Abstract
In animals, males and females can display markedly different longevity (also called sex gaps in longevity, SGL). Sex chromosomes contribute to establishing these SGLs. X-hemizygosity and toxicity of the Y chromosomes are two mechanisms that have been suggested to reduce male longevity (Z-hemizygosity and W toxicity in females in ZW systems). In plants, SGLs are known to exist, but the role of sex chromosomes remains to be established. Here, by using adult sex ratio as a proxy for measuring SGLs, we explored the relationship between sex chromosomes and SGLs across 43 plant species. Based on the knowledge accumulated in animals, we specifically asked whether: (i) species with XY systems tend to have female-biased sex ratios (reduced male longevity) and species with ZW ones tend to have male-biased sex ratios (reduced female longevity); and (ii) this pattern was stronger in heteromorphic systems compared to homomorphic ones. Our results tend to support these predictions although we lack statistical power because of a small number of ZW systems and the absence of any heteromorphic ZW system in the dataset. We discuss the implications of these findings, which we hope will stimulate further research on sex differences in lifespan and ageing across plants.
This article is part of the theme issue ‘Sex determination and sex chromosome evolution in land plants’.
Keywords: sex chromosomes, sex differences, ageing, longevity, sex ratio, plants
1. Introduction
In many animal species, males and females have different mortality patterns and longevities. This phenomenon has been observed in mammals, birds and in invertebrates (reviewed in [1]). For example, mammalian females generally outlive males, although some exceptions have been documented [2]. Studying sex differences in lifespan (also called sex gaps in longevity, SGL) is important for deciphering the physiological and genetic determinants of the ageing process and also for developing sex-specific anti-ageing interventions in humans [3,4]. Three non-exclusive possible evolutionary hypotheses underlying SGL have been proposed (see [1,5]): (i) the sex-specific long-term costs of sexual selection, (ii) the mother's curse, and (iii) the effects of sex chromosomes.
First, in species where sexual selection strongly acts on males, the evolution of marked sexual dimorphism, sex-specific physiologies (e.g. high androgen levels in males) and conspicuous behaviours (e.g. sexual display, aggressiveness) can affect age-specific mortality patterns in a sex-specific way and lead to a reduced lifespan for males [6,7]. Evidence accumulated so far has revealed that the level of sexual selection can—to some extent—explain the degree of SGL [6,8], although this relationship can be largely modulated by environmental conditions [2]. The mother's curse relies on the asymmetric transmission of the mitochondrial genome (through females). Selection is thus completely blind to all the deleterious mutations that affect males only, which will favour accumulation of such mutations in the mitochondrial genomes and could potentially be detrimental for the survival prospects of males [9]. However, while most empirical investigations of the mother's curse have revealed detrimental effects of the accumulation of mtDNA mutations for males' health and fertility (e.g. [10,11]), impacts in terms of reduced male's lifespan remain poorly documented (but see [12]).
In theory, sex chromosome content could also affect ageing and longevity [13]. In highly heteromorphic systems, the Y chromosome is highly degenerated, and most genes have an X copy only. Males are thus X-hemizygous for most of the genes, and they will thus express all the deleterious mutations (the so-called unguarded X effect, [13]). In females, recessive mutations on one X will often be masked by the other X. Moreover, the Y itself can have a toxic effect and reduce male lifespan (reviewed in [1]). Y chromosomes often include a large portion of DNA repeats including transposable elements (TEs). These elements can jump from one place to another in the genome causing somatic mutations and are often silenced via epigenetic mechanisms. The epigenetic marks can fade with time and in ageing males. TEs can then resume activity causing mutation rate acceleration, which can possibly lead to a faster ageing and a reduced lifespan [1]. While evidence suggests that all mechanisms are probably at work [1,4], their relative contributions and how they may vary from one species to another are yet to be quantified.
The impact of sex chromosomes on SGLs is a new area of research. Before 2015, this possibility was considered purely theoretical, and some authors doubted that it even existed [5,13]. In 2015, Pipoly et al. documented a compelling association between adult sex ratios (used as a proxy for SGLs) and sex chromosome types (XY or ZW) in tetrapods [14]. They found that the adult sex ratio was biased towards the homogametic sex (XX females or ZZ males) in amphibians, snakes and lizards, mammals and birds, suggesting a higher mortality for the heterogametic sex (XY males or ZW females) in agreement with both unguarded X and toxic Y mechanisms. This observation was later confirmed by a study using various metrics of longevity and conducted on a wider taxonomic range (i.e. vertebrate and invertebrate species, [15]). From these studies, however, it is not clear whether the unguarded X or the toxic Y effects are at work and what their relative contribution is. In Drosophila melanogaster, females outlive males, and several studies have shown that the unguarded X has limited or no effect [16,17] (but see [18]). The toxic Y is probably the main cause of the SGLs in D. melanogaster [19]. TE activity increases faster in ageing males compared to ageing females, and this correlates with chromosome compactness changes (loss of epigenetic marks typical of heterochromatin). Moreover, longevity is correlated with sex chromosome content not phenotypic sex: longevity is reduced in female XXY flies and is increased in male XO flies (compared to female XX and male XY flies respectively, [19]). The toxic Y effect has been confirmed in Drosophila miranda [20]. A correlational study suggests that the toxic Y effect may also be widespread in tetrapods [21]. This study indeed observed (but using a relatively small dataset, ca 20 species) that Y chromosome size (relative to genome size) is correlated to male longevity in agreement with the toxic Y (but not expected under the unguarded X). This raises the possibility of an interplay between sex chromosome size and SGLs. Selection for male viability and longevity may favour shortening of the Y chromosome, an interesting possibility given that why old and degenerated Y (or W) chromosomes in animals are tiny remains a mystery [22,23].
On the other hand, SGLs have been poorly studied in plants. We know that plants can exhibit extreme differences in longevity among species, with herbs often living 1 year and some trees being several hundreds or even thousands of years old (e.g. [24–26]). Most flowering plants are hermaphrodites, but 6% are dioecious, representing about 15 000 species [27]. A study of adult sex ratios on 243 of these plants has revealed that they probably exhibit SGLs [28]. Growth forms (herb, shrub, tree, etc…) are one of the major determinants of the variation in SGLs among plants, with herbs tending to display balanced sex ratios, while trees exhibit strongly male-biased sex ratios, probably because female trees suffer from a substantial survival cost of reproduction. Interestingly, sex chromosomes were found to be associated with adult sex ratios. Species with documented sex chromosomes had a female-biased sex ratio, with the strongest biases in species with heteromorphic sex chromosomes. Whether XY and ZW systems show different correlations with sex ratios (as observed in animals [14,15] is currently unknown. The study of SGLs in dioecious plants is important because a global view on ageing and its evolution ought to include plants. Moreover, plants are interesting because dioecy is derived, contrary to the situation in animals (where the ancestor was gonochoristic and hermaphroditism is derived). Dioecious plants are thus good systems to study emerging SGLs. Also, sexual dimorphism is weak in plants [29], which make them potentially good systems to study the other mechanisms underlying SGLs. Of note, the mother's curse may be strong as both mitochondria and plastids are usually transmitted maternally in flowering plants [30]. Finally, dioecious plants are over-represented among crops (13% (R. Ming 2021, personal communication)), understanding and managing SGLs may be useful for agronomical purposes.
In the dataset of Field et al. [28], information on sex chromosomes was available for only 22 species. No information was available on sex chromosome type (XY and ZW), precluding any analysis using this information. Since 2013, a number of studies have reported and characterized sex chromosomes in dioecious plants (see reviews from [31–33]). We thus updated the dataset of Field et al. [28] with this information. Our goal was to conduct an analysis on the sex ratios contrasting XY and ZW systems and to test the following predictions: under the toxic Y or unguarded X effects, the heterogametic sex should have a reduced lifespan, XY systems should show female-biased sex ratios, ZW ones should show male-biased sex ratios. These biases are supposed to be stronger in heteromorphic systems compared to homomorphic ones.
2. Methods
(a) . Dataset
We extracted adult sex ratio values from [28] (dataset available from Dryad: https://doi.org/10.5061/dryad.28kd1). Adult sex ratios (ASR) were computed as the number of males (individuals with male flowers) divided by the total number of individuals (males and females). Only populations with greater than 10 sampled individuals were conserved for computing the adult sex ratios as in [28]. We assumed that adult sex ratios were good proxies for SGLs. In other words, a female-biased mortality should lead to an adult sex ratio biased towards males (i.e. ASR > 0.5), while a male-biased mortality should lead to an adult sex ratio biased towards females (i.e. ASR < 0.5). We updated the information on sex chromosomes for all species included in the dataset. In particular, we sought information about presence of sex chromosomes and sex chromosome type (XY/ZW and homomorphic/heteromorphic). We screened all papers using cytology/genetics/genomics (entered as keywords) published after 2012 on sex chromosomes and sex determination for the 243 species included in the dataset of Field et al. [28] using Pubmed and Google scholar. We also looked for species for which both sex ratios and information about sex chromosomes were published since 2012 but found only one species (Amborella trichopoda), which was added to the dataset. All data used in our analysis are provided in the electronic supplementary material.
(b) . Statistical procedure
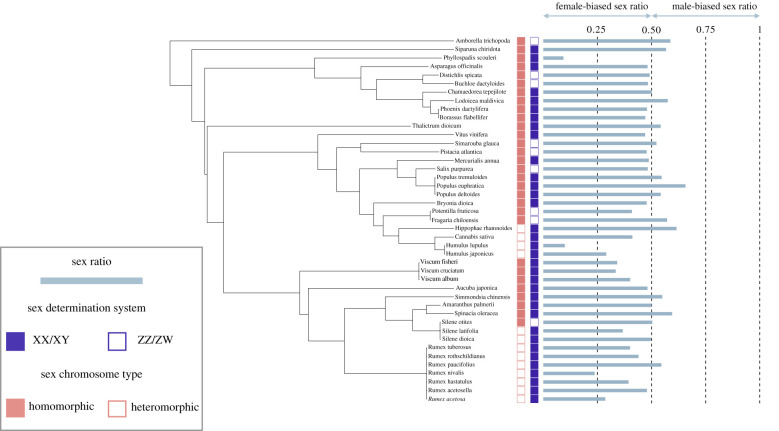
To avoid any statistical issue owing to phylogenetic inertia [34], we used phylogenetic generalized least-squares (PGLS) models. The strength of the phylogenetic signal on the error structure of each model was assessed with Pagel's λ [35]. Pagel's λ was estimated with maximum-likelihood by using the PGLS command from the R-package caper [36] and was further incorporated into the models to control for the phylogenetic dependence among species. The λ classically varies between 0 (no phylogenetic signal) and 1 (the observed pattern is predicted by the phylogeny; similarity among species scales proportionally to their shared evolutionary time following a Brownian motion model; see [35]). For all the phylogenetically controlled analyses, data were linked to the phylogenetic tree built by [28]. We modified this phylogenetic tree by pruning out the species without sex chromosomes and by adding A. trichopoda at the basis of the tree (A. trichopoda is known to be the sister species of all other extent angiosperms, [37,38]) and obtained the tree shown in figure 1. It is important to note that for all the models described below, λ was not statistically different from 0 and analyses performed without accounting for phylogenetic dependence among species lead to qualitatively similar results (data not shown).
Figure 1.
Phylogenetic tree of the species with sex chromosomes included in the dataset and used for the PGLS analysis.
To explore the association between ASR and sex chromosomes, we first analysed whether the variation in adult sex ratios observed among plants was explained by the genetic sex determination system by fitting PGLS models with ASR as the response variable in all models and the sex chromosome type (XY/ZW) as a predictor. Then, we tested whether the variation in adult sex ratios observed among plants differed between heteromorphic and homomorphic systems by fitting PGLS models with ASR as the response variable in all models, and the level of heteromorphy (heteromorphic/homomorphic) as a predictor. No heteromorphic ZW system was present in our dataset, and we thus compared ASR values between the three following group of plants: homomorphic XY, heteromorphic XY and homomorphic ZW. All PGLS models were performed with R 4.0.0 (R Core Development Team) using the packages ape [39] and caper [36]. Unless otherwise stated, parameter estimates are given as ±95% confidence interval (CI).
3. Results
Table 1 shows a summary of our work to update the dataset of Field et al. [28] with information on sex chromosomes. We doubled the number of species for which this information is available, and the dataset now includes information on sex chromosome type.
Table 1.
Summary of the updating of the dataset of Field et al. [28] on dioecious plant sex ratios.
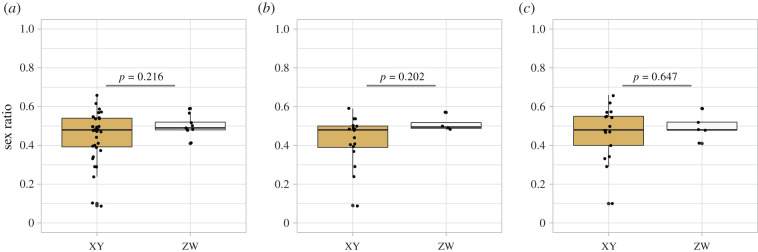
Our first analysis revealed that ASR did not differ between XX/XY and ZZ/ZW plants (table 2a and figure 2). However, we observed that species with XY systems have a slightly biased female-biased sex ratio (mean ASR = 0.44, 95% CI [0.40; 0.49]; median ASR = 0.48, n = 34), while those with a ZW system have a balanced sex ratio (mean ASR = 0.5, 95% CI [0.46; 0.54]; median ASR = 0.49, n = 9). Similar to what was observed with the whole dataset, ASR did not differ between XX/XY and ZZ/ZW in herb-like species (table 2a and figure 2). In herb-like species, species with XY systems have a slightly female-biased sex ratio (XY: mean ASR = 0.42, 95% CI [0.36; 0.49], median ASR = 0.48, n = 17), while ASR did not deviate from 0.5 in ZW species (mean ASR = 0.51, 95% CI [0.44; 0.57]; median ASR = 0.50, n = 4). However, in trees, ASR CI includes 0.5 in both groups (XY: mean ASR = 0.47, 95% CI [0.39; 54]; median ASR = 0.48, n = 17; ZW: mean ASR = 0.49, 95% CI [0.41; 0.58]; median ASR = 0.48, n = 5) highlighting an absence of deviation from a balanced sex ratio.
Table 2.
Parameters of the models discussed in the text. (In all models, the λ-value was not statistically different from 0, indicating that the phylogeny did not influence the relationship between sex chromosomes and sex ratio.)
| n | estimate | s.e. | T | p | λ [95% CI] | ||
|---|---|---|---|---|---|---|---|
| (a) | |||||||
| all plants | intercept | 0.44 | 0.02 | 21.64 | <0.01 | 0 [n.a.; 0.82] | |
| sex chromosome type ‘ZW’ | 43 | 0.06 | 0.045 | 1.27 | 0.216 | ||
| herb-like species | intercept | 0.42 | 0.03 | 15.27 | <0.01 | 0 [n.a.; 0.82] | |
| sex chromosome type ‘ZW’ | 21 | 0.09 | 0.06 | 1.32 | 0.202 | ||
| tree-like speciesa | intercept | 0.47 | 0.03 | 15.07 | <0.01 | n.a. | |
| sex chromosome type ‘ZW’ | 22 | 0.03 | 0.06 | 0.46 | 0.647 | ||
| (b) | |||||||
| all plants | intercept | 0.39 | 0.03 | 12.32 | <0.01 | 0 [n.a.; 0.72] | |
| sex chromosome type/heteromorphy level ‘homomorphic XY’ | 43 | 0.09 | 0.04 | 2.2 | 0.032 | ||
| sex chromosome type/heteromorphy level ‘homomorphic ZW’ | 0.11 | 0.05 | 2.25 | 0.03 | |||
| herb-like species | intercept | 0.4 | 0.04 | 11.14 | <0.01 | 0 [n.a.; 0.81] | |
| sex chromosome type/heteromorphy level ‘homomorphic XY’ | 21 | 0.05 | 0.06 | 0.84 | 0.42 | ||
| sex chromosome type/heteromorphy level ‘homomorphic ZW’ | 0.1 | 0.07 | 1.53 | 0.15 | |||
| tree-like species | intercept | 0.33 | 0.07 | 4.83 | <0.01 | 0 [n.a.; 0.85] | |
| sex chromosome type/heteromorphy level ‘homomorphic XY’ | 22 | 0.15 | 0.08 | 2.07 | 0.052 | ||
| sex chromosome type/heteromorphy level ‘homomorphic ZW’ | 0.16 | 0.09 | 1.85 | 0.08 | |||
aThe estimate corresponds to an ordinary least regression, instead of PGLS (owing to lack of model convergence). In (a), the sex chromosome type ‘XY’ has been used as a reference, and in (b), the sex chromosome type/heteromorphy level ‘heteromorphic XY’ has been used as a reference.
Figure 2.
Sex ratios and sex chromosomes. (a) All species, (b) herb-like plants, and (c) tree-like plants. Herb-like plants include annual and perennial herbs, and tree-like plants include shrubs, trees, vines and mistletoes according to [28]. p-values are indicated. (Online version in colour.)
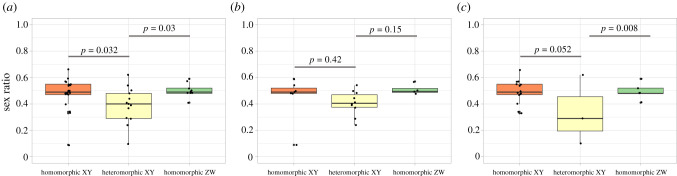
We then analysed separately homomorphic and heteromorphic systems. In the heteromorphic XY systems, we found a strongly female-biased sex ratio (mean ASR = 0.39, 95% CI [0.30; 0.47]; median ASR = 0.40, n = 13). We found the same for herbs (mean ASR = 0.40, 95% CI [0.34; 0.47]; median ASR = 0.41, n = 10), while in trees, the sample size was too small to draw any definitive conclusion, despite a female-biased sex ratio (mean ASR = 0.33, 95% CI [−0.31; 0.99]; median ASR = 0.29, n = 3). Homomorphic systems on the other hand exhibit close-to-balanced sex ratios (mean ASR = 0.49, 95% CI [0.45; 0.52]; median ASR = 0.49, n= 30; figure 3). As expected, our analyses revealed that the level of heteromorphy influences ASR (figure 3). ASR values were significantly lower in heteromorphic XY than in homomorphic sex chromosomes (table 2b). When analysed separately, a similar pattern was found in tree-like plants and in herb-like plants, although it was less clear in the latter (table 2b).
Figure 3.
Sex ratios and sex chromosome heteromorphy. (a) All species, (b) herb-like plants, and (c) tree-like plants. Herb-like plants include annual and perennial herbs and tree-like plants include shrubs, trees, vines and mistletoes according to [28]. p-values are indicated. (Online version in colour.)
4. Discussion
Our results support a contribution of sex chromosomes in establishing SGLs in plants. The heterozygous sex seems to have a reduced lifespan. This is clear for XY systems and less clear for ZW ones, possibly owing to lower representation of the latter in our dataset. The patterns are stronger for heteromorphic systems, although this could be observed for XY systems only, as our dataset only included homomorphic ZW systems. There is, to our knowledge, no documented heteromorphic ZW system in plants yet. Patterns look similar in herbs and trees. This was somewhat surprising as Field et al. [28] found that overall sex ratio biases have different directions in herbs and trees: female-biased in the former, male-biased in the latter [28]. We observe that sex ratios were more female-biased in herbs and possibly in trees with heterogametic XY systems, suggesting that such sex chromosomes have a strong impact on male survival and possibly ageing in trees.
Our dataset only included 43 species, which limits its statistical power. Moreover, adult sex ratios are rough proxies for SGLs. They are correlated to a number of factors, perhaps the main one being growth form, which we took into account here. However, they are also affected by sex ratios at germination, and adult sex ratios are valuable proxies for SGLs only if sex ratio at germination is 1 : 1. Biased sex ratios at germination are known to exist in plants (discussed in [28]), although there are also reports of unbiased ratios in many species (e.g. [40,42]). Also, theoretical work has shown that selection among pollen in plants with very young sex chromosome systems (not yet strongly degenerated) might generate male-biased sex ratio [43]. Future studies should ideally use larger datasets (including more ZW systems) and other proxies (demographic data).
Our study aims at stimulating more research on the effect of sex chromosomes on sex-specific differences in longevity in plants. Sex chromosomes are being characterized in a growing number of plant species [31–33], which should help in updating the dataset of Field et al. [28] further and also enrich it with information on the size of the non-recombining region, the level of Y degeneration, the age of the sex chromosome system. Getting more sex ratio data (or longevity data) would also be useful. These are lacking for some interesting species. Coccinia grandis for example is the dioecious plant with the strongest documented sex chromosome heteromorphy [44], and a strong SGL is expected for this plant, but no sex ratio data are currently available in this species.
Acknowledgements
We thank editors Susanne Renner and Niels Müller for their positive feedback about this work and the possibility to contribute to this special issue. We thank Sally Otto and Susanne Renner for their careful reviewing of this manuscript and suggestions to improve it. We thank all the members of the Longevity ANR grant for discussions.
Data accessibility
The data are provided in the electronic supplementary material [45].
Authors' contributions
G.A.B.M.: conceptualization, data curation, funding acquisition, investigation, visualization, writing—original draft, writing—review and editing; J-F.L.: formal analysis, funding acquisition, visualization, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
G.A.B.M. and J-F.L. acknowledge the financial support of Agence Nationale de la Recherche (grant no. ANR-20-CE02–0015).
References
- 1.Marais GAB, Gaillard JM, Vieira C, Plotton I, Sanlaville D, Gueyffier F, Lemaître JF. et al. 2018. Sex gap in aging and longevity: can sex chromosomes play a role? Biol. Sex Differ. 9, 33. ( 10.1186/s13293-018-0181-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemaître JF, et al. 2020. Sex differences in adult lifespan and aging rates of mortality across wild mammals. Proc. Natl Acad. Sci. USA 117, 8546-8553. ( 10.1073/pnas.1911999117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austad SN, Fischer KE. 2016. Sex differences in lifespan. Cell Metab. 23, 1022-1033. ( 10.1016/j.cmet.2016.05.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hägg S, Jylhävä J. 2021. Sex differences in biological aging with a focus on human studies. Elife 10, e63425. ( 10.7554/eLife.63425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maklakov AA, Lummaa V. 2013. Evolution of sex differences in lifespan and aging: causes and constraints. Bioessays 35, 717-724. ( 10.1002/bies.201300021) [DOI] [PubMed] [Google Scholar]
- 6.Tidiere M, Gaillard JM, Muller DW, Lackey LB, Gimenez O, Clauss M, Lemaître JF. 2015. Does sexual selection shape sex differences in longevity and senescence patterns across vertebrates? A review and new insights from captive ruminants. Evolution 69, 3123-3140. ( 10.1111/evo.12801) [DOI] [PubMed] [Google Scholar]
- 7.Brooks RC, Garratt MG. 2017. Life history evolution, reproduction, and the origins of sex-dependent aging and longevity. Ann. NY Acad. Sci. 1389, 92-107. ( 10.1111/nyas.13302) [DOI] [PubMed] [Google Scholar]
- 8.Clutton-Brock TH, Isvaran K. 2007. Sex differences in ageing in natural populations of vertebrates. Proc. R. Soc. B 274, 3097-3104. ( 10.1098/rspb.2007.1138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank SA. 2012. Evolution: mitochondrial burden on male health. Curr. Biol. 22, R797-R799. ( 10.1016/j.cub.2012.07.066) [DOI] [PubMed] [Google Scholar]
- 10.Nakada K, Sato A, Yoshida K, Morita T, Tanaka H, Inoue S, Yonekawa H, Hayashi JI. 2006. Mitochondria-related male infertility. Proc. Natl Acad. Sci. USA 103, 15 148-15 153. ( 10.1073/pnas.0604641103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milot E, Moreau C, Gagnon A, Cohen AA, Brais B, Labuda D. 2017. Mother's curse neutralizes natural selection against a human genetic disease over three centuries. Nat. Ecol. Evol. 1, 1400-1406. ( 10.1038/s41559-017-0276-6) [DOI] [PubMed] [Google Scholar]
- 12.Camus MF, Clancy DJ, Dowling DK. 2012. Mitochondria, maternal inheritance, and male aging. Curr. Biol. 22, 1717-1721. ( 10.1016/j.cub.2012.07.018) [DOI] [PubMed] [Google Scholar]
- 13.Trivers R. 1985. Social evolution. San Francisco, CA: Benjamin Cummings. [Google Scholar]
- 14.Pipoly I, Bokony V, Kirkpatrick M, Donald PF, Szekely T, Liker A. 2015. The genetic sex-determination system predicts adult sex ratios in tetrapods. Nature 527, 91-94. ( 10.1038/nature15380) [DOI] [PubMed] [Google Scholar]
- 15.Xirocostas ZA, Everingham SE, Moles AT. 2020. The sex with the reduced sex chromosome dies earlier: a comparison across the tree of life. Biol. Lett. 16, 20190867. ( 10.1098/rsbl.2019.0867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sultanova Z, Andic M, Carazo P. 2018. The ‘unguarded-X’ and the genetic architecture of lifespan: inbreeding results in a potentially maladaptive sex-specific reduction of female lifespan in Drosophila melanogaster. Evolution 72, 540-552. ( 10.1111/evo.13426) [DOI] [PubMed] [Google Scholar]
- 17.Brengdahl M, Kimber CM, Maguire-Baxter J, Friberg U. 2018. Sex differences in life span: females homozygous for the X chromosome do not suffer the shorter life span predicted by the unguarded X hypothesis. Evolution 72, 568-577. ( 10.1111/evo.13434) [DOI] [PubMed] [Google Scholar]
- 18.Carazo P, Green J, Sepil I, Pizzari T, Wigby S. 2016. Inbreeding removes sex differences in lifespan in a population of Drosophila melanogaster. Biol. Lett. 12, 20160337. ( 10.1098/rsbl.2016.0337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown EJ, Nguyen AH, Bachtrog D. 2020. The Y chromosome may contribute to sex-specific ageing in Drosophila. Nat. Ecol. Evol. 4, 853-862. ( 10.1038/s41559-020-1179-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen AH, Bachtrog D. 2021. Toxic Y chromosome: Increased repeat expression and age-associated heterochromatin loss in male Drosophila with a young Y chromosome. PLoS Genet. 17, e1009438. ( 10.1371/journal.pgen.1009438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sultanova Z, Downing PA, Carazo P. 2020. Genetic sex determination and sex-specific lifespan in tetrapods–evidence of a toxic Y effect. bioRxiv.
- 22.Charlesworth D, Charlesworth B, Marais G. 2005. Steps in the evolution of heteromorphic sex chromosomes. Heredity 95, 118-128. ( 10.1038/sj.hdy.6800697) [DOI] [PubMed] [Google Scholar]
- 23.Bachtrog D. 2013. Y-chromosome evolution: emerging insights into processes of Y-chromosome degeneration. Nat. Rev. Genet. 14, 113-124. ( 10.1038/nrg3366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones OR, et al. 2014. Diversity of ageing across the tree of life. Nature 505, 169-173. ( 10.1038/nature12789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pujol B, Marrot P, Pannell JR. 2014. A quantitative genetic signature of senescence in a short-lived perennial plant. Curr. Biol. 24, 744-747. ( 10.1016/j.cub.2014.02.012) [DOI] [PubMed] [Google Scholar]
- 26.Miryeganeh M. 2021. Senescence: the compromised time of death that plants may call on themselves. Genes 12, 143. ( 10.3390/genes12020143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renner SS. 2014. The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 101, 1588-1596. ( 10.3732/ajb.1400196) [DOI] [PubMed] [Google Scholar]
- 28.Field DL, Pickup M, Barrett SC. 2013. Comparative analyses of sex-ratio variation in dioecious flowering plants. Evolution 67, 661-672. ( 10.1111/evo.12001) [DOI] [PubMed] [Google Scholar]
- 29.Barrett SC, Hough J. 2013. Sexual dimorphism in flowering plants. J. Exp. Bot. 64, 67-82. ( 10.1093/jxb/ers308) [DOI] [PubMed] [Google Scholar]
- 30.Wicke S, Schneeweiss GM, dePamphilis CW, Müller KF, Quandt D. 2011. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol. Biol. 76, 273-297. ( 10.1007/s11103-011-9762-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muyle A, Shearn R, Marais GA. 2017. The evolution of sex chromosomes and dosage compensation in plants. Genome Biol. Evol. 9, 627-645. ( 10.1093/gbe/evw282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlesworth D. 2019. Young sex chromosomes in plants and animals. New Phytol. 224, 1095-1107. ( 10.1111/nph.16002) [DOI] [PubMed] [Google Scholar]
- 33.Renner SS, Müller NA. 2021. Plant sex chromosomes defy evolutionary models of expanding recombination suppression and genetic degeneration. Nat. Plants 7, 392-402. ( 10.1038/s41477-021-00884-3) [DOI] [PubMed] [Google Scholar]
- 34.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1-15. ( 10.1086/284325) [DOI] [Google Scholar]
- 35.Pagel M. 1999. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst. Biol. 48, 612-622. ( 10.1080/106351599260184) [DOI] [Google Scholar]
- 36.Orme D, et al. 2013. The caper package: comparative analysis of phylogenetics and evolution in R. R package version 5. See https://CRAN.R-project.org/package=caper.
- 37.Qiu YL, et al. 1999. The earliest angiosperms: evidence from mitochondrial, plastid and nuclear genomes. Nature 402, 404-407. ( 10.1038/46536) [DOI] [PubMed] [Google Scholar]
- 38.Soltis DE, Bell CD, Kim S, Soltis PS. 2008. Origin and early evolution of angiosperms. Ann. NY Acad. Sci. 1133, 3-25. ( 10.1196/annals.1438.005) [DOI] [PubMed] [Google Scholar]
- 39.Paradis E. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289-290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 40.Anger N, Fogliani B, Scutt CP, Gâteblé G. 2017. Dioecy in Amborella trichopoda: evidence for genetically based sex determination and its consequences for inferences of the breeding system in early angiosperms. Ann. Bot. 119, 591-597. ( 10.1093/aob/mcw278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Käfer J, et al. 2021. A derived ZW chromosome system in Amborella trichopoda, representing the sister lineage to all other extant flowering plants. New Phytol. 233, 1636-1642. ( 10.1111/nph.17662) [DOI] [PubMed] [Google Scholar]
- 42.Morgan EJ, Kaiser-Bunbury CN, Edwards PJ, Scharmann M, Widmer A, Fleischer-Dogley F, Kettle CJ. et al. 2020. Identification of sex-linked markers in the sexually cryptic coco de mer: are males and females produced in equal proportions? AoB Plants 12, plz079. ( 10.1093/aobpla/plz079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott MF, Otto SP. 2017. Haploid selection favors suppressed recombination between sex chromosomes despite causing biased sex ratios. Genetics 207, 1631-1649. ( 10.1534/genetics.117.300062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sousa A, Fuchs J, Renner SS. 2013. Molecular cytogenetics (FISH, GISH) of Coccinia grandis : a ca. 3 myr-old species of Cucurbitaceae with the largest Y/autosome divergence in flowering plants. Cytogenet Genome Res. 139, 107-118. ( 10.1159/000345370) [DOI] [PubMed] [Google Scholar]
- 45.Marais GAB, Lemaître J-F. 2022. Sex chromosomes, sex ratios and sex gaps in longevity in plants. Figshare. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are provided in the electronic supplementary material [45].