Abstract
Abundant evidence indicates that both genetic and environmental factors contribute to the etiology of autism spectrum disorders (ASDs). However, limited knowledge is available concerning these contributing factors. An epidemiology study reported a link between increased incidence of autism and living closely to major highways, suggesting a possible role for pollutants from highway traffic. We investigated whether maternal exposure to diesel exhaust particles (DEP) negatively affects fetal development leading to autism-like phenotype in mice. Female mice and their offspring were exposed to DEP during pregnancy and nursing. Adult male offspring were then tested for behaviors reflecting the typical symptoms of ASD patients. Compared to control mice, DEP-exposed offspring exhibited higher locomotor activity, elevated levels of self-grooming in the presence of an unfamiliar mouse, and increased rearing behaviors, which may be relevant to the restricted and repetitive behaviors seen in ASD patients. However, the DEP-exposed mice did not exhibit deficits in social interactions or social communication which are the key features of ASD. These results suggest that early life exposure to DEP could have an impact on mouse development leading to observable changes in animal behaviors. Further studies are needed to reveal other environmental insults and genetic factors that would lead to animal models expressing key phenotypes of the autism spectrum disorders.
Keywords: diesel exhaust particles, early-life exposure, autism, repetitive behaviors
Introduction
Autism Spectrum Disorders (ASDs) are neurodevelopmental disorders characterized by deficits in sociability, impaired social communication, and restricted and repetitive behaviors [American Psychiatric Association, 2000]. Evidence from twin studies has indicated the contribution of genetic and environmental factors to ASD, as shown by almost 90% concordance rates in monozygotic twins [Bailey et al., 1995; Hallmayer et al., 2011; Lichtenstein et al., 2010; Ronald et al., 2006; Taniai et al., 2008]. Although genetic and linkage studies have identified many candidate genes, the exact genetic causes have not been determined for a large majority of autism cases. A number of epidemiological studies indicate gene–environment interaction as possible contributors in ASD [Hallmayer et al., 2011; Hultman, Sparen, & Cnattingius, 2002; Newschaffer et al., 2007]. Events during pre- and post-natal stages can adversely affect normal development of the fetus [Perera & Herbstman, 2011]. Prenatal stress, combined with a reduced serotonin transporter expression, has been shown to produce deficits in social interaction, an important feature of autism [Jones et al., 2010]. Similarly, insults during early pregnancy via exposure to chemical and biological agents have been linked to autism etiology in a subset of cases [Chess, Fernandez, & Korn, 1978; Williams et al., 2001].
Diesel exhaust particle (DEP), a component of air pollution, is one such environmental factor that has increasingly been associated with negative health outcomes [Silverman et al., 2012; Sydbom et al., 2001]. Prenatal exposure to DEP may produce abnormalities in reproductive functions and changes in immune responses [Niedzwiecki et al., 2012; Watanabe & Ohsawa, 2002; Yoshida et al., 2006]. Exposure to DEP during pregnancy can also cause an increased frequency of fetal DNA deletions [Reliene et al., 2005]. High concentration (1000 μg/m3) exposure to DEP in utero results in elevated levels of serum testosterone and also causes a decrease in sperm production [Yoshida et al., 2006]. A similar level of exposure during prenatal period has also been shown to produce a significant decrease in dopamine turnover within the striatum and reduce locomotor activity in mice [Yokota et al., 2009]. A recent epidemiological study also found an association between residential proximity to freeways, a proxy for traffic-related pollutants and incidences of autism [Volk et al., 2011]. The aim of this study is to test the hypothesis that high concentration exposure of DEP to mice during gestation and nursing could adversely impair fetal brain development leading to behaviors relevant to autism spectrum disorders.
Materials and Methods
Animals
Eight-week-old B6C3F1 male and female mice (Jackson laboratories; Bar Harbor, ME) were allowed to acclimate for 1 week at the New York University (NYU) animal facility. Two 9-week-old females were then paired with a single male for up to 4 days and checked for the presence of vaginal plugs to confirm mating. The successfully mated female mice were exposed to DEP as described below. The DEP exposure continued for both the dams and pups during nursing and ended 1 week after birth. All procedures conducted at NYU were approved by the NYU School of Medicine Institutional Animal Care and Use Committee (IACUC). The weaned male and female offspring were transferred from NYU to the Ohio State University (OSU). Animals were maintained on a 12-hour dark/light cycle with ad libitum access to water and food. At the age of 6 weeks, behavioral experiments were performed on both offspring sexes. All animal procedures were approved by The Ohio State University Internal laboratory Animals Use Committee.
Diesel Exhaust Exposure
Diesel exhaust was produced by a 5500-watt single cylinder diesel engine generator (Yanmar YDG 5500EE-6EI; Osaka, Japan) that contains a 418-cc displacement engine (Model LE100EE-DEGY6) as previously described [Lin et al., 2010]. The engine was operated using an ultra-low-sulfur diesel fuel, and the diesel exhaust particles (DEP) were diluted to a desirable level through a serial dilution system with High Efficiency Particulate Air-filtered ambient air, and routed to a 1 m3 flow-through exposure chamber. Mice were exposed at a concentration of 1000 μg/m3 or filtered air for 4 hr/day, (5 days/week) from the beginning of gestation until the first week after birth. Since mice are likely to be less sensitive to pollutants than humans, we and other investigators choose the DEP concentration of 1000 μg/m3. This concentration is much higher than human exposures to particulate matter (PM10) from diesel exhaust which is approximately 50 μg/m3 in major US cities [National Ambient Air Quality Standards].
Behavioral Assay
Open field locomotion.
Spontaneous locomotor activity was measured in a 40 cm × 40 cm apparatus placed in a sound-attenuating chamber for 20 min [Brielmaier et al., 2012; Fonken et al., 2011]. Animals were placed in the center of the apparatus at the beginning of the test. Total distance traveled and time spent in the center (20 cm × 20 cm) of the box were measured using tracking software (ANYmaze, Stoelting Co.) Grooming and rearing behaviors were also scored in the same paradigm by an observer blind to treatment groups.
Elevated plus maze.
The elevated plus maze consists of two open arms (35 × 6 cm2) and two closed arms (35 × 6 × 22 cm3) at right angles to each other. Mice were placed at the intersection of the two arms and allowed to explore the maze for a total of 5 min [Holmes et al., 2003]. Time spent in the open vs. closed arms and the number of entries into the arm were measured using tracking software. Arm entry was defined as the placement of all fours limbs in the arm.
Social approach task.
Social approach was measured in a three-chambered apparatus using a previously validated procedure [Crawley, 2007]. The test is divided into three 10 min stages. In the first 10 min stage, mice were allowed to freely explore the apparatus containing novel wire cages in the side chambers. This is followed by another 10 min test; wherein an unfamiliar mouse (stranger 1) is enclosed under the wire cage in one of the side chambers. The third phase involves the introduction of another unfamiliar mouse (stranger 2) which is placed under the wire cage that was previously empty. Time spent in the chambers during all three stages was measured. Cylindrical wire cages (Galaxy Cup, Spectrum Diversified Designs, Streetsboro, OH) were used to hold the stranger mice. The location of the stranger mice and wire cage were counterbalanced between test animals. Age matched C57BL/6j mice were used as unfamiliar stranger mice and were habituated to the wire cages for 15 min/day for 3 days prior to the test.
Reciprocal social interaction.
Reciprocal social interaction was recorded for 30 min in an apparatus measuring 30 × 15.5 cm. Two animals from the same treatment group (but different home cages) were placed in apparatus containing fresh bedding [McFarlane et al., 2008; Peca et al., 2011]. The following behaviors were scored for 15 min and quantified by a person blind to the treatment groups. These behaviors included: nose-to-nose sniffing, nose-to-anogenital sniffing, and fighting behavior.
Grooming and rearing behaviors.
Grooming and rearing behaviors of each of the two mice were also scored from video recordings of reciprocal social interactions described above. Time spent self-grooming and number of grooming episodes were scored [Pearson et al., 2011]. A grooming bout is defined as a single episode of uninterrupted sequence of body grooming. Similarly, time spent rearing and number of rearing episodes were recorded and scored. A rearing episode is defined as number of times the mouse stands upright on its hind limbs. Both behaviors were scored and quantified by an experimenter blind to the treatment groups.
Social transmission of food preference.
Social transmission of food preference was performed using a method published previously [Kogan et al., 1997; McFarlane et al., 2008; Rampon et al., 2000; Wrenn et al., 2003]. Animals were randomly divided into two cohorts within each treatment group and designated as either demonstrators or observers. Demonstrator mice were habituated to flavored food (1% cinnamon or 2% cocoa) mixed with powdered mouse chow in a jar (Dyets Inc., PA) 2 days prior to the experiment. Mice designated as demonstrators were then food deprived for 18 hr. The following day, half the animals in the demonstrator group received 1% cinnamon (Kroger, Cincinnati, OH) and the rest received 2% cocoa (Hershey’s cocoa, Hershey, PA) in their home cage for an hour. Amount of food consumed was noted and mice that failed to consume more than 0.1 g were removed from the study. This phase was followed immediately by a one-on-one interaction with observer mice from the same treatment group for 30 min. The demonstrator and observer mice in each treatment group were socially naïve and from different cages. Mice from both cohorts were returned to their respective cages after the 30-min interaction session. At this stage, food from the observer animals was removed. Twenty-four hours later, observer mice were given a choice between cocoa and cinnamon flavored food. The jars were then weighed, and the amount of food consumed was noted.
Statistical Analyses
Data analysis was performed using SPSS software (version 19). Repeated measures analysis of variance (ANOVA) were used to compare locomotion across time between the two treatment groups (control vs. DEP) in the open field and time spent in chambers (stranger vs. empty, stranger 1 vs. stranger 2) in the social approach task. One-way ANOVA was used to analyze time spent in the open arm and number of entries in the elevated plus maze, grooming and rearing behaviors, and percent cued food consumed in the social transmission of food preference task.
Results
DEP Exposure
To investigate the effect of DEP exposure during development, animals were exposed to DEP during pregnancy and nursing. The DEP treatment did not affect litter sizes which averaged ~9 pups per litter. The body weights of animals in the two treatment groups were also similar (data not shown) and no obvious defects in the physical health of animals were observed as a result of treatment. The weaned mice were then shipped to OSU and were allowed to habituate to the new environment for a week. All animals were tested in the following order, open field locomotion at age 5–6 weeks; social behavior tests at age 6 weeks; reciprocal social interaction, self-grooming, and rearing behaviors at week 7; social transmission of food preference was tested at week 8 and elevated plus maze at week 9.
Open Field Locomotor Activity
Open field locomotor activity was measured in a locomotor box for 20 min. Figure 1 shows the distance traveled in an open field apparatus over time (Fig. 1A), total distance traveled (Fig. 1B) and the percentage of time spent in the center of the chamber (Fig. 1C) by the control and DEP-treated mice. Repeated measures ANOVA reveals a significant difference in locomotor activity in the first 5 min of testing between the DEP-treated group (n = 24) and the controls (n = 24) (Fig. 1A, F1, 46 = 10.919, P = 0.002). One-way ANOVA showed a significant main effect of treatment on overall locomotor activity (distances traveled in 20 min) (Fig. 1B, F1, 46 = 5.997, P = 0.018). No significant differences was observed in the amount of time spent in the center area of the open field apparatus between control and DEP-treated animals (Fig. 1C, F1, 46 = 0.723, P = 0.400).
Figure 1.
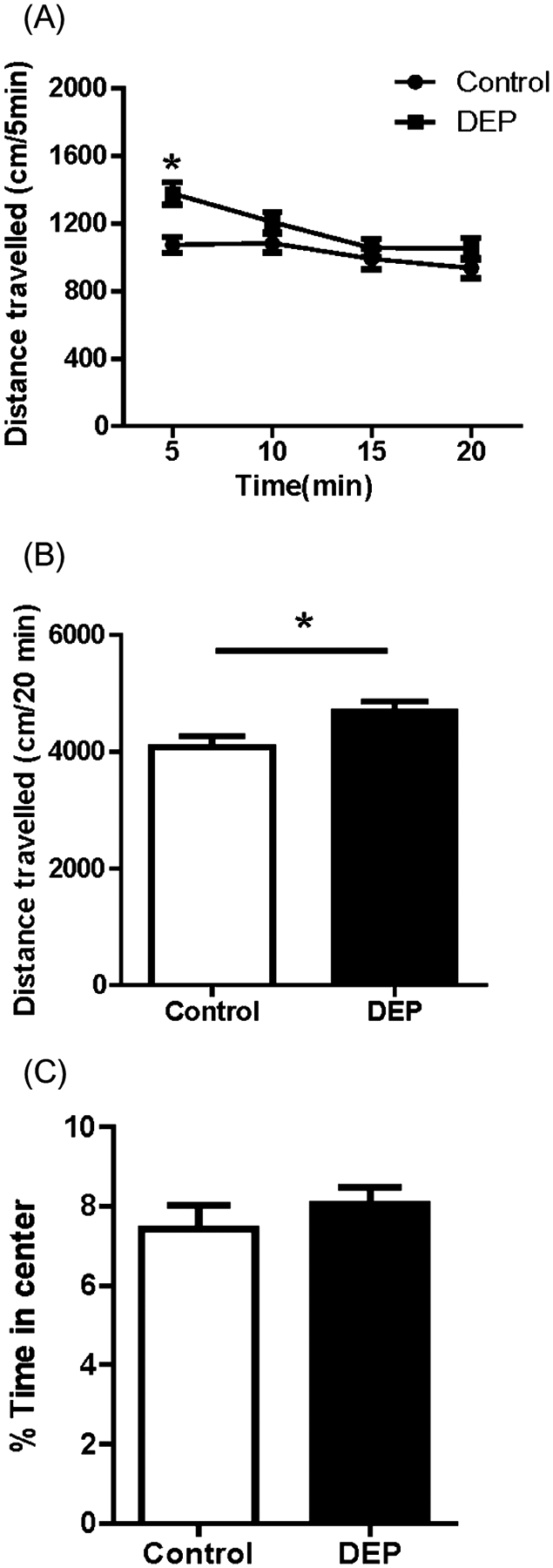
Open field locomotor activity. (A) Distance traveled over time. Repeated measures analysis of variance (ANOVA) reveals a significant difference in locomotor activity in the first 5 min of testing between the diesel exhaust particles (DEP)-treated group (n = 24) and the controls (n = 24) (F1,46 = 10.919, P = 0.002). (B) Total distance traveled in 20 min. One way ANOVA showed a significant main effect of treatment on locomotor activity (F1,46 = 5.997, P = 0.018). No significant difference was observed in the amount of time spent in the center area of the open field apparatus between control and DEP-treated animals (F1,46 = 0.723, P = 0.400). Data are presented as mean ± SEM.
Elevated Plus Maze
Figure 2 shows the time spent in the open arms (Fig. 2A) and the total number of entries into the open arms (Fig. 2B) of the elevated plus maze by the control and DEP-treated groups. DEP-treated animals (n = 24) did not differ from the control group (n = 24) in the amount of time spent in the open arms (Fig. 2A, F1, 45 = 0.153, P = 0.697). Similarly, the number of entries made into the open arm also did not differ significantly across treatment groups (Fig. 2B, F1, 45 = 0.318, P = 0.576).
Figure 2.
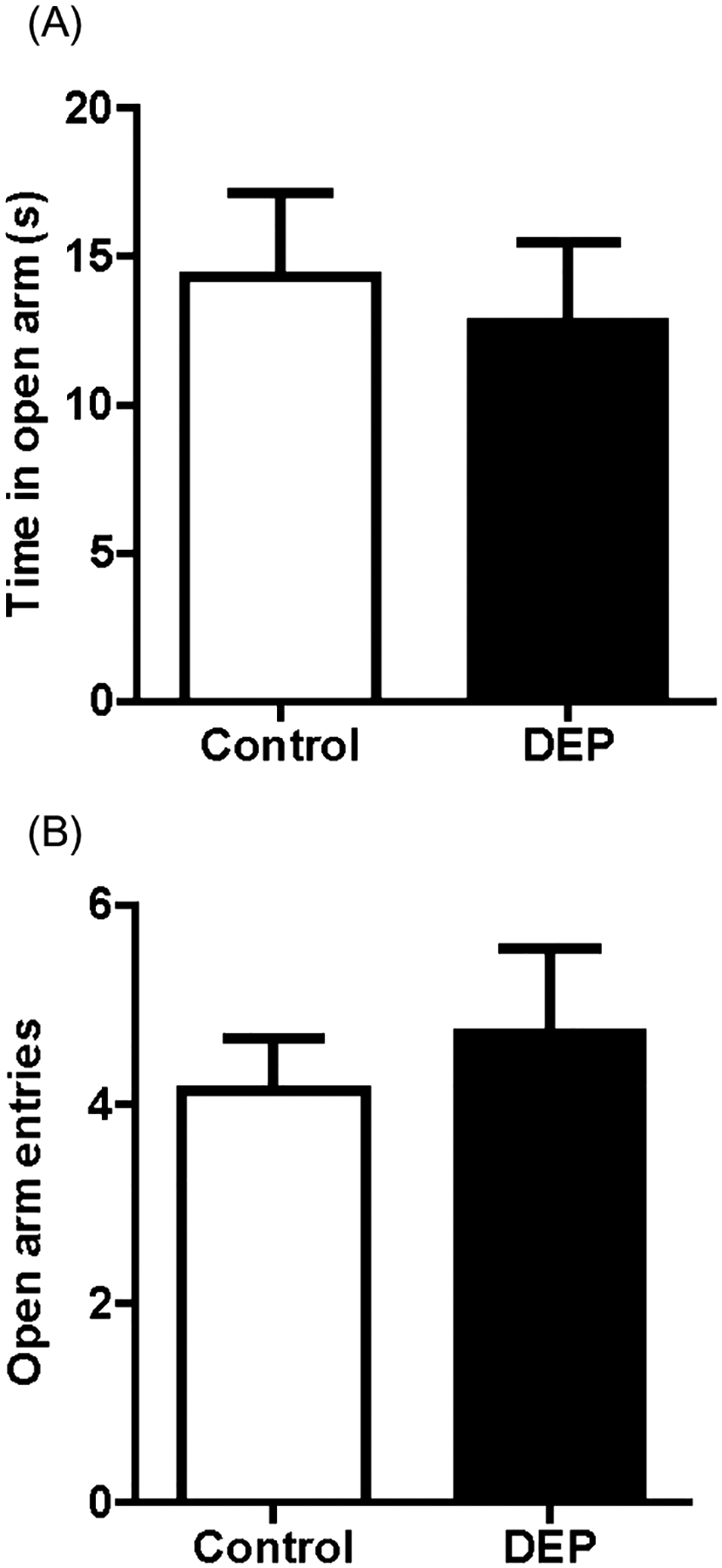
Elevated plus maze. (A) Time spent in the open arm in a 5 min test. Diesel exhaust particles (DEP)-treated animals (n = 24) did not differ from the control group (n = 24) in the amount of time spent in the open arm (F1,45 = 0.153, NS). (B) Number of entries into the open arm. No significant difference was observed in the number of entries into the open arm between treatment groups. (F1,45 = 0.318, NS). Data are presented as mean ± SEM.
Social Approach Tests
Social approach was tested using a three-chambered apparatus, and the test mice did not show preference for either of the side chambers (data not shown). Figure 3A shows that the DEP-treated mice exhibited significant preference (n = 24, *P < 0.05, one-way ANOVA) for the unfamiliar mice (stranger 1) over the empty wire cage just like controls (n = 24, *P < 0.05). There was no significant main effect of treatment (DEP exposure) on the time spent in the chamber containing stranger 1 over the chamber containing an empty wire cage as measured by repeated measures ANOVA (F1,46 = 1.231, P = 0.273). In the third 10-min phase of the test, a new stranger mouse (stranger 2) was placed in the side chamber under the previously empty wire cage. The test mouse was then monitored and time spent with the now familiar mouse (Stranger 1) and unfamiliar mouse (Stranger 2) were recorded and shown in Figure 3B. Both groups of mice showed preference for the new unfamiliar mice, but there was no significant difference between the DEP-treated and control groups (repeated measures ANOVA, (F1,46 = 0.129, P = 0.721).
Figure 3.
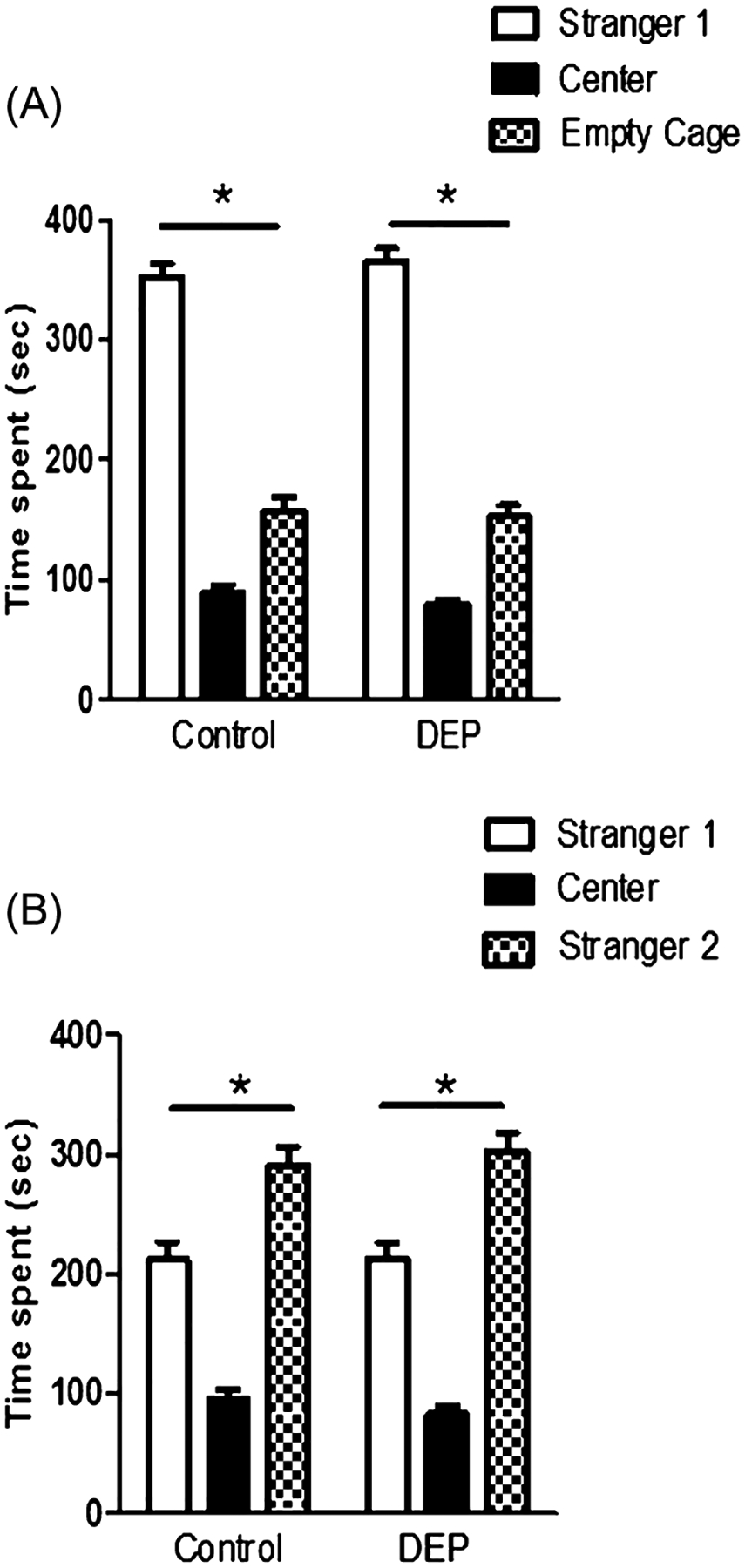
Social approach task. (A) Time the test mice spent in the center chamber, the side chamber containing an unfamiliar mouse (stranger 1) enclosed in a wire cage or the side chamber having an empty wire cage. Diesel exhaust particles (DEP)-treated animals (n = 24, *P < 0.05) displayed normal sociability, similar to their controls (n = 24, *P < 0.05). (B) Time spent in each of the three chambers when a new unfamiliar mouse (stranger 2) was placed under a previously empty wire cage. DEP treated animals showed similar preference for social novelty as their control counterparts.
Reciprocal Social Interaction
Figure 4 shows the number of episodes of nose-to-nose sniffing (Fig. 4A) and nose-to-anogenital sniffing (Fig. 4B) between pairs of control mice and pairs of DEP-treated mice. There appears to be a reduced frequency of nose-to-nose and nose-to-anogenital sniffs between DEP treated mice (8 pairs, n = 8) compared to the control group (n = 12). However, the differences are not statistically significant for the nose-to-nose sniffing (Fig. 4A, (F1,18 = 0.925, P = 0.349) or nose-to-anogenital episodes (Fig. 4B, (F1,18 = 1.921, P = 0.183).
Figure 4.
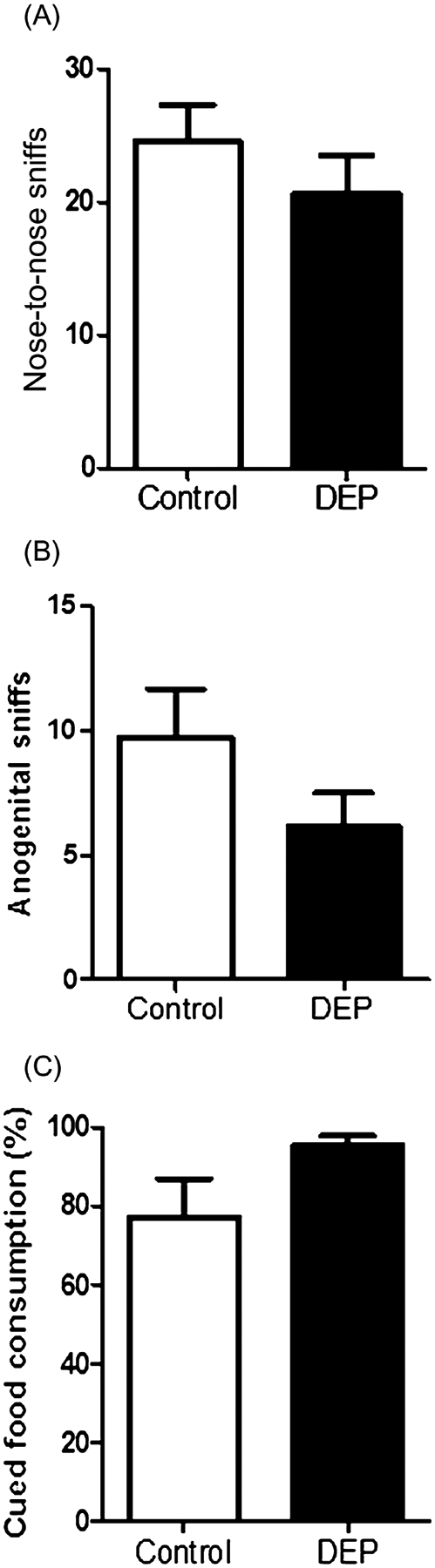
Reciprocal social interaction. Two mice from the same treatment groups but from different home cages were placed in a novel environment and number of nose-to-nose sniffing episodes, (A) and number of nose to anogenital sniffing episodes (B) were recorded. No significant differences were observed between control (n = 12) and diesel exhaust particles (DEP)-treated (n = 8) animals on number of episodes of nose-to-nose sniffing (F1,18 = 0.925, P = 0.349) or nose-to-anogenital sniffing(F1,18 = 1.921, P = 0.183). (C) Social transmission of food preference. No significant differences were observed between the control (n = 11) and DEP-treated animals (n = 12) in the cued food consumed (F1,11.125 = 3.27, NS). Data are presented as mean ± SEM.
Social Transmission of Food Preference
Two food flavors were tested, and no preference for either flavor was observed for both treatment groups (data not shown). Figure 4C shows that the observer mice from both groups consumed substantially more food mixed with a flavor (cued food) that the demonstrator mice consumed over a non-cued food suggesting effective “communication” between mice. Levene’s test revealed unequal variances between the two groups (P = 0.004), hence data were analyzed using one-way ANOVA with Welch correction. However, there was no significant difference in the percentages of cued food consumed between the control (n = 11) and DEP-treated (n = 12) mice (F1, 11.125 = 3.27, P = 0.098).
Self-Grooming and Rearing Behavior
Self-grooming and rearing behaviors were scored during the reciprocal social interaction paradigm when two animals where placed together in the apparatus. Figure 5 shows time spent self-grooming (Fig. 5A) and number of rearing episodes (Fig. 5B) in 15 min. Data are presented as mean ± SEM. DEP-treated animals (n = 16) spent significantly longer time self-grooming compared to controls (n = 24) (Fig. 5A; F1,18 = 8.282, P = 0.010). There was also a significant increase in the number of rearing episodes in the DEP-treated animals compared to control animals (Fig. 5B; F1,18 = 4.698, P = 0.04). Additionally, self-grooming and rearing behaviors were also scored during the open field locomotor activity task when a single mouse was in the apparatus. Figure 5C shows time spent grooming, and Figure 5D shows rearing episodes in a 15-min session. There was no significant difference in time spent self-grooming in DEP-treated mice (n = 24) compared to controls (n = 24) (Fig. 5C; F1,46 = 0.084, P = 0.774). However, DEP-treated mice displayed significantly higher number of rearing episodes compared to controls (F1,46 = 4.527, P = 0.039).
Figure 5.
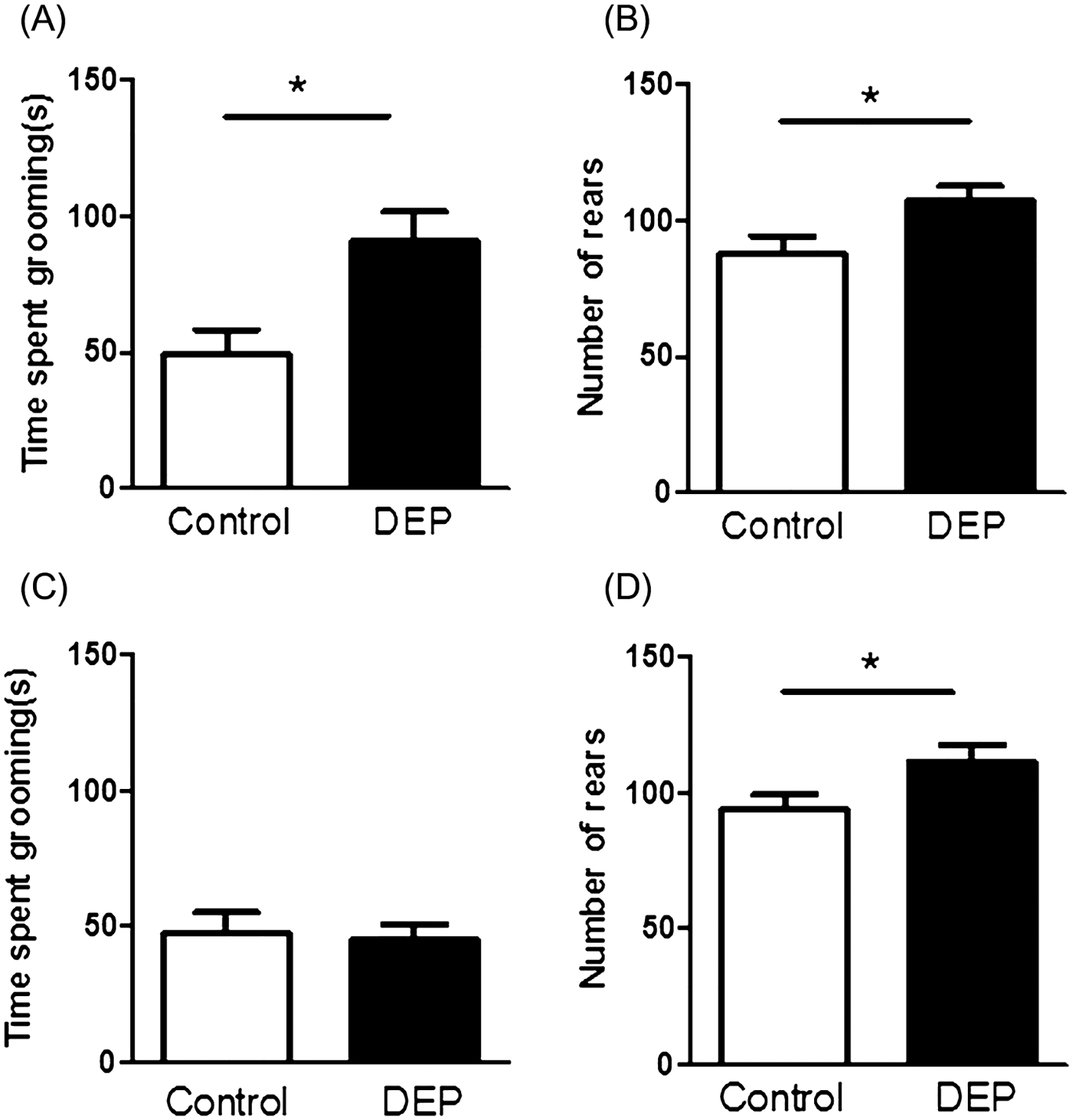
Self-grooming and rearing behavior. (A and B) Two mice were placed in a novel environment and grooming and rearing behaviors of each animal were scored. (A) Diesel exhaust particles (DEP)-treated (n = 16) animals spent significantly longer time self-grooming compared to controls (n = 24), (F1,18 = 8.282, P = 0.010). (B) Number of rearing episodes. There was a significant increase in number of rearing episodes in the DEP-treated animals compared to control animals, (F1,18 = 4.698, P = 0.04). (C and D) Single mouse was placed in test apparatus and self-grooming and rearing behaviors were scored. (C) No significant differences were observed between control (n = 24) and DEP-treated mice (n = 24) in the amount of time spent self-grooming (F1,46 = 0.084, P = 0.774). (D) Significant increase in rearing episodes was observed in DEP-treated mice compared to controls (F1,46 = 4.527, P = 0.039). Data are presented as mean ± SEM.
Discussion
In the current study, we have investigated the possibility that exposures to diesel exhaust particles during gestation and early life may impair brain development leading to autism-like symptoms. We report that mice exposed to diesel exhaust particles during pre- and postnatal development exhibit an increase in locomotor activity and rearing behaviors. These mice also display elevated levels of repetitive self-grooming only in the presence of an unfamiliar mouse. However, these animals did not exhibit deficits in social interaction or anxiety-like behavior and display normal social communication. These results suggest that exposure to diesel exhaust particles during early development may not have a very strong impact on mouse development leading to very obvious deficits in social behaviors. However, significant increases in stereotyped behaviors were observed which may be relevant to the repetitive/compulsive behaviors seen in autistic patients. These findings support the hypothesis that, besides genetic factors, pre- and postnatal environment can contribute to behaviors similar to those observed in an autistic phenotype.
Animal studies have shown that exposure to DEP, a common component of air pollution, particularly in urban environments, can alter endocrine function and cause an increase in pro-inflammatory cytokines within the central nervous system [Levesque et al., 2011]. In utero exposure of DEP has been shown to produce deficits in learning and memory [Hougaard et al., 2008]. However, no study has reported the effect of DEP on social behaviors. A recent epidemiological study has shown for the first time a causal link between autistic incidences and residential proximity to freeways as a proxy for traffic-related pollutants, suggesting that environmental pollutants could be possible risk factors for autism-like behavior [Volk et al., 2011]. Since there are no biochemical markers to test for autism, we investigated animal behaviors that have face validity to some of the core symptoms of autism. We tested our animals in a variety of behavioral paradigms that measures sociability, social communication, and stereotyped behavior. Our results indicate that animals treated with DEP spend more time with an unfamiliar mouse than a novel object, similar to the control mice, indicating that prenatal exposure to DEP does not alter social behaviors in mice. Besides measuring the animal’s sociability in a three-chambered apparatus, we also recorded parameters such as nose-to-nose sniffing and nose-to-anogenital sniffing. Although the data show a trend of decrease in these behaviors in the DEP-treated animals compared to the controls, the difference failed to reach statistical significance. Social transmission of food preference task (STFP) was used to assess social communication in these animals. The test measures animal’s ability to use social cues acquired via interaction with another mouse to then make a choice. The test has been reported to have potential face validity to certain features of autism [Crawley, 2007]. However, significant differences between control and DEP-exposed animals on the STFP task was not observed here. Taken together, these findings suggest that prenatal and early-life exposure to DEP do not have adverse impact on mouse development that results in a significant deficit in social behavior or communication.
Repetitive and restrictive behaviors are one of the diagnostic criteria for autism spectrum disorders [American Psychiatric Association, 2000]. Mouse models of autism have previously been shown to display excessive self-grooming rearing and other stereotyped behaviors [Blundell, Blaiss et al. 2010; Chao et al., 2010, Etherton, Blaiss et al. 2009; Peca et al., 2011]. A dysfunctional fronto-striatal circuitry has been suggested to contribute to restrictive behavior [Langen et al., 2012]. Increased striatal volume has also been positively correlated with repetitive behavior in autistic patients [Hollander et al., 2005]. In utero exposure to low concentrations of DEP also results in enhanced dopamine concentration within the pre-frontal cortex and reduced dopamine turnover in the striatum and nucleus accumbens [Suzuki et al., 2010]. We found that DEP-exposed mice when compared to controls, exhibited elevated levels of self-grooming only in the presence of an unfamiliar mouse. Interestingly, there was no difference between DEP-treated mice and controls in time spent self-grooming when the mice were alone. The increased self-grooming in DEP-treated mice might be due to anxiety induced by the presence of an unfamiliar mouse. These results are similar to previous reports that show higher grooming times during social interaction in a mouse model of fragile X syndrome [McNaughton et al., 2008; Mines, Yuskaitis, King, Beurel, & Jope, 2010]. Previous studies have also shown that in small population of children diagnosed with autism, higher anxiety levels are associated with a greater frequency of repetitive behaviors [Gillott, Furniss, & Walter, 2001; Rodgers, Glod, Connolly, & McConachie, 2012a; Rodgers, Riby, Janes, Connolly, & McConachie, 2012b]. However, mice may groom and rear more for a variety of reasons which may or may not be related to autism [van Erp et al., 1994; Han et al., 2012]. Additional experiments such as reversal learning and marble burying are needed to further characterize how perinatal DEP exposures affect repetitive behaviors and restricted interests. A subset of children diagnosed with autism spectrum disorders display a hyperactive phenotype [Sinzig, Walter, & Doepfner, 2009]. Hyperactivity has also been reported in a few mouse models of autism [Penagarikano et al., 2011]. In our study, DEP-exposed animals display an increased locomotor activity in an open field environment compared to controls, suggesting some effect of early-life DEP exposure on the development of brain structures underlying motor behavior. Another recent study also showed that prenatal DEP exposure has an impact on locomotor activities in mice [Suzuki et al., 2010]. However, this study reported a decrease in locomotor activity as a consequence of DEP exposure. There are a few differences between the two studies. First, the strains of mice used in the two studies are different, we used an inbred strain B6C3F1, whereas they used ICR mice, an outbreed strain. Second, the durations of DEP exposure in the two studies are different. We exposed pregnant dams during pregnancy and nursing for 4 hr/day, 5 days/week, whereas the other group exposed the mice for 8 hr/day, 5 days/week from day 2 through day 17 of gestation. These disparities could have contributed to the observed differences between the two studies. B6C3F1 mice are thought to be more sensitive to toxic materials and thus are commonly used in toxicological studies [King-Herbert, Sills, & Bucher, 2010]. There are no obvious abnormality reported for this strain in its baseline behaviors, health, and physiology. One study reports that B6C3F1 appeared to display a more anxious phenotype compared to C57 mice [Benatti et al., 2011]. However, we did not observe any obvious anxiety-like phenotype.
A small proportion of patients diagnosed with ASD present with symptoms that are commonly associated with psychological disorders like attention deficit hyperactivity disorder (ADHD) and anxiety [Brereton, Tonge, & Einfeld, 2006; Esbensen et al., 2003; Goldstein & Schwebach, 2004; Leyfer et al., 2006; Muris et al., 1998; Simonoff et al., 2008]. Some of the anxiety symptoms manifest as simple phobias, separation anxiety disorders, or agoraphobia [Muris et al., 1998]. In order to test for anxiety-like behavior, we performed elevated plus maze and open field test. We observed no significant differences between the two groups of mice in the amount of time spent in the center of the open field, a measure of anxiety-like behavior in animals. This was supported by no differences observed in the time spent in the open arm or the number of transitions into the open arm of an elevated plus maze.
In summary, exposures to DEP prenatally and during nursing appear to have an impact on the development of the central nervous system leading to observable differences in animal behaviors. Specifically, mice with early-life DEP exposure exhibited increased locomotor activity and elevated levels of self-grooming and rearing which are relevant to the restricted and repetitive behaviors seen in ASD patients. However, the same treatment did not cause elevated anxiety or did not result in significant deficits in social interaction or social communication, the key symptoms of ASD patients. Together, these results suggest that early-life exposure to DEP could have an impact on mouse development leading to observable changes in animal behaviors. It is likely that multiple environmental insults in combination with genetic factors would have stronger impact and result in animals with other key phenotypes of the autism spectrum disorders.
Acknowledgments
The authors would like to acknowledge Dawn Han for her assistance.
Grant sponsor:
National Institute on drug abuse; Grant number: DA014610 and in part by the NYU NIEHS Center Grant No. ES000260.
References
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders-text revision (DSM-IV-TR), 4e. Washington, DC: American Psychiatric Association Press, Inc. [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, et al. (1995). Autism as a strongly genetic disorder: evidence from a British twin study. Psychological Medicine, 25, 63–77. [DOI] [PubMed] [Google Scholar]
- Benatti C, Alboni S, Montanari C, Caggia F, Tascedda F, et al. (2011). Central effects of a local inflammation in three commonly used mouse strains with a different anxious phenotype. Behavioural Brain Research, 224, 23–34. [DOI] [PubMed] [Google Scholar]
- Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, et al. (2010). Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. Journal of Neuroscience, 30, 2115–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brereton AV, Tonge BJ, & Einfeld SL (2006). Psychopathology in children and adolescents with autism compared to young people with intellectual disability. Journal of Autism and Developmental Disorders, 36, 863–870. [DOI] [PubMed] [Google Scholar]
- Brielmaier J, Matteson PG, Silverman JL, Senerth JM, Kelly S, et al. (2012). Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS ONE, 7, e40914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, & Chahrour M (2010). Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature, 468, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess S, Fernandez P, & Korn S (1978). Behavioral consequences of congenital rubella. The Journal of Pediatrics, 93, 699–703. [DOI] [PubMed] [Google Scholar]
- Crawley JN (2007). Mouse behavioral assays relevant to the symptoms of autism. Brain Pathology, 17, 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbensen AJ, Rojahn J, Aman MG, & Ruedrich S (2003). Reliability and validity of an assessment instrument for anxiety, depression, and mood among individuals with mental retardation. Journal of Autism and Developmental Disorders, 33, 617–629. [DOI] [PubMed] [Google Scholar]
- Etherton MR, Blaiss CA, Powell CM, Sudhof TC, et al. (2009). Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proceedings of the National Academy of Sciences of the United States of America, 106, 17998–18003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Xu X, Weil ZM, Chen G, Sun Q, et al. (2011). Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Molecular Psychiatry, 16, 987–995, 973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillott A, Furniss F, & Walter A (2001). Anxiety in high-functioning children with autism. Autism, 5, 277–286. [DOI] [PubMed] [Google Scholar]
- Goldstein S, & Schwebach AJ (2004). The comorbidity of pervasive developmental disorder and attention deficit hyperactivity disorder: results of a retrospective chart review. Journal of Autism and Developmental Disorders, 34, 329–339. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, et al. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry, 68, 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, et al. (2012). Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature, 489, 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, et al. (2005). Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biological Psychiatry, 58, 226–232. [DOI] [PubMed] [Google Scholar]
- Holmes A, Kinney JW, Wrenn CC, Li Q, Yang RJ, et al. (2003). Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology, 28, 1031–1044. [DOI] [PubMed] [Google Scholar]
- Hougaard KS, Jensen KA, Nordly P, Taxvig C, Vogel U, et al. (2008). Effects of prenatal exposure to diesel exhaust particles on postnatal development, behavior, genotoxicity and inflammation in mice. Particle and Fibre Toxicology, 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman CM, Sparen P, & Cnattingius S (2002). Perinatal risk factors for infantile autism. Epidemiology, 13, 417–423. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith RM, Edwards KS, Givens B, Tilley MR, & Beversdorf DQ (2010). Combined effect of maternal serotonin transporter genotype and prenatal stress in modulating offspring social interaction in mice. International Journal of Developmental Neuroscience, 28, 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Herbert AP, Sills RC, & Bucher JR (2010). Commentary: update on animal models for NTP studies. Toxicologic Pathology, 38, 180–181. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Blendy JA, Coblentz J, Marowitz Z, et al. (1997). Spaced training induces normal long-term memory in CREB mutant mice. Current Biology, 7, 1–11. [DOI] [PubMed] [Google Scholar]
- Langen M, Leemans A, Johnston P, Ecker C, Daly E, et al. (2012). Fronto-striatal circuitry and inhibitory control in autism: Findings from diffusion tensor imaging tractography. Cortex, 48, 183–193. [DOI] [PubMed] [Google Scholar]
- Levesque S, Surace MJ, McDonald J, & Block ML (2011). Air pollution & the brain: Subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. Journal of Neuroinflammation, 8, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, et al. (2006). Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders, 36, 849–861. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Carlstrom E, Rastam M, Gillberg C, & Anckarsater H (2010). The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. The American Journal of Psychiatry, 167, 1357–1363. [DOI] [PubMed] [Google Scholar]
- Lin L, Zhu H, Quan C, Grunig G, Ballaney M, et al. (2010). Prenatal allergen and diesel exhaust exposure and their effects on allergy in adult offspring mice. Allergy and Asthma Clinical Immunology, 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, & Crawley JN (2008). Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes, Brain, and Behavior, 7, 152–163. [DOI] [PubMed] [Google Scholar]
- McNaughton CH, Moon J, Strawderman MS, Maclean KN, Evans J, & Strupp BJ (2008). Evidence for social anxiety and impaired social cognition in a mouse model of fragile X syndrome. Behavioral Neuroscience, 122, 293–300. [DOI] [PubMed] [Google Scholar]
- Mines MA, Yuskaitis CJ, King MK, Beurel E, & Jope RS (2010). GSK3 influences social preference and anxiety-related behaviors during social interaction in a mouse model of fragile X syndrome and autism. PLoS ONE, 5, e9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Steerneman P, Merckelbach H, Holdrinet I, & Meesters C (1998). Comorbid anxiety symptoms in children with pervasive developmental disorders. Journal of Anxiety Disorders, 12, 387–393. [DOI] [PubMed] [Google Scholar]
- National Ambient Air Quality Standards. http://www.epa.gov/airtrends/pm.html#pmnat
- Newschaffer CJ, Croen LA, Daniels J, Giarelli E, & Grether JK (2007). The epidemiology of autism spectrum disorders. Annual Review of Public Health, 28, 235–258. [DOI] [PubMed] [Google Scholar]
- Niedzwiecki M, Zhu H, Corson L, Grunig G, Factor PH, et al. (2012). Prenatal exposure to allergen, DNA methylation, and allergy in grandoffspring mice. Allergy, 67, 904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BL, Pobbe RL, Defensor EB, Oasay L, Bolivar VJ, et al. (2011). Motor and cognitive stereotypes in the BTBR T+tf/J mouse model of autism. Genes, Brain, and Behavior, 10, 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peca J, Feliciano C, Ting JT, Wang W, Wells MF, et al. (2011). Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature, 472, 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, et al. (2011). Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell, 147, 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F, & Herbstman J (2011). Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol, 31, 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, & Tsien JZ (2000). Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nature Neuroscience, 3, 238–244. [DOI] [PubMed] [Google Scholar]
- Reliene R, Hlavacova A, Mahadevan B, Baird WM, & Schiestl RH (2005). Diesel exhaust particles cause increased levels of DNA deletions after transplacental exposure in mice. Mutation Research, 570, 245–252. [DOI] [PubMed] [Google Scholar]
- Rodgers J, Glod M, Connolly B, & McConachie H (2012a). The relationship between anxiety and repetitive behaviours in autism spectrum disorder. Journal of Autism and Developmental Disorders, 42, 2404–2409. [DOI] [PubMed] [Google Scholar]
- Rodgers J, Riby DM, Janes E, Connolly B, & McConachie H (2012b). Anxiety and repetitive behaviours in autism spectrum disorders and Williams syndrome: a cross-syndrome comparison. Journal of Autism and Developmental Disorders, 42, 175–180. [DOI] [PubMed] [Google Scholar]
- Ronald A, Happe F, Bolton P, Butcher LM, Price TS, et al. (2006). Genetic heterogeneity between the three components of the autism spectrum: a twin study. Journal of the American Academy of Child and Adolescent Psychiatry, 45, 691–699. [DOI] [PubMed] [Google Scholar]
- Silverman DT, Samanic CM, Lubin JH, Blair AE, Stewart PA, et al. (2012). The diesel exhaust in miners study: A nested case-control study of lung cancer and diesel exhaust. Journal of the National Cancer Institute, 104, 855–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 921–929. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Walter D, & Doepfner M (2009). Attention deficit/hyperactivity disorder in children and adolescents with autism spectrum disorder: Symptom or syndrome? Journal of Attention Disorders, 13, 117–126. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Oshio S, Iwata M, Saburi H, Odagiri T, et al. (2010). In utero exposure to a low concentration of diesel exhaust affects spontaneous locomotor activity and monoaminergic system in male mice. Particle and Fibre Toxicology, 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydbom A, Blomberg A, Parnia S, Stenfors N, Sandstrom T, & Dahlen SE (2001). Health effects of diesel exhaust emissions. The European Respiratory Journal, 17, 733–746. [DOI] [PubMed] [Google Scholar]
- Taniai H, Nishiyama T, Miyachi T, Imaeda M, & Sumi S (2008). Genetic influences on the broad spectrum of autism: Study of proband-ascertained twins. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 147B, 844–849. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Kruk MR, Meelis W, & Willekens-Bramer DC (1994). Effect of environmental stressors on time course, variability and form of self-grooming in the rat: Handling, social contact, defeat, novelty, restraint and fur moistening. Behavioural Brain Research, 65, 47–55. [DOI] [PubMed] [Google Scholar]
- Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, & McConnell R (2011). Residential proximity to freeways and autism in the CHARGE study. Environmental Health Perspectives, 119, 873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, & Ohsawa M (2002). Elevated serum immunoglobulin E to Cryptomeria japonica pollen in rats exposed to diesel exhaust during fetal and neonatal periods. BMC Pregnancy and Childbirth, 2, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G, King J, Cunningham M, Stephan M, Kerr B, & Hersh JH (2001). Fetal valproate syndrome and autism: Additional evidence of an association. Developmental Medicine and Child Neurology, 43, 202–206. [PubMed] [Google Scholar]
- Wrenn CC, Harris AP, Saavedra MC, & Crawley JN (2003). Social transmission of food preference in mice: Methodology and application to galanin-overexpressing transgenic mice. Behavioral Neuroscience, 117, 21–31. [PubMed] [Google Scholar]
- Yokota S, Mizuo K, Moriya N, Oshio S, Sugawara I, & Takeda K (2009). Effect of prenatal exposure to diesel exhaust on dopaminergic system in mice. Neuroscience Letters, 449, 38–41. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ono N, Tsukue N, Oshio S, Umeda T, et al. (2006). In utero exposure to diesel exhaust increased accessory reproductive gland weight and serum testosterone concentration in male mice. Environmental Sciences, 13, 139–147. [PubMed] [Google Scholar]


