia, toxic insoluble tau and neuronal loss. 8 Although precise mechanisms remain controversial, the effect of a dysregulated immune response to HIV infection contributes to HIV‐associated neurocognitive disorders. 9 While other chronic viral infections are likely to trigger microglial responses, whether, when and in whom such infections might affect progression to clinical dementia is unknown.
The incidence of herpes zoster (HZ), a painful, blistering, dermatomal rash that occurs due to varicella zoster virus reactivation, rises sharply with age as cell‐mediated immunity declines. HZ may lead to localised post‐herpetic neuralgia, as well as a range of, albeit uncommon, acute ophthalmic, visceral and neurological complications such as stroke. 10 It is less clear whether HZ has other long‐term sequelae including effects on neurodegeneration. A small propensity score matched cohort from Taiwan (N = 3384) demonstrated a threefold increase in dementia risk after ophthalmic zoster, 11 but findings from two matched cohort studies using health insurance claims data from Taiwan and Korea suggested that any increased risk of dementia associated with HZ is likely to be modest (11%–12%). 12 , 13 Recent results from a Korean case control study showed no evidence for an association. 14 Subgroup analyses from a multi‐centre Northern European cohort focussed on antiviral medication use after herpes viruses also failed to support these findings. 15 It is unclear whether differences between populations or within population subgroups could explain the contrasting findings. If a robust, replicable effect were present among certain groups, this could have important implications for HZ vaccination and treatment policy, especially in settings of high infectious burden.
We therefore aimed to investigate whether HZ was associated with subsequent dementia diagnosis overall and among specific population subgroups using routine clinical health data from the United Kingdom.
Methods
Data sources
We used electronic health records data from the Clinical Practice Research Datalink (CPRD). The CPRD Gold database contains anonymised primary care records from approximately 7% of the UK population who are broadly representative of the general population by age, sex and ethnicity. 16 Longitudinal records are generated through routine care and include demographic information, clinical events such as diagnoses and symptoms, prescriptions, investigations, referrals and some lifestyle factors. Data are recorded by general practice staff using Read codes, a hierarchical clinical classification system, along with numerical data on additional clinical measures such as height, weight and blood pressure, and prescriptions issued by the GP with a product name and British National Formulary code. Health information received by the practise from other sources such as secondary care is also coded and entered into the patient record. We also used linked small area deprivation data defined by the index of multiple deprivation (IMD) at general practise level. IMD is a relative measure of deprivation calculated for lower‐layer super output areas, which is based upon seven domains (income; employment; education, skills and training; health deprivation and disability; crime; barriers to housing and services; living environment deprivation). 17
Study design
We conducted a historical cohort study using a matched design. Eligible patients identified as having incident herpes zoster diagnosed during the study period were matched to up to four eligible patients who had not been diagnosed with herpes zoster. Patients were matched on age (within 1 year), sex, general practise and calendar time. Exposure was time‐updated: patients who were selected as an unexposed match could subsequently enter the study as an exposed patient if they developed herpes zoster; their follow‐up as an unexposed match was censored at diagnosis of herpes zoster.
Study population and follow up
Patients were eligible for inclusion if they were aged 40 years or over and had no history of prior dementia or prior herpes zoster. The study period ran from 1 January 2000 to 31 December 2017. Eligible follow‐up time began at the latest of: 1 January 2000, 40th birthday, or having 12 months of eligible follow‐up time within CPRD (when the patient was registered at the practise and practise data were considered to meet acceptable quality standards). Patients were required to be registered with the practise for at least 12 months prior to study entry to prevent historical recording of diagnoses at registration. Follow up began at the index date, which was the date of herpes zoster diagnosis for exposed patients or the date of diagnosis of the exposed match for unexposed patients. Follow‐up ended at the earliest of: dementia diagnosis, death, end of CPRD follow‐up (transfer out of the general practise or last collection date from the practise) or 31 December 2017.
Exposure definition
Exposure was defined as incident herpes zoster at any site ascertained through diagnosis and referral codes in GP records with the date of the first record taken as the index date. In secondary analyses, we investigated the effect of ophthalmic zoster versus zoster at any other site. Ophthalmic zoster was defined either by the presence of a specific code for ophthalmic zoster in the year following first diagnosis, or by a non‐specific zoster code plus a diagnosis of, or treatment for, acute eye infection (such as keratitis or conjunctivitis) within 2 weeks of zoster onset, or from records of first‐ever‐specific chronic eye conditions known to be associated with zoster (such as, conjunctival scarring or episcleritis), within 3 months of zoster using an approach defined in previous studies. 18 We also explored any association between antiviral treatment for zoster, defined as a prescription for an antiviral agent in the 7 days following the index date, and dementia.
Outcome definition
The primary outcome was incident all‐cause dementia defined using diagnostic and referral codes in primary care records, with the date taken as the date of first record. In a sensitivity analysis, we expanded the outcome definition to include administrative as well as diagnosis and referral codes, taking the first of any type of code as dementia date. In this analysis, this wider definition was also used to exclude patients with prior dementia. Secondary outcomes were dementia subtypes (Alzheimer's disease, vascular dementia, other specified dementia, mixed/unspecified dementia). For individuals whose records contained more than one specified dementia subtype, the subtype was recoded to ‘mixed’.
Covariate definitions
Potential confounders were identified in primary care electronic health records used Read codes based upon previous literature and a priori knowledge of risk factors for exposure and outcome. Codelists are available on LSHTM Data Compass (https://doi.org/10.17037/DATA.00002672). Age, sex, general practise and calendar time were matching factors and also potential confounders. Year of study entry, when included in models, was grouped into 2000–2003, 2004–2006, 2007–2009, 2010–2013, 2013–2017. Additional confounders defined at study entry were: frailty, as measured by the electronic frailty index (eFI), 19 grouped into four categories of increasing frailty, grouped frequency of consultations with the general practise in the past 3 years as a measure of health seeking behaviour, harmful alcohol use, body mass index (BMI; grouped into underweight (<18.5), normal (18.5–25), overweight (25–30) and obese (>30) using the record closest to the index date), smoking (never, current and former), uncontrolled diabetes (measure of glycated haemoglobin of 7.5% or above in the last 2 years) and prior diagnosis of: chronic kidney disease, asthma, autoimmune disease, chronic obstructive pulmonary disease, depression in the last 2 years, diagnosed hypertension, ischaemic heart disease, liver disease, stroke, traumatic brain injury and herpes simplex virus. Immunosuppression was determined through diagnoses, referrals and prescriptions (HIV, organ transplant or conditions leading to permanent immune deficiency or aplastic anaemia ever diagnosed, haematological malignancy or bone marrow transplant within the last 2 years, impaired cell‐mediated immunity, chemotherapy or biologics within the last year, or two prescriptions of other oral steroids in the last year). Potential confounding by ethnicity (Black, White, South Asian, Mixed/Other) was assessed in sensitivity analysis only. Socio‐economic status at practise level was measured by quintile of the 2015 index of multiple deprivation (IMD).
Statistical analysis
We first described the characteristics of the cohort by exposure group at study entry. Age‐ and sex‐adjusted rates of incident dementia were calculated by exposure group. We fitted Cox proportional hazards regression models using age as the underlying timescale to estimate cause‐specific hazards of dementia among those with and without HZ exposure. The initial model was adjusted for age (as the timescale) and sex and stratified by practise. Fully adjusted models additionally included the pre‐specified individual‐level confounders detailed above. Robust standard errors clustered by patient were used to account for patients potentially appearing as both exposed and unexposed. The proportional hazards assumption was assessed graphically and using tests based on Schoenfeld residuals. Violations of the assumption were handled via stratified Cox models, if occurring in confounder variables.
We carried out several sensitivity analyses to explore missing covariate data on BMI and smoking, and compared results with the primary complete case analysis. These were: (i) we removed BMI and smoking from the primary analysis model; (ii) we assumed that patients missing smoking information were non‐smokers and patients missing BMI data had a normal BMI; (iii) we imputed missing data for BMI and smoking using chained equations under the missing at random assumption. Ten imputed datasets were created. Continuous BMI was imputed using predictive mean matching, with five nearest neighbours, and smoking using a multinomial logistic regression. Imputation was performed separately by exposure group. Imputation models included the Nelson‐Aalen estimate of cumulative hazard for dementia, the indicators for death and dementia, as well as the exposure and all potential confounders. Cause‐specific Cox models were fitted in each imputed dataset; estimates were combined using Rubin's rules. Due to the large proportion of missing ethnicity data (59%), we did not include it in the primary model. In other sensitivity analyses, we: (iv) carried out a complete case analysis with ethnicity added to our primary analysis model; (v) imputed ethnicity assuming that those with missing ethnicity were White.
Additional sensitivity analyses included (vi) exploring biases resulting from differential follow up in the exposed and unexposed by censoring follow up within a matched set at the first of: the end of the exposed patient's follow‐up, or the last of the end of the unexposed patients' follow‐up; (vii) exploring possible reverse causation by excluding the first year of follow up. Entire matched sets were excluded from this analysis if the exposed patient experienced the outcome within the first year or all unexposed matches experienced the outcome within the first year. Similarly, the first 2, 3 and 4 years of follow up were excluded; (viii) expanding the outcome definition to increase its sensitivity, as the primary outcome was likely to have high specificity but lower sensitivity. First, matched sets were excluded if the exposed patient had a history of dementia using the wider definition or all controls had a history of dementia. Second, the outcome was also defined using the wider definition. These two analyses were undertaken sequentially.
Cause‐specific hazards of death and the composite of death or dementia were estimated using the primary analysis approach described above, censoring follow up at the competing event. We plotted cumulative incidence functions under exposure and no exposure by fitting cause‐specific hazards models for death and dementia using Royston Parmar smooth parametric proportional hazards models and marginalising over the cause‐specific hazards.
Secondary analyses included restricting the exposure definition to ophthalmic zoster only. In this analysis, matched sets were excluded if the exposed patient did not have ophthalmic zoster. We also repeated analyses for the secondary outcomes Alzheimer's disease, vascular dementia, mixed or unspecified dementia and other specified causes, censoring if a different dementia subtype occurred. In other secondary analyses, we explored interactions between HZ and sex, frailty and receipt of antivirals post‐exposure.
Finally, following evidence of non‐proportionality, log‐logistic model and generalised gamma models were also fitted, which do not make the proportional hazards assumption. Model fit was compared using the AIC. Analyses were carried out using Stata version 16 (StataCorp. 2017. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
Ethical approval
This study was approved by the Independent Scientific Advisory Committee of the CPRD (18_134R) and the LSHTM ethics committee (15991).
Results
Description of study population
Figure 1 shows the flowchart of patients included in the study. Of 177,581 eligible patients with an incident HZ diagnosis, 437 (0.3%) were unable to be matched. Four unexposed matches were found for most exposed patients. For 296 sets, three matches were found, for 283 sets, two matches were found and for 271 sets, only one match was identified. The final matched cohort included 177,144 exposed patients and 706,901 matched unexposed patients.
Figure 1.
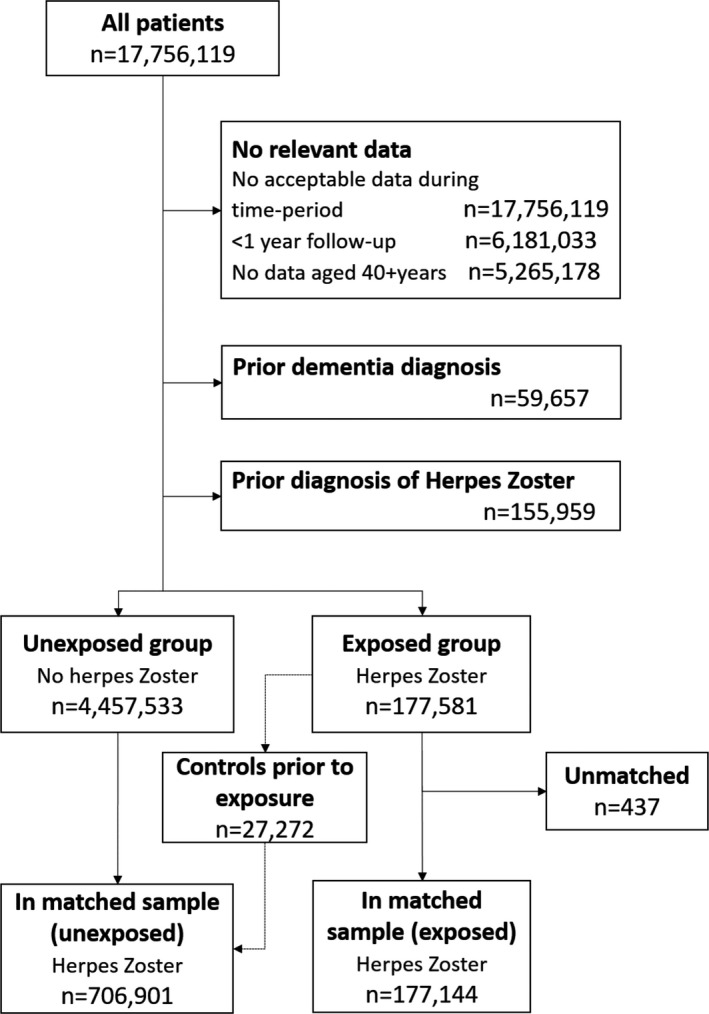
Flowchart of study population.
Table 1 shows baseline characteristics of included patients by exposure status. While the age, sex and practise deprivation quintile distributions were similar due to the matched design, the HZ‐ exposed group had a slightly higher rate of primary care consultations (mean annual consultation rate 10.9 vs. 8.7) and were more likely to be classified as frail than the unexposed group (40.7% vs. 33.1%). BMI distribution and smoking rates were similar across exposure groups, but the unexposed had more missing data (8.8% vs. 5.4%; 2.9% vs. 0.6%), perhaps reflecting lower contact with health services. The HZ‐exposed group generally had a slightly higher proportion of comorbidities, with the exception of traumatic brain injury and stroke. Only 5% of HZ cases were identified as ophthalmic zoster, with site unspecified in 95% of cases. Most exposed patients (63%) received antivirals in the week following diagnosis.
Table 1.
Baseline characteristics by exposure group.
| Characteristic | No Herpes zoster | Herpes zoster |
|---|---|---|
| N = 706,901 | N = 177,144 | |
| Years of follow up: | ||
| Mean (SD) | 5.4 (4) | 5.5 (4.1) |
| Median (25th, 75th ptile) | 4.5 (2,8) | 4.7 (2.1,8.3) |
| Age (years): | ||
| Mean (SD) | 65.1 (12.8) | 65.1 (12.9) |
| Median (25th, 75th ptile) | 65 (55.1,74.9) | 65 (55.1,75) |
| Grouped age: | ||
| 40‐ < 50 | 99,866 (14.1) | 24,967 (14.1) |
| 50‐ < 60 | 161,779 (22.9) | 40,444 (22.8) |
| 60‐ < 70 | 184,997 (26.2) | 46,163 (26.1) |
| 70‐ < 80 | 160,827 (22.8) | 40,320 (22.8) |
| 80+ | 99,432 (14.1) | 25,250 (14.3) |
| Male | 282,061 (39.9) | 70,690 (39.9) |
| Annual consultation rate | ||
| Median (25th, 75th ptile) | 6.3 (2.9,11.7) | 8.3 (4.3,14.3) |
| Consultation rate (contacts/yr): | ||
| < 10 | 482,332 (68.2) | 103,060 (58.2) |
| 10‐ < 20 | 159,423 (22.6) | 50,184 (28.3) |
| 20‐ < 30 | 43,054 (6.1) | 15,447 (8.7) |
| 30+ | 22,092 (3.1) | 8453 (4.8) |
| Frailty Index (eFI) | ||
| Median (25th, 75th ptile) | 0.1 (0,0.2) | 0.1 (0.1,0.2) |
| Frailty Index (eFI) group | ||
| Fit | 473,020 (66.9) | 105,095 (59.3) |
| Mild frailty | 160,726 (22.7) | 47,729 (26.9) |
| Moderate frailty | 56,356 (8.0) | 18,445 (10.4) |
| Severe frailty | 16,799 (2.4) | 5875 (3.3) |
| BMI | ||
| Mean (SD) | 27.2 (5.3) | 27.2 (5.3) |
| Median (25th, 75th ptile) | 26.4 (23.5,29.9) | 26.4 (23.6,29.9) |
| BMI category: | ||
| Underweight | 11,594 (1.6) | 2884 (1.6) |
| Normal Weight | 231,470 (32.7) | 59,262 (33.5) |
| Overweight | 243,383 (34.4) | 63,878 (36.1) |
| Obese | 157,956 (22.3) | 41,483 (23.4) |
| Missing | 62,498 (8.8) | 9637 (5.4) |
| Smoking status: | ||
| No | 264,121 (37.4) | 66,004 (37.3) |
| Yes | 155,237 (22.0) | 35,960 (20.3) |
| Ex | 267,329 (37.8) | 74,081 (41.8) |
| Missing | 20,214 (2.9) | 1099 (0.6) |
| Harmful alcohol use | 24,601 (3.5) | 6071 (3.4) |
| Ethnicity | ||
| White | 271,882 (38.5) | 71,625 (40.4) |
| South Asian | 6302 (0.9) | 1376 (0.8) |
| Black | 3485 (0.5) | 559 (0.3) |
| Other | 2530 (0.4) | 615 (0.3) |
| Mixed | 946 (0.1) | 194 (0.1) |
| Unknown | 421,756 (59.7) | 102,775 (58.0) |
| Deprivation group: | ||
| 1 (least deprived) | 138,157 (19.5) | 34,604 (19.5) |
| 2 | 121,460 (17.2) | 30,434 (17.2) |
| 3 | 144,900 (20.5) | 36,320 (20.5) |
| 4 | 142,616 (20.2) | 35,736 (20.2) |
| 5 (most deprived) | 159,768 (22.6) | 40,050 (22.6) |
| Prior history of: | ||
| Asthma | 87,768 (12.4) | 26,956 (15.2) |
| COPD | 63,536 (9.0) | 19,931 (11.3) |
| Diabetes | 75,524 (10.7) | 20,725 (11.7) |
| Uncontrolled diabetes (2 years) | 32,671 (4.6) | 9271 (5.2) |
| Hypertension (diagnosed) | 232,748 (32.9) | 62,436 (35.2) |
| Ischaemic heart disease | 117,646 (16.6) | 34,429 (19.4) |
| Stroke | 25,152 (3.6) | 6595 (3.7) |
| Traumatic brain injury | 5310 (0.8) | 1454 (0.8) |
| Immunosuppressed | 45,957 (6.5) | 19,623 (11.1) |
| Autoimmune disease | 53,030 (7.5) | 18,015 (10.2) |
| Liver disease | 4820 (0.7) | 1359 (0.8) |
| Chronic kidney disease | 51,882 (7.3) | 15,181 (8.6) |
| Depression (2 years) | 14,310 (2.0) | 4316 (2.4) |
| HSV | 23,607 (3.3) | 8535 (4.8) |
| Details of exposure | ||
| Antivirals (in 7 days) | 186 (<1) | 110,997 (62.7) |
| Site of Zoster: | ||
| Ophthalmic | 8775 (5.0) | |
| Non‐truncal (exclud. HZO) | 984 (0.6) | |
| Site unspecified | 167,385 (94.5) | |
| Outcome events: | ||
| Death | 87,371 (12.4) | 25,685 (14.5) |
| Dementia of which (%) of dementia) | 21,213 (3.0) | 5372 (3.0) |
| Alzheimer's | 6571 (31.0) | 1674 (31.2) |
| Vascular | 4876 (23.0) | 1324 (24.7) |
| Other specified | 808 (3.8) | 203 (3.8) |
| Mixed/ unspecified | 8958 (42.2) | 2171 (40.4) |
| Dementia (wider definition)a | 22,902 (3.2) | 5839 (3.3) |
Smaller denominator; matched sets discarded if the exposed or all unexposed patients had pre‐study dementia by this definition.
Among HZ patients, 25,685 (14.5%) died during follow up and 5372 (3%) experienced incident dementia during follow‐up compared to 87,371 (12.4%) who died and 21,213 (3%) who experienced incident dementia in the unexposed group. Follow‐up periods were similar between groups (median 4.7 years in the exposed v 4.5 years in the unexposed).
Dementia incidence rates
The overall dementia incidence rate was 5.58 (95% CI 5.51–5.65) per 1000 person years. Figure 2 shows estimated incidence rates of dementia by age and sex. As expected, incidence rates increase steeply with age, before declining among people aged 95 years or more. Females had a higher dementia incidence than males apparent from 80 years and over. Figure 3 further stratifies by HZ status and shows little difference in age‐ and sex‐stratified dementia incidence rates between patients with and without HZ until estimates diverge in the oldest (>95 years) age group.
Figure 2.
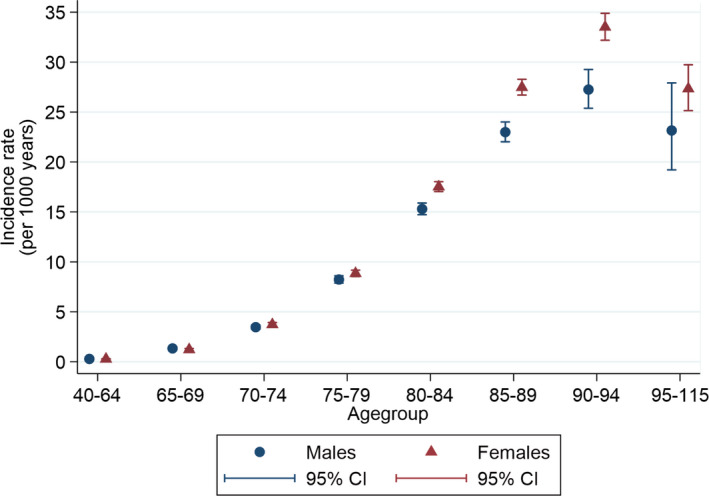
Incidence rates of dementia by age and sex. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
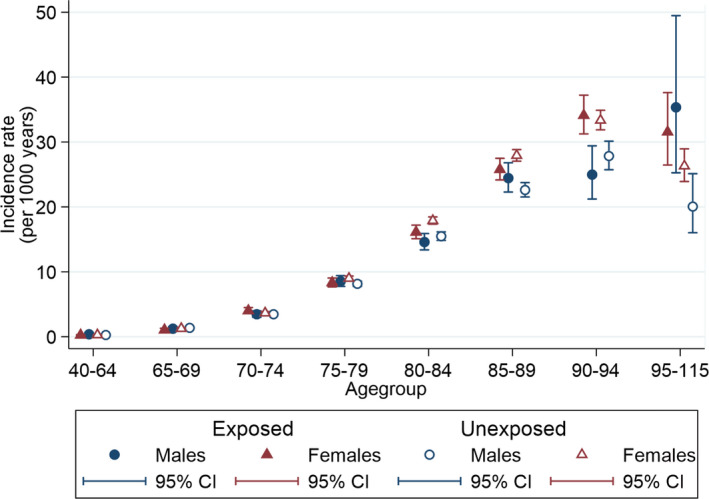
Incidence rates of dementia by age, sex and herpes zoster. [Colour figure can be viewed at wileyonlinelibrary.com]
Association between HZ and incident dementia and sensitivity analyses
Findings from the primary analysis and sensitivity analyses are shown in Table 2. HZ was not associated with incident dementia in an age‐, sex‐ and practice‐adjusted model (HR 0.97, 95% CI 0.94–1.00). Further adjustment for all confounders gave an adjusted HR of 0.92, 95% CI 0.89–0.95. Sensitivity analyses designed to deal with missing covariate data made little difference to the effect estimates: removing BMI and smoking from the model gave very similar estimates to both single and multiple imputation of these variables as well as single imputation of ethnicity data. Adjusting for ethnicity in a complete case analysis resulted in almost identical estimates to the primary analysis (adjusted HR 0.92, 95% C.I 0.88–0.97). Both truncating follow up (adjusted HR 0.90, 95% CI 0.87–0.93) and delaying entry to the study (adjusted HR 0.89, 95% CI 0.86–0.92 for 1 year delay) resulted in estimates that were slightly further from the null value. Using a broader definition of dementia as the outcome did not materially alter results (adjusted HR 0.92, 95% CI 0.89–0.95).
Table 2.
Estimated hazard ratios from Cox models for primary analysis and sensitivity analyses.
| Type of analysis | HR (95% CI) |
|---|---|
| Primary analysis | |
|
Sex‐, age‐ and GP‐adjusted Fully adjusted modela |
0.97 (0.94, 1.00) 0.92 (0.89, 0.95) |
| Sensitivity analyses | |
|
0.93 (0.90, 0.96) 0.93 (0.90, 0.96) 0.93 (0.90, 0.96) 0.92 (0.88, 0.97) 0.93 (0.90, 0.96) 0.89 (0.86, 0.92) 0.87 (0.84, 0.90) 0.86 (0.83, 0.89) 0.86 (0.82, 0.90) 0.90 (0.87, 0.93) 0.92 (0.89, 0.94) 0.92 (0.89, 0.95) |
Adjusted for sex, age, practice, year of study entry, frailty index, prior consultation rate, harmful alcohol use, BMI, smoking, chronic kidney disease, asthma, autoimmune disease, COPD, depression, hypertension, ischaemic heart disease, severe immunosuppression, liver disease, stroke, traumatic brain injury, herpes simplex, uncontrolled diabetes.
Competing risks analysis: association between HZ and dementia, death and dementia or death
Table 3 shows estimated hazard ratios from the primary analysis model, for outcomes of dementia, death and their composite. There was a small increase in risk of death among individuals with HZ (adjusted HR 1.02, 95% CI 1.01–1.04) while the composite outcome of death and dementia showed no association with HZ (adjusted HR 1.00, 95% CI 0.99–1.02).
Table 3.
Estimated HRs for the three outcomes of dementia, death and their composite.
| HR (95% CI) | |||
|---|---|---|---|
| Variable | Dementia | Death | Composite |
| Herpes Zoster | 0.92 (0.89, 0.95) | 1.02 (1.01, 1.04) | 1.00 (0.99, 1.02) |
| Male | 0.90 (0.87, 0.92) | 1.49 (1.47, 1.51) | 1.35 (1.33, 1.37) |
| Year of study entry: | |||
| 2000–2003 | 0.94 (0.90, 0.98) | 1.16 (1.13, 1.18) | 1.11 (1.09, 1.13) |
| 2004–2006 | 0.96 (0.93, 1.00) | 1.12 (1.10, 1.14) | 1.08 (1.07, 1.10) |
| 2007–2009 | Reference | Reference | Reference |
| 2010–2013 | 1.02 (0.98, 1.06) | 0.87 (0.85, 0.89) | 0.90 (0.88, 0.92) |
| 2013–2017 | 1.02 (0.97, 1.08) | 0.78 (0.75, 0.80) | 0.82 (0.80, 0.84) |
| Grouped frailty index: | |||
| Fit | Reference | Reference | Reference |
| Mild frailty | 1.23 (1.19, 1.27) | 1.35 (1.33, 1.37) | 1.33 (1.31, 1.35) |
| Moderate frailty | 1.43 (1.36, 1.50) | 1.68 (1.64, 1.73) | 1.63 (1.59, 1.66) |
| Severe frailty | 1.73 (1.62, 1.86) | 2.15 (2.07, 2.22) | 2.04 (1.98, 2.10) |
| Grouped consultation rate (contact/year): | |||
| < 10 | Reference | Reference | Reference |
| 10‐ < 20 | 1.15 (1.11, 1.18) | 1.17 (1.15, 1.19) | 1.16 (1.15, 1.18) |
| 20‐ < 30 | 1.30 (1.24, 1.37) | 1.40 (1.36, 1.43) | 1.38 (1.35, 1.41) |
| 30+ | 1.34 (1.26, 1.43) | 1.78 (1.73, 1.84) | 1.69 (1.64, 1.74) |
| Harmful alcohol use | 1.36 (1.25, 1.47) | 1.69 (1.64, 1.75) | 1.65 (1.60, 1.70) |
| BMI group: | |||
| Underweight | 1.31 (1.21, 1.40) | 1.90 (1.83, 1.97) | 1.77 (1.71, 1.83) |
| Normal | Reference | Reference | Reference |
| Overweight | 0.84 (0.82, 0.86) | 0.84 (0.83, 0.85) | 0.84 (0.83, 0.85) |
| Obese | 0.75 (0.72, 0.78) | 0.95 (0.93, 0.97) | 0.91 (0.90, 0.92) |
| Smoking status | |||
| Never | Reference | Reference | Reference |
| Current | 1.09 (1.05, 1.13) | 1.76 (1.72, 1.79) | 1.61 (1.58, 1.63) |
| Former | 1.05 (1.02, 1.08) | 1.15 (1.13, 1.17) | 1.13 (1.11, 1.14) |
| History of: | |||
| Chronic kidney disease | 0.94 (0.91, 0.99) | 1.12 (1.09, 1.14) | 1.07 (1.05, 1.10) |
| Asthma | 0.97 (0.94, 1.01) | 0.94 (0.92, 0.96) | 0.94 (0.93, 0.96) |
| Autoimmune disease | 0.97 (0.93, 1.01) | 1.01 (0.99, 1.03) | 1.00 (0.98, 1.02) |
| COPD | 0.94 (0.90, 0.98) | 1.32 (1.29, 1.34) | 1.24 (1.22, 1.27) |
| Depression (last 2 years) | 1.56 (1.45, 1.68) | 1.18 (1.14, 1.23) | 1.25 (1.21, 1.30) |
| Hypertension diagnosis | 0.89 (0.86, 0.91) | 0.99 (0.98, 1.01) | 0.97 (0.96, 0.98) |
| IHD diagnosis | 0.98 (0.95, 1.01) | 1.10 (1.08, 1.12) | 1.08 (1.06, 1.09) |
| Severe immunosuppression | 0.89 (0.84, 0.93) | 1.63 (1.60, 1.67) | 1.48 (1.45, 1.51) |
| Liver disease | 0.93 (0.76, 1.14) | 1.81 (1.69, 1.94) | 1.68 (1.57, 1.80) |
| Stroke | 1.30 (1.24, 1.36) | 1.33 (1.29, 1.36) | 1.32 (1.29, 1.35) |
| Traumatic brain injury | 1.26 (1.10, 1.46) | 0.92 (0.85, 1.00) | 0.98 (0.92, 1.05) |
| HSV | 1.00 (0.93, 1.07) | 0.82 (0.79, 0.86) | 0.86 (0.83, 0.89) |
| Uncontrolled diabetes (last 2 years) | 1.15 (1.09, 1.21) | 1.35 (1.31, 1.38) | 1.31 (1.29, 1.34) |
Figure 4 shows estimated cumulative incidence function for dementia by HZ status; at 100 years, the estimated difference remains less than 2% points.
Figure 4.
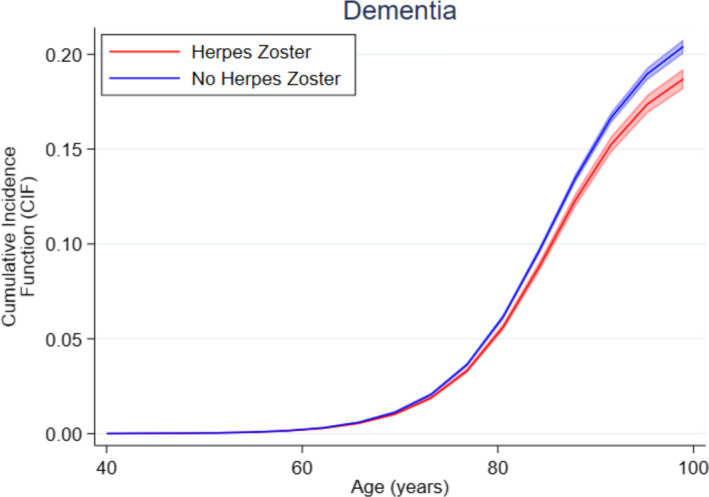
Cumulative cause‐specific incidence function for dementia, by herpes zoster status. [Colour figure can be viewed at wileyonlinelibrary.com]
Secondary analyses
-
i
Ophthalmic zoster. Similar results were seen when restricting exposure to HZO only (adjusted HR 0.88, 95% CI 0.78–0.99) for dementia.
-
ii
Dementia subtypes. There was no association between HZ and Alzheimer's disease (adjusted HZ 0.94, 95% CI 0.89–1.00), vascular dementia (adjusted HR 0.95, 95% C.I, 0.90–1.02) or other specified dementia subtypes (adjusted HR 0.91, 95% CI 0.77–1.07). A protective association was seen between HZ and dementia classified as mixed or unspecified (adjusted HZ 0.88, 95% CI 0.83–0.92).
-
iii
Interactions by sex, frailty and antiviral status. Table 4 shows estimated HRs by sex, frailty and receipt of antiviral drugs. There was some evidence of an interaction of HZ with sex, with no association for males but a protective association for females. There was strong evidence of an interaction with frailty, with the protective association absent in the fittest patients at baseline and increasing with increasing frailty. There was no evidence of a difference in association according to antiviral treatment status.
Table 4.
Estimated HRs for dementia, including interactions (individually) with sex, frailty and antivirals post‐exposure.
| Variable | HR (95% CI) | Interaction p‐value |
|---|---|---|
| Sex | 0.036 | |
| Female | 0.89 (0.86, 0.93) | |
| Male | 0.96 (0.91, 1.01) | |
| Grouped frailty index: | <0.001 | |
| Fit | 0.97 (0.92, 1.02) | |
| Mild frailty | 0.91 (0.86, 0.96) | |
| Moderate frailty | 0.88 (0.82, 0.95) | |
| Severe frailty | 0.82 (0.73, 0.92) | |
| Antivirals | 0.774 | |
| No | 0.91 (0.87, 0.96) | |
| Yes | 0.92 (0.89, 0.95) |
Model checking
There was no evidence against the proportional hazards assumption for the main exposure of interest, HZ, in any of these Cox models. However, the proportional hazards assumption was violated for several coefficients and stratifying the Cox model did not resolve all these problems. Stratifying by general practise, sex, calendar year, frailty, consultation rate, BMI group and history of stroke (the key variables leading to PH violations) made little difference to the hazard ratio for HZ (adjusted HR 0.94, 95% CI 0.90–0.98). When AICs for various model types were compared, the generalised gamma model, an accelerated failure time model which does not rely upon the proportional hazards assumption, fit best––see Table 5. This model estimates that the median time to dementia diagnosis is 1.009 times longer (i.e. increased by approximately 1%) in the HZ group compared to the unexposed group, which is consistent with findings from the proportional hazards models suggesting a small overall reduction in hazard of dementia diagnosis associated with HZ.
Table 5.
AICs and estimated point estimates (Hazard ratio for PH models or time ratios for AFT models) for Herpes Zoster from various survival models.
| Model | Model type | AIC | Point estimate (95% CI) exponentiated |
|---|---|---|---|
| Cox | PH | 543,745.1 | 0.915 (0.887, 0.944) |
| Weibull | PH and AFT | 43,607.56 | 0.908 (0.880, 0.936) |
| Log‐logistic | AFT | 42,572.3 | 1.009 (1.006, 1.012) |
| Generalised gamma | AFT | 42,327.88 | 1.009 (1.006, 1.012) |
PH Proportional hazards; AFT accelerated failure time.
Note: models all excluded general practise; this made little difference to the Cox model estimates.
Discussion
Summary of findings
We found no evidence that HZ was associated with an increased risk of subsequent dementia diagnosis in a large UK population‐based cohort. A small protective association between HZ and dementia was seen only for frail individuals and females, and for mixed/unspecified dementia. There was no difference by receipt of antiviral medications. HZ was not associated with a composite outcome of dementia or death. These findings were robust across various sensitivity analyses.
Comparison with other studies
Our results were consistent with recent findings from other cohorts for example, from the SAIL Databank in Wales in which both diagnosed and treated individuals with HZ, as well as diagnosed and untreated individuals with HZ, had a 9%–10% lower risk of dementia diagnosis recording than individuals without HZ. 15 In the same study, individuals with treated HZ from Denmark had a 10% lower dementia diagnosis risk, while there was no difference in dementia diagnoses in Germany among those diagnosed and treated for HZ. 15 These findings contrast with studies using insurance claims data from Taiwan and Korea, three of which showed an increase in dementia risk after herpes zoster 11 , 12 , 13 and two reporting a large reduction in dementia among those with HZ treated with antiviral medications. 12 , 13 Our sample size was at least twice as large as all previous studies, except for the Denmark cohort in which information on HZ diagnoses were not available and had to be inferred from antiviral dosage. Compared to the studies with contrasting findings, we adjusted for additional covariates; nevertheless, even without adjustment, we did not see differences in rates of dementia diagnoses between patients exposed and unexposed to HZ. We also used robust approaches to addressing missing covariate data which had little impact on the findings. Unique to our study was the use of different modelling approaches with varying underlying assumptions; consistency across these models adds confidence to our findings. We additionally identified that a small apparent protective association between HZ (with or without antiviral use) and dementia was confined to frail individuals and to females, and was seen only for mixed or unspecified dementia.
Differences between our study and findings from Taiwan and Korea may reflect methodological differences: previous studies either used future information to define covariates 12 or the timing of covariate assessment was unclear; 11 , 13 in addition, these studies were unable to adjust for lifestyle factors and did not explore the role of frailty. The definitions of the dementia outcome in previous studies also differed for example, by requiring either several instances of a dementia code in outpatient data, 11 , 12 or 30 days of medication, 13 dementia recording may have been more likely among those who sought care frequently, contributing to ascertainment bias. Finally, a contribution of genetic differences between populations cannot be excluded.
Strengths and limitations
Strengths of our study include the large population size: we captured all patients with a record of HZ in a representative real‐world UK population over a 17‐year time period. We matched patients on age, sex, practise (which relates both to geographic region and socio‐economic status) and calendar time, and comprehensively controlled for confounders. We also carried out a range of sensitivity analyses to increase confidence in our estimates. Nevertheless, there are some limitations. HZ is potentially under‐recorded in EHRs: figures from the US suggest that around 90% of adults aged 50 years or over report seeking medical care for an episode of HZ. 20 While younger patients who tend to have more mild disease are least likely to attend, these patients are also the least likely to have long‐term sequelae, including dementia, so the impact on our study is estimated to be small.
The positive predictive value (PPV) of a dementia diagnosis in routinely collected EHRs is relatively high 21 and we focussed on all‐cause dementia as the primary outcome as the PPV of a dementia diagnosis varies by dementia subtype. 22 However, in the UK only around two thirds of patients with dementia have a diagnosis recorded in primary care. We nevertheless saw similar results when expanding our outcome definition to include administrative as well as diagnostic and referral codes. These have been shown to improve detection of dementia in primary care records compared with diagnosis codes alone. 23
Previous studies have identified characteristics associated with the likelihood of having a recorded dementia diagnosis in primary care among those with objective evidence of dementia. 24 Diagnoses are more common among those with severe memory impairment and those living with a partner; conversely, people with extensive cardiovascular comorbidities, people aged 90 years or over and those living in residential care settings were less likely to be diagnosed. In our study, the apparent protective association between HZ and dementia was only apparent for frail individuals, who are likely to have many physical comorbidities. It is therefore possible that this group was disproportionately affected by under‐recording of dementia diagnoses, perhaps because of a focus on treating other physical conditions rather than prioritising memory clinic referrals. Finally, we controlled for health seeking behaviour based on mean GP consultations over the past 3 years, although if recording of dementia were differential by HZ status, this would typically tend to overestimate, rather than underestimate, associations between HZ and dementia.
Other limitations include the difficulty investigating risk factors for syndromes with long latency periods that potentially extend over decades: it is possible that, despite our longitudinal design, the follow‐up time was insufficient to capture any upstream effects of HZ that is, inducing early neuropathological changes that may eventually contribute towards a clinical dementia diagnosis. Nevertheless, the absence of evidence for any increase in dementia diagnosis with HZ, and apparent small protective association in certain population subgroups, suggests that HZ is unlikely to lead to a clinically significant increase in the risk of dementia diagnosis. Finally, as with any EHR‐based study there is potential for residual confounding by unmeasured factors such as those related to genetic risk. However, in the general population, genetic risk factors for HZ and dementia are likely to explain only a small proportion of the variation in risk. 25 , 26
Implications
We found no evidence that HZ was associated with increased risk of dementia diagnosis in a large population‐based cohort from the UK. The apparent small protective association seen only in frail individuals may be partly explained by a competing risk of death in those individuals, in addition to preferential under‐recording of dementia among persons with multiple physical comorbidities. Despite epidemiological evidence and biological mechanisms linking HZ and acute cerebrovascular events, a lack of association between HZ and increased dementia risk may be unsurprising: HZ usually occurs as a single clinical episode provoking a transient immune response in immunocompetent individuals, 27 which may not affect the long trajectory of dementia development.
Nevertheless, the relationship between persisting viruses and brain health over the life course remains underexplored. Future research should triangulate information across large, prospectively collected datasets from different populations to investigate associations further, taking careful steps to reduce biases for example, by including laboratory‐confirmed infection definitions, repeated regular assessments of outcome and comprehensive confounder control. Improved understanding of the complex interplay between infections, immune system modulation and chronic disease risk could help both to characterise susceptible individuals and identify routes for future intervention.
Author Contributions
Conception and design of the study: CWG, EW, NP, JMB, SIS and LS. Acquisition and analysis of data: EW, SIS and JBH. Drafting manuscript and figures: CWG and EW. Reviewing manuscript for important intellectual content and approving the final version: CWG, EW, SIS, JBH, NP, JMB and LS.
Conflict of Interest
SIS is now employed by the Clinical Practice Research Datalink (CPRD), a division of the UK Medicines and Healthcare products Regulatory Agency (MHRA), but the views expressed in this publication are his own and do not represent the official position of either the CPRD or the MHRA. All other authors report no conflict of interest.
Acknowledgements
This study is based in part on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the authors alone. CWG is supported by a Wellcome Intermediate Clinical Fellowship (201440/Z/16/Z). Open Access funding enabled and organized by Projekt DEAL.
Funding Statement
This work was funded by Wellcome grant 201440/Z/16/Z.
References
- 1. Patterson C World Alzheimer Report 2018 [Internet]. : Alzheimer's Disease International; 2018. [cited 2021 Jan 25]. Available from: https://www.alzint.org/resource/world‐alzheimer‐report‐2018/ [Google Scholar]
- 2. Mukadam N, Sommerlad A, Huntley J, Livingston G. Population attributable fractions for risk factors for dementia in low‐income and middle‐income countries: an analysis using cross‐sectional survey data. Lancet Glob Health. 2019;7(5):e596‐e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Warren‐Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory‐confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self‐controlled case series analysis of national linked datasets from Scotland. Eur Respir J. 2018;51(3):1701794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zwaans WaR, , van Winden MEC, Rohde GGU The relevance of respiratory viral infections in the exacerbations of chronic obstructive pulmonary disease—a systematic review. J Clin Virol off Publ Pan Am Soc Clin Virol 2014;61(2):181–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moghoofei M, Azimzadeh Jamalkandi S, Moein M, Salimian J, Ahmadi A. Bacterial infections in acute exacerbation of chronic obstructive pulmonary disease: a systematic review and meta‐analysis. Infection. 2020;48(1):19‐35. [DOI] [PubMed] [Google Scholar]
- 7. Muzambi R, Bhaskaran K, Brayne C, Smeeth L, Warren‐Gash C. Common bacterial infections and risk of incident cognitive decline or dementia: a systematic review protocol. BMJ Open. 2019;9(9):e030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018;21(10):1359‐1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nightingale S, Winston A, Letendre S, et al. Controversies in HIV‐associated neurocognitive disorders. Lancet Neurol. 2014;13(11):1139‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forbes HJ, Williamson E, Benjamin L, et al. Association of herpesviruses and stroke: systematic review and meta‐analysis. PLoS One. 2018;13(11):e0206163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsai M‐C, Cheng W‐L, Sheu J‐J, et al. Increased risk of dementia following herpes zoster ophthalmicus. PLoS One. 2017;12(11):e0188490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen VC‐H, Wu S‐I, Huang K‐Y, et al. Herpes zoster and dementia: a Nationwide population‐based cohort study. J Clin Psychiatry. 2018;79(1):16m11312. [DOI] [PubMed] [Google Scholar]
- 13. Bae S, Yun S‐C, Kim M‐C, Yoon W, Lim JS, Lee S‐O, et al. Association of herpes zoster with dementia and effect of antiviral therapy on dementia: a population‐based cohort study. Eur Arch Psychiatry Clin Neurosci. 2021;271(5):987‐997. [Cited 2021 Apr 15]; Available from: 10.1007/s00406-020-01157-4 [DOI] [PubMed] [Google Scholar]
- 14. Choi HG, Park BJ, Lim JS, Sim SY, Jung YJ, Lee SW. Herpes zoster does not increase the risk of neurodegenerative dementia: a case‐control study. Am J Alzheimers Dis Other Dement 2021;36:15333175211006504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schnier C, Janbek J, Williams L, et al. Antiherpetic medication and incident dementia: observational cohort studies in four countries. Eur J Neurol. 2021;28(6):1840‐1848. [DOI] [PubMed] [Google Scholar]
- 16. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44(3):827‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Department for Communities and Local Government . The English Indices of Deprivation. 2015. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/465791/English_Indices_of_Deprivation_2015_‐_Statistical_Release.pdf [Google Scholar]
- 18. Langan SM, Minassian C, Smeeth L, Thomas SL. Risk of stroke following herpes zoster: a self‐controlled case‐series study. Clin Infect Dis. 2014;58(11):1497‐1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hales CM, Harpaz R, Bialek SR. Self‐reported herpes zoster, pain, and health care seeking in the health and retirement study: implications for interpretation of health care–based studies. Ann Epidemiol. 2016;26(6):441‐446.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGuinness LA, Warren‐Gash C, Moorhouse LR, Thomas SL. The validity of dementia diagnoses in routinely collected electronic health records in the United Kingdom: a systematic review. Pharmacoepidemiol Drug Saf. 2019;28(2):244‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilkinson T, Ly A, Schnier C, et al. Identifying dementia cases with routinely collected health data: a systematic review. Alzheimers Dement [Internet]. 2018. Apr [cited 2018 Jul 19]; Available from:. http://linkinghub.elsevier.com/retrieve/pii/S1552526018300700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ford E, Sheppard J, Oliver S, Rooney P, Banerjee S, Cassell JA. Automated detection of patients with dementia whose symptoms have been identified in primary care but have no formal diagnosis: a retrospective case–control study using electronic primary care records. BMJ Open. 2021;11(1):e039248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aldus CF, Arthur A, Dennington‐Price A, et al. Undiagnosed dementia in primary care: a record linkage study. Health Serv Deliv Res. 2020;8(20):1‐108. [PubMed] [Google Scholar]
- 25. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673‐2734. [DOI] [PubMed] [Google Scholar]
- 26. Tian C, Hromatka BS, Kiefer AK, et al. Genome‐wide association and HLA region fine‐mapping studies identify susceptibility loci for multiple common infections. Nat Commun. 2017;8(1):599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Warren‐Gash C, Forbes H, Maple P, Quinlivan M, Breuer J. Viral load and antibody boosting following herpes zoster diagnosis. J Clin Virol off Publ Pan Am Soc Clin Virol. 2018;103:12‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]


