Introduction
Permanent chemotherapy-induced alopecia (pCIA) is the absence of hair regrowth after more than 6 months of treatment discontinuation.1 pCIA has a profound impact on patients’ quality of life.1 Data on the pathophysiology and treatment of pCIA are scarce.1 We describe a patient who developed pCIA after hematopoietic stem cell transplantation and responded to low-dose oral minoxidil (LDOM) with full hair regrowth.
Case report
A 41-year–old Caucasian woman was diagnosed with non-Hodgkin lymphoma and treated with the EPOCH-RX protocol (etoposide, vincristine, cyclophosphamide, doxorubicin prednisone, and rituximab). After treatment, the patient experienced complete hair loss, which was followed by spontaneous full regrowth. Two years later, the patient developed therapy-related acute myeloid leukemia. After treatment with busulfan and cyclophosphamide failed, a salvage protocol with cytarabine was administered, followed by allogeneic hematopoietic stem cell transplantation. To prevent graft-versus-host disease (GVHD), the patient was given cyclosporine, methotrexate, and antithymocyte globulin. Consequently, she experienced total alopecia with minimal regrowth that did not improve after 18 months, which necessitated the use of a scalp prosthesis. The patient was referred to a dermatologist for evaluation. On examination, there was severe diffuse alopecia without erythema, scaling, skin thickening, or dyspigmentation. pCIA, chronic GVHD, alopecia areata, and androgenetic alopecia (AGA) aggravated by chemotherapy were suggested as differential diagnoses. Aside from alopecia, no clinical or laboratory signs of chronic GVHD were found. A punch biopsy of the scalp skin revealed a few miniaturized follicles and the absence of inflammation or fibrosis (Fig 1). The patient was started on oral prednisone 40 mg daily and topical minoxidil solution (TMS) 2% twice daily. Prednisone was tapered down over 8 weeks and discontinued owing to lack of improvement. TMS was increased to 5% daily for 3 months, then twice daily for 10 months, with minimal improvement.
Fig 1.
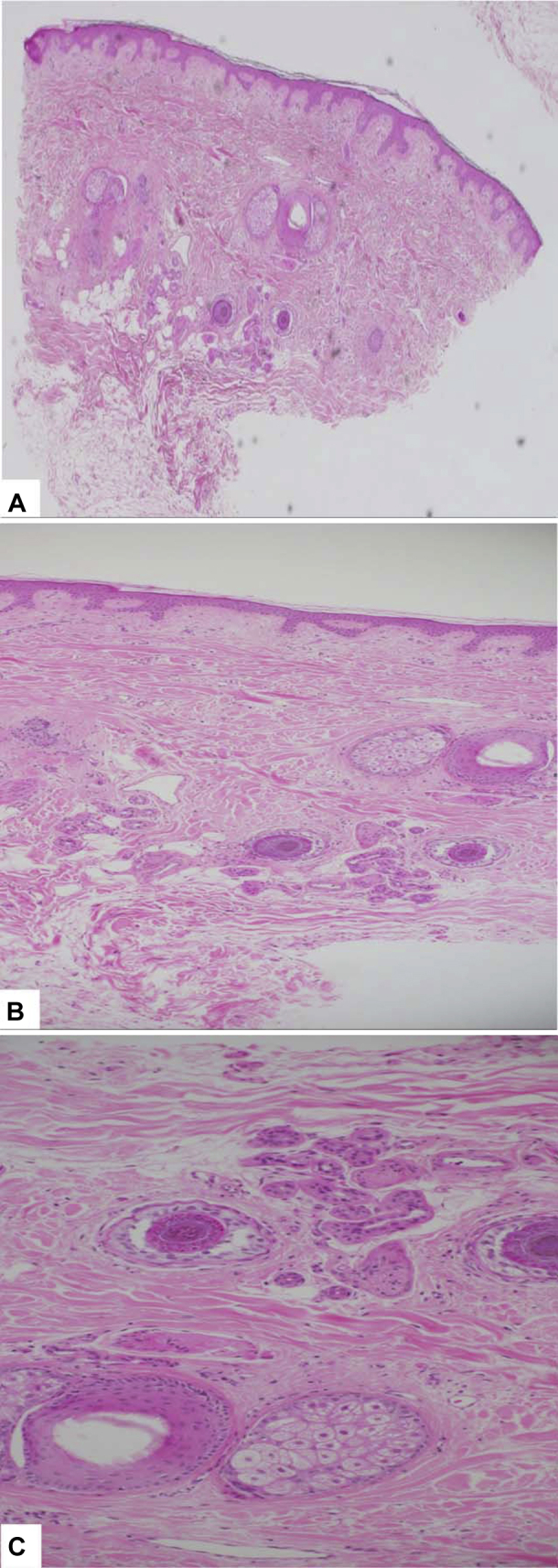
Punch biopsy specimen from the scalp vertex area prior to treatment. Histopathology section showing a nonscarring pattern with reduction in the number of hair follicles, marked follicular miniaturization, and variation in follicle size. There is no inflammatory infiltrate or fibrosis. (A-C, Hematoxylin-eosin stain; original magnifications; A, ×40; B, ×100; C, ×200.)
The patient attended the dermatology clinic 2 years later looking for new treatment options. Aside from a history of hematologic malignancies, her medical history was unremarkable. There had been no prior history of thyroid disease and hair loss. The patient’s only chronic medication was hormone replacement treatment for posttransplant-related menopause. There was no family history of AGA. On examination, she had severe diffuse scalp hair loss (Fig 2). The pull test was positive, showing telogen roots. Trichoscopy revealed intact follicular ostia, increased single-hair pilocebaceous units, anisotrichosis with increased number of vellus hairs, a few yellow dots, and peripilar sign. There was no erythema or scaling. Eyebrows and eyelashes were intact. Laboratory findings, which included a complete blood count, blood chemistry, liver and renal function tests, iron, ferritin, thyroid function, and antinuclear antibodies, were all normal. Based on the history and the clinical and histologic findings, pCIA was diagnosed. With this diagnosis, LDOM at a dose of 1.25 mg daily was initiated. Because of the significant improvement without adverse effects, the dosage was increased to 2.5 mg after 3 months, then to 3.75 mg after 3 months, and to 5 mg after 6 months, resulting in complete regrowth (Fig 3). Four months after starting LDOM, the patient was able to remove the scalp prosthesis because of significant improvement. The patient is now on LDOM 5 mg daily therapy and has remained stable for 9 months, with no side effects. Follow-up trichoscopy demonstrated a considerable improvement in hair density and an increase in hair shaft diameters. Repeated scalp skin biopsy revealed a number of follicular units was within normal range. However, the majority of these were made up of a single hair with varying follicle sizes, which was indicative of AGA in its early stage (Fig 4).
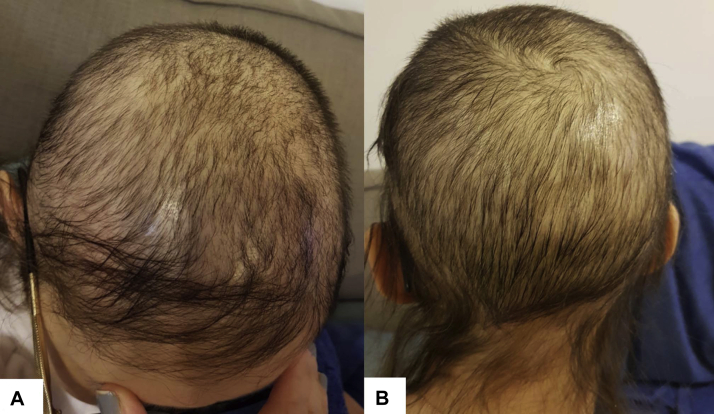
Fig 2.
Clinical photograph of the patient 3 and a half years after hematopoietic stem cell transplantation showing prominent diffuse hair thinning with sparse fragile hair without inflammation or scaling. A, Anterior aspect of the scalp and vertex. B, Posterior aspect of the scalp.
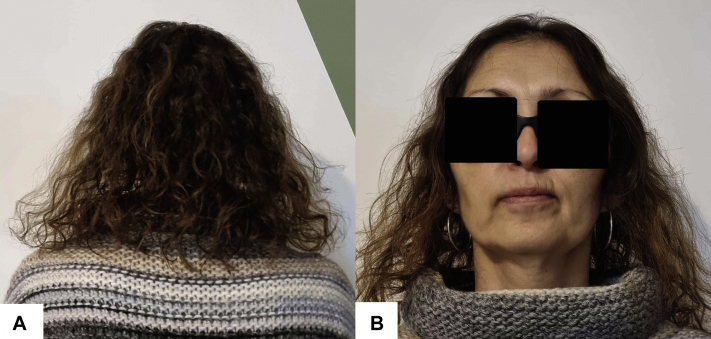
Fig 3.
Clinical photograph of the patient after 1 year of treatment with low-dose oral minoxidil showing full hair regrowth. A, Posterior aspect of the scalp. B, Anterior aspect of the scalp.
Fig 4.
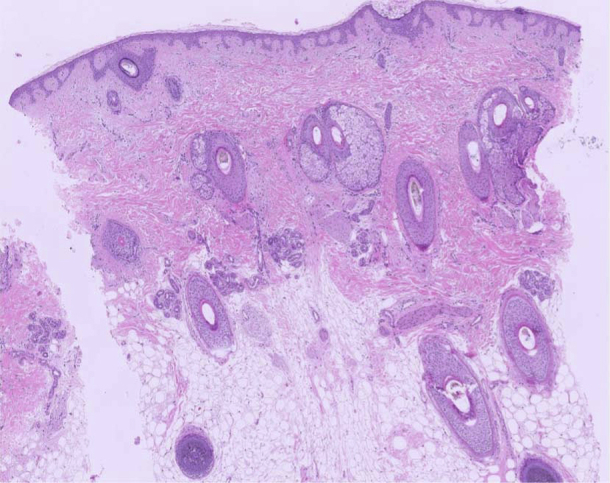
Punch biopsy specimen from the scalp vertex area 20 months after starting therapy with low-dose oral minoxidil. Histopathology section showing a preserved number of hair follicles, normal terminal-to-vellus hair ratio, some hair follicle miniaturization, and variation in follicle size. There is no inflammatory infiltrate or fibrosis. (Hematoxylin-eosin stain; original magnification: ×40).
Discussion
In recent years, there has been an increase in the reports of pCIA, or persistent CIA, as it has been suggested to be renamed.1,2 pCIA was first reported in patients with hematologic malignancies after hematopoietic stem cell transplantation with busulfan therapy, and it has since been linked to a number of chemotherapeutic drugs used to treat a variety of cancers and transplant types.1,3, 4, 5 Apart from busulfan, cyclophosphamide, anthracycline, carboplatin, docetaxel, paclitaxel, and etoposide are the most commonly associated agents.6 pCIA’s clinical spectrum comprises an AGA-like pattern, diffuse or patchy patterns, and total alopecia.3,4,6 Histopathology reveals a nonscarring pattern with reduced follicular density, follicle miniaturization, and no inflammation or fibrosis.4,6 Damage to follicle stem cells, keratinocyte apoptosis, and altered signaling with failure to restore a new cycle because of matrix separation from dermal papillae are suggested pathogenetic mechanisms.2,4 At least 2 chemotherapeutic agents—in our case, busulfan and cyclophosphamide—have been linked to pCIA.1,3 Furthermore, our patient received chemotherapy for a previous hematologic malignancy. It has been suggested that higher cumulative doses and combined treatments may increase the risk of hair follicle toxicity.1,2,4 Chronic GVHD, another possible cause of permanent hair loss after allogeneic transplantation, is clinically similar to lichen planopilaris and histologically characterized by a scarring pattern with perifollicular lichenoid infiltrate and fibrosis, which were not present in our patient.7 Aggravation of AGA, an additional differential diagnosis, is also less plausible because of the lack of previous AGA history and the diffuse pattern with occipital involvement.
The data on pCIA prevention and treatment are limited and based mainly on case reports, case series, and expert opinion. There have been reports of TMS and oral calcitriol being used as preventive agents, with disappointing results.4 TMS and oral spironolactone are the most commonly reported treatment options, with limited response in selected cases.8 Topical bimatoprost has had some positive responses, mostly for eyelash and eyebrow involvement.8 Lately, LDOM has shown promise in the treatment of a variety of hair loss conditions, including AGA, persistent telogen effluvium, traction alopecia, loose anagen syndrome, alopecia areata, monilethrix, and scarring alopecia.9 A few cases of pCIA improvement after LDOM treatment have been published.2,8, 9, 10 LDOM therapy resulted in complete hair regrowth in our case, which, to our knowledge, has not been documented previously.
Given the growing number of cancer survivors, the significant impact of pCIA on the quality of life, and the lack of a well-established treatment, future research on the efficacy of LDOM in this patient population is warranted.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Tallon B., Blanchard E., Goldberg L.J. Permanent chemotherapy-induced alopecia: case report and review of the literature. J Am Acad Dermatol. 2010;63(2):333–336. doi: 10.1016/j.jaad.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 2.Bhoyrul B., Asfour L., Lutz G., et al. Clinicopathologic characteristics and response to treatment of persistent chemotherapy-induced alopecia in breast cancer survivors. JAMA Dermatol. 2021;157(11):1335–1342. doi: 10.1001/jamadermatol.2021.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palamaras I., Misciali C., Vincenzi C., Robles W.S., Tosti A. Permanent chemotherapy-induced alopecia: a review. J Am Acad Dermatol. 2011;64(3):604–606. doi: 10.1016/j.jaad.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Tran D., Sinclair R.D., Schwarer A.P., Chow C.W. Permanent alopecia following chemotherapy and bone marrow transplantation. Australas J Dermatol. 2000;41(2):106–108. doi: 10.1046/j.1440-0960.2000.00405.x. [DOI] [PubMed] [Google Scholar]
- 5.Baker B.W., Wilson C.L., Davis A.L., et al. Busulphan/cyclophosphamide conditioning for bone marrow transplantation may lead to failure of hair regrowth. Bone Marrow Transplant. 1991;7(1):43–47. [PubMed] [Google Scholar]
- 6.Basilio F.M., Brenner F.M., Werner B., Rastelli G.J. Clinical and histological study of permanent alopecia after bone marrow transplantation. An Bras Dermatol. 2015;90(6):814–821. doi: 10.1590/abd1806-4841.20154013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machado M., Moreb J.S., Khan S.A. Six cases of permanent alopecia after various conditioning regimens commonly used in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40(10):979–982. doi: 10.1038/sj.bmt.1705817. [DOI] [PubMed] [Google Scholar]
- 8.Freites-Martinez A., Shapiro J., van den Hurk C., et al. Hair disorders in cancer survivors. J Am Acad Dermatol. 2019;80(5):1199–1213. doi: 10.1016/j.jaad.2018.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vañó-Galván S., Pirmez R., Hermosa-Gelbard A., et al. Safety of low-dose oral minoxidil for hair loss: A multicenter study of 1404 patients. J Am Acad Dermatol. 2021;84(6):1644–1651. doi: 10.1016/j.jaad.2021.02.054. [DOI] [PubMed] [Google Scholar]
- 10.Yang X., Thai K.E. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil. Australas J Dermatol. 2016;57(4):e130–e132. doi: 10.1111/ajd.12350. [DOI] [PubMed] [Google Scholar]