Abstract
“Getting a good night’s sleep” seems a panacea for improving mood and cognition. These subjective impressions are supported by countless studies exploring impacts of sleep (and sleep loss) on mental health, metabolism, and immune function. Similarly, being “out of phase” with local time, commonly experienced by shift workers of jetlagged air travelers, demonstrates that there are both neural and physiologic effects of internal circadian (daily) time being misaligned with external environmental time. This chapter reviews these areas contextualized using the model of allostasis and allostatic load that emphasizes the impact of this “wear and tear” on the brain and body.
Keywords: Sleep deprivation, Hippocampus, Allostasis, Allostatic load, Glycogen, Oxidative stress, Pro-inflammatory cytokines, Circadian disruption
INTRODUCTION
Anecdotally, there can be little doubt that sleep plays a role in maintaining a good mood and cognitive acuity. Sleep deprivation one night followed by “getting a good night’s sleep” on the next clearly impacts neurobehavioral function as well as promotes physiologic balance and resilience. These subjective impressions are supported by numerous laboratory studies of endocrine function and metabolism as well as from investigations of sleep deprivation effects on cognitive and neural function, including research on the brain that shows a variety of substantial changes resulting from sleep restriction, with reversal after recovery sleep. Similarly, being “out of phase” with local time, be it from a week of nightshift work following a week of dayshift work, or transmeridian air travel across multiple time zones, demonstrates that there are both neural and physiologic effects of internal circadian (daily) time being misaligned with external environmental time. This article reviews selected aspects of the current state of knowledge in these areas and then evaluates what is known using the model of allostasis and allostatic load that emphasizes the “wear and tear” on the brain and body from coping with stress.
ALLOSTASIS AND ALLOSTATIC OVERLOAD
The maintenance of homeostasis, defined as those aspects of physiology that must remain stable to keep us alive (eg, oxygen tension, body temperature, pH), is an active process requiring coordinated action of many different systems, including the autonomic nervous system and neuroendocrine and immune systems. This active process is called “allostasis” or “maintaining stability through change.”1–3 Allostatic mediators work as a nonlinear, sometimes reciprocating, network (Fig. 1), meaning that too much or too little of each mediator can perturb the entire network, leading to harmful consequences. Take for example the relationship between cytokines and the glucocorticoids. Pro-inflammatory cytokines stimulate the production of cortisol, which then suppresses inflammatory cytokine production.4,5 Similarly, increased activity of the sympathetic nervous system increases pro-inflammatory cytokine production, whereas parasympathetic activity has the opposite effect.6,7 This balance is particularly important, as during an infection, the pro-inflammatory response that is essential to mounting an immune defense is normally contained by cortisol and also by parasympathetic activity.4,6 Inadequate containment can lead to septic shock and death. Treatment with cortisol, or elevation of parasympathetic activity, is a pathway that can reduce the exaggerated inflammatory response.4 However, at the opposite extreme, too much cortisol can suppress pro- inflammatory responses, thus compromising immune defenses.4,8
Fig. 1.
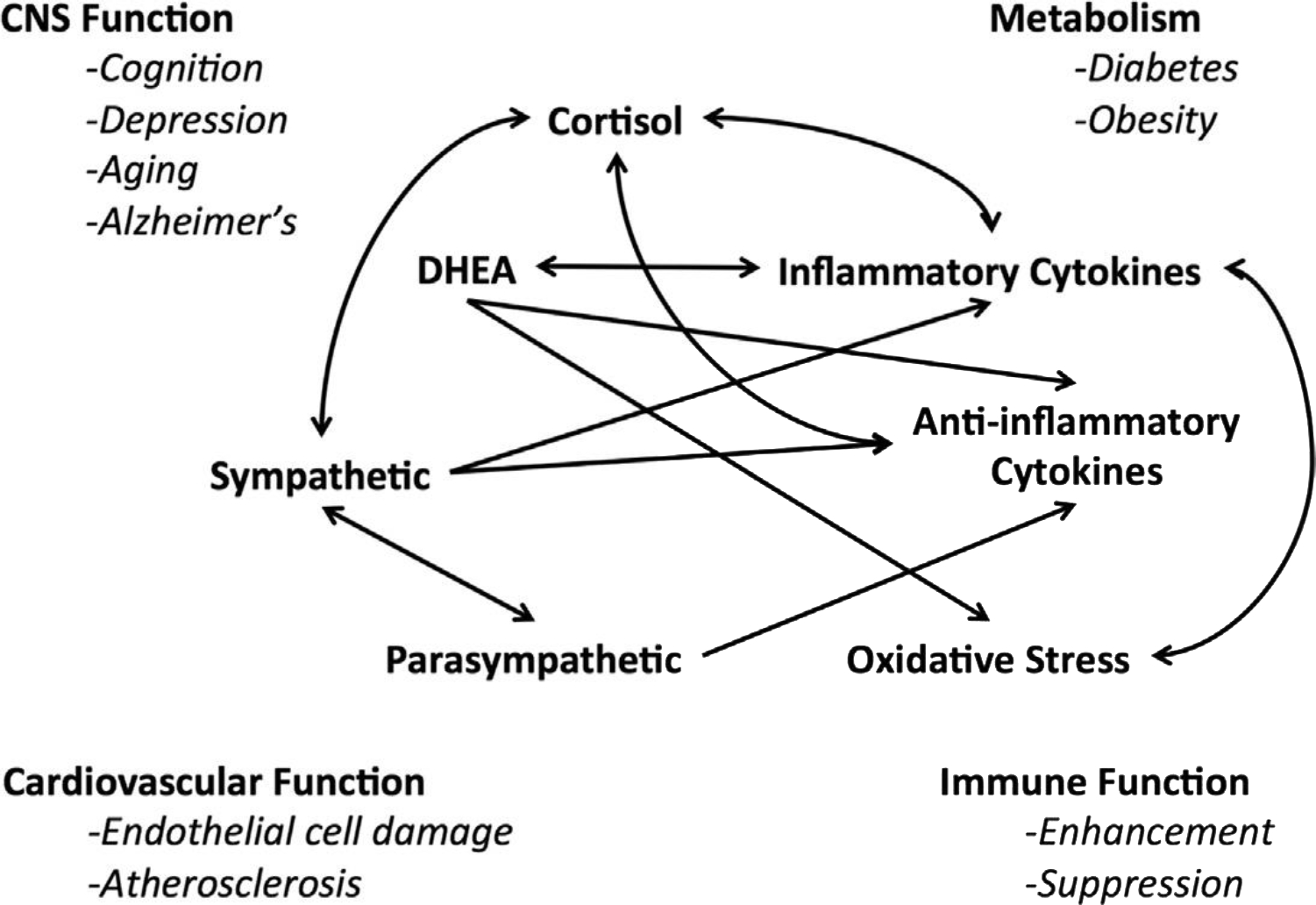
Nonlinear network of mediators of allostasis involved in the stress response. Arrows indicate that each system regulates the others in a reciprocal manner, creating a nonlinear network. Moreover, there are multiple pathways for regulation (eg, inflammatory cytokine production is negatively regulated via anti-inflammatory cytokines as well as via parasympathetic and glucocorticoid pathways), whereas sympathetic activity increases inflammatory cytokine production. Parasympathetic activity, in turn, contains sympathetic activity. CNS, central nervous system; DHEA, dehydroepiandrosterone. (Adapted from Karatsoreos IN, McEwen BS. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn Sci 2011;15:576–84; with permission.)
Allostatic overload, which is wear and tear produced by imbalances in the mediators of allostasis, is perfectly illustrated by these 2 examples: too much or too little activity of certain mediators of allostasis.9 Other examples of allostatic overload include conditions such as hypertension, atherosclerosis, diabetes, and the metabolic syndrome as well as stress-induced remodeling in brain regions that support memory, executive function, and anxiety.3,10 One of the key mediators of allostasis is Cortisol (corticosterone in rodent species), and conditions in which corticosteroid balance is affected lead to many such changes in physiologic function and brain structure. Cushing disease and anxiety/depressive disorders are 2 such conditions that affect multiple allostatic mediators, including the corticosteroids.
Cushing disease, broadly defined as hypercortisolemia induced by organic (eg, pituitary or adrenal tumor) or iatrogenic (eg, high doses of corticosteroids to reduce inflammation) factors, is accompanied by several cognitive and emotional symptoms, including depression. Intriguingly, depressive symptoms can be relieved by surgical correction of hypercortisolemia11. In major depressive illness as well as in Cushing disease, the duration of the illness and not the age of the subject predicts a progressive reduction in hippocampal volume and is observed by structural MRI.12 Moreover, there are a variety of other anxiety-related disorders, such as posttraumatic stress disorder (PTSD), in which atrophy of the hippocampus has been reported,13,14 suggesting that this is a common process reflecting chronic imbalance in the activity of adaptive systems. These chronic imbalances include the hypothalamic-pituitary-adrenal (HPA) axis, and also endogenous neurotransmitters, such as glutamate.12 Metabolic symptoms are also reported in both Cushing and major depression, as both are associated with chronic elevation of cortisol that results in gradual loss of minerals from bone, and increases in abdominal obesity.
CIRCADIAN DISRUPTION AND ALLOSTATIC LOAD AND OVERLOAD
When exploring how the brain and body are affected by stress, it is often overlooked that they may be directly regulated by time of day. All of the systems that are modulators of allostasis show rhythms of activity over the sleep-wake cycle. For instance, Cortisol (corticosterone in rodents; CORT) shows a clear circadian pattern, with the peak of CORT occurring just before waking in both nocturnal animals (such as rats and mice) and diurnal animals (such as humans). Circadian rhythms are observed in almost all physiologic measures, including endocrine and immune mediators.15–17 Many of these factors are also impacted by sleep deprivation, as is discussed later. The circadian system in mammals is centered in the suprachiasmatic nucleus (SCN), with both neural and hormonal projections throughout the brain and body, and impacting many of the systems involved in mediating allostasis; disruption of the circadian system can place the organism into a state of high allostatic load. Indeed, if circadian patterns of CORT are disrupted by adrenalectomy (ADX) and tonic replacement of CORT via a pellet implant providing no diurnal rhythm, this results in a “sluggish” HPA axis response, with poor shutoff of adrenocorticotropic hormone following termination of a stressor, in contrast to the situation in which the ADX animal drinks CORT in the water in a diurnal pattern.18 That CORT is also able to reset peripheral oscillators in other body tissues lends credence to an important relationship between disrupted rhythms and allostatic load; this is important because circadian disruption (CD; eg, shift work and jet lag) and sleep deprivation are not uncommon in the modern world and constitute an increasing health concern.19,20
The master circadian clock is located in the SCN of the hypothalamus and drives all rhythms in physiology and behavior.21–23 In addition to the master SCN clock, “peripheral” circadian clocks, in tissues throughout the body, serve to set local time. These peripheral clocks are synchronized to the SCN by a multitude of signals, including glucocorticoids, which are able to “reset” some peripheral clocks in the brain and body (eg, liver), but not others.24 In the brain, it has been shown that rhythms in glucocorticoids modulate clock protein expression in the oval nucleus of the bed nucleus of the stria terminalis as well as the central amygdala (CEA).25 It is also known that the basolateral nuclei of the amygdala and the dentate gyrus of the hippocampus express opposite diurnal rhythms of PERIOD2 (a core clock component) when compared with the CEA, and that the CEA rhythm is further influenced by ADX.26 The differential regulation of cellular rhythms in different brain regions is of particular interest when considering “healthy” regulation of HPA function requires rhythmic glucocorticoids, as discussed above.18 It is posited that if efficient regulation of the HPA axis is a hallmark of a “healthy” response, then disrupted circadian patterns (or, in this case, a lack of a pattern) can result in an unhealthy regulation of the HPA and thus could contribute to allostatic load.27 Thus, both disruption of the HPA axis and disruption of circadian rhythms could have interacting effects and contribute to shifts in resilience and vulnerability.
Both descriptive and epidemiologic studies show that individuals who suffer from repeated chronic circadian misalignment show negative physiologic, neural, and behavioral effects. In humans, a study of flight crews showed that those who endure more bouts of jet lag (transmeridian, short recovery crews) show shrunken medial temporal lobes, increased reaction time, and poorer performance in visual-spatial cognitive tasks compared with long recovery crews. In addition to the neurobehavioral effects, short- recovery individuals displayed a significant correlation between salivary cortisol levels and medial temporal cortex volume, whereas this effect was not observed in long recovery crews. Thus, rapid and repeated shifting of the circadian clock through jet lag is related to reductions in volume of the medial temporal lobe and shifts in behavioral performance on attentional tasks. Moreover, the brain effects are correlated with plasma cortisol levels. What is the significance of these neural and physiologic changes? It is intriguing to speculate that perhaps such shrunken temporal lobes may impart decreased resilience to negative outcomes of stress, as has been observed in PTSD.28,29 In addition, sleep deprivation seems to increase the amygdalar response to negative emotional stimuli because of an amygdala- prefrontal disconnect.30 Such effects could also exacerbate cardiovascular reactivity, which may contribute to pathophysiology.31–33 Sleep deprivation studies have investigated the effects of chronic deprivation on cognitive performance, in both animal and human subjects, but in many cases, the stressful effects of the methods used to induce sleep deprivation confound these studies. Thus, a different approach must be applied to help disentangle these effects.
To directly probe how disruption of the circadian clock affects neurobehavioral function, new models need to be applied, and new hypotheses need to be tested. Karatsoreos and coworkers34 characterized a mouse model of chronic environmental circadian desynchronization (eCD) that has provided several novel insights. This model induces disruption by housing mice in a light- dark cycle of 20 hours (10 hours light, 10 hours dark) compared with laboratory standard 24-hour cycles. After only 5 weeks of this environmental disruption, eCD mice begin showing metabolic signs of allostatic load, including increased weight, adiposity, and leptin levels. A significant effect is also observed in glucose metabolism, because eCD mice also show an imbalance between insulin and plasma glucose, suggesting development of a prediabetic state. Strikingly, the observed metabolic changes are accompanied by changes in prefrontal cortex cellular morphology, mirroring those observed in chronic stress. eCD animals show shrunken and less complex apical dendritic trees of cells in layer II/III of the medial prefrontal cortex,34 with no apparent effects on basal dendrites. eCD animals further show behavioral abnormalities, with marked cognitive rigidity in a modified Morris water maze. Specifically, eCD mice demonstrate normal performance on the learning phase of the initial task, but are slower to adapt to the hidden platform being moved to a novel location. These changes were mostly due to the mice making more perseverative errors by returning to the original location of the platform.34 In addition to the cognitive flexibility problems, eCD mice display what may be considered an “impulsive”-like phenotype in the light-dark box, while not showing any overt anxiety-like phenotype.34 Taken in a wider context, these effects of eCD are remarkably similar to those observed in 21 days of chronic restraint stress in rodents, which results in morphologic simplification of prefrontal cortical neurons and impairment in prefrontal mediated behaviors, such as attentional set-shifting or other working memory tasks.35–37 When discussing such models of eCD, it is important to note that, just as total hippocampal lesions provided insight into the role of this brain structure in learning and memory, circadian models are purposefully “exotic” (ie, shortened 20-hour days are not expected to become a common occurrence in human society). These models should provide important proof-of-principle concepts that should set the stage for more ecologically relevant and more refined models to be developed.
The specific mechanisms by which disrupted circadian clocks cause changes in brain, behavior, and peripheral physiology remain unknown. One hypothesis is that disrupted light-dark cycles lead to a gradual loss of cohesion between circadian clocks in the brain (eg, the SCN) and those in the body (eg, liver). This loss of cohesion results in central and peripheral oscillators eventually becoming out of phase with each other, creating internal desynchrony. In the brain, this could lead to changes in synchrony between various nodes of a neural circuit (eg, the prefrontal cortex and amygdala drifting out of phase). Over many cycles, such loss of cohesion could lead to changes in neurobehavioral function (as evidenced in34). In addition to long-term, chronic circadian desynchronization (CD), shorter durations of CD could lead to changes in these circuits that make them more vulnerable to further insult and could be a more insidious mechanism of circuit level disruption, setting the stage for other stressors (eg, metabolic stress, immune stress) to overwhelm an already compromised network. Extending this model to the periphery, disruption of peripheral body clocks could lead to changes in the way the stress system responds to environmental or psychological stressors. Together, this model illustrates how CD could lead to neural circuits becoming more vulnerable to insult as well as pathways by which disruption could compromise allostatic responses engaged to help an organism adapt to environmental challenge. In this schema, CD effects may be similar to the diathesis-stress models, which could explain many of the epidemiologic findings of increased risk for development of psychiatric, cardiovascular, or other physiologic syndromes in populations undergoing chronic CD, such as shift workers.20,38–40
METABOLIC AND HORMONAL RESPONSES TO SLEEP DEPRIVATION AND CIRCADIAN DISRUPTION
Sleep deprivation produces an allostatic overload that can have deleterious consequences. Increases in blood pressure, decreases in parasympathetic tone, and increases in evening cortisol are all observed after only 4 hours of sleep deprivation. Metabolic effects of this short-duration deprivation also include increased insulin levels and increased appetite, possibly through the elevation of ghrelin, a pro-appetitive hormone, and decreased levels of leptin.41–43 These short- term effects of sleep deprivation are significant when one considers epidemiologic evidence showing that reduced sleep duration is associated with increased body mass and obesity in the NHANES study.44 However, these relationships are by no means simple. For instance, in adolescents, sleep reduction is associated with measurable changes in insulin sensitivity,45 whereas durations of sleep longer than average are not associated with such changes. Similarly, a study by St-Onge and colleagues46 in adults suggests that short-term sleep deprivation is not the cause of altered insulin sensitivity per se, but instead may contribute to overeating, potentially via different mechanisms in men and women. An important study by Vetrivelan and colleagues47 showed that chronic partial sleep loss in a rat model of spontaneous short sleep did not lead to changes in metabolism, with the authors suggesting that observed effects on metabolism in sleep deprivation studies may instead be due to other factors, such as CD, changes in diet, or reduced activity levels. This work is clearly supported by the earlier eCD work of Karatsoreos and McEwen,27 in which mice that were circadian-disrupted, but not explicitly sleep-deprived, gained weight and had altered levels of plasma insulin and leptin.
Sleep deprivation also has marked effects on other circulating messengers, in addition to metabolic effects. Specifically, immune mediators are also altered following sleep deprivation. Pro- inflammatory cytokine levels are increased, along with deterioration in performance as measured by tests of psychomotor vigilance, and this has been reported to result from a modest sleep restriction to 6 hours per night.48 In humans, poor sleep and sleep deprivation in aging women have been associated with elevated levels of interleukin (IL)-6.49 Indeed, recent work has demonstrated that eCD can lead to significant changes in immune challenges while not dramatically altering basal inflammatory tone.92,93 Thus, clear links exist between sleep deprivation, CD, and neurobehavioral, metabolic, and immune function.
SLEEP DEPRIVATION AND CIRCADIAN DISRUPTION IN MOOD DISORDERS
There is wide agreement that psychiatric illness, including depression, involves CD of body temperature, mood, and sleep.50,51 Moreover, acute sleep deprivation is effective in 40% to 60% of depressed subjects in improving mood within 24 to 48 hours, in contrast to antidepressant medications that typically require 2 to 8 weeks to have an effect.52,53 New research also indicates that sleep deprivation in patients with major depressive disorder can lead to clear plasticity in the cortex.94 The mechanisms underlying the mood- improving effects of this sudden acute circadian “shock” are as yet unknown, but suggest that directly manipulating the sleep-circadian system may be a pathway to explore. Along these lines, light (photic) therapy is another form of circadian manipulation that is effective in some depressed patients, and there are pharmaceutical agents for mood disorders, such as agomelatine, that interact with the melatonin system54 as well as the use of melatonin itself for circadian phase shifting in seasonal affective disorder.50
Gross CDs, as measured by changes in sleep- activity cycles, are not the only rhythms that are perturbed in mood disorders. Psychotic major depression involves elevated evening cortisol levels, whereas the nonpsychotic form does not,55 and elevated evening cortisol as well as CD are linked to metabolic syndrome.56 Moreover, glucocorticoids are able to reset and synchronize the circadian clocks of peripheral tissues, including the liver, although the central pacemaker clock of the SCN does not respond to glucocorticoid.24 Because almost every cell in the body expresses clock genes and glucocorticoid receptors are ubiquitous, the effects of CD can be profound: for example, in hamsters living in a light:dark cycle 2 hours different from the period of their normal clock, cardiac hypertrophy and renal failure are observed.57
NEURAL RESPONSES TO SLEEP DEPRIVATION
The brain is the master regulator of the neuroendocrine, autonomic, and immune systems. It is important to remember that it is also the master regulator of behaviors that contribute to unhealthy or healthy lifestyles, which, in turn, influence the physiologic processes of allostasis.3 Therefore, chronic stress can therefore have direct and indirect effects on cumulative allostatic overload. There are many disparate changes driven by allostatic overload resulting from chronic stress. In animal models, chronic stress causes atrophy of neurons in the prefrontal cortex and hippocampus, brain regions involved in executive function, selective attention, and memory. On the other hand, chronic stress leads to hypertrophy of neurons in the amygdala, a region involved in fear, anxiety, and aggression.58 Thus, chronic stress compromises the ability of an organism to learn, remember, and make decisions, as well as increases levels of anxiety and aggression.
Although not as much work has been conducted, it is obvious that the results of sleep deprivation, and perhaps to a lesser extent CD, share certain characteristics with chronic stress. For instance, there is recent evidence not only for cognitive impairment resulting from sleep restriction in animal models but also for altered levels of cytokines, oxidative stress markers, glycogen levels, and structural changes in the form of reduced dentate gyrus neurogenesis. Specifically, increases in brain levels of mRNA of the pro-inflammatory cytokine IL-1b are reported following sleep deprivation by gentle handling and is reported to be higher in daytime (during the normal sleep period in rodents) than in darkness (during the normal activity time for rodents).59 Closely related to inflammatory processes, through the actions of NADPH oxidase,60,61 is oxidative stress involving the generation of free radicals. Seventy-two hours of “flower pot” or platform sleep deprivation has been reported to increase oxidative stress in the mouse hippocampus, as measured by increased lipid peroxidation and increased ratios of oxidized to reduced glutathione.62 Glycogen, found predominantly in white matter, is also profoundly affected by sleep deprivation and is reported to decrease by as much as 40% in rats deprived of sleep for 24 hours by novelty and gentle handling, an effect reversed by recovery sleep.63,64 The specific consequences of this effect are not yet elucidated, although it is noteworthy that glycogen in astrocytes is able to sustain axon function during glucose deprivation in central nervous system white matter.65
With respect to memory and cognitive performance, there are numerous reports of impairments following sleep deprivation. For example, sleep deprivation by the platform (or flower pot) method leads to impaired performance of spatial memory in the Morris water maze,66 and reduced CA1 hippocampal long-term potentiation.67 Sleep deprivation using these methods also impairs retention of passive avoidance memory, a context-dependent, fear-memory task.62 A different method of sleep deprivation, by gentle stimulation or novelty, following contextual fear conditioning impairs memory consolidation.68 A 6-hour period of total sleep deprivation by novelty exposure impaired acquisition of a spatial task in the Morris water maze.69 A 4-hour period of sleep deprivation by gentle stimulation impaired the late phase long term potentiation (LTP) in the dentate gyrus 48 hours later but had the opposite effect to enhance late-phase LTP in the prefrontal cortex.70 Some of these effects might be related to changes in beta-adrenergic activity, at least in the hippocampus.95 There is also good evidence suggesting that sleep deprivation can affect heterosynaptic metaplasticity in mouse hippocampal slices.96 while new work also suggests that epigenetic modifications might play a critical role in synaptic plasticity following sleep deprivation.97 Emotional behaviors are also affected by sleep deprivation. Increases in fighting behavior are observed following REM sleep deprivation71; there is also a report of increased aggression in the form of phencyclidine-induced muricide after sleep deprivation.72,73 These findings harken to the results of increased aggression among cage mates in rats subjected to 21 days of 6h per day of chronic restraint stress during the resting period when some sleep deprivation may occur.74 Thus, the effects of sleep deprivation are not simple and are influenced by their type, intensity, duration, and the types of behavioral outcomes measured.
The neural mechanisms of the effects of sleep deprivation on behavior are still not understood. However, sleep deprivation in rats using a treadmill for 96 hours has been reported to decrease proliferation of cells in the dentate gyrus of the hippocampal formation by as much as 50%.75 In addition, there are significant changes in other measures of synaptic plasticity in these models, including changes in LTP.98 A confound in this type of experiment is that the effects of activity are difficult to disentangle from the effects of the deprivation. A similar neural effect has also been reported by keeping rats in a slowly rotating drum, but, here again, there is a question of how much physical activity and physical stress may have contributed to the suppression of cell proliferation.76 Nevertheless, sleep restriction by novelty exposure, a more subtle method, prevented the increased survival of new dentate gyrus neurons promoted by spatial training in a Morris water maze.77 Recently, clear effects of sleep and sleep deprivation on hippocampal function has been demonstrated, showing that hippocampal network activity changes during sleep-dependent memory consolidation, and that sleep deprivation can affect hippocampal synaptic plasticity during defined timeframes.78,79 Work in the visual cortex also shows that sleep affects memory consolidation,80–82 suggesting these effects are not merely limited to the hippocampus. As always, untangling the contributions of sleep loss from the effects of misalignment of circadian patterns of sleep with the environmental light-dark cycle remains a critical question that should be assessed in both basic research as well as in the clinic.
INTEGRATION AND SUMMARY
Sleep is thought to be a neural state during which consolidation of declarative memories takes place.83 Sleep deprivation, even for the course of the active period of the day in diurnal animals, increases the homeostatic drive to sleep, with resulting changes in pro-inflammatory cytokines and glycogen levels. Relatively brief deprivation of sleep promotes an exacerbation of these processes with progressively more severe physiologic, neurobiological, and behavioral consequences as the sleep deprivation is prolonged.
CD is a broader aspect of the problem of sleep disruption, with disruption of the circadian clock contributing to changes in sleep patterns, and sleep patterns potentially influencing the circadian clock. Shift work and jet lag are 2 common practices that have measurable effects on the brain and body.84,85 For instance, long distance air travel with short turnaround has been reported to be associated with smaller volume of the temporal lobe and impaired performance on a visual-spatial cognitive task.86
The long-term consequences of sleep deprivation and CD constitute a form of allostatic load, with consequences involving hypertension, reduced parasympathetic tone, increased pro-inflammatory cytokines, increased oxidative stress, and increased evening cortisol and insulin. As noted above, reduced sleep and CD are associated with increased chances of cardiovascular disease and diabetes. Indeed, shorter sleep times have been associated with increased obesity.44 Moreover, diabetes is associated with impaired hippocampal function,87 decreased hippocampal volume,88 and increased risk for Alzheimer disease89,90, are observed.
In addition to the inflammatory and cardio- metabolic changes that are observed, depressive illness is almost universally associated with disturbed sleep.91 Thus, there are not only linkages between the multiple, interacting mediators that are involved in allostasis and allostatic load/overload, as summarized in Fig. 1, but also overlaps (ie, comorbidities) between disorders, such as diabetes, hypertension, cardiovascular disease, and depression, that are associated with excessive stress and with the dysregulation of the systems that normally promote allostasis and successful adaptation.
Sleep has important functions in maintaining homeostasis and sleep deprivation. Sleep deprivation or other forms of circadian desynchronization and disruption are stressors that have consequences for the brain as well as many body systems. Whether the sleep deprivation or circadian disruption is due to anxiety, depression, jetlag, shift work, or other aspects of a hectic lifestyle, there are consequences that contribute to allostatic load throughout the body. Taken together, these changes in brain and body are further evidence that circadian disruption predisposes an individual to altered responses to stressors as well as impaired cognitive function and metabolic dysregulation. Sleep deprivation can be described as a chronic stressor that can cause allostatic overload, including mood and cognitive impairment and autonomic and metabolic dysregulation.
KEY POINTS.
Allostatic load/overload refers to the cumulative wear and tear on body systems caused by too much stress and/or inefficient management of the systems that promote adaptation through allostasis.
Circadian disruption is a broad problem that alters allostasis and elevates allostatic load, affecting brain and body systems. Sleep deprivation is an all-too-common example of a process that includes circadian disruption.
Even a few days of sleep deprivation or circadian misalignment in young healthy volunteers have been reported to increase appetite and caloric intake, increase levels of pro-inflammatory cytokines, decrease parasympathetic and increase sympathetic tone, increase blood pressure, increase evening cortisol levels, as well as elevate insulin and blood glucose.
Chronic circadian disruption and reduced sleep time are associated with elevated cortisol, increased obesity, and reduced volume of the temporal lobe.
Mood disorders involve disrupted circadian rhythmicity and altered sleep-wake patterns; yet, acute sleep deprivation can have rapid antidepressant effects and manipulating the timing of the secretion or exogenous administration of melatonin can be beneficial in mood disorders.
Repeated stress in animal models causes brain regions involved in memory and emotions, such as hippocampus, amygdala, and prefrontal cortex, to undergo structural remodeling with the result that memory is impaired and anxiety and aggression are increased. Structural and functional MRI studies in depression and Cushing disease, as well as anxiety disorders and in air crews with jet lag, provide evidence that the human brain may be similarly affected.
Brain regions such as the hippocampus are sensitive to glucose and insulin, and both type I and type II diabetes are associated with cognitive impairment and (for type II diabetes) an increased risk for Alzheimer disease. Insofar as poor sleep and circadian disruption also exacerbate metabolic dysregulation as well as contribute to other aspects of physiologic dysregulation, they must be considered contributors to risk for dementia.
Animal models of chronic sleep deprivation indicate that memory is impaired along with depletion of glycogen stores and increases in oxidative stress and free-radical production.
ACKNOWLEDGEMENT
Dr. Bruce McEwen sadly passed away in January, 2020, and is posthumously included in this chapter as he was a trailblazer and thought leader in understanding brain-body interactions in the context of stress and allostasis, who was equally invested in integrating sleep and circadian rhythms into the broader models of adaptive homeostasis. He was a dear mentor to Dr. Karatsoreos who he encouraged to work at the interface of stress, allostatic load, circadian rhythms, sleep, and health.
Footnotes
The authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of life stress, cognition and health. New York: John Wiley & Sons; 1988. p. 629–49. [Google Scholar]
- 2.McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Intern Med 1993;153:2093–101. [PubMed] [Google Scholar]
- 3.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 1998;338:171–9. [DOI] [PubMed] [Google Scholar]
- 4.Munck A, Guyre PM. Glucocorticoids and immune function. In: Ader R, Felten DL, Cohen N,editors. Psychoneuroimmunology. San Diego (CA): Academic Press; 1991. p. 447–74. [Google Scholar]
- 5.Sapolsky RM. Physiological and pathophysiological implications of social stress in mammals. Coping with the environment: neural and endocrine mechanisms. New York: Oxford University Press; 2000. p. 517–32. [Google Scholar]
- 6.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405: 458–62. [DOI] [PubMed] [Google Scholar]
- 7.Bierhaus A, Wolf J, Andrassy M, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A 2003;100: 1920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 2000;21:55–89. [DOI] [PubMed] [Google Scholar]
- 9.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav 2003; 43:2–15. [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS. Structural plasticity of the adult brain: how animal models help us understand brain changes in depression and systemic disorders related to depression. Dialogues Clin Neurosci 2004;6:119–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEwen BS. Mood disorders and allostatic load. Biol Psychiatry 2003;54:200–7. [DOI] [PubMed] [Google Scholar]
- 12.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry 2003;54:338–52. [DOI] [PubMed] [Google Scholar]
- 13.Pitman RK. Hippocampal diminution in PTSD: more (or less?) than meets the eye. Hippocampus 2001; 11:73–4. [DOI] [PubMed] [Google Scholar]
- 14.Bremner JD. Neuroimaging studies in posttraumatic stress disorder. Curr Psychiatry Rep 2002;4:254–63. [DOI] [PubMed] [Google Scholar]
- 15.Butler MP, Kriegsfeld LJ, Silver R. Circadian regulation of endocrine functions. In: Pfaff D, Arnold A, Etgen A, et al. , editors. Hormones, brain and behavior. 2nd edition. San Diego (CA): Academic Press; 2009. p. 473–505. [Google Scholar]
- 16.Cearley C, Churchill L, Krueger JM. Time of day differences in ILIbeta and TNFalpha mRNA levels in specific regions of the rat brain. Neurosci Lett 2003;352:61–3. [DOI] [PubMed] [Google Scholar]
- 17.Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N YAcad Sci 2010;1193:48–59. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson L, Akana SF, Cascio CS, et al. Circadian variations in plasma corticosterone permit normal termination of adrenocorticotropin responses to stress. Endocrinology 1988;122:1343–8. [DOI] [PubMed] [Google Scholar]
- 19.Boivin DB, Tremblay GM, James FO. Working on atypical schedules. Sleep Med 2007;8:578–89. [DOI] [PubMed] [Google Scholar]
- 20.Knutsson A Health disorders of shift workers. Occup Med (Lond) 2003;53:103–8. [DOI] [PubMed] [Google Scholar]
- 21.Moore-Ede MC. Physiology of the circadian timing system: predictive versus reactive homeostasis. Am J Physiol 1986;250:R735–52. [DOI] [PubMed] [Google Scholar]
- 22.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 1972;42:201–6. [DOI] [PubMed] [Google Scholar]
- 23.Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus: the mind’s clock. New York: Oxford University Press; 1991. [Google Scholar]
- 24.Balsalobre A, Brown SA, Marcacci L, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 2000;289:2344–7. [DOI] [PubMed] [Google Scholar]
- 25.Segall LA, Perrin JS, Walker CD, et al. Glucocorticoid rhythms control the rhythm of expression of the clock protein, Period2, in oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neuroscience 2006;140:753–7. [DOI] [PubMed] [Google Scholar]
- 26.Lamont EW, Robinson B, Stewart J, et al. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci U S A 2005; 102:4180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karatsoreos IN, McEwen BS. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn Sci 2011;15:576–84. [DOI] [PubMed] [Google Scholar]
- 28.Gilbertson MW, Paulus LA, Williston SK, et al. Neurocognitive function in monozygotic twins discordant for combat exposure: relationship to posttraumatic stress disorder. J Abnorm Psychol 2006;115:484–95. [DOI] [PubMed] [Google Scholar]
- 29.Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002;5:1242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoo SS, Gujar N, Hu P, et al. The human emotional brain without sleep-a prefrontal amygdala disconnect. Curr Biol 2007;17:R877–878. [DOI] [PubMed] [Google Scholar]
- 31.Gianaros PJ, Jennings JR, Sheu LK, et al. Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension 2007;49:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage 2009;47: 922–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gianaros PJ, Sheu LK, Matthews KA, et al. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci 2008;28:990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karatsoreos IN, Bhagat S, Bloss EB, et al. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A 2011; 108:1657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry 2000; 48:721–31. [DOI] [PubMed] [Google Scholar]
- 36.McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci 2006;8:367–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEwen BS, Coirini H, Westlind-Danielsson A, et al. Steroid hormones as mediators of neural plasticity. J Steroid Biochem Mol Biol 1991;39:223–32. [DOI] [PubMed] [Google Scholar]
- 38.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst 2001;93:1557–62. [DOI] [PubMed] [Google Scholar]
- 39.Suwazono Y, Dochi M, Sakata K, et al. A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity (Silver Spring) 2008;16:1887–93. [DOI] [PubMed] [Google Scholar]
- 40.Lowden A, Moreno C, Holmback U, et al. Eating and shift work - effects on habits, metabolism and performance. Scand J Work Environ Health 2010;36: 150–62. [DOI] [PubMed] [Google Scholar]
- 41.Leproult R, Copinschi G, Buxton O, et al. Sleep loss results in an elevation of cortisol levels the next evening. Sleep 1997;20:865–70. [PubMed] [Google Scholar]
- 42.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999;354:1435–9. [DOI] [PubMed] [Google Scholar]
- 43.Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846–50. [DOI] [PubMed] [Google Scholar]
- 44.Gangwisch JE, Malaspina D, Boden-Albala B, et al. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep 2005;28:1289–96. [DOI] [PubMed] [Google Scholar]
- 45.Matthews KA, Dahl RE, Owens JF, et al. Sleep duration and insulin resistance in healthy black and white adolescents. Sleep 2012;35:1353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.St-Onge MP, O’Keeffe M, Roberts AL, et al. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep 2012;35:1503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vetrivelan R, Fuller PM, Yokota S, et al. Metabolic effects of chronic sleep restriction in rats. Sleep 2012;35:1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 2004;89:2119–26. [DOI] [PubMed] [Google Scholar]
- 49.Friedman EM, Hayney MS, Love GD, et al. Social relationships, sleep quality, and interleukin-6 in aging women. Proc Natl Acad Sci U S A 2005;102: 18757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewy AJ, Lefler BJ, Emens JS, et al. The circadian basis of winter depression. Proc Natl Acad Sci U S A 2006;103:7414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karatsoreos IN. Links between circadian rhythms and psychiatric disease. Front Behav Neurosci 2014;8:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vogel GW, Thompson FC Jr, Thurmond A, et al. The effect of REM deprivation on depression. Psychosomatics 1973;14:104–7. [DOI] [PubMed] [Google Scholar]
- 53.Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: what do we know, where do we go? Biol Psychiatry 1999;46:445–53. [DOI] [PubMed] [Google Scholar]
- 54.Morley-Fletcher S, Mairesse J, Soumier A, et al. Chronic agomelatine treatment corrects behavioral, cellular, and biochemical abnormalities induced by prenatal stress in rats. Psychopharmacology 2011; 217:301–13. [DOI] [PubMed] [Google Scholar]
- 55.Keller J, Flores B, Gomez RG, et al. Cortisol circadian rhythm alterations in psychotic major depression. Biol Psychiatry 2006;60:275–81. [DOI] [PubMed] [Google Scholar]
- 56.Rintamaki R, Grimaldi S, Englund A, et al. Seasonal changes in mood and behavior are linked to metabolic syndrome. PLoS One 2008;3:e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martino TA, Oudit GY, Herzenberg AM, et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol 2008;294:R1675–83. [DOI] [PubMed] [Google Scholar]
- 58.McEwen BS, Chattarji S. Molecular mechanisms of neuroplasticity and pharmacological implications: the example of tianeptine. Eur Neuropsychopharmacol 2004;14:S497–502. [DOI] [PubMed] [Google Scholar]
- 59.Taishi P, Chen Z, Obal FJ, et al. Sleep-associated changes in interleukin-1beta mRNA in the brain. J Interferon Cytokine Res 1998;18:793–8. [DOI] [PubMed] [Google Scholar]
- 60.Clark RA, Valente AJ. Nuclear factor kappa B activation by NADPH oxidases. Mech Ageing Dev 2004; 125:799–810. [DOI] [PubMed] [Google Scholar]
- 61.Tang J, Liu J, Zhou C, et al. Role of NADPH oxidase in the brain injury of intracerebral hemorrhage. J Neurochem 2005;94:1342–50. [DOI] [PubMed] [Google Scholar]
- 62.Silva RH, Abilio VC, Takatsu AL, et al. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology 2004;46:895–903. [DOI] [PubMed] [Google Scholar]
- 63.Kong J, Shepel PN, Holden CP, et al. Brain glycogen decreases with increased periods to wakefulness: implications for homeostatic drive to sleep. J Neurosci 2002;22:5581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown AM. Brain glycogen re-awakened. J Neurochem 2004;89:537–52. [DOI] [PubMed] [Google Scholar]
- 65.Wender R, Brown AM, Fern R, et al. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J Neurosci 2000;20:6804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Youngblood BD, Zhou J, Smagin GN, et al. Sleep deprivation by the “flower pot” technique and spatial reference memory. Physiol Behav 1997;61: 249–56. [DOI] [PubMed] [Google Scholar]
- 67.Kim EY, Mahmoud GS, Grover LM. REM sleep deprivation inhibits LTP in vivo in area CA1 of rat hippocampus. Neurosci Lett 2005;388:163–7. [DOI] [PubMed] [Google Scholar]
- 68.Graves LA, Heller EA, Pack AI, et al. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem 2003;10: 168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guan Z, Peng X, Fang J. Sleep deprivation impairs spatial memory and decreases extracellular signalregulated kinase phosphorylation in the hippocampus. Brain Res 2004;1018:38–47. [DOI] [PubMed] [Google Scholar]
- 70.Romcy-Pereira R, Pavlides C. Distinct modulatory effects of sleep on the maintenance of hippocampal and medial prefrontal cortex LTP. Eur J Neurosci 2004;20:3453–62. [DOI] [PubMed] [Google Scholar]
- 71.de Paula HM, Hoshino K. Correlation between the fighting rates of REM sleep-deprived rats and susceptibility to the ‘wild running’ of audiogenic seizures. Brain Res 2002;926:80–5. [DOI] [PubMed] [Google Scholar]
- 72.Musty RE, Consroe PF. Phencyclidine produces aggressive behavior in rapid eye movement sleep- deprived rats. Life Sci 1982;30:1733–8. [DOI] [PubMed] [Google Scholar]
- 73.Russell JW, Singer G. Relations between muricide, circadian rhythm and consummatory behavior. Physiol Behav 1983;30:23–7. [DOI] [PubMed] [Google Scholar]
- 74.Wood GE, Young LT, Reagan LP, et al. Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm Behav 2003;43:205–13. [DOI] [PubMed] [Google Scholar]
- 75.Guzman-Marin R, Suntsova N, Stewart DR, et al. Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J Physiol 2003;549(2):563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roman V, Van der Borght K, Leemburg SA, et al. Sleep restriction by forced activity reduces hippocampal cell proliferation. Brain Res 2005;1065:53–9. [DOI] [PubMed] [Google Scholar]
- 77.Hairston IS, Little MT, Scanlon MD, et al. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol 2005;94:4224–33. [DOI] [PubMed] [Google Scholar]
- 78.Prince TM, Wimmer M, Choi J, et al. Sleep deprivation during a specific 3-hour time window post-training impairs hippocampal synaptic plasticity and memory. Neurobiol Learn Mem 2014;109:122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ognjanovski N, Maruyama D, Lashner N, et al. CA1 hippocampal network activity changes during sleep- dependent memory consolidation. Front Syst Neuro-sci 2014;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aton SJ, Seibt J, Dumoulin M, et al. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron 2009;61:454–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aton SJ, Broussard C, Dumoulin M, et al. Visual experience and subsequent sleep induce sequential plastic changes in putative inhibitory and excitatory cortical neurons. Proc Natl Acad Sci U S A 2013;110:3101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aton SJ, Suresh A, Broussard C, et al. Sleep promotes cortical response potentiation following visual experience. Sleep 2014;37:1163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gais S, Born J. Declarative memory consolidation: mechanisms acting during human sleep. Learn Mem 2004;11:679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Atkinson G, Davenne D. Relationships between sleep, physical activity and human health. Physiol Behav 2007;90:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karlson B, Eek FC, Hansen AM, et al. Diurnal Cortisol pattern of shift workers on workday and a day off. Scand J Work Environ Health 2006;Suppl (2):27–34. [Google Scholar]
- 86.Cho K Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci 2001;4:567–8. [DOI] [PubMed] [Google Scholar]
- 87.Hendrickx H, McEwen BS, van der Ouderaa F. Metabolism, mood and cognition in aging: the importance of lifestyle and dietary intervention. Neurobiol Aging 2005;26S:S1–5. [DOI] [PubMed] [Google Scholar]
- 88.Gold SM, Dziobek I, Sweat V, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 2007;50:711–9. [DOI] [PubMed] [Google Scholar]
- 89.Arvanitakis Z, Wilson RS, Bienias JL, et al. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 2004; 61:661–6. [DOI] [PubMed] [Google Scholar]
- 90.Rasgon N, Jarvik L. Insulin resistance, affective disorders, and Alzheimer’s disease: review and hypothesis. J Gerontol 2004;59A:178–83. [DOI] [PubMed] [Google Scholar]
- 91.Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry 2005;66:1254–69. [DOI] [PubMed] [Google Scholar]
- 92.Pearson GL, Savenkova M, Barnwell JJ, Karatsoreos IN. Circadian desynchronization alters metabolic and immune responses following lipopolysaccharide inoculation in male mice. Brain Behav Immun. 2020. Aug;88:220–229. doi: 10.1016/j.bbi.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Phillips DJ, Savenkova MI, Karatsoreos IN. Environmental disruption of the circadian clock leads to altered sleep and immune responses in mouse. Brain Behav Immun. 2015. Jul;47:14–23. doi: 10.1016/j.bbi.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 94.Kuhn M, Maier JG, Wolf E, Mainberger F, Feige B, Maywald S, Bredl A, Michel M, Sendelbach N, Normann C, Klöppel S, Eckert A, Riemann D, Nissen C. Indices of cortical plasticity after therapeutic sleep deprivation in patients with major depressive disorder. J Affect Disord. 2020. Dec 1;277:425–435. doi: 10.1016/j.jad.2020.08.052. [DOI] [PubMed] [Google Scholar]
- 95.Lu HJ, Lv J. β-adrenergic Receptor Activity in the Hippocampal Dentate Gyrus Participates in Spatial Learning and Memory Impairment in Sleep-deprived Rats. Exp Neurobiol. 2021. Apr 30;30(2):144–154. doi: 10.5607/en20058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vecsey CG, Huang T, Abel T. Sleep deprivation impairs synaptic tagging in mouse hippocampal slices. Neurobiol Learn Mem. 2018. Oct;154:136–140. doi: 10.1016/j.nlm.2018.03.016. Epub 2018 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wong LW, Chong YS, Wong WLE, Sajikumar S. Inhibition of Histone Deacetylase Reinstates Hippocampus-Dependent Long-Term Synaptic Plasticity and Associative Memory in Sleep-Deprived Mice. Cereb Cortex. 2020. Jun 1;30(7):4169–4182. doi: 10.1093/cercor/bhaa041. [DOI] [PubMed] [Google Scholar]
- 98.Rajizadeh MA, Esmaeilpour K, Haghparast E, Ebrahimi MN, Sheibani V. Voluntary exercise modulates learning & memory and synaptic plasticity impairments in sleep deprived female rats. Brain Res. 2020. Feb 15;1729:146598. doi: 10.1016/j.brainres.2019.146598. [DOI] [PubMed] [Google Scholar]


