Introduction
Patients with acquired immune deficiency syndrome or immunosuppression due to solid organ transplantation are at increased risk of treatment-resistant cutaneous warts, also known as recalcitrant warts. There are numerous treatments for cutaneous warts, with varying efficacy and recurrence rates; overall, recurrence is common. We present the case of a 66-year-old woman with recalcitrant warts successfully treated with systemic administration of the 9-valent human papillomavirus (HPV) vaccine (Gardasil 9, Merck & Co Inc) prior to renal transplant.
Case report
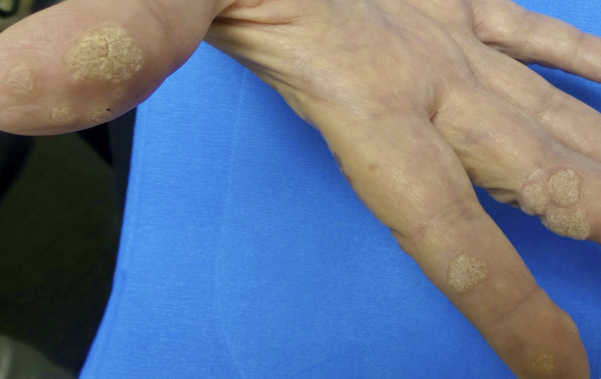
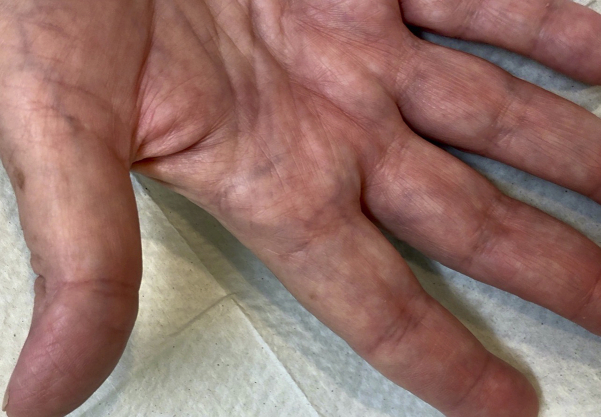
A 66-year-old woman with a past medical history of chronic renal failure, type 2 diabetes, hypertension, and thyroid cancer presented with 10 recalcitrant cutaneous warts on both hands that had been present for 10 years. Despite countless treatments with liquid nitrogen, the warts never improved (Fig 1). Before undergoing renal transplant, the patient was referred to our service for treatment. Given the recent reported success of using systemic1,2 and intralesional3,4 administration of HPV vaccines for recalcitrant warts and cancers, we offered the systemic 9-valent HPV vaccine to this patient. After informed consent, the patient received 3 intramuscular vaccine injections at 0, 2, and 6 months. The period of observation was from 2018-2021. The warts began to improve after the first dose of the vaccine and completely resolved after the third dose. The 9-valent HPV vaccine was well tolerated, the patient did not experience any adverse events, and she successfully underwent renal transplantation in 2019. She has not experienced post-transplant recurrence of the warts (Fig 2).
Fig 1.
Before treatment.
Fig 2.
Two and half years after treatment.
Discussion
Although cutaneous warts are physically benign and typically self-resolve in immunocompetent patients within a year,5 they can have a significantly negative impact on quality of life, which may prompt the desire for treatment. There are several classes of wart treatments available, including destructive therapies (eg, salicylic acid, cryotherapy, lasers), virucidal therapies, antiproliferative therapies (eg, 5-fluorouracil), immunotherapies (eg, imiquimod), as well as complementary and alternative therapies (eg, duct tape). Despite a plethora of available treatment options, recurrence is common, and affects up to 50% of the patients.6
In immunocompromised patients, such as solid organ transplant recipients, warts are especially prevalent. In one study of renal transplant recipients, after more than 5 years of living with the transplant, 92% of the participants had warts, and 65% of the participants had more than 5 warts.7 With a low rate of spontaneous wart regression in this population, medical treatment is often necessary to achieve remission.8
The 9-valent HPV vaccine is predominantly used to prevent anogenital warts and malignancies caused by infection with HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58. This vaccine was designed as a prophylactic, rather than a therapeutic, vaccine, but recent evidence suggests that it may have therapeutic potential as well.1,2 In the past decade, there have been several reported cases of recalcitrant wart resolution following administration of HPV vaccines.1,2 Given this evidence, the lack of success with multiple rounds of cryotherapy, and the difficulty of treating warts in transplant recipients due to immunosuppression,9 our patient consented to this treatment.
The patient’s warts completely resolved after all 3 injections, and she successfully underwent a renal transplant less than 3 months after the last injection. The warts have not recurred. In our patient, the HPV vaccine, which is normally used to prevent anogenital warts, was an effective treatment for recalcitrant cutaneous warts. Although the HPV types that typically cause cutaneous warts are not directly covered by this vaccine, it has been hypothesized that there may be some antigenic epitope cross-reactivity with the vaccine’s HPV coverage.2
The use of this vaccine may be considered in all potential transplant candidates, with or without preexisting recalcitrant cutaneous warts, given the therapeutic challenge that warts present once patients become immunocompromised. However, like other wart treatments, the HPV vaccine is not a panacea for all warts in all patients. This was highlighted by a recent case series that found no significant increase in wart clearance after vaccination.10 There is a need for randomized placebo-controlled trials before broad recommendations can be made, but it may still be prudent to offer this relatively low-side-effect option to patients preparing for organ transplantation.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Ferguson S.B., Gallo E.S. Nonavalent human papillomavirus vaccination as a treatment for warts in an immunosuppressed adult. JAAD Case Rep. 2017;3(4):367–369. doi: 10.1016/j.jdcr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venugopal S.S., Murrell D.F. Recalcitrant cutaneous warts treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16, and 18) in a developmentally delayed, 31-year-old white man. Arch Dermatol. 2010;146(5):475–477. doi: 10.1001/archdermatol.2010.71. [DOI] [PubMed] [Google Scholar]
- 3.Nofal A., Marei A., Ibrahim A.M., Nofal E., Nabil M. Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J Am Acad Dermatol. 2020;82(1):94–100. doi: 10.1016/j.jaad.2019.07.070. [DOI] [PubMed] [Google Scholar]
- 4.Nichols A.J., De Bedout V., Fayne R.A., Burke G.W., Kirsner R.S., Ioannides T. Systemic and intratumoral 9-valent human papillomavirus vaccine treatment for squamous cell carcinoma in situ in a renal transplant recipient. JAAD Case Rep. 2020;6(4):289–291. doi: 10.1016/j.jdcr.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruggink S.C., Eekhof J.A., Egberts P.F., van Blijswijk S.C., Assendelft W.J., Gussekloo J. Natural course of cutaneous warts among primary schoolchildren: a prospective cohort study. Ann Fam Med. 2013;11(5):437–441. doi: 10.1370/afm.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bencini P.L., Guida S., Cazzaniga S., et al. Risk factors for recurrence after successful treatment of warts: the role of smoking habits. J Eur Acad Dermatol Venereol. 2017;31(4):712–716. doi: 10.1111/jdv.14086. [DOI] [PubMed] [Google Scholar]
- 7.Dyall-Smith D., Trowell H., Dyall-Smith M.L. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991;30(11):785–789. doi: 10.1111/j.1365-4362.1991.tb04787.x. [DOI] [PubMed] [Google Scholar]
- 8.Schmook T., Nindl I., Ulrich C., Meyer T., Sterry W., Stockfleth E. Viral warts in organ transplant recipients: new aspects in therapy. Br J Dermatol. 2003;149(suppl 66):20–24. doi: 10.1046/j.0366-077x.2003.05627.x. [DOI] [PubMed] [Google Scholar]
- 9.D'Souza G.F., Zins J.E. Severe plantar warts in an immunocompromised patient. N Engl J Med. 2017;377(3):267. doi: 10.1056/NEJMicm1616238. [DOI] [PubMed] [Google Scholar]
- 10.Kost Y., Deutsch A., Zhu T.H., Hulur I., Blasiak R.C. Vaccination against human papillomavirus is not associated with resolution of verruca vulgaris in immunocompetent 9- to 21-year olds. J Am Acad Dermatol. 2021;S0190-9622(21):02344–02346. doi: 10.1016/j.jaad.2021.08.016. [DOI] [PubMed] [Google Scholar]