Abstract
Treatment outcomes for migraine and other chronic headache and pain conditions typically demonstrate modest results. A greater understanding of underlying pain mechanisms may better inform treatments and improve outcomes. Increased GABA+ has been identified in recent studies of migraine, however, it is unclear if this is present in other headache, and pain conditions. We primarily investigated GABA+ levels in the posterior cingulate gyrus (PCG) of people with migraine, whiplash-headache and low back pain compared to age- and sex-matched controls, GABA+ levels in the anterior cingulate cortex (ACC) and thalamus formed secondary aims. Using a cross-sectional design, we studied people with migraine, whiplash-headache or low back pain (n = 56) and compared them with a pool of age- and sex-matched controls (n = 22). We used spectral-edited magnetic resonance spectroscopy at 3T (MEGA-PRESS) to determine levels of GABA+ in the PCG, ACC and thalamus. PCG GABA+ levels were significantly higher in people with migraine and low back pain compared with controls (eg, migraine 4.89 IU ± 0.62 vs controls 4.62 IU ± 0.38; P = .02). Higher GABA+ levels in the PCG were not unique to migraine and could reflect a mechanism of chronic pain in general. A better understanding of pain at a neurochemical level informs the development of treatments that target aberrant brain neurochemistry to improve patient outcomes.
Perspective:
This study provides insights into the underlying mechanisms of chronic pain. Higher levels of GABA+ in the PCG may reflect an underlying mechanism of chronic headache and pain conditions. This knowledge may help improve patient outcomes through developing treatments that specifically address this aberrant brain neurochemistry.
Keywords: Pain, GABA+, Proton magnetic resonance spectroscopy, MRS, 1-HMRS
Chronic pain is the leading cause of disability worldwide, when considering both migraine, and musculoskeletal pain conditions such as low back pain.82 Randomized controlled trials in musculoskeletal pain40,81 and headache conditions,13,71,78 have investigated numerous approaches to reduce the burden of chronic pain. However, results remain modest with some patients failing to respond to interventions.17,34,68,74 It is not fully understood why some people fail to recover from an initial onset of pain. However, gaining a better understanding of potential underlying neurochemical mechanisms may offer a further insight into the development and persistence of chronic pain.
One proposed mechanism of chronic pain is dysfunction in the metabolism of GABA, the main inhibitory neurometabolite of the central nervous system.20 Chronic pain has been proposed to be associated with reduced levels of GABA+. Here, the term GABA+ is used rather than GABA to acknowledge the unwanted co-edited macromolecules and homocarnosine that are typically edited alongside GABA in GABA-edited magnetic resonance spectroscopy (MRS). This reduced GABA+ is considered reflective of a state of hyperexcitability.23 The hyperexcitability, or loss of inhibition in combination with altered thalamocortical connectivity is said to give rise to a constant perception of pain.16,33,39 However, the reduction in GABA+ has not been observed across all pain conditions. Two contrasting examples are migraine and low back pain. Increased GABA+ was found in adults and children with migraine compared to controls, using MRS sequences optimized to detect GABA+2,5 and macromolecule suppressed GABA respectively4. It was hypothesized that increased GABA/GABA+ levels could reflect a migraine-specific mechanism such as cortical spreading depression, neurogenic inflammation or vasodilation.2,43,60 In contrast, no differences in GABA+ levels were detected in people with low back pain compared to healthy controls. This may suggest that the dysfunction in hyperexcitability could be driven by an increase in excitatory neurometabolites such as glutamate rather than the inhibitory GABA.31 Alternatively, it might reflect that the MRS parameters were not sufficiently optimized to detect GABA+ or GABA in these studies. Grachev et al28 used a non-edited STEAM sequence- ie, not optimized to measure GABA or GABA+, and Janetzki et al42 failed to report which parameters were used. Taken together, an increase in GABA+ may be a unique underlying mechanism of migraine, however, further studies optimized to detect GABA+ are required in other pain conditions.
To date a direct comparison of GABA+ levels in headache and pain groups has not been completed in the same GABA+ optimized MRS study. A recent meta-analysis of studies reporting GABA+ levels in pain conditions concluded that GABA+ levels appeared increased in migraine but variable in other pain conditions.62 This suggests that GABA+ levels could possibly be specific to the pain condition and might reflect a migraine specific process such as cortical spreading depression, rather than more generic pain states such as central sensitization which can be seen across all chronic pain conditions. In addition to the limitation that pain groups were not compared within a single study, the quality of the methods of MRS acquisitions varied significantly.
In recent years, methods to quantify GABA+ have significantly evolved. High-quality studies use optimized sequences (MEscher GArwood point resolved spectroscopy; MEGA-PRESS48) and acquisition parameters specifically designed for detecting GABA+.54 However, none of the 4 studies29,42,67 of musculoskeletal pain included in the meta-analysis62 reported utilizing these parameters. Furthermore, there was substantial variation in the brain region studied; a variable considered a potential confounder in the measurement of GABA+.30,65,80,83 Consequently, it is unclear whether the variation in GABA+ levels seen across pain conditions are reflective of a true between-group difference or rather a reflection of the studies’ methodological quality or brain region examined.
A number of brain regions have been proposed to be involved in the modulation of pain. The posterior cingulate gyrus formed our primary aim following our previous work which showed increased GABA+ in this region in people with migraine, which was associated with higher levels of central sensitisation.1,2 The anterior cingulate cortex, and thalamus formed secondary aims due to their well-documented role in the pain experience.16,39,84
Therefore, the aim of this study was to establish whether the increased levels of GABA+ reported in high quality studies of migraine were present in other headache or pain conditions. The results of the study will provide further insight into mechanisms or consequences of chronic pain conditions, which in turn may direct future treatment options for those with persistent chronic pain.
Materials and Methods
Study Design
The study used a cross-sectional, case-control design to measure levels of GABA+ determined using MRS in people with migraine, other pain conditions and healthy control subjects. Ethical approval was granted from Western Sydney Local Health District (WSLHD) study number HREC/17/WMEAD/429. Written consent from participants was gained in line with the principles of the Declaration of Helsinki.
Participants
Inclusion/Exclusion Criteria
We aimed to recruit a total of sixty participants with a pain condition (n = 20 migraine, n = 20 whiplash-headache and n = 20 low back pain) to be age- and sex-matched to an individual from a pool of pain-free controls (n = 22) (Fig 1). Participants with migraine were included if they satisfied the conditions according to the International classification of headache disorders (ICHD)-341 criteria for migraine with or without aura (https://ichd-3.org/1-migraine/), had experienced migraines for at least 3 months, and had a Headache Impact Test (HIT-6) score over 50 (exceeding moderate headache-related disability). Participants with whiplash-headache were included if they satisfied ICHD-3 for ‘whiplash with persistent headache’, had symptoms for at least 3 months, and had moderate headache-related disability (HIT-6 >50). Participants with low back pain were included if they had pain in the region between T12 to S1 in accordance with the International Classification of Diseases (ICD) diagnosis for low back pain,86 had experienced symptoms for at least 3 months, and had an Oswestry Disability Index (ODI) score over 20% (exceeding moderate low-back-pain-related disability). Pain-free controls were included if they had not experienced a previous migraine, regular headaches or a pain condition in the last 3 months or had not experienced a previous pain condition that had lasted for over 3 months.
Figure 1.
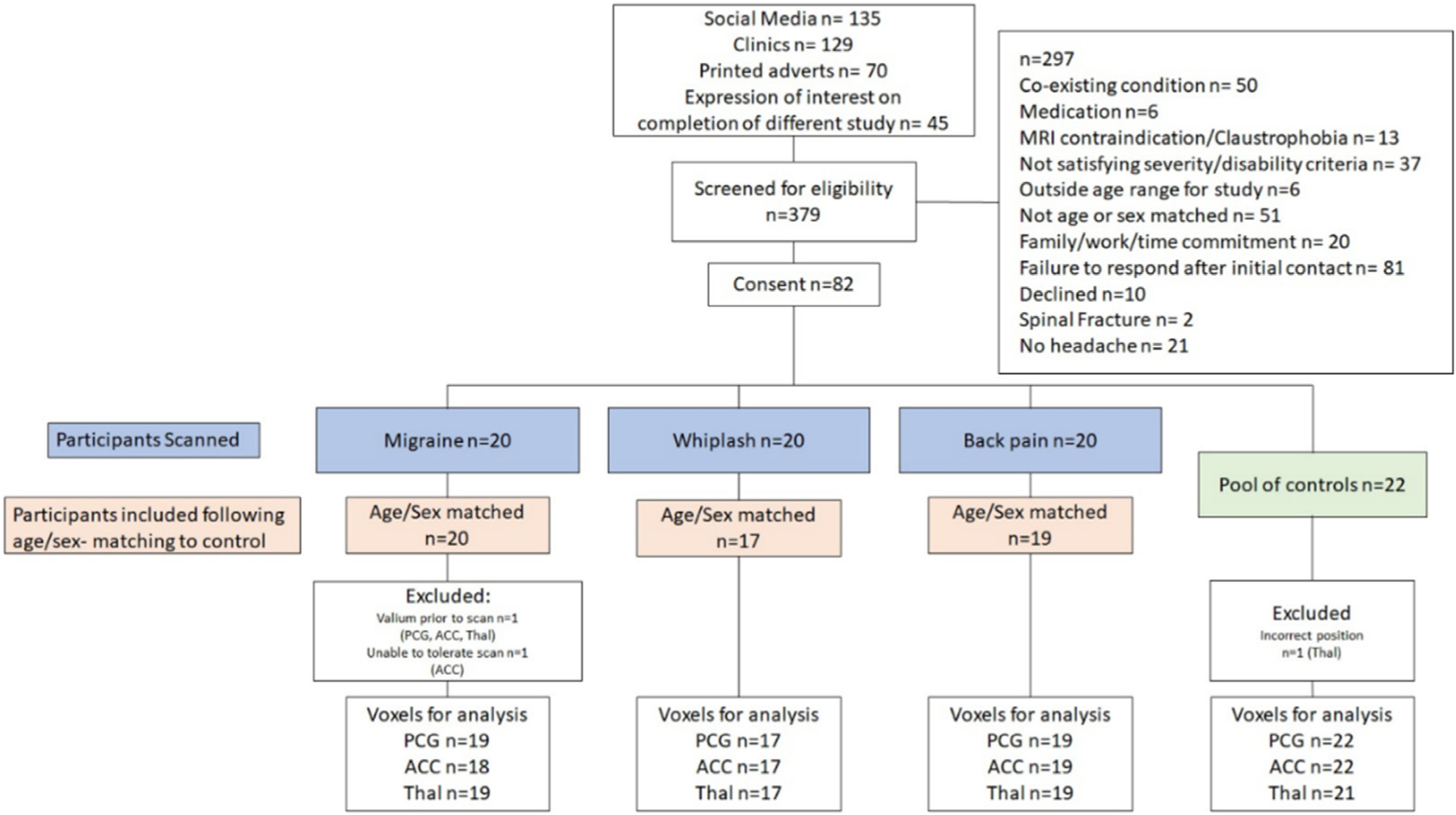
Flow of participants through the study.
Participants were excluded from the study if they had any contraindications for MRI or conditions that compromised MR spectroscopy such as pregnancy, metal implants, claustrophobia, or metal braces. Participants were also excluded if they experienced severe depression, had a diagnosed neurological or psychiatric condition or took medications which might influence GABA or glutamate levels in the brain such as gabapentin, pre-gabalin, topiramate, diazepam or glutamate. Furthermore, participants were not included in the study if they had symptoms in common with the other groups (eg, both migraine and low back pain). Additional exclusion criteria applying to all groups included not being independently ambulant, health complaints in the last 5 days and the inability to communicate in the English language. Further exclusion criteria were applied to the whiplash-headache group, including: any self-reported pre-existing headache condition, or if the road traffic crash was major (eg, involved spinal fracture or occupant fatality).
Age and Sex Matching
Both age and sex matching are important in the analysis of GABA+. There is a growing body of evidence suggesting GABA+ is lower in females than males57,69 and decreases with age, although the rate of this has yet to be established.25,63,73 To address the impact of age without age-restricting, a pair-wise sampling approach was adopted. Participants with pain were matched to a participant from a pool of twenty-two controls (within 5 years of age and the same sex). The pool of controls was established through age- and sex-matching to the migraine group with the addition of 2 participants matched to accommodate the whiplash-headache group.
Recruitment
Participants with headache and pain conditions were recruited from primary and secondary care settings. These included physiotherapy, general practitioner and neurology clinics as well as hospital outpatient departments. Potential participants either responded to an advertisement placed in a participating clinic, or clinicians working within the clinic gained consent from the potential participant to be contacted by the research team. Controls were recruited through advertisements placed on University, hospital and community noticeboards and on social media. Potential participants were first screened by telephone and eligible participants were given detailed information about the study before providing written informed consent. Participants then completed self-reported measures of pain and disability online and underwent MRS examination.
Participant Characteristics
Participant demographic and clinical characteristics were collected using the online survey platform, REsearch Data Capture (REDCap)37. Demographic data included age, gender, height, weight, and educational level. Symptom data collected at baseline were pain duration and intensity and a self-reported description of symptoms. Participants also completed self-reported measures of pain, disability, and psychological distress at baseline (detailed below).
Self-report Measures of Pain and Disability
The Numeric Rating Scale (NRS) is the most widely used measure of pain and has been validated for use in multiple pain conditions. The score range is 0–100 with higher scores reflecting higher levels of pain.22,85 The Central Sensitization Inventory (CSI) is a validated 100- point scale, where higher scores are associated with higher levels of hypersensitivity to both noxious and non-noxious stimuli.47,56 Scores over 22.5 have been associated with migraine,1 and scores over 40 have been associated with the presence of central sensitization syndromes such as fibromyalgia.56 The Headache Impact Test (HIT-6) is a validated tool measuring headache-related disability. Scores range from 36 to 78 with higher scores demonstrating higher disability, and scores over 50/78 are considered high.11,87,88 The World Health Organization Disability Assessment Schedule (WHODAS 2.0–12) is a validated measure of general disability. The score ranges from 12 to 60 with higher levels indicating greater disability.4,14,64 The Depression, Anxiety and Stress Scale (DASS- 21) is a validated tool to measure depression, anxiety and stress, with sub-scores over 7/21, 6/21 and 10/21, respectively, indicating moderate levels of distress.59 The Post Traumatic Stress Disorder (PTSD) Checklist identifies those who are experiencing PTSD; it is scored from 0 to 80, and with scores over 31 indicating the likely diagnosis of PTSD8.
Magnetic Resonance Spectroscopy
Brain neurometabolite concentration was assessed using a J-difference-edited proton magnetic resonance spectroscopy (MRS) sequence, with the metabolite of interest in this study being GABA, the primary inhibitory neurotransmitter in the human brain. J-difference editing is a widely used method to study coupled signals from low-concentration molecules such as GABA that are overlaid by larger signals from other metabolites. The most commonly used method for GABA detection, MEGA-PRESS,26,48 consists of 2 experiments: In the first (“ON”) experiment, a frequency-selective radiofrequency pulse is applied to refocus the evolution of the scalar coupling in the GABA molecule; in the second (“OFF”) experiment, this coupling is allowed to evolve freely. Subtracting the OFF from the ON spectrum yields the GABA-edited difference spectrum with a prominent GABA peak at 3.02 ppm, while the overlaying strong signals from creatine and phosphocreatine are cancelled out. Due to the finite selectivity of the editing pulses, the edited peak contains contaminations from co-edited macromolecules and homocarnosine, and is therefore termed “GABA+” in the literature.15,54 The levels of GABA+ were reported in institutional units, ie, relative to the internal concentration reference of tissue water. GABA+ levels were corrected for magnetization relaxation effects of tissue water, and for differing concentration of GABA between grey and white matter.36 Potential confounders of GABA+ concentration have been reported. In order to reduce the impact of these potential pharmacological confounders, participants were not allowed to consume pain medications, nicotine, alcohol or caffeine on the day of scanning.6,10,53,58
MRI and MRS Acquisitions
Structural imaging and spectroscopic data were acquired on a clinical Siemens PRISMA 3T scanner (Siemens Healthineers, Erlangen, Germany, software version VE11C) at a large teaching hospital in Sydney, Australia, using a 64-channel head/neck array coil for RF signal reception. Anatomical images were acquired as follows. Firstly, a high-resolution 3D T1-weighted isotropic scan (MPRAGE) was acquired sagittally to guide voxel placement using all 3 reformatted planes for accurate positioning (repetition time (TR) = 2400 ms; echo time (TE) = 2.21 ms; inversion time (TI) = 1150ms; voxel size = 1. 0 × 1.0 × 1.0mm3; iPAT = 3; FOV = 256mm; matrix = 256 × 256; acquisition time = 4min 35sec). Secondly, an axial 2D T2-weighted series (TR = 7490ms; TE = 99ms; voxel size = 0.6 × 0.3mm3; iPAT = 2; FOV = 220mm; matrix = 384 × 288; acquisition time = 2min 24sec) was also acquired to further aid voxel placement. Both the T1-W and T2-W series were sent for review and reporting by a consultant radiologist to allow for identification and follow up of any incidental findings.
Following the sequence Application Guide and Release Notes,12 flip angle calibration was then performed for each voxel location (TR = 2000ms; TE= 30ms, 1 average). The RF power value identified to achieve the optimal flip angle resulting in maximal signal-to-noise ratio (SNR) and subsequent peak height was then used for the MEscher-Garwood Point RESolved Spectroscopy (MEGA-PRESS)48 acquisition for GABA+ detection. MEGA-PRESS data were then acquired with the following parameters: TR = 2000ms; TE = 68 ms; 192 averages; 2048 data points; spectral width = 2000 Hz; editing pulse frequencies set to 1.9 ppm and 7.5 ppm for editing of GABA; editing pulse bandwidth = 70 Hz; center of slice selection frequency (“delta frequency”) = −1.7 ppm relative to water for optimal co-localization of the 3.0 ppm GABA signal with the prescribed voxel. Finally, water-unsuppressed MEGA-PRESS data were acquired (1 average; delta frequency = 0.0 ppm for optimal co-localization of the unsuppressed water signal).
Brain Regions
The primary brain region of interest was the posterior cingulate gyrus (PCG) because it has been shown to be implicated in migraine2 and associated with reports of increased central sensitization.1 Further, posterior regions of the brain have demonstrated good reliability for GABA+ measurements.65,72 Secondary brain regions of interest were the anterior cingulate cortex (ACC) and thalamus. The ACC was selected for its potential association with the affective and psychosocial aspects of pain.84 The thalamus has been shown to be critically involved in central pain processing networks using fMRI, volumetric studies and MR spectroscopy.16,39 Whilst the insula has also been identified as a potential contributor to pain mediation,75,79 it was not deemed feasible to be included due to protocol time restrictions. Imaging was limited to 1 hour, in order to ensure participant compliance and reduce the chance of movement-related artifacts.
Voxel Placement
The PCG voxel measured 25(AP) × 40 (RL) × 25 (CC) mm3 and was positioned closely aligned parallel to the superior posterior border of the splenium of the corpus callosum, inferior to the cingulate sulcus and antero-superior to the parieto-occipital sulcus, covering the PCG (and a portion of the inferior precuneus; Fig 2E). The ACC voxel measured 40 (AP) × 25 (RL) × 25 (CC) mm3 and was aligned parallel to the superior anterior border of the genu of the corpus callosum and inferior to the supero-anterior portion of the cingulate sulcus (Fig 2F). The thalamic voxel measured 25(AP) × 40 (RL) × 25 (CC) mm3 and was angled slightly over the thalamus on para-sagittal images to encompass maximal thalamic tissue. (Fig 2G). The long axis of the voxel was aligned anterior-to-posterior in the ACC and right-to-left in the PCG and thalamus.
Figure 2.
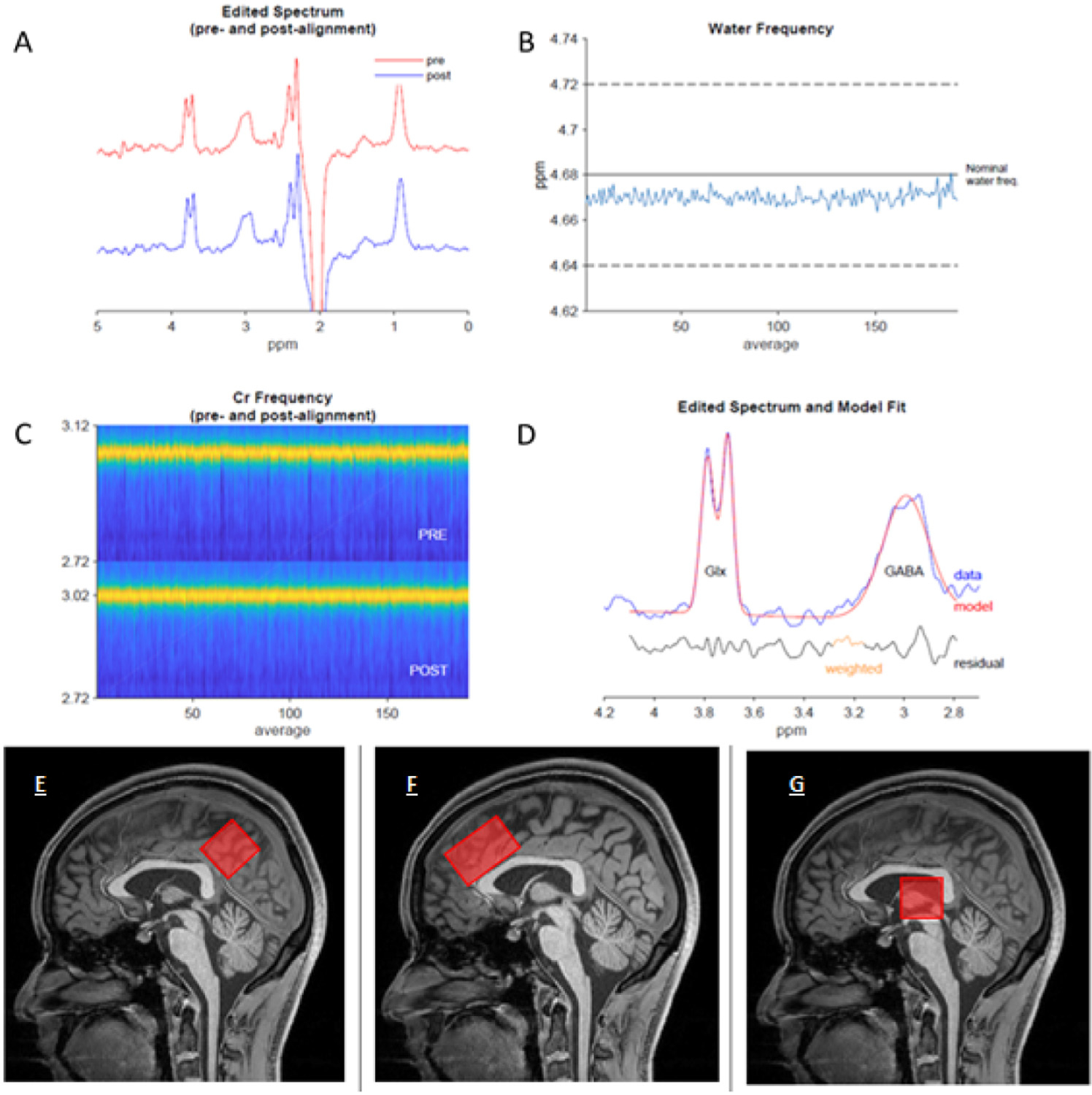
Example spectra from the PCG of a participant following fitting, and visual representation of voxel positioning. Example Gannet output taken from a migraine participant. (A) Demonstrates the processed GABA-edited difference spectrum before frequency and phase correction (red) and after (blue) (B) Shows the residual water signal plotted against time, demonstrating the stability of the experiment with respect to eg, field drift, or motion, (C) Shows the creatine signal (yellow) over the duration of the experiment, pre- refers to before frequency-and-phase correction and post refers to after. (D) Shows the output from the GannetFit module. The GABA-edited spectrum is shown in blue, the model in red, and the fit residual in black below. Schematic diagram to demonstrate the voxel placement for (E) PCG, (F) ACC and (G) thalamus.
MRS Processing
MEGA-PRESS data were processed using the MATLAB-based toolbox Gannet (version 3.1.3).18 Gannet consists of several modules: GannetLoad applied zero-filling, 3-Hz exponential line broadening, and frequency-and-phase-correction of individual averages using the spectral registration algorithm.55 A new, improved method of spectral alignment called “RobustSpecReg”, has been found to reduce subtraction artefacts in a majority of datasets, and was therefore used alongside spectral registration in order to minimize fit error.52 The aligned transients are then averaged to yield the edit-ON and edit-OFF spectra, which are subsequently subtracted to create the GABA-edited MEGA-PRESS difference spectrum. Each dataset was processed using both alignment algorithms, and a decision as to which result to use for further analysis was made based on visual inspection by a researcher with over 8 years of experience working on spectral editing (GO) who was blinded to group allocation. Spectral fitting was performed by the GannetFit module, which applies a single Gaussian model to fit the 3.02 ppm GABA+ signal and models the creatine peak in the “OFF” spectrum as well as the water signal in the water-unsuppressed data as Lorentzian peaks. The GannetCoRegister and GannetSegment modules call SPM1224 to determine the tissue volume fractions of grey matter, white matter and cerebrospinal fluid. Gan-netQuantify then returns GABA levels in institutional units (IU), corrected for effects of tissue water content and relaxation effects as well as for GABA being present in grey matter at approximately twice the concentration of that in white matter.36
MRS Quality Assessment
The quality of the spectra was inspected by an independent investigator (GO) who was blinded to participant group. The spectra were analyzed visually for artifacts and insufficient water suppression. Where the fit error (standard deviation of the fit residual divided by the amplitude of the model) exceeded 10%, data were processed, and fitted using spectral registration rather than Robust spectral registration.52 In cases where the fit error improved, this new analysis was retained. Should fit error exceed 12%; the cut off typically recommended in GABA spectroscopy papers,18,65,66 the scan was excluded from the analysis.
Statistical Analysis
A sample size of 17 per group was calculated a priori. This was based on our previous study2 and allows detection of a between-group GABA+ level difference of 0.2 IU with 80% power. Participants were age- and sex- matched within a maximum of 5 years in order to account for potential age-related GABA changes that have been observed.25,45,46,63 (See above section 2.2.2. Age and Sex matching).
Data were tested for normality of distribution using the Kolmogorov-Smirnov test and the Shapiro-Wilk test. Descriptive statistics (mean, standard deviation (SD) or median, interquartile range (IQR)) were used to report normally and non-normally distributed participant demographics and pain characteristics respectively. Between-group differences in participant characteristics were assessed using a Chi-square test for data presented as proportions (for example sex), and analysis of variance (ANOVA) or Kruskal-Wallis for normally and non-normally distributed data, respectively. Significance values were then adjusted by the Bonferroni correction for multiple tests.
In order to detect the difference in GABA+ levels between the pain groups and their paired age- and sex- matched controls, we used Wilcoxon signed rank tests. This test is recommended for pair-wise comparisons when data are non-parametric,44 was used in our previous study2 and allows for the close age- and sex-matching required for the analysis of GABA+ which is not possible when using a comparison of means such as an ANOVA.
In order to adjust for multiple comparisons, tests were split into 3 groups dependent on brain region and adjusted for using the Holm-Bonferroni procedure.70
To determine the difference in GABA+ levels between the different pain groups, the same analysis was used. We calculated pair-wise differences in 3 age- and sex- matched comparisons 1) migraine versus whiplash-headache, 2) whiplash-headache versus back pain, 3) back pain versus migraine. Whilst this method has the benefit of allowing for pair-wise comparison between similar individuals in terms of age and sex between the groups, it did limit the number of pairs that could be included in the analysis between pain groups.
To assess data quality between groups we also investigated group differences in fit error, SNR, frequency drift, and the full-width half-maximum (FWHM) of the modelled water peak using the Kruskal-Wallis nonparametric test.
A post-hoc analysis investigating the correlation between PCG GABA+ levels and pain duration was conducted by determining Pearson’s r correlation coefficient.
Statistical analyses were conducted using SPSS version 26 (SPSS Inc, Chicago, IL). A P-value of <.05 was deemed significant across the analyses.
Data Availability
Gannet 3.1.3 code is available from: https://github.com/richardedden/Gannet3.1/releases/tag/v3.1.3. The data supporting the study’s findings are available from the corresponding author, upon reasonable request following approval from the University of Sydney.
Results
Participants
Three hundred and seventy-nine participants were screened. Of these 297 were excluded, and 82 (migraine n = 20, whiplash-headache n = 20, low back pain n = 20 and controls n = 22) were enrolled. Following age- and sex-matching, 56 participants with a pain condition (migraine n = 20, whiplash-headache n = 17, low back pain n = 19) and 22 controls were included in the final analysis. Recruitment was halted in April 2020 due to research restrictions related to the 2020 COVID-19 pandemic leaving 4 unmatched participants (whiplash-headache n = 3, low back pain n = 1). These participants were excluded from the analysis. One participant in the migraine group was excluded after scanning because they took diazepam prior to the scan. Data were missing from the ACC voxel of a single migraine participant and the thalamic voxel of a single control participant due to acquisition error (Fig 1).
Baseline Characteristics
All groups were similar with respect to age, sex, body-mass index (BMI), and educational status (Table 1). The pain groups were all similar with respect to pain intensity (eg, migraine mean ± SD 66.05 ± 22.9, whiplash-headache mean 58.8 ± 24.4 and low back pain mean 57.88 ± 20.26). The whiplash-headache group had significantly higher scores for DASS anxiety (median 30, interquartile range [IQR] 22 to 76) and depression (10, [4 to 24]) compared to the other migraine and back pain groups. The low back pain group had significantly lower CSI (mean ± SD 31.26 ± 14.74) and HIT-6 compared to both the whiplash and headache groups (Table 1). Participants were asked to rate their pain at time of scanning, pain levels were similar across the pain groups, all pain participants reported pain that related to their specified pain condition (eg, current headache levels in the migraine group and back pain in the back pain group), controls reported 4/100 pain on the NRS which related to inconsequential aches from everyday living (eg, post-exercise soreness).
Table 1.
Characteristics of Participants.
| Migraine (n = 20) | Whiplash (n= 17) | Back Pain (n = 19) | Controls (n = 22) | P value | Post-hoc- P < .05 | |
|---|---|---|---|---|---|---|
|
| ||||||
| Age | 39.65 ± 9.95 | 41.12 ± 12.1 | 38.68 ± 12.79 | 39.27 ± 11.49 | 0.78 b | - |
| Sex (female n, %) | 16, 80 | 11,65 | 14, 74 | 16, 73 | 0.78c | - |
| BMI | 26.1 (22.2 – 32) | 25.3(20.8 – 33) | 25 (22.8 – 27.6) | 25.7(23.4 – 26.5) | 0.91 a | - |
| Educational level (University n, %) | 12, 60 | 7, 41 | 8, 42.1 | 16, 72.7 | 0.13 c | - |
| Pain Characteristics | ||||||
| Duration-ys | 18(10.5 – 25) | 2.67(1.5 – 4) | 5(2 – 12) | N/A | 0.01a | ^ || |
| Average pain intensity in last wk (NRS 0 – 100) | 66.05 ± 22.9 | 59.18 ± 21.29 | 54.58 ± 20.05 | N/A | 0.27b | - |
| Pain/Symp-ms | ||||||
| Pain at time of scan (NRS 0–100) | 40(5 – 62) | 31 (21 – 50) | 30 (20 – 38) | 0(0 – 5) | 0.01a | ǂ § † |
| CSI | 48.65 ± 16.5 | 51.24 ± 16.77 | 31.26 ± 14.74 | 9 ± 9.1 | 0.01b | ǂ § † || ≠ |
| Disability | ||||||
| WHODAS 2.0 | 26.9 ± 18.4 | 31.71 ± 18.84 | 19.74 ± 10.84 | 0.64 ± 1.56 | 0.01b | ǂ § † |
| HIT-6 (36 – 78) | 68 (62 – 70) | 65 (61 – 67) | 40 (36 – 44) | 36 (36 – 38) | 0.01a | ǂ § || ≠ |
| Psychological Status | ||||||
| DASS | 20(12 – 32) | 30 (22 – 76) | 16 (8 – 34) | 2 (0 – 6) | 0.01a | ǂ § † |
| Depression | 2 (0 – 9) | 10 (4 – 24) | 4 (2 – 8) | 0 (0 – 0) | 0.01a | § † ^ |
| Anxiety | 6 (2 – 12) | 6 (4 – 16) | 2 (0 – 6) | 0 (0 – 2) | 0.01a | ǂ § ≠ |
| Stress | 10(8 – 17) | 16(10 – 24) | 6 (4 – 20) | 0 (0 – 4) | 0.01a | ǂ § † |
| PTSD Checklist | 9 (5 – 16) | 21 (9 – 36) | 6(2 – 17) | 0 (0 – 4) | 0.01a | ǂ § † |
Normally distributed data presented as Mean ± SD, and non-normally distributed data presented as median and (IQR). Between group analysis are denoted as follows: 1) Kruskal-Wallis test; 2)ANOVA; 3) Chi-Squared. Symbols represent significant post-hoc comparisons (P < .05) following Bonferroni correction as follows
Migraine versus Whiplash
Migraine versus Low back pain
Migraine versus Control
Whiplash versus Low back pain
Whiplash versus Control
Low back pain versus Control.
Spectroscopy Quality
The fit error reflecting spectroscopy quality was under 10% for 209/212 (98%) of voxels with a mean fit error of 4.64% ± 1.08% for the PCG, 4.93% ± 0.96% for the ACC and 5.59% ± 1.8% for the thalamus (Fig 2). Where fit error exceeded 10% (3 voxels) the alternative alignment methods of robust spectral registration was applied (n = 1 control ACC, n = 1 control thalamus, n = 1 whiplash-headache thalamus). After this procedure, all spectra were modelled with a fit error below the recommended value,18,66 and were in line with those reported in the largest MRS dataset available.49 Therefore, all spectra were included in the analysis (Fig 1). Average frequency offset, representing frequency drift was −0.02 ppm ± 0.01 in the PCG, −0.01 ppm ± 0.02 in the ACC and −0.02 ppm ± 0.01 in the thalamus, which is also in line with the Big GABA dataset.49 Spectral quality in terms of fit error, SNR, frequency drift, and full-width half maximum (FWHM) of modelled water signal were the same across all groups and in line with the largest collected GABA+ dataset49,51 (Supplement 1).
Primary Analysis
Comparison Between Individual Pain Groups and Controls in the PCG
GABA+ levels were significantly higher in people with migraine (mean ± SD 4.89 ± 0.62 IU) compared with controls (4.62 ± 0.38 IU) in the PCG (mean difference ± SD 0.28 ± 0.63 IU, P = .02; Table 2, Fig 3B). Similarly, GABA+ levels were significantly higher in people with low back pain (mean ± SD 4.88 ± 0.46 IU) compared with controls (4.58 ± 0.34 IU) in the PCG (mean difference 0.32 ± 0.50 IU, P = .02). Both of these results maintained their significance when corrected for multiple family-wise comparisons (migraine P = .04, low back pain P = .03). In contrast, whilst there was a trend towards an increase in GABA+ levels in people with whiplash-headache (mean ± SD 4.78 ± 0.43) compared to controls (4.63 ± 0.44 IU), it did not reach statistical significance (mean difference 0.16 ± 0.33, P = .07). The majority of participants had higher GABA+ compared to controls irrespective of pain group (73.5% of the migraine group, 70.5% of the whiplash-headache group and 78.9% of the back pain group).
Table 2.
Pair-wise Comparisons Between Pain Group and Control.
| N | Pain GABA+ IU (Means SD) | Control GABA+ IU (Means SD) | Mean differences ± SD | Zscore | P-value | |
|---|---|---|---|---|---|---|
|
| ||||||
| PCG | ||||||
| Migraine | n = 19 | 4.89 ± 0.62 | 4.62 ± 0.38 | 0.28 ± 0.63 | −2.29 | .02* |
| Whiplash | n = 17 | 4.78 ± 0.43 | 4.63 ± 0.44 | 0.16 ± 0.33 | −1.82 | .07 |
| Back Pain | n = 19 | 4.88 ± 0.44 | 4.6 ± 0.32 | 0.31 ± 0.47 | −2.67 | .01* |
| ACC | ||||||
| Migraine | n = 19 | 4.51 ± 0.38 | 4.59 ± 0.51 | −0.06 ± 0.64 | −0.28 | .77 |
| Whiplash | n = 17 | 4.47 ± 0.79 | 4.69 ± 0.99 | −0.22 ± 1.33 | −0.73 | .46 |
| Back Pain | n = 19 | 4.60 ± 0.47 | 4.47 ± 0.5 | 0.13 ± 0.73 | −0.85 | .40 |
| Thalamus Migraine |
n = 19 | 6.16 ± 1.23 | 5.75 ± 0.78 | 0.41 ± 1.05 | −1.49 | .14 |
| Whiplash | n = 17 | 6.08 ± 0.79 | 5.61 ± 0.91 | 0.38 ± 1.47 | −1.40 | .16 |
| Back Pain | n = 18 | 6.55 ± 1.78 | 5.53 ± 0.80 | 1.01 ± 1.81 | −2.11 | .04* |
denotes statistical significance (P−value <.05).
Figure 3.
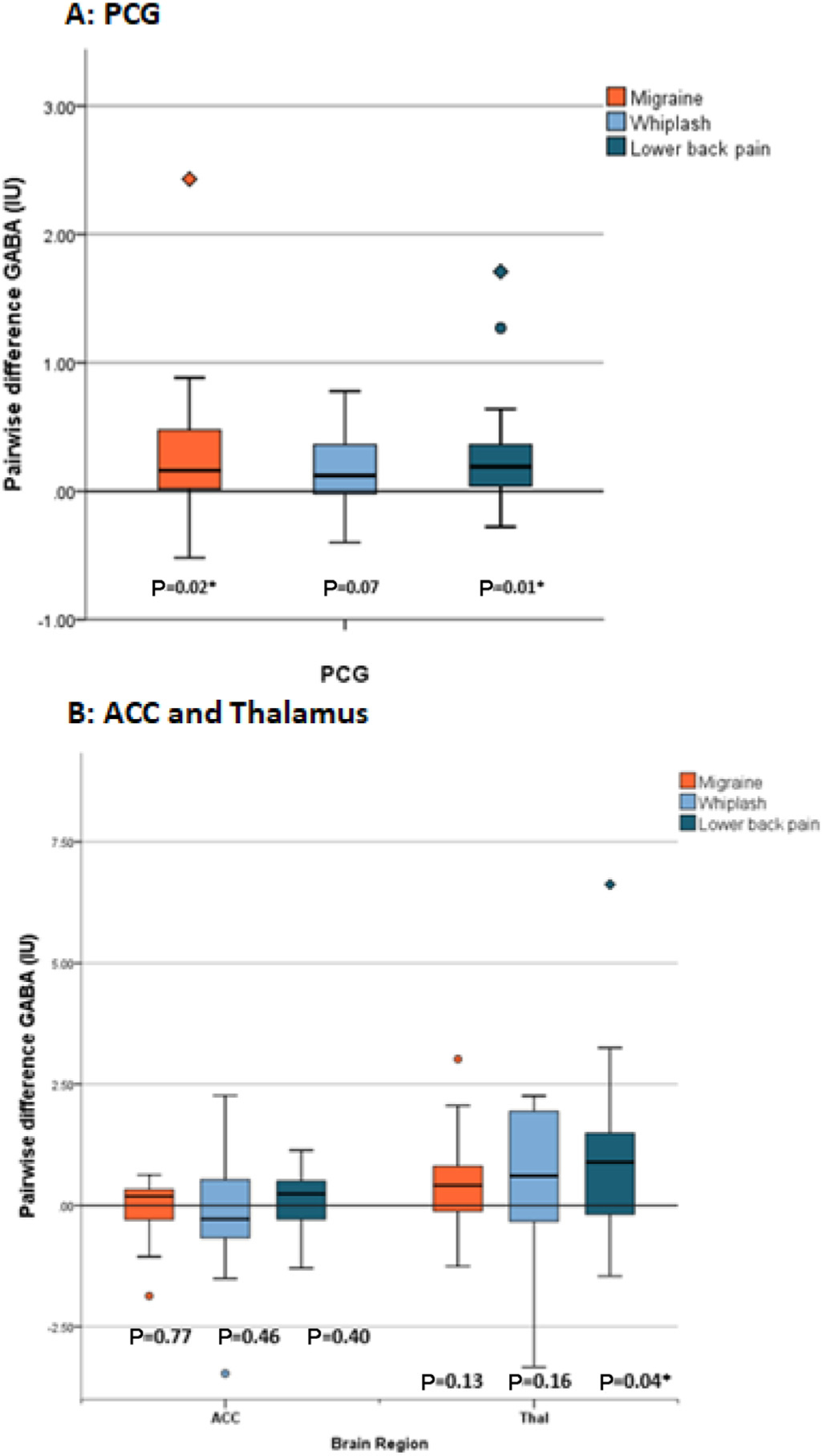
Pair-wise difference in GABA+ (IU) between pain groups and control. (A) Pairwise difference GABA+ (IU) in pain groups compared to control for the PCG. (B) Pairwise difference GABA+ (IU) in pain groups compared to control for the ACC and thalamus. Data are presented as box plots where the boxes represent the interquartile range (IQR), the line within the box demonstrate the median, and the outer whiskers represent the range of data. Outliers are plotted as circles where they reach >1.5 IQR below or above the 25th or 75th percentile and diamonds where they reach >3 IQR below or above the 25th and 75th percentile. P values with stars indicate the statistically significant difference in GABA+ levels between pain group and control.
Secondary Analysis
Comparison Between Individual Pain Groups and Controls in the Thalamus and ACC
In the thalamus, GABA + levels were significantly higher in people with back pain (mean ± SD 6.55 ± 1.78 IU) compared with controls (5.53 ± 0.80 IU; mean difference ± SD 1.01 ± 1.81 IU, P = .04). However, when corrected for multiple family-wise comparisons significance was lost (P = .12). There were no other statistically significant differences between people with migraine or whiplash-headache compared with controls. (Table 2, Figure 3B)
In the ACC, GABA+ levels were not significantly different in any of the pain groups compared to controls. (Table 2, Figure 3B).
Comparison Between Pain Groups
Both age and sex matching were possible in 14 pairs of migraine-whiplash participants, 16 pairs of whiplash-back pain participants and 16 pairs of back pain-migraine participants. There were no significant differences in GABA+ levels between any of the pain groups in any of the brain regions (Table 3).
Table 3.
Pair-wise Comparisons Comparing Difference in GABA+ Levels Between Age- and Sex- matched Individuals From Different Pain Groups.
| Region | Pain A Mean± SD | Pain B Mean± SD | Pairwise Difference ± SD | Zscore | P value | |
|---|---|---|---|---|---|---|
|
| ||||||
| Migraine (A) versus Whiplash (B) n = 14 | PCG | 4.83 ± 0.64 | 4.69 ± 0.40 | 0.14 ± 0.65 | −0.47 | .38 |
| ACC | 4.43 ± 0.36 | 4.62 ± 0.78 | −0.18 ± 0.78 | −0.72 | .38 | |
| Thal | 5.61 ± 1.02 | 6.27 ± 0.88 | −0.66 ± 1.43 | −1.48 | .38 | |
| Whiplash (A) versus Back Pain (B) n = 16 | PCG | 4.66 ± 0.42 | 4.91 ± 0.47 | −0.25 ± 0.54 | −1.60 | .38 |
| ACC | 4.70 ± 0.75 | 4.63 ± 0.50 | 0.07 ± 1.01 | −0.21 | .38 | |
| Thal | 6.31 ± 0.78 | 6.56 ± 1.93 | −0.24 ± 1.68 | −0.21 | .38 | |
| Back Pain (A) versus Migraine (B) n = 16 | PCG | 4.86 ± 0.67 | 4.89 ± 0.48 | −0.03 ± 0.41 | 0.01 | 1.00 |
| ACC | 4.5 ± 0.40 | 4.68 ± 0.44 | −0.17 ± 0.62 | −0.88 | .38 | |
| Thal | 5.69 ± 1.01 | 6.56 ± 1.92 | −0.87 ± 1.87 | −1.76 | .08 | |
Discussion
This study found that GABA+ in the PCG was significantly increased in people with migraine and low back pain compared to controls. Whilst not statistically significant, the whiplash-headache group also showed a trend for increased GABA+ compared to controls. In addition, we found that the increase in GABA+ observed in the PCG was not present in the other brain regions examined, except for the thalamus in the low back pain group.
Increased GABA+ levels observed across all pain groups in this study suggest that the mechanism may be reflective of a chronic pain condition rather than being unique to migraine. Although not statistically significant in the whiplash-headache group, GABA+ levels were found to be higher in 70% of these participants compared to their controls. The secondary analysis revealed no significant difference between the pain groups, suggesting each pain group displays similar GABA+ levels. Together, these findings propose that the increase in GABA+ is not unique to migraine and may be more reflective of a mechanism seen across chronic pain conditions.
We can speculate that the increased level of GABA+ observed in the pain groups could potentially be explained by 2 proposed mechanisms. Firstly, increased GABA+ could be reflective of an adaptive response developed over time in response to pain, as proposed by Bigal et al7 and supported by Bell et al.5 All the included participants were experiencing chronic pain of over 3 months in duration: migraine (median [IQR], 18 years, [10.5 to 25 years]), whiplash-headache (2.7 years [1.5 to 4 years]) and low back pain (5 years [2 to 12 years]). It is therefore possible that the central nervous system has affected the metabolism of GABA, the main inhibitory neurometabolite of the central nervous system, in response to the ongoing levels of pain experienced by the participant. Whilst our post-hoc analysis shows a negligible correlation between PCG GABA+ and pain duration (r = 0.05, P = .63) it is hypothesized that GABA+ levels may be different in a different cohort experiencing acute pain.
Secondly, increased GABA+ may be reflective of a homeostatic mechanism perhaps to counteract increases in excitability that has been observed in some pain conditions.21,27,38,61,89 Whilst the results from this cross-sectional study cannot determine cause or effect, they do challenge hypotheses from previous work that theorized that GABA+ levels could be associated with migraine specific processes such as cortical spreading depression, cortical vasodilation43 or sensitization of the trigeminovascular system.2,43 Therefore, a mechanism of chronic pain in general is more likely to underpin the higher GABA+ levels seen in this study.
Our findings suggest GABA+ is associated with chronic pain, however, these results differ somewhat with the conclusions of our recent meta-analysis.62 To further elucidate proposed mechanisms, we need to review what is already known about neurometabolite behavior in each pain group. Firstly, the higher GABA+ levels observed in the migraine group compared with controls in this study were hypothesized a priori and are consistent with previous data. Previous data demonstrating this same increase in GABA/GABA+ in migraine includes pooled data from our meta-analysis, a recent high-quality study in a pediatric cohort5 and our previous cohort study.2 Conversely, decreased GABA+ has been reported in a single study of people with migraine9 and related to headache severity7 in another. It could be argued the differences might reflect a different subgroups of participants with a different nature of symptoms, or alternatively it may reflect that neither Bridge et al,9 nor Bigal et al7 used a sequence fully optimized for the detection of GABA+. The increase in GABA+ seen in this study and corroborated by other studies that were optimized to detect GABA/GABA+ gives us confidence that this may reflect a true biological finding.
In contrast to migraine and low back pain (LBP), there are no studies that have examined GABA+ levels in a whiplash-headache group, meaning our data cannot be interpreted with known data. We consciously included a musculoskeletal group who also experienced headaches (whiplash-headache) to explore whether the increased GABA+ levels observed in the migraine group were unique to migraine or also present in other headache types. We are confident that the whiplash-headache group are representative of the group in general, as the baseline profile includes higher levels of psychological distress and central sensitization symptoms, typically seen in people with whiplash.76,77 Whilst the differences in GABA+ levels between the whiplash-headache group and controls did not reach statistical significance, the group appears to be clinically similar to the migraine and LBP groups. Firstly, the increased GABA+ concentration (Mean ± SD 4.78 ± 0.43 IU) in the headache-whiplash group and secondly, that similar percentage of people in all groups demonstrating increased GABA+ compared with controls (70.5% of the whiplash-headache group versus 73.5% and 78.9% in the migraine and back pain group respectively). If we accept that the whiplash-headache group appears to behave similarly to the other pain groups, it would strengthen the conclusion that GABA+ is reflective of a pain mechanism rather than a headache mechanism.
In considering our findings in relation to the reports of GABA+ in chronic pain, there is some variation. Our meta-analysis included all studies of GABA+ in chronic pain at the time of print,62 results from the included studies showed mixed findings, with 4 of 21 results demonstrating a decrease in GABA.23,33,35,39 However, these studies differed from ours in terms of type of pain conditions (non-musculoskeletal eg, neuropathic pain,39 spinal cord injury,32,33 pelvic pain35 and fibromyaglia23), study methodology (smaller sample sizes, low signal to noise as a result of small voxel sizes: 8 mL vs 25 mL) and brain region studied (thalamus32,39). Overall, we are confident our data was derived from high quality spectra, using parameters optimized for GABA+.
Our data demonstrated higher quality spectra in the PCG and the ACC compared to the thalamus. The PCG was our primary region of interest due to previous studies demonstrating change within this region, whilst the ACC and thalamus were selected for their known involvement in chronic pain.16,39,84 Since all spectra had an acceptable line width, the larger confidence interval demonstrated in the data from the thalamus, may reflect the region’s small volume and deep location making it less suitable for high signal-to-noise (SNR) data acquisition (Fig 3B). The difficulties associated with reliably scanning the thalamus using MEGA-PRESS at 3T are also seen in other brain regions proposed to be involved in chronic pain such as the insula and the amygdala3. Whilst these regions along with others such as the pre-frontal cortex may demonstrate GABA+ changes, the sequence would require specific optimization to ensure adequate reliability. One method of improving reliability in these regions is to increase the number of averages acquired in these regions.50 However, the resultant increase in scan time increases the chance of movement in regions that are already susceptible to motion artifact and was not feasible in a chronic pain cohort.
The nature of MR spectroscopy presents some further limitations namely the manual localization of the region of interest, the comparably large voxel size required for MEGA-PRESS acquisitions, and contamination of the modelled signal with co-edited macromolecules. Firstly, to address the limitation of manual localization, senior technologists who are experts in both anatomy and MRS positioned the voxel following a strict protocol, guided by anatomical landmarks. Secondly, the reasonably largest voxel size maintaining regional specificity was chosen to enhance SNR. Finally, the decision to not use a macromolecule-suppressed sequence, was made in light of their vulnerability to frequency drift, reflected in better reliability of the observed signal in non-macromolecule suppressed studies.19,49,54
The presence of increased GABA+ in pain conditions provides an insight into a potential underlying mechanism of chronic pain; ongoing research is now required to elucidate the mechanism. Firstly, we need to understand how GABA+ correlates with clinical measures of pain sensitivity associated with chronic pain conditions such as pressure pain thresholds, and other quantitative measures of pain such as heat/cold pain thresholds. This will allow better understanding of the complexity of chronic pain and identify which domains are associated with neurometabolite changes, and further explore whether aberrant neurochemistry is present in everyone experiencing chronic pain condition. Secondly, longitudinal studies are required to investigate the cause or effect relationship between pain and higher GABA+ levels to determine whether increased GABA+ is a cause of chronic pain or an adaptive response to living with chronic pain. Finally, the addition of glutamate metabolism to future studies may allow to further pinpoint the underlying mechanisms. Answering these questions opens future avenues which can explore the matching of treatments to neurometabolite imbalances in chronic pain in an attempt to improve patient outcomes.
Conclusion
In conclusion, the results from this study suggest altered metabolism of GABA+ in migraine, and low back pain. GABA+ is more likely an underlying mechanism of chronic pain in general rather than a potential underlying neurometabolite marker of migraine. Studying the PCG using a method optimized for the detection of GABA+ provides a reliable method to quantify GABA+ levels in chronic pain populations.
Supplementary Material
Acknowledgements
We would like to acknowledge the research assistants working on the project Mi Hoang Amanda Dinh and Alexis Curtis, and the team at Westmead Hospital Radiology department, Australia. In addition, Edward J. Auerbach, Ph.D. and Malgorzata Marjanska, Ph.D. (Center for Magnetic Resonance Research and Department of Radiology, University of Minnesota, USA) for the development of the pulse sequences for the Siemens platform which were provided by the University of Minnesota under a C2P agreement.
Disclosures:
This research was funded by the National Health and Medical Research Council (NHMRC) Centre of Research Excellence in Recovery following Road Traffic Injuries (CRERTI) APP1079022, NHMRC (Career Development Fellowship) APP1161467 and The University of Sydney Research Accelerator (SOAR) Fellowship.
Footnotes
The authors report no conflict of interests.
Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jpain.2021.06.005.
References
- 1.Aguila M-ER, Rebbeck T, Leaver AM, Lagopoulos J, Brennan PC, Hübscher M, Refshauge KM: The association between clinical characteristics of migraine and brain GABA levels: An exploratory study. J Pain 17:1058–1067, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Aguila ME, Lagopoulos J, Leaver AM, Rebbeck T, Hubscher M, Brennan PC, Refshauge KM: Elevated levels of GABA+ in migraine detected using (1) H-MRS. NMR Biomed 28:890–897, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Ahmad AH, Abdul Aziz CB: The brain in pain. Malays J Med Sci 21:46–54, 2014 [PMC free article] [PubMed] [Google Scholar]
- 4.Baron M, Schieir O, Hudson M, Steele R, Kolahi S, Berk-son L, Couture F, Fitzcharles MA, Gagne M, Garfield B, Gut-kowski A, Kang H, Kapusta M, Ligier S, Mathieu JP, Menard H, Starr M, Stein M, Zummer M: The clinimetric properties of the world health organization disability assessment schedule II in early inflammatory arthritis. Arthritis Rheum 59:382–390, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Bell T, Stokoe M, Khaira A, Webb M, Noel M, Amoozegar F, Harris AD: Gaba and glutamate changes in pediatric migraine. PAIN 162, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ: Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am J Psychiatry 161:368–370, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bigal ME, Hetherington H, Pan J, Tsang A, Grosberg B, Avdievich N, Friedman B, Lipton RB: Occipital levels of GABA are related to severe headaches in migraine. Neurology 70:2078–2080, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA: Psychometric properties of the PTSD Checklist (PCL). Behav ResTher 34:669–673, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Bridge H, Stagg CJ, Near J, Lau CI, Zisner A, Cader MZ: Altered neurochemical coupling in the occipital cortex in migraine with visual aura. Cephalalgia 35:1025–1030, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Cai K, Nanga RP, Lamprou L, Schinstine C, Elliott M, Hariharan H, Reddy R, Epperson CN: The impact of gaba-pentin administration on brain GABA and glutamate concentrations: a 7T 1H-MRS study. Neuropsychopharmacology 37:2764–2771, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castien RF, Blankenstein AH, Windt DA, Dekker J: Minimal clinically important change on the headache impact test-6 questionnaire in patients with chronic tension-type headache. Cephalalgia 32:710–714, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Center for Magnetic Resonance Research UoM: Application Guide and Release Notes. 2017
- 13.Chaibi A, Tuchin PJ, Russell MB: Manual therapies for migraine: a systematic review. J Headache Pain 12:127–133, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chwastiak LA, Von Korff M: Disability in depression and back pain: evaluation of the world health organization disability assessment schedule (WHO DAS II) in a primary care setting. J Clin Epidemiol 56:507–514, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Deelchand DK, Marjanska M, Henry PG, Terpstra M: MEGA-PRESS of GABA+: Influences of acquisition parameters. NMR in Biomedicine 34:e4199, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Pietro F, Macey PM, Rae CD, Alshelh Z, Macefield VG, Vickers ER, Henderson LA: The relationship between thalamic GABA content and resting cortical rhythm in neuropathic pain. Hum Brain Mapp 39:1945–1956, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diamond M, Cady R: Initiating and optimizing acute therapy for migraine: the role of patient-centered stratified care. Am J Med 118:18–27, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ: Gan- net: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging 40:1445–1452, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edden RAE, Oeltzschner G, Harris AD, Puts NAJ, Chan KL, Boer VO, Schar M, Barker PB: Prospective frequency correction for macromolecule-suppressed GABA editing at 3T. J Magn Reson Imaging 44:1474–1482, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enna SJ, McCarson KE: The Role of GABA in the mediation and perception of pain. Adv Pharmacol 54:1–27, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Fayed N, Andres E, Viguera L, Modrego PJ, Garcia-Cam- payo J: Higher glutamate+glutamine and reduction of N- acetylaspartate in posterior cingulate according to age range in patients with cognitive impairment and/or pain. Acad Radiol 21:1211–1217, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP: Validity of four pain intensity rating scales. Pain 152:2399–2404, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Foerster BR, Petrou M, Edden RA, Sundgren PC, Schmidt-Wilcke T, Lowe SE, Harte SE, Clauw DJ, Harris RE: Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum 64:579–583, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friston KJ: Statistical Parametric Mapping the Analysis of Functional Brain Images. Amsterdam; Boston., Elsevier/Academic Press, 2007 [Google Scholar]
- 25.Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, Wang X, Barker PB: Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 78:75–82, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA: Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med 55:1219–1226, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Gonzales de la Aleja JG, Ramos A, Mato-Abad V, Marti- nez-Salio A, Hernandez-Tamames JA, Molina JA, Hernan- dez-Gallego J, Alvarez-Linera J: Higher glutamate to glutamine ratios in occipital regions in women with migraine during the interictal state. Headache 53:365–375, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Grachev I, Fredrickson B, Apkarian A: Brain chemistry reflects dual states of pain and anxiety in chronic low back pain. J Neural Transm 109:1309–1334, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Grachev ID, Fredrickson BE, Apkarian AV: Abnormal brain chemistry in chronic back pain: An in vivo proton magnetic resonance spectroscopy study. Pain 89:7–18, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Grewal M, Dabas A, Saharan S, Barker PB, Edden RA, Mandal PK: GABA quantitation using MEGA-PRESS: Regional and hemispheric differences. J Magn Reson Imaging 44:1619–1623, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gussew A, Borys C, Janetzki L, Cleve M, Malessa R, Habenicht U, Straus B, Reichenbach J: Altered regional and interregional interrelations of glutamate and GABA in patients with chronic low back pain-A 1H-MR spectroscopic study. Clin Neurophysiol 126:e109–e110, 2015 [Google Scholar]
- 32.Gustin S, Wrigley P, Youssef A, McIndoe L: Thalamic activity and biochemical changes in individuals with neuropathic pain after spinal cord injury. Pain, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gustin SM, Wrigley PJ, Youssef AM, McIndoe L, Wilcox SL, Rae CD, Edden RAE, Siddall PJ, Henderson LA: Thalamic activity and biochemical changes in individuals with neuropathic pain after spinal cord injury. Pain 155:1027–1036, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hancock MJ, Hill JC: Are small effects for back pain interventions really surprising? J Orthop Sports Phys Ther 46:317–319, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Harper DE, Ichesco E, Schrepf A, Halvorson M, Puiu T, Clauw DJ, Harris RE, Harte SE: Relationships between brain metabolite levels, functional connectivity, and negative mood in urologic chronic pelvic pain syndrome patients compared to controls: A MAPP research network study. NeuroImage: Clinical 17:570–578, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris AD, Puts NA, Barker PB, Edden RA: Spectral-editing measurements of GABA in the human brain with and without macromolecule suppression. Magn Reson Med 74:1523–1529, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG: Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, Clauw DJ: Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis & Rheumatism 60:3146–3152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henderson LA, Peck CC, Petersen ET, Rae CD, Youssef AM, Reeves JM, Wilcox SL, Akhter R, Murray GM, Gustin SM: Chronic pain: Lost inhibition? J Neurosci 33:1754–1782, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikemoto T, Miki K, Matsubara T, Wakao N: Psychological treatment strategy for chronic low back pain. Spine Surg Relat Res 3:199–206, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.International Headache Society: Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia 38:1–211, 2018 [DOI] [PubMed] [Google Scholar]
- 42.Janetzki L, Gussew A, Malessa R, Habenicht U, Reichenbach JR, Strauss B, Borys C: Cerebral metabolic changes and chronic back pain: Study taking into consideration clinical and psychological parameters. Schmerz 30:134–140, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Kocharyan A, Fernandes P, Tong XK, Vaucher E, Hamel E: Specific subtypes of cortical GABA interneurons contribute to the neurovascular coupling response to basal forebrain stimulation. J Cereb Blood Flow Metab 28:221–231, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Laake P, Fagerland MW: Statistical Inference, in Laake P, Benestad HB, Olsen BR, (eds): Research in Medical and Biological Sciences, Second Edition, Amsterdam, Academic Press, 2015, pp 379–430 [Google Scholar]
- 45.Maes C, Hermans L, Pauwels L, Chalavi S, Leunissen I, Levin O, Cuypers K, Peeters R, Sunaert S, Mantini D, Puts NAJ, Edden RAE, Swinnen SP: Age-related differences in GABA levels are driven by bulk tissue changes. Hum Brain Mapp 39:3652–3662, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marenco S, Meyer C, van der Veen JW, Zhang Y, Kelly R, Shen J, Weinberger DR, Dickinson D, Berman KF: Role of gamma-amino-butyric acid in the dorsal anterior cingulate in age-associated changes in cognition. Neuropsychopharmacology 43:2285–2291, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer TG, Neblett R, Cohen H, Howard KJ, Choi YH, Williams MJ, Perez Y, Gatchel RJ: The development and psychometric validation of the central sensitization inventory. Pain Pract 12:276–285, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R: Simultaneous in vivo spectral editing and water suppression. NMR Biomed 11:266–272, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Mikkelsen M, Barker PB, Bhattacharyya PK, Brix MK, Buur PF, Cecil KM, Chan KL, Chen DY, Craven AR, Cuypers K, Dacko M, Duncan NW, Dydak U, Edmondson DA, Ende G, Ersland L, Gao F, Greenhouse I, Harris AD, He N, Heba S, Hoggard N, Hsu TW, Jansen JFA, Kangarlu A, Lange T, Lebel RM, Li Y, Lin CE, Liou JK, Lirng JF, Liu F, Ma R, Maes C, Moreno-Ortega M, Murray SO, Noah S, Noeske R, Noseworthy MD, Oeltzschner G, Prisciandaro JJ, Puts NAJ, Roberts TPL, Sack M, Sailasuta N, Saleh MG, Schallmo MP, Simard N, Swinnen SP, Tegenthoff M, Truong P, Wang G, Wilkinson ID, Wittsack HJ, Xu H, Yan F, Zhang C, Zipunnikov V, Zollner HJ, Edden RAE: Big GABA: Edited MR spectroscopy at 24 research sites. Neuroimage 159:32–45, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mikkelsen M, Loo RS, Puts NAJ, Edden RAE, Harris AD: Designing GABA-edited magnetic resonance spectroscopy studies: Considerations of scan duration, signal-to-noise ratio and sample size. J Neurosci Methods 303:86–94, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mikkelsen M, Rimbault DL, Barker PB, Bhattacharyya PK, Brix MK, Buur PF, Cecil KM, Chan KL, Chen DY, Craven AR, Cuypers K, Dacko M, Duncan NW, Dydak U, Edmondson DA, Ende G, Ersland L, Forbes MA, Gao F, Greenhouse I, Harris AD, He N, Heba S, Hoggard N, Hsu TW, Jansen JFA, Kangarlu A, Lange T, Lebel RM, Li Y, Lin CE, Liou JK, Lirng JF, Liu F, Long JR, Ma R, Maes C, Moreno-Ortega M, Murray SO, Noah S, Noeske R, Noseworthy MD, Oeltzschner G, Porges EC, Prisciandaro JJ, Puts NAJ, Roberts TPL, Sack M, Sailasuta N, Saleh MG, Schallmo MP, Simard N, Stoffers D, Swinnen SP, Tegenthoff M, Truong P, Wang G, Wilkinson ID, Wittsack HJ, Woods AJ, Xu H, Yan F, Zhang C, Zipunnikov V, Zollner HJ, Edden RAE: Big GABA II: Water-referenced edited MR spectroscopy at 25 research sites. Neuroimage 191:537–548, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mikkelsen M, Tapper S, Near J, Mostofsky SH, Puts NAJ, Edden RAE: Correcting frequency and phase offsets in MRS data using robust spectral registration. NMR in Biomedicine e4368, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore CM, Wardrop M, de BFB, Renshaw PF: Topiramate raises anterior cingulate cortex glutamine levels in healthy men; a 4.0 T magnetic resonance spectroscopy study. Psychopharmacol 188:236–243, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Mullins PG, McGonigle DJ, O’Gorman RL, Puts NA, Vidyasagar R, Evans CJ, Cardiff Symposium on MRSoG, Edden RA: Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage 86:43–52, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P: Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med 73:44–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neblett R, Cohen H, Choi Y, Hartzell MM, Williams M, Mayer TG, Gatchel RJ: The Central Sensitization Inventory (CSI): establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J Pain 14:438–445, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Gorman RL, Michels L, Edden RA, Murdoch JB, Martin E: In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. J Magn Reson Imaging 33:1262–1267, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oeltzschner G, Zollner HJ, Jonuscheit M, Lanzman RS, Schnitzler A, Wittsack HJ: J-difference-edited MRS measures of gamma-aminobutyric acid before and after acute caffeine administration. Magn Reson Med 80:2356–2365, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osman A, Wong JL, Bagge CL, Freedenthal S, Gutierrez PM, Lozano G: The depression anxiety stress scales—21 (DASS-21): Further examination of dimensions, scale reliability, and correlates. J Clin Psychol 68:1322–1338, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Palmer AM, Marion DW, Botscheller ML, Bowen DM, DeKosky ST: Increased transmitter amino acid concentration in human ventricular CSF after brain trauma. Neuroreport 6:153–156, 1994 [DOI] [PubMed] [Google Scholar]
- 61.Pearl PL, Hartka TR, Cabalza JL, Taylor J, Gibson MK: Inherited disorders of GABA metabolism. Future Neurol 1:631–636, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peek AL, Rebbeck T, Puts NA, Watson J, Aguila ME, Leaver AM: Brain GABA and glutamate levels across pain conditions: A systematic literature review and meta-analysis of 1H-MRS studies using the MRS-Q quality assessment tool. Neuroimage 116532, 2020. [DOI] [PubMed] [Google Scholar]
- 63.Porges EC, Woods AJ, Edden RA, Puts NA, Harris AD, Chen H, Garcia AM, Seider TR, Lamb DG, Williamson JB, Cohen RA: Frontal gamma-aminobutyric acid concentrations are associated with cognitive performance in older adults. Biol Psychiatry 2:38–44, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Posl M, Cieza A, Stucki G: Psychometric properties of the WHODASII in rehabilitation patients. Qual Life Res 16:1521–1531, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Puts NA, Edden RA: In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog Nucl Magn Reson Spectrosc 60:29–41, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Puts NAJ, Heba S, Harris AD, Evans CJ, McGonigle DJ, Tegenthoff M, Schmidt-Wilcke T, Edden RAE: GABA levels in left and right sensorimotor cortex correlate across individuals. Biomedicines 6:24, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reckziegel D, Raschke F, Cottam WJ, Auer DP: Cingulate GABA levels inversely correlate with the intensity of ongoing chronic knee osteoarthritis pain. Mol Pain 12:1–9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ritchie C, Hendrikz J, Kenardy J, Sterling M: Derivation of a clinical prediction rule to identify both chronic moder- ate/severe disability and full recovery following whiplash injury. Pain® 154:2198–2206, 2013 [DOI] [PubMed] [Google Scholar]
- 69.Saleh MG, Near J, Alhamud A, Van Der Kouwe AJW, Meintjes EM: Effects of tissue and gender on macromolecule suppressed gamma-aminobutyric acid. Int J Imaging SystTechnol 27:144–152, 2017 [Google Scholar]
- 70.Salkind N: Encyclopedia of Research Design. Thousand Oaks, California, sAgE Publications, 2010 [Google Scholar]
- 71.Shearer HM, Carroll LJ, Wong JJ, Côté P, Varatharajan S, Southerst D, Sutton DA, Randhawa KA, Yu H, Mior SA, van der Velde GM, Nordin MC, Stupar M, Taylor-Vaisey AL: Are psychological interventions effective for the management of neck pain and whiplash-associated disorders? A systematic review by the Ontario protocol for traffic injury management (OpTIMa) collaboration. Spine J 16:1566–1581, 2016 [DOI] [PubMed] [Google Scholar]
- 72.Shungu DC, Mao X, Gonzales R, Soones TN, Dyke JP, van der Veen JW, Kegeles LS: Brain gamma-aminobutyric acid (GABA) detection in vivo with the J-editing (1) H Mrs technique: a comprehensive methodological evaluation of sensitivity enhancement, macromolecule contamination and test-retest reliability. NMR in Biomedicine 29:932–942, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simmonite M, Carp J, Foerster BR, Ossher L, Petrou M, Weissman DH, Polk TA: Age-related declines in occipital GABA are associated with reduced fluid processing ability. Acad Radiol 26:1053–1061, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sowden G, Hill JC, Morso L, Louw Q, Foster NE: Advancing practice for back pain through stratified care (STarT Back). BrazJ PhysTher 22:255–264, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, Coghill RC: Roles of the insular cortex in the modulation of pain: insights from brain lesions. J Neurosci 29:2684–2694, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sterling M, Jull G, Vicenzino B, Kenardy J, Darnell R: Physical and psychological factors predict outcome following whiplash injury. Pain 114:141–148, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Sterling M, Kenardy J, Jull G, Vicenzino B: The development of psychological changes following whiplash injury. Pain 106:481–489, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Sullivan A, Cousins S, Ridsdale L: Psychological interventions for migraine: a systematic review. J Neurol 263:23692377, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O: Structure and function of the human insula. J Clin Neurophysiol 34:300–306, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van der Veen JW, Shen J: Regional difference in GABA levels between medial prefrontal and occipital cortices. J Magn Reson Imaging 38:745–750, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Middelkoop M, Rubinstein SM, Kuijpers T, Verha- gen AP, Ostelo R, Koes BW, van Tulder MW: A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non-specific low back pain. Eur Spine J 20:19–39, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, Charlson F, Davis A, Degenhardt L, Dicker D, Duan L, Erskine H, Feigin VL, Ferrari AJ, Fitzmaurice C, Fleming T, Graetz N, Guinovart C, Haagsma J, Hansen GM, Hanson SW, Heuton KR, Higashi H, Kassebaum N, Kyu H, Laurie E, Liang X, Lofgren K, Lozano R, MacIntyre MF, Moradi-Lakeh M, Naghavi M, Nguyen G, Odell S, Ortblad K, Roberts DA, Roth GA, Sandar L, Serina PT, Stanaway JD, Steiner C, Thomas B, Vollset SE, Whiteford H, Wolock TM, Ye P, Zhou M, Avila MA, Aasvang GM, Abbafati C, Ozgo- ren AA, Abd-Allah F, Aziz MIA, Abera SF, Aboyans V, Abraham JP, Abraham B, Abubakar I, Abu-Raddad LJ, Abu- Rmeileh NME, Aburto TC, Achoki T, Ackerman IN, Adele- kan A, Ademi Z, Adou AK, Adsuar JC, Arnlov J, Agardh EE, Al Khabouri MJ, Alam SS, Alasfoor D, Albittar MI, Alegretti MA, Aleman AV, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Alla F, Allebeck P, Allen PJ, AlMazroa MA, Alsharif U, Alvarez E, Alvis-Guzman N, Ameli O, Amini H, Ammar W, Anderson BO, Anderson HR, Antonio CAT, Anwari P, Apfel H, Arsenijevic VSA, Artaman A, Asghar RJ, Assadi R, Atkins LS, Atkinson C, Badawi A, Bahit MC, Bakfalouni T, Balakrishnan K, Balalla S, Banerjee A, Barker-Collo SL, Barquera S, Barregard L, Barrero lH, Basu S, Basu A, Baxter A, Beardsley J, Bedi N, Beghi E, Bekele T, Bell ML, Benjet C, Bennett DA, Bensenor IM, Benzian H, Bernabe E, Beyene TJ, Bhala N, Bhalla A, Bhutta Z, Bienhoff K, Bikbov B, Abdulhak AB, Blore JD, Blyth FM, Bohensky MA, Basara BB, Borges G, Bornstein NM, Bose D, Boufous S, Bourne RR, Boyers LN, Brainin M, Brauer M, Brayne CEG, Brazinova A, Breitborde NJK, Brenner H, Briggs ADM, Brooks PM, Brown J, Brugha TS, Buchbinder R, Buckle GC, Bukhman G, Bulloch AG, Burch M, Burnett R, Cardenas R, Cabral NL, Nonato IRC, Campuzano JC, Carapetis JR, Carpenter DO, Caso V, Castaneda-Orjuela CA, Catala-Lopez F, Chadha VK, Chang J-C, Chen H, Chen W, Chiang PP, Chimed-Ochir O, Chowdhury R, Christensen H, Christophi CA, Chugh SS, Cirillo M, Coggeshall M, Cohen A, Colistro V, Colquhoun SM, Contreras AG, Cooper LT, Cooper C, Cooperrider K, Coresh J, Cortinovis M, Criqui MH, Crump JA, Cuevas-Nasu L, Dandona R, Dandona L, Dansereau E, Dantes HG, Dargan PI, Davey G, Davitoiu DV, Dayama A, De la Cruz-Gongora V, de la Vega SF, De Leo D, del Pozo- Cruz B, Dellavalle RP, Deribe K, Derrett S, Des Jarlais DC, Dessalegn M, deVeber GA, Dharmaratne SD, Diaz-Torne C, Ding EL, Dokova K, Dorsey ER, Driscoll TR, Duber H, Durrani AM, Edmond KM, Ellenbogen RG, Endres M, Ermakov SP, Eshrati B, Esteghamati A, Estep K, Fahimi S, Farzadfar F, Fay DFJ, Felson DT, Fereshtehnejad S-M, Fernandes JG, Ferri CP, Flaxman A, Foigt N, Foreman KJ, Fowkes FGR, Franklin RC, Furst T, Futran ND, Gabbe BJ, Gankpe FG, Garcia-Guerra FA, Geleijnse JM, Gessner BD, Gibney KB, Gillum RF, Ginawi IA, Giroud M, Giussani G, Goenka S, Goginashvili K, Gona P, de Cosio TG, Gosselin RA, Gotay CC, Goto A, Gouda HN, Guerrant Rl, Gugnani HC, Gunnell D, Gupta R, Gupta R, Gutierrez RA, Hafezi- Nejad N, Hagan H, Halasa Y, Hamadeh RR, Hamavid H, Hammami M, Hankey GJ, Hao Y, Harb HL, Haro JM, Hav- moeller R, Hay RJ, Hay S, Hedayati MT, Pi IBH, Heydar- pour P, Hijar M, Hoek HW, Hoffman HJ, Hornberger JC, Hosgood Hd, Hossain M, Hotez PJ, Hoy DG, Hsairi M, Hu H, Hu G, Huang JJ, Huang C, Huiart L, Husseini A, Iannar- one M, Iburg KM, Innos K, Inoue M, Jacobsen KH, Jassal SK, Jeemon P, Jensen PN, Jha V, Jiang G, Jiang Y, Jonas JB, Joseph J, Juel K, Kan H, Karch A, Karimkhani C, Kar- thikeyan G, Katz R, Kaul A, Kawakami N, Kazi DS, Kemp AH, Kengne AP, Khader YS, Khalifa SEAH, Khan eA, Khan G, Khang Y-H, Khonelidze I, Kieling C, Kim D, Kim S, Kimokoti RW, Kinfu Y, Kinge JM, Kissela BM, Kivipelto M, Knibbs L, Knudsen AK, Kokubo Y, Kosen S, Kramer A, Kravchenko M, Krishnamurthi RV, Krishnaswami S, Defo BK, Bicer BK, Kuipers EJ, Kulkarni VS, Kumar K, Kumar GA, Kwan GF, Lai T, Lalloo R, Lam H, Lan Q, Lansingh VC, Larson H, Larsson A, Lawrynowicz AEB, Leasher JL, Lee JT, Leigh J, Leung R, Levi M, Li B, Li Y, Li Y, liang J, Lim S, Lin H-H, Lind M, Lindsay MP, Lipshultz SE, Liu S, Lloyd BK, Ohno SL, Logroscino G, Looker KJ, Lopez AD, Lopez- Olmedo N, Lortet-Tieulent J, Lotufo PA, Low N, Lucas RM, Lunevicius R, Lyons RA, Ma J, Ma S, Mackay MT, Maj- dan M, Malekzadeh R, Mapoma CC, Marcenes W, March LM, Margono C, Marks GB, Marzan MB, Masci JR, Mason- Jones AJ, Matzopoulos RG, Mayosi BM, Mazorodze TT, McGill NW, McGrath JJ, McKee M, McLain A, McMahon BJ, Meaney PA, Mehndiratta MM, Mejia-Rodriguez F, Mekonnen W, Melaku YA, Meltzer M, Memish ZA, Mensah G, Meretoja A, Mhimbira FA, Micha R, Miller TR, Mills EJ, Mitchell PB, Mock CN, Moffitt TE, Ibrahim NM, Mohammad KA, Mokdad AH, Mola GL, Monasta L, Montico M, Montine TJ, Moore AR, Moran AE, Morawska L, Mori R, Moschandreas J, Moturi WN, Moyer M, Mozaffarian D, Mueller UO, Mukaigawara M, Murdoch ME, Murray J, Murthy KS, Naghavi P, Nahas Z, Naheed A, Naidoo KS, Naldi L, Nand D, Nangia V, Narayan KMV, Nash D, Nejjari C, Neu- pane SP, Newman LM, Newton CR, Ng M, Ngalesoni FN, Nhung NT, Nisar MI, Nolte S, Norheim OF, Norman RE, Norrving B, Nyakarahuka L, Oh IH, Ohkubo T, Omer SB, Opio JN, Ortiz A, Pandian JD, Panelo CIA, Papachristou C, Park E-K, Parry CD, Caicedo AJP, Patten SB, Paul VK, Pavlin BI, Pearce N, Pedraza LS, Pellegrini CA, Pereira DM, Perez- Ruiz FP, Perico N, Pervaiz A, Pesudovs K, Peterson CB, Petzold M, Phillips MR, Phillips D, Phillips B, Piel FB, Plass D, Poenaru D, Polanczyk GV, Polinder S, Pope CA, Popova S, Poulton RG, Pourmalek F, Prabhakaran D, Prasad nM, Qato D, Quistberg DA, Rafay A, Rahimi K, Rahimi-Movaghar V, Rahman Su, Raju M, Rakovac I, Rana SM, Razavi H, Refaat A, Rehm J, Remuzzi G, Resnikoff S, Ribeiro AL, Riccio PM, Richardson L, Richardus JH, Riederer AM, Robinson M, Roca A, Rodriguez A, Rojas-Rueda D, Ronfani L, Rothenbacher D, Roy N, Ruhago GM, Sabin N, Sacco RL, Ksoreide K, Saha S, Sahathevan R, Sahraian MA, Sampson U, Sanabria JR, San- chez-Riera L, Santos IS, Satpathy M, Saunders JE, Sawhney M, Saylan MI, Scarborough P, Schoettker B, Schneider IJC, Schwebel DC, Scott JG, Seedat S, Sepanlou SG, Serdar B, Ser- van-Mori EE, Shackelford K, Shaheen A, Shahraz S, Levy TS, Shangguan S, She J, Sheikhbahaei S, Shepard DS, Shi P, Shi- buya K, Shinohara Y, Shiri R, Shishani K, Shiue I, Shrime MG, Sigfusdottir ID, Silberberg DH, Simard EP, Sindi S, Singh JA, Singh L, Skirbekk V, Sliwa K, Soljak M, Soneji S, Soshni- kov SS, Speyer P, Sposato LA, Sreeramareddy CT, Stoeckl H, Stathopoulou VK, Steckling N, Stein MB, Stein DJ, Steiner TJ, Stewart A, Stork E, Stovner LJ, Stroumpoulis K, Sturua L, Sunguya BF, Swaroop M, Sykes BL, Tabb KM, Takahashi K, Tan F, Tandon N, Tanne D, Tanner M, Tavakkoli M, Taylor HR, Te Ao BJ, Temesgen AM, Have MT, Tenkorang EY, Ter- kawi AS, Theadom AM, Thomas E, Thorne-Lyman AL, Thrift AG, Tleyjeh IM, Tonelli M, Topouzis F, Towbin JA, Toyosh- ima H, Traebert J, Tran BX, Trasande L, Trillini M, Truelsen T, Trujillo U, Tsilimbaris M, Tuzcu EM, Ukwaja KN, Undurraga EA, Uzun SB, van Brakel WH, van de Vijver S, Dinge- nen RV, van Gool CH, Varakin YY, Vasankari TJ, Vavilala MS, Veerman LJ, Velasquez-Melendez G, Venketasubrama- nian N, Vijayakumar L, Villalpando S, Violante FS, Vlassov VV, Waller S, Wallin MT, Wan X, Wang L, Wang J, Wang Y, Warouw TS, Weichenthal S, Weiderpass E, Weintraub RG, Werdecker A, Wessells KRR, Westerman R, Wilkinson JD, Williams HC, Williams TN, Woldeyohannes SM, Wolfe CDA, Wong JQ, Wong H, Woolf AD, Wright JL, Wurtz B, Xu G, Yang G, Yano Y, Yenesew MA, Yentur GK, Yip P, Yone- moto N, Yoon S-J, Younis M, Yu C, Kim KY, Zaki MES, Zhang Y, Zhao Z, Zhao Y, Zhu J, Zonies D, Zunt JR, Salomon JA, Murray CJL: Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386:743–800, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Waddell KW, Zanjanipour P, Pradhan S, Xu L, Welch EB, Joers JM, Martin PR, Avison MJ, Gore JC: Anterior cingulate and cerebellar GABA and Glu correlations measured by 1H J- difference spectroscopy. Magn Reson Imaging 29:19–24, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Widerstrom-Noga E, Pattany PM, Cruz-Almeida Y, Felix ER, Perez S, Cardenas DD, Martinez-Arizala A: Metabolite concentrations in the anterior cingulate cortex predict high neuropathic pain impact after spinal cord injury. Pain 154:204–212, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williamson A, Hoggart B: Pain: a review of three commonly used pain rating scales. J Clin Nurs 14:798–804, 2005 [DOI] [PubMed] [Google Scholar]
- 86.World Health Organization: ICD-10 : International Statistical Classification of Diseases and Related Health Problems: Tenth Revision. Geneva, World Health Organization, 2004 [Google Scholar]
- 87.Yang M, Rendas-Baum R, Varon SF, Kosinski M: Validation of the headache impact test (HIT-6TM) across episodic and chronic migraine. Cephalalgia 31:357–367, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zandifar A, Banihashemi M, Haghdoost F, Masjedi SS, Manouchehri N, Asgari F, Najafi MR, Ghorbani A, Zandifar S, Saadatnia M, White MK: Reliability and validity of the persian hit-6 questionnaire in migraine and tension-type headache. Pain Practice 14:625–631, 2014 [DOI] [PubMed] [Google Scholar]
- 89.Zielman R, Wijnen JP, Webb A, Onderwater GLJ, Ronen I, Ferrari MD, Kan HE, Terwindt GM, Kruit MC: Cortical glutamate in migraine. Brain 140:1859–1871, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Gannet 3.1.3 code is available from: https://github.com/richardedden/Gannet3.1/releases/tag/v3.1.3. The data supporting the study’s findings are available from the corresponding author, upon reasonable request following approval from the University of Sydney.


