Abstract
In flowering plants, male and female functions are usually closely associated in the same flowers, as predicted by resource allocation theory. However, the benefits of outbreeding can lead to unisexual flowers and the physiological control of their distribution across the plant (monoecy). Monoecy is thought to be a major route to dioecy (separation of sexual function of different individuals). The developmental and functional problems associated with unisexual flowers may thus be solved at the level of the evolution of monoecy. Consequently, the evolution of dioecy from monoecy requires mutations in only a single gene. Here various scenarios (conceptual models) are presented for the evolution of monoecy and dioecy, including scenarios consistent with known cases of single-gene control of dioecy, such as in Populus, and the artificial breeding of dioecy from monoecy experimentally achieved in Zea and Cucumis. Attention is also drawn here to the phenomenon of pleogamy, the minor or occasional occurrence of additional sex morphs within a species, which may provide important information about the genetic and developmental control of various sexual systems.
This article is part of the theme issue ‘Sex determination and sex chromosome evolution in land plants’.
Keywords: dioecy, monoecy, Populus, pleogamy, iterative development
1. Introduction
The iterative development that is characteristic of flowering plants leads to sexual function being widely distributed over what can be a very large organism. The distributed nature of sexual function can lead to patterning in diverse ways, some of which are probably highly adaptive [1]. Consequently, the dimension of sexual patterning that is concerned with flowers and their types is considered a very important one in botany, and has resulted in a good deal of terminology. Sexual organs in angiosperms are produced in evolutionarily conserved developmental units called flowers, which are of great functional significance. So it is not just the distribution of sexual function in individuals that is salient but also the distribution within flowers. Here, my aim is to introduce and discuss this patterning and suggest evolutionary scenarios, consistent with known examples, for transitions between monocliny (all flowers bisexual), monoecy (unisexual flowers of both sexes on the same individual) and dioecy (unisexual flowers on different individuals). Both the origin of dioecy from monoecy and the origin of dioecy from gynodioecy (female and monoclinous individuals) have been the subject of important theoretical work [2,3]. However, I focus on the monoecy pathway to dioecy here, as not only has it been comparatively neglected, but it is also likely to be the most important [4–6]. Monoecy is relatively common in the world flora with one estimate suggesting it is ‘slightly higher’ than the 5–6% frequency of dioecy [7]. The frequency of gynodioecy on the other hand has been estimated at less than 1% [8]. Also suggestive is the relatively high generic overlap between monoecy and dioecy, but lower between gynodioecy and dioecy [5,7].
(a) . Diversity of distribution of sexual function within flowering plants
Whether the ancestral state of the flower is unisexual or bisexual remains unknown [9], but in the extant angiosperm crown group, at least, it is believed to be bisexual (monoclinous). However, derived unisexual (diclinous) flowers are common. Individual plants can be male, female or cosexual (i.e. having both sexes however distributed). Among cosexual individuals five different sex types can be distinguished based on the varying presence of three different flower types male (M), female (F) and monoclinous (H). These are monoclinous individuals (all H), monoecious (M + F), gynomonoecious (H + F), andromonoecious (H + M) and trimonoecious (H + F + M). Unisexual individuals exist obviously as either male (M) or female (F). A mixture of these sex types can then be represented by individuals of a species to give different sexual systems: male and female (dioecy), female and monoclinous (gynodioecy), male and monoclinous (androdioecy), male, female and monoclinous (trioecy), female only (unisexual females with agamospermy), monoclinous only (monocliny).
Botanical terminology of floral sex can be confusing so it may be useful here to remind the reader how the terms came about. Linnaeus saw the flower correctly as a site for mating, and embodied this view in his systema sexualis [9] using the anthropocentric metaphor of a marital bed. Most flowers he called monoclinous (one bed, from klinē, a bed). These are also called by the imperfect terms ‘hermaphrodite’ or ‘perfect’ flowers (the former ambiguous because of the different use of the term in zoology and the latter typological). Of these flowers he wrote ‘husbands and wives enjoy one and the same bed’ (mariti & uxores uno eodemque thalamo gaudent).
He contrasted these with diclinous flowers (separate bed, or unisexual, flowers), continuing his metaphor he noted that these flowers allow that ‘men and women enjoy separate beds' (mariti & feminae distinctis thalamis gaudent). Diclinous plants could be either monoecious or dioecious. Finally, he used the term ‘polygamous’ (literally ‘with many [types of] marriages') for plants that mix monoclinous and diclinous flowers either on the same plant (polygamomonoecious) or different plants (polygamodioecious and trioecious) (table 1).
Table 1.
Classification of flowering plant sexual systems. The terms ‘polygamia monoecia’, ‘polygamia dioecia’ and ‘polygamia trioecia’, were used by Linnaeus for andro/gynomonecy, andro/gynodioecy and trioecy, respectively. These were replaced by more precise terms in the nineteenth century. As an extension of polygamy, the term ‘pleogamy’ was introduced in the nineteenth century (rare or teratomorphic occurrences of unexpected floral types, e.g. the occurrence of occasional cosexual individuals in a dioecious species). Other relevant terms (cosexual, hermaphrodite, heterodichogamy and paradioecy) are explained in the text.
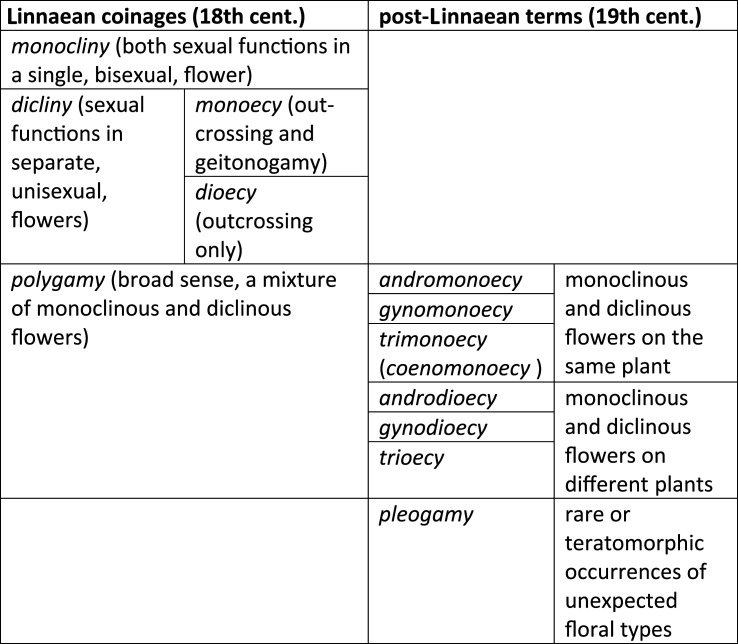 |
There the matter stood until the nineteenth century when scientists, including Darwin, realized that the polygamous class covered a lot of distinct variants that were important to distinguish for the growing discipline of floral biology, but which were not adequately covered by the sexual system. Thus, ‘polygamy’ was quietly dropped in favour of gyno- and andromonoecy, gyno- and androdioecy, trimonoecy and trioecy. The term polygamy does however persist to the present day as a useful catch-all term for any mixture of monoclinous and diclinous flowers. The Linnaean system also omitted the many temporal systems of apportioning sexual function such as heterodichogamy (sexual dimorphism from reciprocal temporal switching of floral sexes), coined in 1878 [7,10]. Generally though, the Linnaean terms have stood the test of time and the vast majority of plants fit fairly comfortably within them. However, there are numerous minor or teratomorphic (i.e. developmentally abnormal) exceptions that come under the term ‘pleogamy’, which is discussed below.
(b) . ‘Quantitative gender’ and the variation of sexual function between individuals
As discussed in the previous section, many cosexual seed plants are prone to produce numbers of unisexual flowers iteratively across the plant body. The precise numbers of male versus female flowers can vary according to environment (e.g. weather, plant age) or genotype, and hence individual plants commonly vary by degree in their maleness and femaleness in a way that is unusual for animals. This phenomenon was studied in a series of highly influential papers by David Lloyd, notably in his paper ‘Sexual strategies in plants III. A quantitative method for describing the gender of plants’ [11]. Lloyd, noting that ‘Gender (maleness or femaleness) is a quantitative phenomenon in plants … ’ [11, p. 103] coined the term ‘quantitative gender’ (eschewing the term ‘quantitative sex’ although this would have the same meaning). He defines ‘gender’ in the following terms: ‘The functional gender of a plant estimates the proportions of its genes which are transmitted through pollen (its maleness) or through ovules (its femaleness)’. Gender is therefore described by a formula (Lloyd's original or the numerous subsequent modifications) where Gi is the femaleness gender of an individual and by extension Ai (maleness gender) is 1 − Gi. There is no point using quantitative gender outside the ‘cosex’ (or cosexual, another Lloyd coining) systems that vary for G (whether monoecious, polygamous or pleogamous). Strict dioecy has Lloydian gender coefficients of 1 and 0. Quantitative gender is thus a developmental state of a cosexual individual that determines the balance of sexual function (defined in terms of gene transmission by gametes). Although generally applied to individuals, Lloydian gender coefficients can be applied, by extension, to inflorescences and populations. It should be pointed out that Lloyd's work has had enormous influence in moving the study of plant sex expression away from qualitative thinking about individual morphologies of sex expression, to quantitative treatments of plant sex at the population level, thereby allowing connections with quantitative population biology and evolutionary genetics. However, underlying the sex expression of plants is evolutionary developmental biology and developmental molecular genetics, and as the science expands in these more mechanistic aspects, particular phenotypes and individual expressions of sexual function are likely to return to prominence.
The importance and influence of Lloyd's papers go some way to explaining another difference between plant and animal systems, which is a quirk of terminology. This is the frequent use of the term ‘gender’ instead of ‘sex’ in botany, in contexts where it would not be used in zoology, except in the sociological literature [12,13]. ‘Gender’ in Lloyd's sense is defined by the transmission of genes via eggs versus sperm, and this is something that is traditionally described under the term sex. So gender and sex are probably interchangeable terms here, and Gi could equally well be called a ‘coefficient of sexual function’.
(c) . Monocliny and monoecy
As has been noted above, flowering plants are, rather interestingly, prone to producing their male and female sexual organs close together in modules called monoclinous flowers. Monoclinous flowers can share reproductive costs that aid both male and female sex function, such as attraction, and this has been shown theoretically (by allocation theory) to favour bisexual flowers [14]. Animals occasionally package their sexual organs in this way, as in the ovotestis of some molluscs, but generally animals are monoecious or dioecious and have their sexual function separated, probably owing to the physical constraints of mating mechanisms.
Monoecy in plants refers to a separation of sexes across the organism, specifically to the production of unisexual flowers that are spatially (and often temporally) separated. In monoecious animals, the spatial position of the sex organs is usually produced by unitary programmed development from the embryo (although not all animals have unitary development: the formation of sex organs in the gut of coelenterates in response to weather may be mentioned). The iterative and modular development of plants allows for a rather different realization of monoecy. As floral modules are produced, their development can respond to ‘environment’ (considered here broadly as both the external environment and the internal environment of physiological and positional cues). These cues trigger the expression of feminizing or masculinizing genes to produce flowers of the appropriate sex in different parts of the plant [15]. This is an interaction of development by environmental/physiological cues (D × E). It is true that many animals determine sex of individuals by the environment (e.g. temperature or age) and sex changes are frequent. However, only plants have such widespread division of the organism into iteratively determined sexual sectors, which makes monoecious plant development akin to a constantly unfolding sexual decision tree. Considering how common monoecy is, at a frequency ‘slightly higher’ than the frequency of dioecy, and perhaps as high as 7% of angiosperm species [7], it is surprisingly understudied.
There are many possible scenarios for transitions between sexual systems, involving few or many genetic changes. The purpose of the next sections is to show how certain transitions can result from changes in one or few genes and to point out examples where such pathways are consistent with examples for which the molecular basis is known.
(d) . A two-gene model for the origin of plant monoecy from monocliny
The evolution of monoecy from monocliny requires at least two mutations: a feminizing mutation (F) to produce female flowers and a masculinizing (M) one to produce male flowers. These may be in separate loci (genetically FFMM; figure 1) but also could (in theory at least) involve a single gene with two genetic changes in transcriptional regulation. Take for example a stamen developmental gene (M), gaining function in a dominant mutation to be expressed over the entire floral meristem, masculinizing the flowers. This gene could then acquire a cis-regulatory element in the promoter allowing repression by a factor only expressed in part of the plant. This factor would thereby become the feminizing factor F, but the mutation would have occurred in the same single gene (M). Importantly, these genes cannot act through loss of function (i.e. recessive) sterility mutations that compromise sexual function, as monoecious plants have both sexes fully functional, which is why we talk of masculinizing and feminizing mutations (more likely to be gain of function, i.e. dominant), and not sterility mutations.
Figure 1.
A scenario for the origin of monoecy from monocliny. If there is further evolution to dioecy, this type of monoecy will lead to XY dioecy (the sexes may be reversed, leading to ZW dioecy). (a) Population of four cosexual plants with monoclinous (hermaphrodite) flowers; (b) dominant, gain-of-function feminizing (gynoecious) mutation arises, F; (c) dominant, gain-of-function masculinizing mutation arises, M, spatially restricted, epistatically dominant to female; (d) population of four monoecious plants (default female type). Orange = hermaphrodite flowers; red = female flowers; blue = male flowers. Dash (–) = any allele. This particular scenario involves a gynoecious intermediate but similar scenarios involving other intermediates (androecious, andromonoecious and gynomonoecious) may be envisioned (as in the electronic supplementary material, figure S1A–D). (The flower icons are by Nefronus (modified) under CC BY-SA 4.0.)
Female flowers require the expression of F and male flowers the expression of M. Finally, as these genes may act in the same developmental pathway, one may be epistatically dominant to the other. Thus, where F and M are both expressed, we have flowers of the epistatically dominant sex. This means that only one of the genes (the epistatically dominant one) needs to be spatially regulated in order to have a mixture of flowers of the two sexes (the other gene can be expressed constitutively).
Although we do not yet have any precisely worked out evolutionary pathway to monoecy at the molecular level, such a scenario given in figure 1 is consistent with our knowledge of the molecular control of monoecy in maize (Zea mays) and cucurbits (Cucurbitaceae), from where much of our most detailed knowledge comes. Even in these systems, our knowledge is incomplete [16,17]. Both systems are complex, but the interplay of feminizing and masculinizing genes is a common feature. In maize for example, TASSEL SEED1&2 are masculinizing genes and SILKLESS is a female-promoting gene, acting by protecting developing pistils from TASSEL SEED (and therefore epistatically dominant to TASSEL SEED). They are consistent with the basic FFMM genetic model, as are the equivalent MF genes in melon, WIP1 and ACS11 [18]. Working out the molecular developmental pathways to monoecy is important, as monoecy is a widespread sexual strategy in plants, and the gene architecture of monoecy will be the ‘toolbox’ used in the evolution of dioecy through the monoecy pathway, a pathway thought to be particularly common [4,5].
(e) . A single-gene origin of dioecy from monoecy
As discussed above, monoecy offers an important staging post for the evolution of dioecy as unisexual flowers have already evolved. Monoecy specifies unisexual flowers according to a D × E interaction, E referring to the physiological/hormonal environment within the plant. What is needed is for sexual specification to become genetically fixed (i.e. under the control of a segregating gene) and so decoupled from environment (a D × E to D × G transition). The precise nature of the transition to dioecy will depend on the genes provided by the molecular developmental genetics of monoecy. Although more complex explanations for the evolution of dioecy could involve mutations in multiple genes, the simplest explanation is that of two mutations in a single gene. As this simplicity is rather remarkable, I will give the scenario here. The monoecious state has feminizing and masculinizing genes, FFMM and figure 2 shows a scenario whereby the evolution of dioecy requires two mutations in one (and only one) of these genes. The XY case requires a strong allele of M (M+) which makes a male (FFMM+). To make a female requires a weak or null allele of M (M−) in homozygous condition (FFM−M−). FFM−M will be monoecious and selfing will give some homozygotes. Then FFM−M+ × FFM−M− will segregate for the single locus M in an XY pattern. For a ZW system, the process is identical except with the sexes flipped with the relevant mutations in F.
Figure 2.
A scenario for the origin of dioecy from the type of monoecy shown in figure 1, via an initial gain of function androecious mutation (although the gynoecious loss of function mutation could come first instead). Dioecy is controlled by a single gene M, with a strong allele (superscript plus; M+), and null allele (superscript minus; M−). This will give an XY sex-determination system, as in Populus tremula. A variant of this type of dioecy has been artificially bred in maize and melon (electronic supplementary material, figure S1F). The pattern of sexes can be reversed in the ancestral monoecious condition to give a ZW system (as in Populus alba; electronic supplementary material, figure S1E). (a) Population of four monoecious plants (of a default female type); (b) dominant, gain-of-function mutation arises in M, abolishing spatial restriction of masculinizing factor (M+); (c) loss-of-function mutation (M−) arises abolishing male factor entirely; plant is then female by default) (M−M−); (d) Population of four dioecious plants (default female, XY). Red = female flowers; blue = male flowers.
Taking the model of monoecy presented in figure 1, it will be seen that there is already a default sex (set by the constitutively expressed gene). The gene promoting the other sex is epistatically dominant to the constitutive gene, so can act as a single-gene sex switch. If it is present, it enforces the sex it promotes. If it is absent or repressed, then the other, default, sex is expressed. The epistatically dominant gene will determine the heterogametic sex, but there is no particular reason why the order of appearance of male versus female phenotypes should be set by it, as either the loss of function mutation or the gain of function could appear first. An advantage of the single-gene model is that there is no requirement to bring two genes into linkage, as there is in a two-gene model. Single-gene control is also present in both cases where dioecy has been artificially bred from monoecy [18,19]. In artificially dioecious maize (Z. mays), for instance, dioecy is controlled by segregation of a single copy of functional TASSEL SEED2 to give males, the male thus being the heterogametic sex [19].
Is there any evidence that this model reflects what happens in nature? Aspens and poplars (Populus spp.) provide an example that is consistent with single-gene control [20,21]. In Populus tremuloides (XY), the M+ allele is a negative regulator (ΨARR17-IR) of the female function gene (popARR17) and the M− allele is the null allele (it is hemizygous). Trees are FFM+M− (male) or FFM−M− (female). Populus alba (ZW) is somewhat different. This segregates the female function gene itself, as F−F−MM (male) and F+F−MM (female: hemizygous for popARR17). Are these mutations plausible and common in nature? They are likely to be. The M− or F− mutations are the loss of function mutations, easily achieved by a premature stop codon or a deletion: such mutations occur regularly. The M+ or F+ mutations may be as simple as abolishing spatial control of expression and allowing the gene to be expressed in all floral primordia (instead of a subset based on environment or developmental context, as in monoecy). This could be achieved via a single promoter mutation. The simplicity of these mutations coupled with the frequent occurrence of monoecy in nature (‘slightly higher’ than the frequency of dioecy [7]) may make this single-gene sex determination via monoecy more common than is currently realized [4].
(f) . Nature and sequence of mutations
The pathway described above could be called ‘segregational capture’ as it involves transferring control by the spatial regulation of a gene (monoecy) to control by the segregation of a gene within a population (dioecy). It can be rapid as it involves major mutations with complete penetrance, such as the hemizygosity of a dominant control factor. This is quite distinct from the monoecy-paradioecy pathway originally posited by Lloyd [22,23]. Paradioecy is defined as ‘the inconstant presence of male or female flowers in the males and females of dioecious species, with the inconstancies being of similar magnitude in both sexes' [24, p. 700], or as Lloyd put it: a ‘pattern of inconstancies of similar magnitude in the two sexes of a dimorphic population with unisexual flowers’ [22, p. 130]. In theory, divergent evolution could increase the sexual differentiation until full dioecy is achieved. Such a mechanism has many attractions as it allows for evolution by many genes of small effect, a well-attested evolutionary mode. However, convincing paradioecious systems are rare, perhaps implying that this pathway is also rare. The discovery of the molecular genetics of more systems will be needed for the relative frequency of different pathways to become clearer.
There remains the problem of whether, in the sequence of feminizing and masculinizing mutations, one is more likely to precede the other. Gynonomonoecy and gynodioecy can arise as devices for outbreeding, but this is not true for andromonoecy and androdioecy [2,3]. However, andromonoecious and androdioecious species exist, indicating that other (and potentially even more interesting) biological factors may be in play [25,26]. Andromonoecy may be a way of dealing with pollen limitation when the development of individual flowers is too canalized to increase stamen number or anther size. Alternatively, it may be a way of increasing rewards to pollen-harvesting pollinators without increasing female reproductive investment to supraoptimal levels. These specific biological factors (whatever they might be), would, as has previously been suggested, ‘make studies of andromonoecy particularly valuable’ [3, p. 149].
In Asteraceae, gynomonoecy is a particular feature of capitulum biology and very common [27], thus skewing the overall ratio. When Asteraceae is excluded, then andromonoecy appears to be at least twice as common as gynomonoecy. Taking the figures of Yampolsky & Yampolsky [28], but excluding Asteraceae, then 128 genera of angiosperms are noted as andromonoecious, while only 19 are gynomonoecious. These occurrences are not random, with ‘hotspots’ being in the Poaceae and Apiaceae [29] for andromonoecy, and in the ‘chenopods’ (Amaranthaceae s.l.) for gynomonoecy. It may be that monoecy commonly evolves via andromonoecy, with the genetic change for male flowers leading, followed by one for female flowers [26]. In the case of dioecy, however, with the reasonable assumption that it is a mechanism for outbreeding, the female mutation is more likely to lead, although it is worth considering whether there may be ecological or resource allocation factors that would allow an androecious mutation to persist in a monoecious population.
(g) . Developmental lability and instability in plants and pleogamy
As discussed elsewhere [30], the development of animals is generally unitary, somewhat independent of environment, and tends to be canalized by cell lineage. In plants by contrast, development is modular and iterative, results from continual environmental feedback and tends to be canalized by cell position rather than cell lineage. These important distinctions allow plants a good deal of plasticity with respect to environment. A tree on a windswept mountain or coastal headland will not be the same size and shape as a tree in a sheltered valley. Similar developmental plasticity can apply, to a lesser extent, to the reproductive structures. Rare or teratomorphic floral forms are often encountered because of this lability (or instability) of developmental processes in plants. The lability may result from mutations in developmental genes predisposing plants to an abnormal form [31], hybridity or developmental irregularities owing to environmental insult (e.g. hot and cold shock), epigenetic shock following hybridization or abnormal methylation patterns as in the peloric Linaria studied by Linnaeus [32]. Vertebrates are generally more highly canalized developmentally than plants, but even here sexual lability can occur (especially in certain fishes, amphibia, reptiles and birds [33]).
In plants, this phenomenon of developmental instability, when it affects sexual function, as it frequently does, can lead to a mixing of the usual mating systems described above. For instance, a normally dioecious species may have an occasional plant that produces some hermaphrodite or opposite sex flowers. This is well known in willows (Salix spp.) and poplars (Populus spp.) for example [34]. Such mixed systems are usually obviously aberrant although in some cases can be more stable. They were extensively studied by Löw [35] and Schulz [36,37] in the nineteenth century, and the name pleogamy was coined, as summarized by Knuth [38]. Pleogamy was formed from pleíōn (pléōn): more, extra, supernumerary and gamia: marriage (in the Linnean sense, forming a natural pair with the Linnean term polygamy). Polygamy refers to a mixing of monoclinous and diclinous flowers, whereas pleogamy refers to further mixing in supernumerary ways. The definition I adopt is: ‘the rare occurrence of unexpected floral forms in plants of a given mating system’.
A good example is provided by Asparagus officinalis which is dioecious, but between 0 and 1% of male plants are ‘andromonoecious’ (i.e. they have some monoclinous, or at least less well masculinized flowers). Floral feminization may be slight, or all the way to fully hermaphrodite with fertile ovules [39]. Hermaphrodite flowers can also occasionally be found on female plants so making the plants technically ‘gynomonoecious’, but in these, fertile pollen is never developed [39]. It is not helpful to refer to ‘andromonoecy’ and ‘gynomonoecy’ in plants that are clearly male and female but with aberrant hermaphrodite flowers. Rather we should reserve andro- and gynomonoecy for cases where this is the usual and stable mating system. The aberrant occurrences are therefore pleogamy and we may talk of female pleogamy, where the pleogamy is effectively feminizing (i.e. the production of female flowers on monoclinous individuals, or hermaphrodite flowers on male individuals), or ‘male pleogamy’ when the situation is reversed and pleogamy is masculinizing.
Pleogamous variation is of great interest. First, it allows the formation of supersexes [30], and second, it may give important insights into the developmental origin of the major mating systems. Ehlers & Bataillon [40] have addressed the issue of pleogamy in an evolutionary context, although referring to the phenomenon as ‘inconstant sex’ rather than pleogamy. They found numerous examples of female pleogamy (the occurrence of hermaphrodite flowers on ‘male’ individuals), associated with animal pollination. Models show that a feminizing modifier (causing female pleogamy) could invade populations under certain conditions, such as pollen limitation [40]. This shows that pleogamy may not be just accidental developmental aberration (although it may be) but that some level of pleogamy may be selected for under certain conditions. This may be a route to stable trioecy, or just a failure to attain absolute dioecy.
More information on pleogamous systems would be very valuable in order to understand its evolutionary developmental origin and molecular genetic basis. However, in recent times, this information has not been collected particularly well or systematically. Part of the problem is that whereas these cases are common enough to come upon from time to time, they are rare enough that it is unrewarding to go looking for them. To quote Löw [35]: ‘Progress in this sphere is only possible by correlated and systematized work, conducted by many investigators.’
2. Concluding remarks
It should be clear from the above that greater knowledge of the molecular control of reproductive development in diverse systems is highly desirable, especially in monoecious systems, where maleness and femaleness are iterated sequentially according to developmental rules and environmental feedbacks. The superbly obliging model organism, Arabidopsis thaliana, that has served botany so well, is monoclinous and fails us when we ask questions about other systems of sexual development. In dioecious plants, sex determination involves both a ‘trigger’ and a downstream ‘developmental mechanism’. The triggers we already know are immensely diverse, but it is possible that a common downstream developmental pathway may be involved in multiple seed plant systems. Now would be a good time to move the study of plant sex determination, and plant sex in general, from ‘evolutionary genetics' to ‘evolutionary developmental genetics’.
Acknowledgements
I thank Susanne Renner and Niels Müller for much stimulating discussion, for organizing the meeting ‘Sex determination, sex chromosome evolution and the role of sexual differentiation in land plants', and for inviting the contribution presented here. I also thank participants at the meeting, notably Crispin Jordan and Michael Scott for many helpful, stimulating and challenging comments. Two anonymous reviewers are appreciated for their valuable comments and insights.
Electronic supplementary material
Electronic supplementary material, figure S1. Alternative evolutionary scenarios for reproductive transitions. 1A: A scenario for the evolution of monoecy from monocliny via a gynomonoecious mutation; 1B: evolution of monoecy from monocliny via an andromonoecious mutation; 1C: monoecy from monocliny via a gynoecious mutation; 1D: monoecy from monocliny via an androecious mutation; 1E: ZW dioecy from monoecy via a gynoecious mutation; 1F: XY dioecy from monoecy; 1G: evolution of dioecy via an androecious mutation followed by a gynoecious mutation; 1H: a scenario for the evolution of an XY system (as in Populus tremula) from a ZW system (as in Populus alba).
Data accessibility
This article has no additional data.
Authors' contributions
Q.C.: conceptualization, investigation, methodology, writing—original draft and writing—review and editing.
Competing interests
I declare I have no competing interests.
Funding
Work in my laboratory is funded by the Canadian Natural Sciences and Engineering Research Council (NSERC) Discovery Grants program (grant no. RGPIN-2014-05820), support from which is gratefully acknowledged.
References
- 1.Barrett SCH, Hough J. 2013. Sexual dimorphism in flowering plants. J. Exp. Bot. 64, 67-82. ( 10.1093/jxb/ers308) [DOI] [PubMed] [Google Scholar]
- 2.Charlesworth B, Charlesworth D. 1978. Model for evolution of dioecy and gynodioecy. Am. Nat. 112, 975-997. ( 10.1086/283342) [DOI] [Google Scholar]
- 3.Charlesworth D, Charlesworth B. 1978. Population genetics of partial male sterility and evolution of monoecy and dioecy. Heredity 41, 137-153. ( 10.1038/hdy.1978.83) [DOI] [Google Scholar]
- 4.Renner SS, Ricklefs RE. 1995. Dioecy and its correlates in the flowering plants. Am. J. Bot. 82, 596-606. ( 10.1002/j.1537-2197.1995.tb11504.x) [DOI] [Google Scholar]
- 5.Renner SS, Müller NA. 2021. Plant sex chromosomes defy evolutionary models of expanding recombination suppression and genetic degeneration. Nat. Plants 7, 392-402. ( 10.1038/s41477-021-00884-3) [DOI] [PubMed] [Google Scholar]
- 6.Renner SS. 2016. Pathways for making unisexual flowers and unisexual plants: moving beyond the ‘two mutations linked on one chromosome’ model. Am. J. Bot. 103, 587-589. ( 10.3732/ajb.1600029) [DOI] [PubMed] [Google Scholar]
- 7.Renner SS. 2014. The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 101, 1588-1596. ( 10.3732/ajb.1400196) [DOI] [PubMed] [Google Scholar]
- 8.Godin VN, Demyanova EI. 2013. On the distribution of gynodioecy in flowering plants. Botanicheskiy Zhurnal 93, 1465-1487. [Google Scholar]
- 9.Renner SS, Müller NA. 2022. Sex determination and sex chromosome evolution in land plants. Phil. Trans. R. Soc. B 377, 20210210. ( 10.1098/rstb.2021.0210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renner SS. 2001. How common is heterodichogamy? Trends Ecol. Evol. 16, 595-597. ( 10.1016/S0169-5347(01)02280-7) [DOI] [Google Scholar]
- 11.Lloyd DG. 1980. Sexual strategies in plants III. A quantitative method for describing the gender of plants. New Zealand J. Bot. 18, 103-108. ( 10.1080/0028825X.1980.10427235) [DOI] [Google Scholar]
- 12.Haig D. 2000. Of sex and gender. Nat. Genet. 25, 373. ( 10.1038/78033) [DOI] [PubMed] [Google Scholar]
- 13.Haig D. 2004. The inexorable rise of gender and the decline of sex: social change in academic titles, 1945–2001. Arch. Sex. Behav. 33, 87-96. ( 10.1023/B:ASEB.0000014323.56281.0d) [DOI] [PubMed] [Google Scholar]
- 14.Charlesworth D, Morgan MT. 1991. Allocation of resources to sex functions in flowering plants. Phil. Trans. R. Soc. B 332, 91-102. ( 10.1098/rstb.1991.0036) [DOI] [Google Scholar]
- 15.Golenberg EM, West NW. 2013. Hormonal interactions and gene regulation can link monoecy and environmental plasticity to the evolution of dioecy in plants. Am. J. Bot. 100, 1022-1037. ( 10.3732/ajb.1200544) [DOI] [PubMed] [Google Scholar]
- 16.Martínez C, Jamilena M. 2021. To be a male or a female flower, a question of ethylene in cucurbits. Curr. Opin. Plant Biol. 59, 101981. ( 10.1016/j.pbi.2020.101981) [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Liu B. 2017. Genetic regulation of maize flower development and sex determination. Planta 245, 1-14. ( 10.1007/s00425-016-2607-2) [DOI] [PubMed] [Google Scholar]
- 18.Boualem A, et al. 2015. A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science 350, 688-691. ( 10.1126/science.aac8370) [DOI] [PubMed] [Google Scholar]
- 19.Jones DF. 1934. Unisexual maize plants and their relation to dioecism in other organisms. Proc. Natl Acad. Sci. USA 20, 39-41. ( 10.1073/pnas.20.1.39) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cronk Q, Müller NA. 2020. Default sex and single gene sex determination in dioecious plants. Front. Plant Sci. 11, 1162. ( 10.3389/fpls.2020.01162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller NA, et al. 2020. A single gene underlies the dynamic evolution of poplar sex determination. Nat. Plants 6, 630-637. ( 10.1038/s41477-020-0672-9) [DOI] [PubMed] [Google Scholar]
- 22.Lloyd DG. 1980. The distributions of gender in four angiosperm species illustrating two evolutionary pathways to dioecy. Evol. Int. J. Org. Evol. 34, 123-134. ( 10.1111/j.1558-5646.1980.tb04795.x) [DOI] [PubMed] [Google Scholar]
- 23.Dorken ME, Barrett SCH. 2004. Sex determination and the evolution of dioecy from monoecy in Sagittaria latifolia (Alismataceae). Proc. R. Soc. Lond. B 271, 213-219. ( 10.1098/rspb.2003.2580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renner SS, Won H. 2001. Repeated evolution of dioecy from monoecy in Siparunaceae (Laurales). Syst. Biol. 50, 700-712. ( 10.1080/106351501753328820) [DOI] [PubMed] [Google Scholar]
- 25.Spalik K. 1991. On evolution of andromonoecy and ‘overproduction’ of flowers: a resource allocation model. Biol. J. Linn. Soc. 42, 325-336. ( 10.1111/j.1095-8312.1991.tb00566.x) [DOI] [Google Scholar]
- 26.De Jong TJ, Shmida A, Thuijsman F. 2008. Sex allocation in plants and the evolution of monoecy. Evol. Ecol. Res. 10, 1087-1109. [Google Scholar]
- 27.Torices R, Mendez M, Gomez JM. 2011. Where do monomorphic sexual systems fit in the evolution of dioecy? Insights from the largest family of angiosperms. New Phytol. 190, 234-248. ( 10.1111/j.1469-8137.2010.03609.x) [DOI] [PubMed] [Google Scholar]
- 28.Yampolsky C, Yampolsky H. 1922. Distribution of sex forms in the phanerogamic flora. Biblioteca Genetica 3, 1-62. [Google Scholar]
- 29.Schlessmann MA. 2010. Major events in the evolution of sexual systems in Apiales: ancestral andromonoecy abandoned. Plant Divers. Evol. 128, 233-245. ( 10.1127/1869-6155/2010/0128-0011) [DOI] [Google Scholar]
- 30.Cronk QC. 2022. Some sexual consequences of being a plant . Trans. R. Soc. B 377, 20210213. ( 10.1098/rstb.2021.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scutt CP, Oliveira M, Gilmartin PM, Negrutiu I. 1999. Morphological and molecular analysis of a double-flowered mutant of the dioecious plant white campion showing both meristic and homeotic effects. Dev. Genetics 25, 267-279. () [DOI] [PubMed] [Google Scholar]
- 32.Cubas P, Vincent C, Coen E. 1999. An epigenetic mutation responsible for natural variation in floral symmetry. Nature 401, 157-161. ( 10.1038/43657) [DOI] [PubMed] [Google Scholar]
- 33.Perrin N. 2009. Sex reversal: a fountain of youth for sex chromosomes? Evol. Int. J. Org. Evol. 63, 3043-3049. ( 10.1111/j.1558-5646.2009.00837.x) [DOI] [PubMed] [Google Scholar]
- 34.Stettler RF. 1971. Variation in sex expression of black cottonwood and related hybrids. Silvae Geneticae 20, 42-46. [Google Scholar]
- 35.Loew E. 1894. Blütenbiologische floristik des mitteleren und nördlichen Europa sowie Grönlands. Systematische zusammenstellung des in den letzten zehn jahren veröffentlichten beobachtungsmaterials. Stuttgart, Germany: F. Enke. [Google Scholar]
- 36.Schulz A. 1890. Beiträge zur kenntnis der bestäubungseinrichtungen und Geschlechtsvertheilung bei den Pflanzen, II. (Bibliotheca Botanica 3:17). Kassel, Germany: T. Fischer. [Google Scholar]
- 37.Schulz A. 1888. Beiträge zur kenntnis der bestäubungseinrichtungen und Geschlechtsvertheilung bei den Pflanzen, I. (Bibliotheca botanica 2:10). Kassel, Germany: T. Fischer. [Google Scholar]
- 38.Knuth P. 1906. Handbook of flower pollination, vol. 1 (tr. J.R. Ainsworth Davis). Oxford, UK: Clarendon Press. [Google Scholar]
- 39.Franken AA. 1970. Sex characteristics and inheritance of sex in asparagus (Asparagus officinalis L.). Euphytica 19, 277-287. ( 10.1007/BF01904204) [DOI] [Google Scholar]
- 40.Ehlers BK, Bataillon T. 2007. ‘Inconstant males’ and the maintenance of labile sex expression in subdioecious plants. New Phytol. 174, 194-211. ( 10.1111/j.1469-8137.2007.01975.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.




