Abstract
Optimal treatment strategies for serious infections caused by Staphylococcus aureus have not been fully characterized. The combination of a β-lactam plus an aminoglycoside can act synergistically against S. aureus in vitro and in vivo. MiKasome, a new liposome-encapsulated formulation of conventional amikacin, significantly prolongs serum half-life (t1/2) and increases the area under the concentration-time curve (AUC) compared to free amikacin. Microbiologic efficacy and left ventricular function, as assessed by echocardiography, were compared in animals administered either oxacillin alone or oxacillin in combination with conventional amikacin or MiKasome in a rabbit model of experimental endocarditis due to S. aureus. In vitro, oxacillin, combined with either free amikacin or MiKasome, prevented the bacterial regrowth observed with aminoglycosides alone at 24 h of incubation. Rabbits with S. aureus endocarditis were treated with either oxacillin alone (50 mg/kg, given intramuscularly three times daily), oxacillin plus daily amikacin (27 mg/kg, given intravenously twice daily), or oxacillin plus intermittent MiKasome (160 mg/kg, given intravenously, a single dose on days 1 and 4). The oxacillin-alone dosage represents a subtherapeutic regimen against the infecting strain in the endocarditis model (L. Hirano and A. S. Bayer, Antimicrob. Agents Chemother. 35:685–690, 1991), thus allowing recognition of any enhanced bactericidal effects between oxacillin and either aminoglycoside formulation. Treatment was administered for either 3 or 6 days, and animals were sacrificed after each of these time points or at 5 days after a 6-day treatment course (to evaluate for posttherapy relapse). Left ventricular function was analyzed by utilizing serial transthoracic echocardiography during treatment and posttherapy by measurement of left ventricular fractional shortening. At all sacrifice times, both combination regimens significantly reduced S. aureus vegetation counts versus control counts (P < 0.05). In contrast, oxacillin alone did not significantly reduce S. aureus vegetation counts after 3 days of therapy. Furthermore, at this time point, the two combinations were significantly more effective than oxacillin alone (P < 0.05). All three regimens were effective in significantly decreasing bacterial counts in the myocardium during and after therapy compared to controls (P < 0.05). In kidney and spleen abscesses, all regimens significantly reduced bacterial counts during therapy (P < 0.0001); however, only the combination regimens prevented bacteriologic relapse in these organs posttherapy. By echocardiographic analysis, both combination regimens yielded a significant physiological benefit by maintaining normal left ventricular function during treatment and posttherapy compared with oxacillin alone (P < 0.001). These results suggest that the use of intermittent MiKasome (similar to daily conventional amikacin) enhances the in vivo bactericidal effects of oxacillin in a severe S. aureus infection model and preserves selected physiological functions in target end organs.
Staphylococcus aureus is the most common cause of endovascular infection (e.g., intravascular catheter sepsis) and is the second leading cause of infective endocarditis (1, 8, 29). Previous studies of S. aureus endocarditis (especially in cases of left-sided valvular involvement) have emphasized the difficulties in achieving both microbiologic and physiologic cures with the use of antibiotic therapy alone (8). Efficacy has been limited by primary drug failures, bacteriologic relapse, and ongoing valvular destruction leading to heart failure. Thus, there is an urgent need for new and better approaches to the treatment of severe S. aureus infections, particularly endocarditis.
β-Lactam antibiotics in combination with aminoglycosides are frequently used in the treatment of severe S. aureus infections and, in particular, to take advantage of the in vitro and in vivo synergistic effect between such agents (2, 9, 21). It is well known that aminoglycosides are important anti-infective agents, since they have rapid and concentration-dependent bactericidal effects and long postantibiotic effects (5). However, their use may also be associated with serious ototoxicity and nephrotoxicity. Thus, investigators have attempted to define optimal therapeutic regimens for aminoglycosides, as well as to identify novel aminoglycoside formulations that minimize toxicity and increase overall efficacy.
The liposomes used in MiKasome are small and unilamellar. Amikacin is entrapped within the internal aqueous core (10, 11). Liposomes provide a unique system for antibiotics which can increase their therapeutic index by enhancing efficacy and/or decreasing toxicity. It has been reported that the liposome encapsulation of aminoglycosides significantly alters their pharmacokinetics, with increases in plasma half-life, area under the concentration-time curve, and maximum concentration, as well as decreases in the volume of distribution. Moreover, liposome encapsulation appears to cause a shift in drug accumulation from the kidney to other organs (such as the liver and spleen), thus potentially reducing nephrotoxicity (10, 11, 17, 31, 32). Furthermore, the therapeutic advantage of liposome-encapsulated aminoglycosides has been confirmed by several investigators by using experimental Klebsiella pneumoniae infection models in both neutropenic and normal animals (7, 14). We have previously shown that in experimental pseudomonal endocarditis MiKasome was effective as primary therapy and accumulated extensively within multiple target tissues (e.g., the liver, spleen, and kidney) without causing toxicity in these organs (31, 32). While conventional amikacin therapy resulted in extensive drug accumulation within proximal renal tubular epithelial cells in this model (a major determinant of aminoglycoside nephrotoxicity), MiKasome did not accumulate at this site in the kidney (32).
As described above, it is known that there is a synergistic staphylocidal effect between β-lactams and aminoglycoside agents. However, the combination of liposome-encapsulated aminoglycosides with β-lactam antibiotics has not been evaluated with in vivo models of invasive S. aureus infections. Thus, the present study evaluated the in vivo efficacy of a β-lactam (oxacillin) alone or in combination with either daily administration of conventional amikacin or intermittent administration of MiKasome, a liposome-encapsulated amikacin formulation. We utilized the standard rabbit model of experimental endocarditis due to S. aureus, analyzing both microbiologic endpoints as well as cardiac function by serial echocardiography.
(This study was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998 [abstract B-74].)
MATERIALS AND METHODS
Microorganism.
The S. aureus strain used in this study (VP986β+) was kindly provided by Henry F. Chambers, San Francisco, California, and has been characterized in detail elsewhere (3). The strain, a β-lactamase-producing clinical isolate exhibiting borderline oxacillin resistance, has been previously used in rabbit endocarditis models (3, 15).
Antibiotics.
MiKasome was provided by NeXstar Pharmaceuticals, Inc. (Boulder, Colo.), and had a total lipid concentration of 96.16 mg/ml and an amikacin concentration of 12.34 mg/ml. The mean liposome diameter was 45 nm, as determined by laser light scattering. The free amikacin and the oxacillin were purchased from Bristol-Myers Squibb, Inc. (New Brunswick, N.J.), and Marsam Pharmaceuticals, Inc. (Cherry Hill, N.J.), respectively. All drugs were reconstituted according to the manufacturer’s recommendations.
In vitro susceptibility testing.
The MICs of oxacillin, free amikacin, and MiKasome against the S. aureus strain were determined in cation-supplemented Mueller-Hinton broth (MHB [Difco Laboratories, Detroit, Mich.]) by a microdilution technique done according to National Committee for Clinical Laboratory Standards guidelines with a final S. aureus inoculum of either 105 or 107 CFU/ml. These inocula were chosen since 105 CFU/ml is a standard level for antibiotic susceptibility testing, while 107 CFU/ml represents an S. aureus density regularly achieved within aortic valve vegetations of rabbits with experimental endocarditis (see below). The MIC was defined as the lowest drug concentration preventing visible turbidity after 18 h of incubation at 37°C.
In vitro timed-kill testing.
The timed-kill curve technique was employed for defining the bactericidal interactions of oxacillin with either free amikacin or MiKasome against S. aureus VP986β+. The S. aureus strain was grown overnight and then diluted in antibiotic-containing MHB to achieve a final inoculum of either 5 × 105 or 107 CFU/ml. The final antibiotic concentrations were as follows: (i) oxacillin, free amikacin, or MiKasome each at 5× MICs; (ii) oxacillin plus free amikacin each at 5× MICs; and (iii) oxacillin plus MiKasome each at 5× MICs. Aliquots from each reaction mixture were quantitatively cultured on cation-supplemented MHB agar (also including 2% NaCl) in triplicate for incubations of 0, 2, 4, 6, and 24 h at 37°C. After 24 h of incubation, colonies were counted for each time point, and killing curves were then constructed to delineate bacterial survival (log10 CFU/milliliter) over time. A synergistic interaction was considered present if combinations of oxacillin with either free amikacin or MiKasome caused a >2-log10 decrease in CFU/milliliter at 24 h compared with the most effective single drug (22).
Experimental endocarditis.
New Zealand White female rabbits (2.0 to 3.0 kg; Irish Farms Products and Services, Norco, Calif.) were housed in individual cages and had free access to food and water. Aortic valve endocarditis was induced as described previously (24). Briefly, an indwelling polyethylene catheter was positioned in the left ventricle of each rabbit, with the tip passing across the aortic valve to induce sterile vegetations. The catheter was left in place throughout the study. At 24 h postcatheterization, each rabbit was inoculated intravenously (i.v.) with 107 CFU of S. aureus VP986β+. This inoculum causes experimental endocarditis in >95% of catheterized rabbits (15).
Treatment.
At 24 h postinfection, rabbits were randomized to receive either (i) no therapy (control); (ii) oxacillin at 50 mg/kg, given intramuscularly three times daily (8 a.m., 1 p.m., and 6 p.m.); (iii) oxacillin plus daily free amikacin at 27 mg/kg, given i.v. twice daily (8 a.m. and 6 p.m.); or (iv) oxacillin plus intermittent MiKasome at 160 mg/kg, given i.v., with a single dose on days 1 and 4. Treatment was given for either 3 or 6 days. The oxacillin-alone regimen represented a subtherapeutic regimen against the infecting strain in the endocarditis model (15), thus allowing recognition of any enhanced bactericidal effects between oxacillin and either aminoglycoside formulation. The MiKasome regimen was selected as a result of previous studies which showed that this dose regimen produces a t1/2 of >50 h and prolonged plasma amikacin levels well above the MIC of the current S. aureus strain. Conventional amikacin was given at a dose of 27 mg/kg given twice daily, representing the same total dose as the MiKasome regimen over the treatment period. The pharmacokinetics of oxacillin in the rabbit S. aureus endocarditis model have been well described in the recent literature and were not determined in this study (4). Moreover, the pharmacokinetics of MiKasome and conventional amikacin in experimental endocarditis have also been previously published and so were not determined (31).
Microbiological evaluation.
Control rabbits were sacrificed at 24 h postinfection. Treated rabbits were sacrificed after either 3 or 6 days of therapy to evaluate efficacy or at 5 days after completion of a 6-day therapy course (to evaluate prevention of relapse). Animals were included in the analysis only if the catheters were correctly positioned across the aortic valve and macroscopic vegetations were detected at the time of sacrifice. Rabbits were euthanatized with 100 mg of thiopental given as a rapid i.v. bolus. Postmortem, all aortic valve vegetations, as well as myocardial, kidney, and spleen samples from individual animals, were removed, weighed, and homogenized in 1.0 ml of sterile saline with a tissue homogenizer. For the myocardium, an ∼1-cm3 random tissue sample was removed from the interventricular septum and then processed as described above for vegetations. For the kidney and spleen, multiple visible abscesses were sampled and processed as described above for vegetations. If no overt abscesses were seen in the kidney or spleen, random ∼1-cm3 biopsies were obtained and processed as described above. Tissue homogenates from each sample were quantitatively cultured on Trypticase soy agar (TSA) plates. Since the t1/2 of MiKasome is >50 h in rabbits, polyanethole sulfonic acid (1%) was added to TSA plates to inactivate the amikacin in tissue samples of rabbits receiving the combination of oxacillin with MiKasome (6). Tissue homogenate cultures were grown for 24 h at 37°C, and surviving bacterial densities were expressed as the log10 CFU per gram of tissue. Mean tissue densities in the different treatment groups were statistically compared to evaluate therapeutic efficacy. Culture-negative tissue homogenates were assigned a value of <0.99 to 2.84 log10 CFU/g of tissue, based on the lower detection limit and the actual tissue weight.
Left ventricular function evaluation.
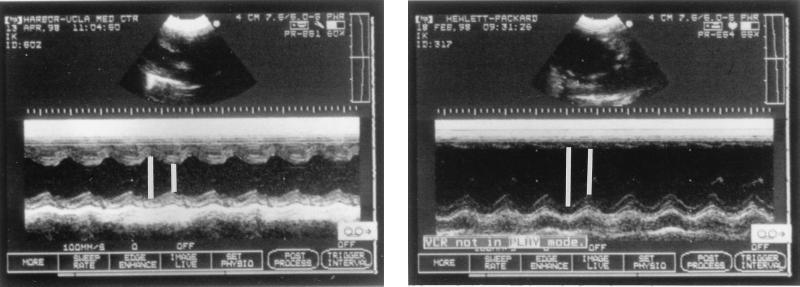
To determine the effects of the various antibiotic regimens on left ventricular function over time, serial transthoracic M-model echocardiography was performed throughout the experimental period. At each evaluation time point, at least seven randomly selected rabbits from the different therapy groups were assessed. The operator (L.I.K.) was blinded as to the therapeutic regimens being used in the evaluated rabbits. Additionally, echocardiographic studies were also performed in 25 healthy rabbits to evaluate left ventricular function in a normal rabbit population. Since untreated controls were sacrificed at 24 h postinfection, serial echocardiography was not performed in this group. Echocardiography was carried out with a 7.5-MHz transducer linked to an ultrasound unit (Sonos Intravascular; Hewlett-Packard, Palo Alto, Calif.). The transducer was placed in the third or fourth left intercostal space to achieve a parasternal long-axis view. In this two-dimensional view, the cursor indicating the M-mode ultrasound beam was positioned between the tips of the mitral valve leaflet and the chordal level so that it was perpendicular to the left ventricular long axis. According to previously published guidelines for assessment of human left ventricular size, the rabbit left ventricular internal dimension (LVID) was measured from the trailing edge of the endocardial echoes of the interventricular septum to the leading edge of the posterior left ventricular endocardial echos, both end-diastole (LVIDd) and end-systole (LVIDs) from the same beat, as illustrated in Fig. 1 (25, 27). From these measurements, the fractional shortening (%ΔD) was derived. The fractional shortening correlates with the ejection fraction as a representation of the overall left ventricular systolic function (19). The calculation of the fractional shortening is based on the following formula: %ΔD = [(LVIDd − LVIDs)/LVIDd] × 100 (25).
FIG. 1.
Transthoracic M-mode echocardiography in a rabbit model. The left panel shows the combination of oxacillin with MiKasome, and the right panel shows oxacillin alone. The short line distance is indicated by a short white line and is the left ventricular internal dimension at end-systole (LVIDs); the long line distance is indicated by a longer white line and is the left ventricular internal dimension at end-diastole.
Statistical analyses.
To compare S. aureus densities in vegetations, myocardium, kidney, and spleen in the different treatment groups, the Kruskal-Wallis test with the Tukey post-hoc modification for multiple comparisons was utilized. The two-way repeated measures analysis of variance was utilized to compare the overall characteristics of left ventricular function among the three different regimens over the treatment and posttreatment time period. A P value of ≤0.05 was considered significant.
RESULTS
In vitro susceptibility.
At an inoculum of 105 CFU/ml, the MICs for oxacillin, free amikacin, and MiKasome were 2, 2, and 8 μg/ml, respectively. At the higher inoculum (107 CFU/ml), the MICs of all the antibiotics were increased twofold. As noted, the MICs of MiKasome were fourfold greater than for free amikacin at both of the inocula tested.
Timed-kill curves.
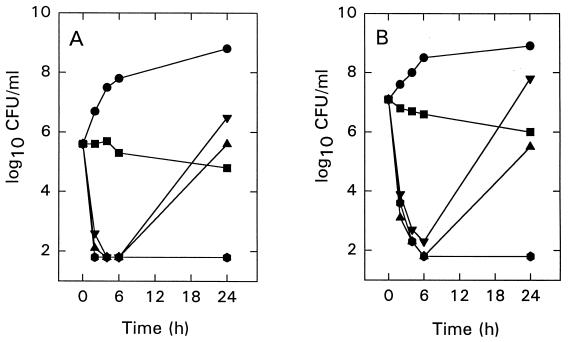
Figure 2 shows the timed-kill curves of oxacillin, free amikacin, and MiKasome alone (all antibiotics were tested at 5× MICs) and the combination of oxacillin with either free amikacin or MiKasome at final inocula of 5 × 105 CFU/ml (Fig. 2A) and 107 CFU/ml (Fig. 2B). Oxacillin alone had a slow bactericidal effect. Free amikacin and MiKasome alone produced a bactericidal effect after 6 h of incubation; however, rapid regrowth was observed at between 6 and 24 h. At 24 h of incubation, oxacillin, combined with either free amikacin or MiKasome, prevented the regrowth phenomenon observed above (data not shown for oxacillin plus free amikacin).
FIG. 2.
Killing curves of oxacillin (■), free amikacin (▴), and MiKasome (▾) alone and the combination of oxacillin with MiKasome ( ) against S. aureus VP986β+ at initial inocula of 5 × 105 CFU/ml (A) and 107 CFU/ml (B). The concentration of each antibiotic was 5× MICs. ●, Control group.
Microbiologic evaluation in endocarditis model.
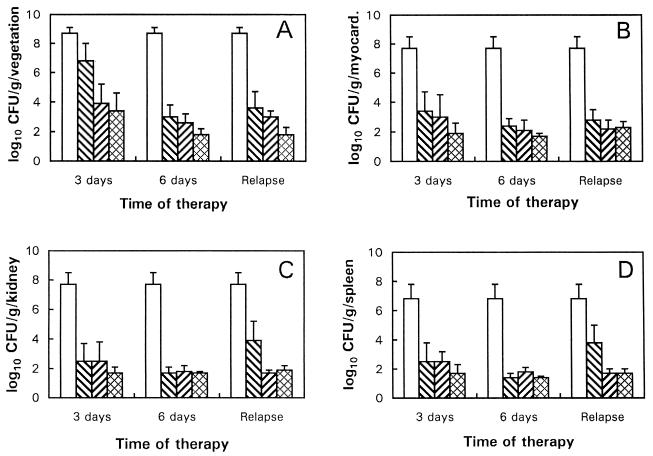
Figure 3 shows the S. aureus densities in vegetations, myocardium, kidney, and spleen in the different therapy regimens. At all sacrifice times (3 or 6 days of therapy and at 5 days posttherapy), both combination regimens significantly reduced S. aureus densities in vegetations versus the untreated controls (P < 0.05) (Fig. 2A). Interestingly, oxacillin monotherapy did not significantly reduce vegetation densities after 3 days of therapy. However, at this time point, both combination regimens were significantly more effective than oxacillin alone (P <0.05) (Fig. 2A). Since myocardial abscesses are a recognized complication of S. aureus endocarditis, the efficacies of the drug regimens against myocardial infection were assessed. All three regimens were effective in significantly decreasing myocardial S. aureus densities compared to the controls (P < 0.05) (Fig. 3B). Oxacillin alone and the combination of oxacillin plus either free amikacin or MiKasome significantly reduced S. aureus densities in renal abscesses versus the controls during therapy (Fig. 3C). However, at 5 days posttherapy, oxacillin alone did not prevent bacteriologic relapse in this tissue. In contrast, the two combination regimens significantly reduced S. aureus densities versus both controls and oxacillin alone at this time point (P < 0.05) (Fig. 3C). Data obtained with splenic abscesses were virtually identical to those observed with renal abscesses (Fig. 3D).
FIG. 3.
Mean vegetation (A), myocardium (B), kidney (C), and
spleen (D) S. aureus densities for regimens with oxacillin
(▧) alone, oxacillin plus free amikacin (▨), and oxacillin plus
MiKasome ( ) in
experimental rabbit endocarditis. The data represent the mean (± the
standard deviation) from at least seven rabbits. □, Untreated control
group.
) in
experimental rabbit endocarditis. The data represent the mean (± the
standard deviation) from at least seven rabbits. □, Untreated control
group.
Evaluation of tissue sterilization and survival rates.
Table 1 shows the sterilization frequencies of vegetations, myocardium, and kidney samples in the various therapeutic regimens. In general, the percentage of sterilizations in all target tissues was somewhat higher in rabbits that had received the oxacillin plus MiKasome combination regimen compared to those receiving oxacillin alone. Similar results were obtained for spleen abscess sterilizations (data not shown). None of these differences reached statistical significance. The proportions of animals surviving during and after therapy with oxacillin alone or in combination with either free amikacin or MiKasome were similar (58, 60, and 62%, respectively).
TABLE 1.
Sterilization of various tissues with oxacillin (50 mg/kg, three times daily) alone or with oxacillin combined with either free amikacin or MiKasome in an experimental S. aureus endocarditis model
| Regimen | No. of sterile samples/total no. of
samples (%)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vegetation
|
Myocardium
|
Kidney
|
|||||||
| 3 days | 6 days | Relapsea | 3 days | 6 days | Relapse | 3 days | 6 days | Relapse | |
| Oxacillin | 0/9 (0) | 2/10 (20) | 0/7 (0) | 1/9 (11) | 4/10 (40) | 1/7 (14) | 2/9 (22) | 7/10 (70) | 0/7 (0) |
| Oxacillin + free amikacin | 0/8 (0) | 2/9 (22) | 2/9 (22) | 1/8 (13) | 4/9 (44) | 3/9 (33) | 4/8 (50) | 7/9 (78) | 7/9 (78) |
| Oxacillin + MiKasome | 1/9 (11) | 5/6 (83) | 5/10 (50) | 4/9 (44) | 6/6 (100) | 2/10 (20) | 5/9 (56) | 6/6 (100) | 8/10 (80) |
Relapse, 5 days posttherapy.
Left ventricular function.
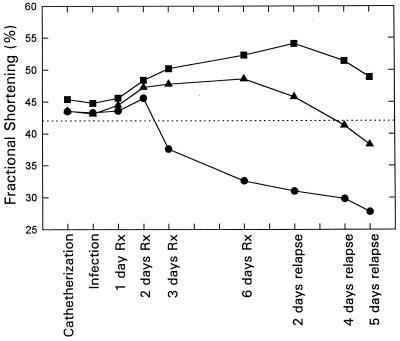
The serial measurements of left ventricular function in all of the treatment regimens are illustrated in Fig. 4. Measurements of fractional shortening in 25 healthy animals revealed a mean value of 42.8%. Both of the aminoglycoside combination regimens significantly preserved normal left ventricular function over the course of the observation period (e.g., between catheterization and relapse time points) compared to the regimen of oxacillin alone (P < 0.001). Furthermore, the combination of oxacillin with conventional amikacin was significantly more effective in terms of the protection of left ventricular function than was oxacillin plus MiKasome (P < 0.001). In contrast, oxacillin alone did not protect the left ventricular function, with significant and progressive declines in function noted after the second day of therapy.
FIG. 4.
Impact of oxacillin alone (●), oxacillin plus free amikacin (■), and oxacillin plus MiKasome (▴) on serial left ventricular function in experimental rabbit endocarditis. The dashed line represents the mean value of fractional shortening of 25 healthy rabbits. P values: oxacillin alone versus oxacillin plus free amikacin, P < 0.001; oxacillin alone versus oxacillin plus MiKasome, P < 0.001; oxacillin plus free amikacin versus oxacillin plus MiKasome, P < 0.001. Rx, therapy.
DISCUSSION
In recent years, many studies have attempted to optimize aminoglycoside therapeutic regimens, as well as to identify novel aminoglycoside formulations for minimizing toxicity while increasing overall efficacy against both intra- and extracellular infections. In this regard, liposome-encapsulated aminoglycosides have received considerable attention, both for their potential for increased efficacy and for the reduction in toxicities in the treatment of experimental intracellular bacterial infections (12, 13, 17, 20, 23, 28). We also reported that a liposome-encapsulated amikacin formulation (MiKasome) was as effective as free amikacin in the treatment of experimental P. aeruginosa endocarditis (an extracellular infection); moreover, that study produced evidence of reduced nephrotoxicity in MiKasome-treated animals compared to those receiving free amikacin (31, 32). However, it is presently unclear what precise role liposomal aminoglycosides will play in combination with β-lactam agents in the treatment of severe extracellular gram-positive infections (e.g., S. aureus).
The present study demonstrated several noteworthy microbiologic findings: (i) all three antibiotic regimens were effective in reducing S. aureus densities in vegetations, myocardium, kidney, and spleen compared to untreated controls during therapy (except for oxacillin alone after 3 days of therapy for vegetation densities); (ii) all three regimens were also effective in preventing bacteriologic relapses in vegetations and myocardium at 5 days posttherapy; (iii) in contrast, oxacillin alone did not prevent bacteriologic relapse in the kidney and spleen at 5 days posttherapy; and (iv) the combination regimens (especially with MiKasome) yielded a somewhat higher frequency of target tissue sterilization than the use of oxacillin alone. It should be pointed out that the infecting S. aureus strain in this study exhibits borderline oxacillin resistance in vitro (3). Therefore, it is not clear at this time whether the superior microbiologic effects demonstrated for the combination of MiKasome plus oxacillin versus free amikacin plus oxacillin in the present study would also be observed in the treatment of a more highly susceptible staphylococcal strain.
Congestive heart failure complicating infective endocarditis has a significant negative impact on the prognosis of this infection (16, 18, 26). In aortic endocarditis, as induced in the current study, left ventricular function during the experimental period is influenced by a number of factors, including (i) the degree of direct myocardial infiltration by the organism, resulting in myocardial inflammation and necrosis; (ii) the extent of valvular destruction and incompetence from vegetative endocarditis, resulting in hemodynamically significant ventricular volume overload; and (iii) adaptive myocardial mechanisms responding to such ventricular volume overload states (30). In previous studies involving small-animal models, M-mode and two-dimensional high-frequency echocardiography studies have provided accurate visualization of the intracardiac structures and have elucidated the precise mechanisms of cardiophysiologic function (16, 18, 26). Therefore, the ability to serially evaluate left ventricular function echocardiographically during the course of experimental endocarditis may provide important data on correlations of microbiologic efficacy (e.g., decreases in intravegetation bacterial densities) with cardiac functional improvements. In the current study, serial echocardiographic assessments of animals treated with the different antibiotic regimens revealed two major findings: (i) an early and progressive decline in left ventricular function in the oxacillin monotherapy group and (ii) preservation of normal ventricular function in the combination therapy regimens. The early decline (by the third day of therapy) of left ventricular function in rabbits given oxacillin monotherapy was associated with higher vegetation bacterial densities in this treatment group compared to the combination regimens at this time point. These observations suggested that this early decline in ventricular function seen in the oxacillin monotherapy group may have been directly correlated with a more severe valvulitis and valvular regurgitation, with resultant ventricular volume overload, compared to the aminoglycoside regimens. In contrast, only minor differences in bacterial counts within the myocardium were found at this time point between the antibiotic regimens. It thus seems unlikely that differences in the extent of myocarditis induced by S. aureus could contribute to the differences in ventricular function noted between the monotherapy and combination therapy regimens. It is also conceivable that oxacillin monotherapy might be associated with a delayed clearance of bacteremia compared to the combination therapy regimens, resulting in sepsis-related myocardial dysfunction. However, pilot studies in our laboratory have indicated that this oxacillin dose regimen rapidly clears the blood stream of this infecting strain, even though this regimen exerts a relatively slow clearance of intravegetation staphylococci (1a). For the combination therapy regimens, a progressive adjustment in ventricular function was noted to occur from the supranormal range during therapy to the physiologically normal range posttherapy. This observation undoubtedly reflects multiple events occurring in vivo, including myocardial regulatory mechanisms adjusting to constant volume overload in the face of endocarditis (30), as well as volume overload related to drug administrations. For example, MiKasome treatment consists of two 30-ml i.v. infusions (days 1 and 4 of therapy) into a vascular system (total blood volume of ∼150 ml in rabbits) that is already somewhat compromised by volume overload related to aortic valve endocarditis.
In summary, in the current study the combination of oxacillin with either conventional amikacin or intermittent MiKasome yielded significant reductions in S. aureus densities in all of the target tissues compared to controls and the use of oxacillin alone both during therapy and posttherapy. Furthermore, the combination regimens, but not the use of oxacillin alone, appeared to protect left ventricular function for the duration of the study. These results suggest that intermittent dose regimens of MiKasome may enhance the in vivo bactericidal effects of oxacillin in severe S. aureus infections and may preserve selected physiological functions in selected target organs during such infections.
ACKNOWLEDGMENTS
This study was supported in part by a research grant from NeXstar Pharmaceuticals, Inc., Boulder, Colo. (to A.S.B.). L.I.K. was supported by a grant of the Deutsche Forschungsgemeinschaft (KU 1155/1-1).
We thank Rodrey White and George Kopchek for technical assistance in the rabbit echocardiographic analyses. We also thank Dorothy Colagiovanni and Jill Adler-Moore for their scientific assistance and Eliza Gaenger, Yin Li, and Suzanne M. Zondler for their excellent technical assistance in the infective endocarditis model. We thank Julie Wolf for excellent assistance in the statistical analyses.
REFERENCES
- 1.Bayer A S. Staphylococcal bacteremia and endocarditis. Arch Intern Med. 1982;142:1169–1177. [PubMed] [Google Scholar]
- 1a.Bayer, A. S. Unpublished data.
- 2.Caron F, Pestel M, Kitzis M D, Lemeland J F, Humbert G, Gutmann L. Comparison of different beta-lactam-glycopeptide-gentamicin combinations for an experimental endocarditis caused by a highly beta-lactam-resistant and highly glycopeptide-resistant isolate of Enterococcus faecium. J Infect Dis. 1995;171:106–112. doi: 10.1093/infdis/171.1.106. [DOI] [PubMed] [Google Scholar]
- 3.Chambers H F, Archer G, Matsuhashi M. Low-level methicillin resistance in strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1989;33:424–428. doi: 10.1128/aac.33.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers H F, Sachdeva M, Kennedy S. Binding affinity for penicillin-binding protein 2a correlates with in vivo activity of β-lactam antibiotics against methicillin-resistant Staphylococcus aureus. J Infect Dis. 1990;162:705–710. doi: 10.1093/infdis/162.3.705. [DOI] [PubMed] [Google Scholar]
- 5.Craig W A, Gudmundson S. Postantibiotic effect. In: Lorian V, editor. Antibiotics in laboratory medicine. London, England: The Williams & Wilkins Co.; 1991. pp. 403–431. [Google Scholar]
- 6.Drugeon H, Le Gallou F, Caillon J. Methods d’etudes de l’activite bactericide. In: Courvalin P, Drugeon H, Flandrou J P, Goldstein F, editors. Bactericidie: aspects theoriques et therapeutiques. Paris, France: Editions Maloine; 1990. pp. 112–126. [Google Scholar]
- 7.Eng E, McAndrews B, Satorius A, Proffitt R T, Adler-Moore J. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Comparative efficacy of amikacin and liposomal amikacin in the treatment of Klebsiella pneumoniaeinfection in mice, abstr. A-26. [Google Scholar]
- 8.Espersen F, Frimodt-Moller N. Staphylococcus aureusendocarditis: a review of 119 cases. Arch Intern Med. 1986;146:1118–1121. [PubMed] [Google Scholar]
- 9.Fantin B, Carbon C. In vivo antibiotic synergism: contribution of animal models. Antimicrob Agents Chemother. 1992;36:907–912. doi: 10.1128/aac.36.5.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fielding R M, Lewis R O, Moon-McDermott L. Altered tissue distribution and elimination of amikacin encapsulated in unilamellar, low-clearance liposomes (MiKasome) Pharmacol Res. 1998;15:1775–1781. doi: 10.1023/a:1011925132473. [DOI] [PubMed] [Google Scholar]
- 11.Fielding R M, Mukwaya G, Sandhaus R A. Clinical and preclinical studies with low-clearance liposomal amikacin (MiKasome) In: Woodle M C, Storm G, editors. Long-circulating liposomes: old drugs, new therapeutics. New York, N.Y: Springer-Verlag; 1998. pp. 213–225. [Google Scholar]
- 12.Fierer J, Hatlen L, Lin J-P, Estrella D, Mihalko P, Yau-Young A. Successful treatment using gentamicin liposomes of Salmonella dublininfections in mice. Antimicrob Agents Chemother. 1990;34:343–348. doi: 10.1128/aac.34.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fountain M W, Weiss S K, Fountain A G, Shen A, Lenk R P. Treatment of Brucella canis and Brucella abortus in vitro and in vivoby stable plurilamellar vesicle-encapsulated aminoglycoside. J Infect Dis. 1985;152:529–535. doi: 10.1093/infdis/152.3.529. [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg R S, Mitilenes G M, Lenk R P, Jedrusiak J A, Savage K, Swenson C E. The impact of liposome encapsulation of gentamicin on the treatment of extracellular Gram-negative bacterial infections. UCLA Symp Mol Cell Biol New Ser. 1988;89:205–214. [Google Scholar]
- 15.Hirano L, Bayer A S. β-Lactam–β-lactamase inhibitor combinations are active in experimental endocarditis caused by β-lactamase-producing oxacillin-resistant staphylococci. Antimicrob Agents Chemother. 1991;35:685–690. doi: 10.1128/aac.35.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoit B D, Khoury S F, Kranias E G, Ball N, Walsh R A. In vitroechocardiographic detection of enhanced left ventricular function in gene-targeted mice with phospholamban deficiency. Circ Res. 1995;77:632–637. doi: 10.1161/01.res.77.3.632. [DOI] [PubMed] [Google Scholar]
- 17.Karlowsky J A, Zhanel G G. Concepts on the use of liposomal antimicrobial agents: applications for aminoglycosides. Clin Infect Dis. 1992;15:654–667. doi: 10.1093/clind/15.4.654. [DOI] [PubMed] [Google Scholar]
- 18.Kupferwasser L I, Darius H, Buerke M, Rupprecht H J, Mohr-Kahaly S, Meyer J. Transesophageal ultrasonographic imaging in rat heart. Visualization of aortic valve vegetations in non-bacterial thrombotic endocarditis. J Am Soc Echocardiogr. 1998;11:201–205. doi: 10.1016/s0894-7317(98)70077-x. [DOI] [PubMed] [Google Scholar]
- 19.Lewis R P, Sandler H. Relationship between changes in left ventricular dimension and the ejection fraction in man. Circulation. 1971;44:548–557. doi: 10.1161/01.cir.44.4.548. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Berestein G. Liposomes as carriers of antimicrobial agents. Antimicrob Agents Chemother. 1987;31:675–678. doi: 10.1128/aac.31.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer R D, Liu S. In vitro synergy studies with ciprofloxacin and selected beta-lactam agents and aminoglycosides against multidrug-resistant Pseudomonas aeruginosa. Diagn Microbiol Infect Dis. 1988;11:151–157. doi: 10.1016/0732-8893(88)90017-x. [DOI] [PubMed] [Google Scholar]
- 22.Moellering R C, Wennersten C, Weinberg A N. Studies on antibiotic synergism against enterococci. I. Bacteriologic studies. J Lab Clin Med. 1971;77:821–828. [PubMed] [Google Scholar]
- 23.Morgan J R, Williams W E. Preparation and properties of liposome-associated gentamicin. Antimicrob Agents Chemother. 1980;17:544–548. doi: 10.1128/aac.17.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perlman B B, Freedman L S. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J Biol Med. 1971;42:394–410. [PMC free article] [PubMed] [Google Scholar]
- 25.Picard M H. M-mode echocardiography: principles and examination techniques. In: Weyman A E, editor. Principles and practice of echocardiography. 2nd ed. New York, N.Y: Lea & Febiger; 1994. pp. 282–301. [Google Scholar]
- 26.Pollick C, Hale S L, Kloner R A. Echocardiographic and cardiac assessment of mice. J Am Soc Echocardiogr. 1995;8:602–610. doi: 10.1016/s0894-7317(05)80373-6. [DOI] [PubMed] [Google Scholar]
- 27.Sahn D J, DeMaria A N, Kisslo J, Weyman A E. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 28.Swenson C E, Stewart K A, Hammett J L, Fitzsimmons W E, Ginsberg R S. Pharmacokinetics and in vivo activity of liposome-encapsulated gentamicin. Antimicrob Agents Chemother. 1990;34:235–240. doi: 10.1128/aac.34.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tunkel A R, Mandell G L. Infecting microorganisms. In: Kaye D, editor. Infective endocarditis. 2nd ed. New York, N.Y: Raven Press, Ltd.; 1992. pp. 85–97. [Google Scholar]
- 30.Wainai Y. Adaptive mechanisms of the aorta and the left ventricle to volume overloading following abrupt aortic regurgitation in rabbits. Cardiovasc Res. 1991;25:463–467. doi: 10.1093/cvr/25.6.463. [DOI] [PubMed] [Google Scholar]
- 31.Xiong Y Q, Adler-Moore J, Zack P, Bayer A S. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Efficacy of MiKasome (a liposomal amikacin formulation) versus free amikacin in experimental endocarditis due to Pseudomonas aeruginosa, abstr. A-30. [Google Scholar]
- 32.Zack P M, Zondler S M, Magallanez A, Xiong Y Q, Adler-Moore J P, Bayer A S. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Treatment with liposomal amikacin reduces myocarditis in the rabbit model of Pseudomonas aeruginosaendocarditis, abstr. B-30a. [Google Scholar]