Abstract
Pseudoxanthoma elasticum (PXE), a prototype of heritable ectopic calcification disorders, affects the skin, eyes, and the cardiovascular system due to inactivating mutations in the ABCC6 gene. There is no effective treatment for the systemic manifestations of PXE. In this study, the efficacy of INS-3001, an analog of phytic acid, was tested for inhibition of ectopic calcification in an Abcc6−/− mouse model of PXE. In prevention study, Abcc6−/− mice, at 6 weeks of age, the time of onset of ectopic calcification, were treated with INS-3001 with 0.16, 0.8, 4, 20 or 100 mg/kg/day administered by subcutaneous implantation of osmotic pumps, as well as 4 mg/kg/day by subcutaneous injection thrice weekly or 14, 4, and 0.8 mg/kg/day once weekly subcutaneous injection. Mice were necropsied at 12 weeks of age. Histologic examination and quantitative calcium assay revealed that mice receiving 6 weeks of continuous INS-3001 administration via osmotic pumps showed dose-dependent inhibition of muzzle skin calcification with complete response at 4 mg/kg/day and a minimum effective dose at 0.8 mg/kg/day. INS-3001 plasma concentrations were dose-dependent and largely consistent during treatment for each dose. Thrice weekly and once weekly subcutaneous injections of INS-3001 also prevented calcification. In established disease study, 12-week-old Abcc6−/− mice with extensive calcification were continuously administered INS-3001 at 4 mg/kg/day for a follow-up of 12 weeks. INS-3001 treatment was found to stabilize existing calcification that had developed at start of treatment. These results suggest that INS-3001 may provide a promising preventive treatment strategy for PXE, a currently intractable ectopic calcification disorder.
Keywords: animal model, ectopic calcification, phytic acid, INS-3001, pseudoxanthoma elasticum
1. BACKGROUND
Pseudoxanthoma elasticum (PXE) is a heritable multisystem ectopic calcification disorder which manifests with profound deposition of calcium phosphate complexes in the extracellular matrix of connective tissues in several organs. The disease presents with late-onset, progressive development of clinical manifestations in the skin, eyes, and cardiovascular system.1–3 Mineral deposition in the skin is primarily of aesthetic concern, but may portend development of serious eye and cardiovascular problems, causes of significant morbidity and occasional mortality. The classic forms of PXE are caused by loss-of-function mutations in the ABCC6 gene encoding a putative efflux transporter protein ABCC6.4–6 ABCC6 mutations can also cause generalized arterial calcification of infancy (GACI) type 27, 8, a severe early-onset vascular calcification disorder that affects the fetus in utero or in infancy. While the substrate of ABCC6 is unknown, recent studies demonstrated that insufficient plasma levels of inorganic pyrophosphate, a potent anti-calcification factor, underlies the pathophysiology in PXE.1, 9, 10 Despite significant progress in understanding the disease pathogenesis, there are no effective treatments for the systematic manifestations of PXE.
Phytic acid (myo-inositol hexakisphosphate; IP6) is the phosphate ester of inositol, a naturally occurring substance found in nuts and seeds, known as a protective agent against pathological calcification.11–14 To overcome the poor pharmacokinetics of IP615, a series of inositol phosphate analogs were synthesized from myo-inositol, through controlled derivatization with oligo (ethylene glycol) chains, sulfate groups and/or phosphate groups, and subsequently screened for their ability to inhibit calcification. INS-3001 was found to be superior to all investigated compounds in delaying human serum calcification propensity, almost 10-fold more potently than IP6.16 In vitro assays also demonstrated that INS-3001 rescued human vascular smooth muscles cells from calciprotein and calcium phosphate-induced calcification.16 INS-3001 displayed an almost 10-fold longer plasmatic half-life compared to IP6 after intravenous bolus injection in rats (78 ± 32 min vs. 8 ± 2 min, respectively). INS-3001 inhibited vitamin D3-induced and adenine diet-induced cardiovascular calcification in Sprague Dawley rats with favorable pharmacokinetic and safety profiles.16 Although inhibition of induced vascular calcification in vitro and in vivo was demonstrated for INS-3001, its efficacy on spontaneous tissue calcification has yet to be demonstrated.
1.1. Question addressed
In this study, we investigated the inhibitory effects of INS-3001 on spontaneous connective tissue calcification in an Abcc6−/− mouse model of PXE.
2. EXPERIMENTAL DESIGN
Abcc6 knock-out mice (Abcc6−/−) on C57BL/6J background were generated as described previously.17 C57BL/6J mice from The Jackson Laboratory (Bar Harbor, ME) were used as wild-type controls (WT). In prevention study, 6-week-old Abcc6−/− mice were administered with INS-3001 by periodic subcutaneous injections or continuous administration via subcutaneous ALZET osmotic pump model 2006 (DURECT Corp, Cupertino, CA) for duration of 6 weeks (Table 1). In established disease study, 12-week-old Abcc6−/− mice were administered INS-3001 at 4 mg/kg/day via osmotic pumps for 12 weeks (Table 1). All mice were maintained on a standard diet and sacrificed at 12 weeks of age, 6 weeks after initiation of treatment (prevention study), or at 24 weeks of age, 12 weeks after initiation of treatment (established disease study). All protocols were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Table 1.
Experimental groups of mice by genotype and treatment regimen
| Mice | Treatment | Delivery route | Dose (mg/kg/day)† | Dose (mg/kg/per injection)† | Frequency | No. of mice (F+M)‡ |
|---|---|---|---|---|---|---|
|
| ||||||
| Prevention study | ||||||
|
| ||||||
| Abcc6 +/+ | ‒ | ‒ | ‒ | ‒ | ‒ | 6+6 |
| Abcc6−/− | ‒ | ‒ | ‒ | ‒ | ‒ | 5+6 |
| Abcc6−/− | INS-3001 | Osmotic pump | 0.16 | ‒ | Continuous | 5+5 |
| Abcc6−/− | INS-3001 | Osmotic pump | 0.8 | ‒ | Continuous | 4+6 |
| Abcc6−/− | INS-3001 | Osmotic pump | 4 | ‒ | Continuous | 3+3 |
| Abcc6−/− | INS-3001 | Osmotic pump | 20 | ‒ | Continuous | 5+4 |
| Abcc6−/− | INS-3001 | Osmotic pump | 100 | ‒ | Continuous | 3+4 |
| Abcc6−/− | INS-3001 | s.c. injection | 4 | 9 | Thrice weekly | 4+4 |
| Abcc6−/− | INS-3001 | s.c. injection | 14 | 100 | Once weekly | 5+5 |
| Abcc6−/− | INS-3001 | s.c. injection | 4 | 28 | Once weekly | 5+5 |
| Abcc6−/− | INS-3001 | s.c. injection | 0.8 | 5.6 | Once weekly | 5+5 |
| Established disease study | ||||||
| Abcc6−/− | ‒ | ‒ | ‒ | ‒ | ‒ | 5+4 |
| Abcc6−/− | INS-3001 | Osmotic pump | 4 | ‒ | Continuous | 0+6 |
INS-3001 was administered in sodium salt but the doses refer to the acid form of the compound.
F, female; M, male; s.c., subcutaneous.
Muzzle skin biopsies (left side) from euthanized mice were collected and fixed in 10% phosphate-buffered formalin and embedded in paraffin. Paraffin sections (6 μm) were stained with Hematoxylin and Eosin or von Kossa using standard procedures. To quantify the mineral deposition, muzzle skin biopsies (right side) were harvested and decalcified with 1.0 M HCl for 48 hours at room temperature. Solubilized calcium was then determined by a colorimetric assay.18, 19 The values were normalized to tissue weight. Whole blood was collected by retro-orbital bleeding or cardiac puncture. For subcutaneously injected mice, whole blood was collected 30 minutes after injection of INS-3001. Whole blood was collected at 2 days, 3 weeks, and 6 weeks after treatment initiation in mice receiving INS-3001. The concentrations of INS-3001 in heparin plasma were analyzed on an ultra-fast liquid chromatography, as described previously.16 The data were analyzed using Kruskal-Wallis nonparametric test. Statistical significance was reached with P < 0.05.
3. RESULTS
Prevention study: Continuous delivery of INS-3001 in young Abcc6−/− mice
The Abcc6−/− mice were administered INS-3001 by subcutaneous implantation of osmotic pumps at doses of 0.16, 0.8, 4, 20, and 100 mg/kg/day. The untreated wild-type and Abcc6−/− mice served as negative and positive controls of ectopic calcification. Treatment was initiated at 6 weeks of age, a time point at the earliest stages of ectopic calcification in the Abcc6−/− mice.17 The extent of ectopic calcification after 6 weeks of treatment was analyzed by two independent assays measuring calcification in the dermal sheath of vibrissae in muzzle skin, an early and reliable biomarker in this mouse model of PXE.17 One piece of muzzle skin was processed for semi-quantitative histological evaluation. A calcium-specific stain, von Kossa staining, revealed robust calcification in the dermal sheath of vibrissae in the untreated Abcc6−/− mice at 12 weeks of age. The Abcc6−/− mice receiving INS-3001 at concentrations at and above 4 mg/kg/day showed little, if any, calcification in the muzzle skin (Fig. 1A). There is no dose correlation with INS-3001 at the range of 4–100 mg/kg/day, as the lowest dose of 4 mg/kg/day completely prevented ectopic calcification to the same extent as the highest dose of 100 mg/kg/day (almost 100% inhibition). Significantly reduced calcification was noted at 0.8 mg/kg/day; by contrast, 0.16 mg/kg/day showed no effects on prevention of muzzle skin calcification (Fig. 1A). The therapeutic effects of INS-3001 were substantiated by the calcium content in the other muzzle skin biopsy measured using a quantitative chemical assay (Fig. 2A). The results showed that the levels of calcium in the INS-3001 treated Abcc6−/− mice at 4 mg/kg/day and above were similar to those of WT mice devoid of ectopic calcification (Fig. 2A). The partial inhibition of ectopic calcification at 0.8 mg/kg/day was corroborated by calcium assay corresponding to 59% inhibition (Fig. 2A), thus 0.8 mg/kg/day was identified as the minimum active dose.
Figure 1. Histopathologic examination of vibrissae calcification in the muzzle skin of Abcc6−/− mice in different groups.
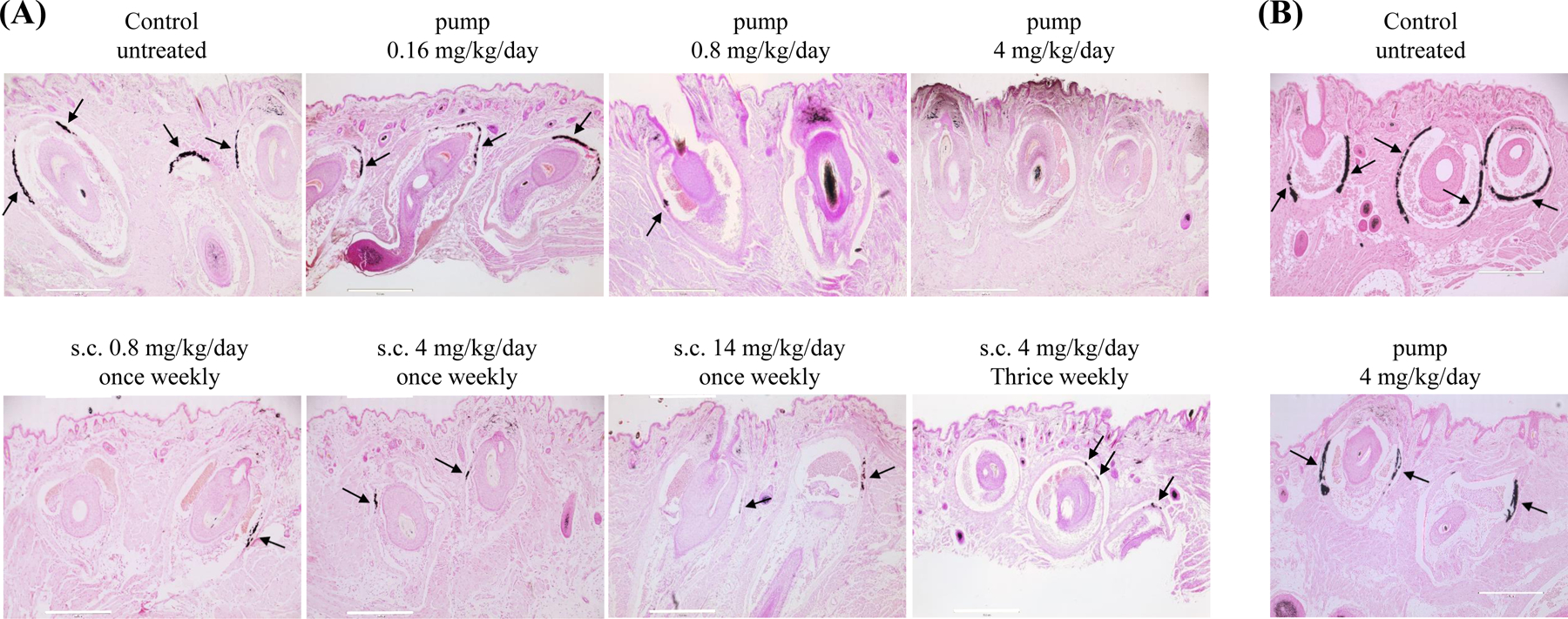
(A), Prevention study. All mice were analyzed at 12 weeks of age. von Kossa stains revealed robust calcification in the dermal sheath of vibrissae in untreated Abcc6−/− mice. The Abcc6−/− mice receiving INS-3001 via osmotic pumps at 4 mg/kg/day had very little, if any, calcification in the vibrissae. The Abcc6−/− mice receiving INS-3001 via osmotic pumps at 0.16 mg/kg/day had no effects on calcification in the vibrissae. Osmotic pump delivery of INS-3001 at 0.8 mg/kg/day had evident but significantly reduced calcification. Subcutaneous injections of INS-3001 at different doses and injection frequencies all resulted in decreased calcification. (B), Established disease study. All mice were analyzed at 24 weeks of age. von Kossa stains revealed extensive vibrissae calcification in untreated Abcc6−/− mice and INS-3001 treatment prevented further calcification which was stabilized at the time of treatment. Arrows indicate ectopic calcification. Scale bar, 0.4 mm.
Figure 2. Chemical assay of calcium in muzzle skin biopsies and plasma concentrations of INS-3001 in mice receiving INS-3001 administration.
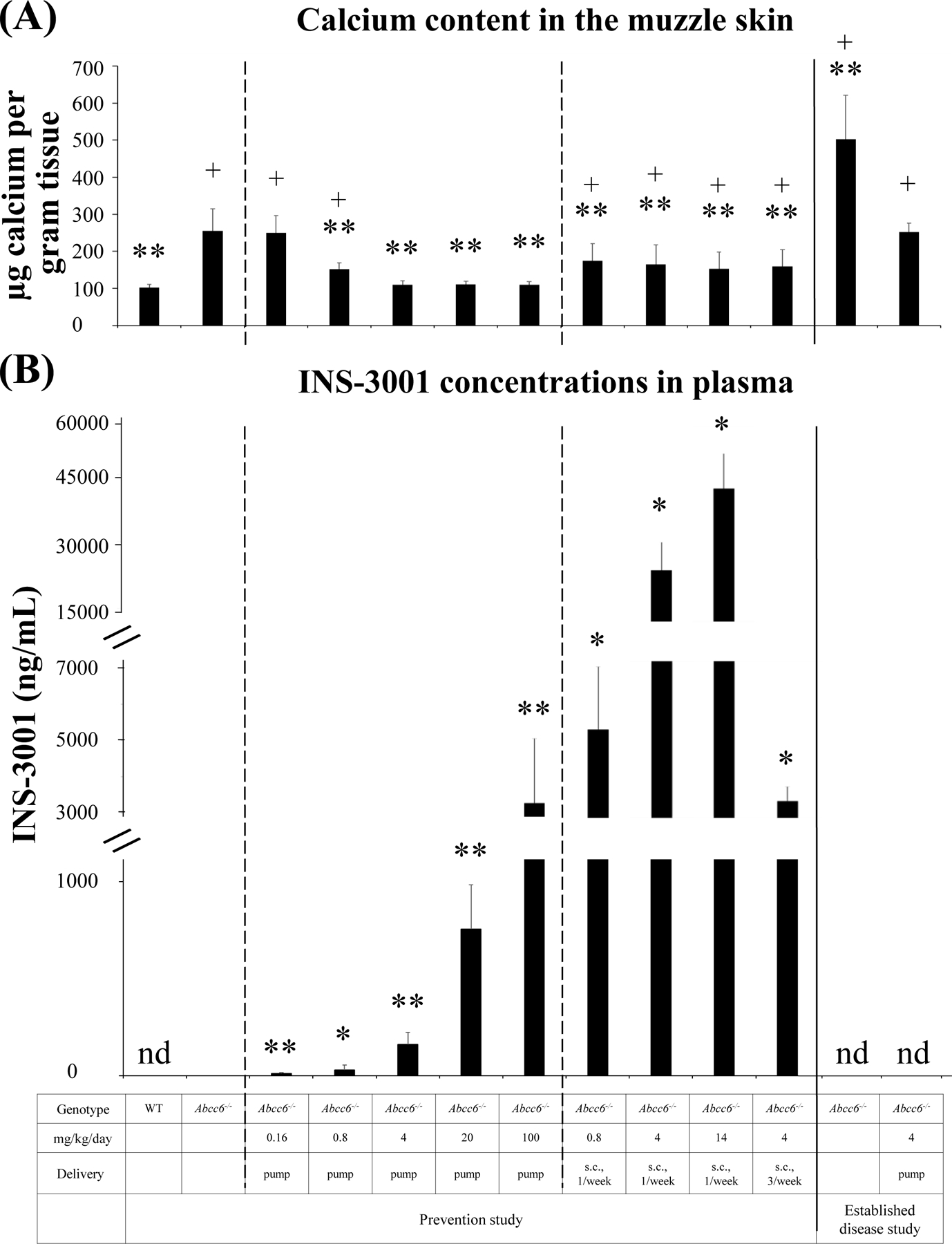
(A), The calcium content in the muzzle skin containing vibrissae. 6–12 mice per group. (B), Plasma concentrations of INS-3001. 5 mice per group. Blood was collected either prior to sacrifice for mice implanted with osmotic pumps or 30 minutes after a subcutaneous injection. Overall, the plasma concentrations scaled relatively well with the dose administered despite variabilities among different groups. The variability may be due to the fact that a single sampling time point was chosen around the anticipated Tmax of 30 minutes, which leads to greater variability than if a complete pharmacokinetic profile had been obtained. * P < 0.05, ** P < 0.01, compared to untreated 12-week-old Abcc6−/− mice; + P < 0.01, compared to untreated WT mice. Data were presented as mean ± SD. nd, not determined.
INS-3001 was undetectable in plasma of untreated Abcc6−/− mice. Administration of INS-3001 at doses from 0.16 to 100 mg/kg/day resulted in dose-dependent plasma concentrations (Fig. 2B). In addition, the levels of INS-3001 remained largely constant at 2 days, 3 weeks, and 6 weeks after implantation of the osmotic pump (Fig. S1), attributed by the nature of the consistent and continuous release of INS-3001 via osmotic pump.
Prevention study: Intermittent subcutaneous injections of INS-3001 in young Abcc6−/− mice
INS-3001, when delivered continuously by osmotic pump at 4 mg/kg/day, completely prevented ectopic calcification in the muzzle skin of Abcc6−/− mice. We next examined whether periodic, bolus subcutaneous injections of INS-3001 at the same dose of 4 mg/kg/day, would have similar therapeutic benefits. Six-week-old Abcc6−/− mice were administered INS-3001 by subcutaneous injections three times per week corresponding to 4 mg/kg/day. These mice showed significantly reduced calcification in the muzzle skin 6 weeks after start of treatment but residual calcification was still evident (Fig. 1A), corresponding to 63% inhibition (Fig. 2A). Thus, subcutaneous injection of INS-3001 is effective, albeit less than with continuous delivery at the same dose of 4 mg/kg/day on inhibition of ectopic calcification.
Three additional dose levels of INS-3001 at weekly subcutaneous injections for a duration of 6 weeks were tested to evaluate the inhibitory effects of INS-3001 (Fig. 1A). The efficacy of weekly injection at 14 mg/kg/day was similar to 4 mg/kg/day three injections per week, suggesting that transient increase of plasma levels of INS-3001 might be beneficial. The Abcc6−/− mice receiving INS-3001 injection at weekly frequency of 4 mg/kg/day showed significantly reduced calcification, similar to the effects with the same dose but at frequency of three injections per week. Weekly injection of INS-3001, at dose as low as 0.8 mg/kg/day, also significantly reduced ectopic calcification, suggesting that INS-3001 is a potent inhibitor of ectopic calcification with pharmacological activity that is maintained over days. The semi-quantitative histologic evaluations of INS-3001 treatment were consistent with the calcium contents in the muzzle skin measured by a chemical assay (Fig. 2A).
The Tmax of INS-3001 in plasma after subcutaneous administration in Sprague Dawley rats was reported to be about 30 minutes after injection16, we therefore measured INS-3001 concentrations in plasma 30 minutes after injection in the Abcc6−/− mice. INS-3001 levels in plasma 30 minutes after injection were much higher than that in the pump administered groups, as expected for a bolus administration (Fig. 2B). INS-3001-mediated prevention of ectopic calcification was more effective with continuous delivery reaching steady-state plasma concentrations, whereas bolus injections resulted in transient increase of INS-3001 concentrations but was still significantly potent when administered periodically.
Established disease study: Continuous delivery of INS-3001 in old Abcc6−/− mice
In established disease study, Abcc6−/− mice were aged to 12 weeks, the time at which ectopic calcification was extensive in the muzzle skin containing vibrissae (Fig. 1B). INS-3001 was administered at 4 mg/kg/day by osmotic pump, a dose and delivery route that resulted in a complete response in the young mice (Fig. 1A). The degree of ectopic calcification was evaluated 12 weeks later. Histopathology and calcium assay of the muzzle skin in the INS-3001 treated mice demonstrated calcification similar to 12-week-old Abcc6−/− control mice (Fig. 1, 2A). These results demonstrated that INS-3001, for a total of 12 weeks of follow-up, stabilized existing calcification and stopped the further growth of calcification.
4. CONCLUSIONS
Our results demonstrated that INS-3001 treatment prevented muzzle skin calcification in the Abcc6−/− mice with concurrent increases in its plasma levels, suggesting that pharmacologic therapy with INS-3001 might provide a promising treatment strategy for spontaneous calcification in PXE and GACI type 2, diseases that currently lack preventive or therapeutic options. The mechanism of action of INS-3001 as a new calcification inhibitor are not through chelation of calcium but rather coating of early nidus of calcification to hinder apposition of further mineralization and deposition of hydroxyapatite crystals.16 This mode of action is similar to that of the natural compound IP6, which was recently reported to stop the progression, albeit without regression, of cardiovascular calcifications in patients on hemodialysis following a 1-year treatment.20 INS-3001 arrested further deposition of mineral at the point when the treatment was initiated, suggesting that if INS-3001 is to be used to treat patients with PXE, it should be started as soon as the diagnosis is made. In contrast to chaperone-based allele-specific therapies21, 22, INS-3001 might have the potential to treat PXE regardless of the types of mutations in ABCC6. Compared to pyrophosphate and bisphosphonates, INS-3001 has high subcutaneous bioavailability, good local tolerability, high potency and no anti-osteoclastic activity.16, 23–27 However, further work is necessary to confirm the compound’s clinical potential.
Supplementary Material
Figure S1. Plasma concentrations of INS-3001 in mice receiving continuous INS-3001 administration via osmotic pump. Blood was collected at 2 days, 3 weeks, and 6 weeks after subcutaneous implantation of osmotic pumps filled with INS-3001 solution to deliver INS-3001 at 0.16, 0.8, 4, 20, and 100 mg/kg/day. 5 mice per group. * P < 0.05, ** P < 0.01, compared to untreated Abcc6−/− mice. Data were presented as mean ± SD.
ACKNOWLEDGEMENTS
This study was supported by Inositec Inc., Zurich, Switzerland. The authors thank Jianhe Huang for his valuable technical assistance. Carol Kelly assisted in manuscript preparation.
Abbreviations:
- PXE
pseudoxanthoma elasticum
- IP6
phytic acid, myo-inositol hexakisphosphate
Footnotes
CONFLICT OF INTEREST
MI is an inventor of patents licensed to Inositec Inc. and a shareholder thereof. INS-3001 is in development by Inositec Inc. The other authors have declared no conflicting interests.
REFERENCES
- 1.Li Q, van de Wetering K, Uitto J. Pseudoxanthoma elasticum as a paradigm of heritable ectopic mineralization disorders: Pathomechanisms and treatment development. Am J Pathol. Feb 2019;189(2):216–225. doi: 10.1016/j.ajpath.2018.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol. 1988;6(1):1–159. [DOI] [PubMed] [Google Scholar]
- 3.Uitto J, Li Q, van de Wetering K, Varadi A, Terry SF. Insights into pathomechanisms and treatment development in heritable ectopic mineralization disorders: Summary of the PXE International Biennial Research Symposium-2016. J Invest Dermatol. 2017;137:790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergen AA, Plomp AS, Schuurman EJ, et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet Jun 2000;25(2):228–31. doi: 10.1038/76109 [DOI] [PubMed] [Google Scholar]
- 5.Le Saux O, Urban Z, Tschuch C, et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet Jun 2000;25(2):223–7. doi: 10.1038/76102 [DOI] [PubMed] [Google Scholar]
- 6.Ringpfeil F, Lebwohl MG, Christiano AM, Uitto J. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci U S A. May 23 2000;97(11):6001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitschke Y, Baujat G, Botschen U, et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet. Jan 13 2012;90:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Brodsky JL, Conlin L, et al. Mutations in the ABCC6 gene as a cause of generalized arterial calcification of infancy: Genotypic overlap with pseudoxanthoma elasticum. J Invest Dermatol. 2014;134:658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borst P, Varadi A, van de Wetering K. PXE, a mysterious inborn error clarified. Trends Biochem Sci. Feb 2019;44(2):125–140. doi: 10.1016/j.tibs.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Favre G, Laurain A, Aranyi T, et al. The ABCC6 transporter: A new player in biomineralization. Int J Mol Sci. Sep 11 2017;18(9)doi: 10.3390/ijms18091941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van den Berg CJ, Hill LF, Stanbury SW. Inositol phosphates and phytic acid as inhibitors of biological calcification in the rat. Clin Sci Sep 1972;43(3):377–83. doi: 10.1042/cs0430377 [DOI] [PubMed] [Google Scholar]
- 12.Buades Fuster JM, Sanchis Cortes P, Perello Bestard J, Grases Freixedas F. Plant phosphates, phytate and pathological calcifications in chronic kidney disease. Nefrologia. Jan - Feb 2017;37(1):20–28. Fosfatos de origen vegetal, fitato y calcificaciones patologicas en la enfermedad renal cronica. doi: 10.1016/j.nefro.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 13.Schlemmer U, Frolich W, Prieto RM, Grases F. Phytate in foods and significance for humans: food sources, intake, processing, bioavailability, protective role and analysis. Mol Nutr Food Res Sep 2009;53 Suppl 2:S330–75. doi: 10.1002/mnfr.200900099 [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol. Mar 2010;5(3):519–30. doi: 10.2215/CJN.06080809 [DOI] [PubMed] [Google Scholar]
- 15.Eiseman J, Lan J, Guo J, Joseph E, Vucenik I. Pharmacokinetics and tissue distribution of inositol hexaphosphate in C.B17 SCID mice bearing human breast cancer xenografts. Metabolism. Oct 2011;60(10):1465–74. doi: 10.1016/j.metabol.2011.02.015 [DOI] [PubMed] [Google Scholar]
- 16.Schantl AE, Verhulst A, Neven E, et al. Inhibition of vascular calcification by inositol phosphates derivatized with ethylene glycol oligomers. Nat Commun Feb 5 2020;11(1):721. doi: 10.1038/s41467-019-14091-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klement JF, Matsuzaki Y, Jiang QJ, et al. Targeted ablation of the Abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol. Sep 2005;25(18):8299–310. doi: 10.1128/MCB.25.18.8299-8310.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Snook AE, Uitto J, Li Q. Adenovirus-mediated ABCC6 gene therapy for heritable ectopic mineralization disorders. J Invest Dermatol. Jan 11 2019;139:1254–1263. doi: 10.1016/j.jid.2018.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Huang J, Pinkerton AB, et al. Inhibition of tissue-nonspecific alkaline phosphatase attenuates ectopic mineralization in the Abcc6(−/−) mouse model of PXE but not in the Enpp1 mutant mouse models of GACI. J Invest Dermatol. Feb 2019;139(2):360–368. doi: 10.1016/j.jid.2018.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raggi P, Bellasi A, Bushinsky D, et al. Slowing progression of cardiovascular calcification with SNF472 in patients on hemodialysis: Results of a randomized phase 2b study. Circulation. Mar 3 2020;141(9):728–739. doi: 10.1161/CIRCULATIONAHA.119.044195 [DOI] [PubMed] [Google Scholar]
- 21.Pomozi V, Brampton C, Fulop K, et al. Analysis of pseudoxanthoma elasticum-causing missense mutants of ABCC6 in vivo; pharmacological correction of the mislocalized proteins. J Invest Dermatol. Apr 2014;134(4):946–53. doi: 10.1038/jid.2013.482jid2013482 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pomozi V, Brampton C, Szeri F, et al. Functional rescue of ABCC6 deficiency by 4-phenylbutyrate therapy reduces dystrophic calcification in Abcc6(−/−) mice. J Invest Dermatol. Mar 2017;137(3):595–602. doi: 10.1016/j.jid.2016.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pomozi V, Brampton C, van de Wetering K, et al. Pyrophosphate supplementation prevents chronic and acute calcification in ABCC6-deficient mice. Am J Pathol. Jun 2017;187(6):1258–1272. doi: 10.1016/j.ajpath.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dedinszki D, Szeri F, Kozak E, et al. Oral administration of pyrophosphate inhibits connective tissue calcification. EMBO Mol Med. Nov 2017;9(11):1463–1470. doi: 10.15252/emmm.201707532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Sundberg JP, Levine MA, Terry SF, Uitto J. The effects of bisphosphonates on ectopic soft tissue mineralization caused by mutations in the ABCC6 gene. Cell Cycle. 2015;14:1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Kingman J, Sundberg JP, Levine M, Uitto J. Etidronate prevents, but does not reverse, ectopic mineralization in a mouse model of pseudoxanthoma elasticum. Oncotarget. 2018;56:30721–30730. doi:doi: 10.18632/oncotarget:10738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kranenburg G, P.A. dJ, Bartstra JW. Etidronate for prevention of ectopic mineralization in patients with pseudoxanthoma elasticum. J Am Coll Cardiol. 2018;71:1117–1126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Plasma concentrations of INS-3001 in mice receiving continuous INS-3001 administration via osmotic pump. Blood was collected at 2 days, 3 weeks, and 6 weeks after subcutaneous implantation of osmotic pumps filled with INS-3001 solution to deliver INS-3001 at 0.16, 0.8, 4, 20, and 100 mg/kg/day. 5 mice per group. * P < 0.05, ** P < 0.01, compared to untreated Abcc6−/− mice. Data were presented as mean ± SD.


