Abstract
Background
Amelogenesis imperfecta (AI) is a tooth development disorder in which the teeth are covered with thin, abnormally formed enamel. This enamel is easily fractured and damaged, which affects the appearance of the teeth, especially if left untreated. Negative psychological outcomes, due to compromised appearance and function, in patients with AI, have been found to compromise a person's attractiveness and reduce social interaction. The treatment used depends on the severity of the problem.
Objectives
To compare the success rates of different restorative materials and techniques used for the restoration of anterior and posterior teeth with AI in terms of patient satisfaction (aesthetics and sensitivity) and function.
Search methods
We searched the Cochrane Oral Health Group's Trials Register (to 18 April 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 3), MEDLINE via OVID (1946 to 18 April 2013), EMBASE via OVID (1980 to 18 April 2013), CINAHL via EBSCO (1980 to 18 April 2013), Abstracts of the Conference Proceedings of the International Association for Dental Research (2001 to 18 April 2013) and reference lists of relevant articles. There were no restrictions on language or date of publication in the electronic searches.
Selection criteria
Randomised controlled trials where children and adolescents with AI who required restoration of teeth were allocated to different restoration techniques would have been selected. Outcomes which would have been evaluated were patient satisfaction, aesthetics, masticatory function and longevity of restorations.
Data collection and analysis
Two review authors would have extracted data and assessed the risk of bias in included studies independently. Disagreement between the two authors would have been resolved by consulting a third review author. First authors were contacted for additional information and unpublished data.
Main results
No studies met the inclusion criteria for this review.
Authors' conclusions
We found no randomised controlled trials of restorative treatments for children and adolescents with AI, and therefore there is no evidence as to which is the best restoration. Well defined randomised controlled trials which recruit children and adolescents and focus on the type and severity of the disorder should be undertaken to determine the best intervention for restoring teeth affected by AI.
Keywords: Adolescent, Child, Humans, Amelogenesis Imperfecta, Amelogenesis Imperfecta/therapy, Treatment Outcome
Plain language summary
Interventions for the restoration of teeth that have been weakened by the absence of enough covering of enamel, caused by amelogenesis imperfecta
Amelogenesis imperfecta (AI) is a tooth development disorder in which the teeth are covered with thin, abnormally formed enamel. The enamel is easily fractured and damaged, which affects the appearance of the teeth, especially if left untreated. Negative psychological outcomes, due to compromised appearance and function, in patients with AI, have been found to affect a person's attractiveness and reduce social interaction. Early and appropriate preventive and restorative care is essential for successful management of AI and for a person's psychological well being.
The treatment used depends on the severity of the problem. Crowns are sometimes used to improve the appearance of the teeth and protect them from damage.
This review undertaken by the Cochrane Oral Health Group set out to compare the success rates of different restorative materials and techniques used for the restoration of front and back teeth affected by AI. The review was to assess patient satisfaction, particularly how the teeth looked, how sensitive they were and how well they functioned.
The most recent search of existing studies was undertaken on 18 April 2013. Randomised controlled trials were sought in which restorations of teeth affected by AI were compared with regard to patient satisfaction and function. Children and adolescents under 18, who had AI and required restorative care, were selected for inclusion irrespective of their nationality. This review found no trials which met the inclusion criteria.
Therefore, there is currently insufficient reliable evidence to support which of these treatments are more effective. Well defined randomised controlled trials which focus on children and adolescents with different types and severity of the disorder should be undertaken to determine the best intervention for restoring teeth affected by AI.
Background
Description of the condition
Amelogenesis imperfecta (AI) is a heterogeneous group of genetic disorders characterised by defects in enamel formation of the teeth in the absence of any generalised or systemic diseases (Kida 2002). Studies presenting the epidemiology of AI have reported varying prevalence from 1:700 in northern Sweden to 1:12‐14,000 in USA (Hoppenreijs 1998). Bäckman 1988 studied 51 families with AI from the county of Västerbotten in northern Sweden. Autosomal dominant inheritance was the likely mode of inheritance in 33 out of 51 families.
Several key problems associated with AI include diagnosis, aesthetics, poor oral hygiene, gingivitis, dental sensitivity, loss of vertical dimension due to a rapid wear of the dentition, and the cost and requirement of lifelong extensive restorative care (Coffield 2005). Moreover, researchers have also found an association between AI and negative psychological outcomes, and that the dental defect in AI can destroy a person's attractiveness and pose a threat to social interaction (Coffield 2005). It has been noted that early recognition followed by appropriate preventive and restorative care is essential for successful management of AI and for a person's psychological well being (Mackie 1991; Ayers 2004; Coffield 2005).
Since 1945, AI classifications were based on two components: the determination of phenotype characteristics (clinical and radiographic features) and the description of the mode of inheritance (autosomal dominant/autosomal recessive, X‐linked and isolated) in each allocating case of AI (Witkop 1988). Different inheritance patterns such as X‐linked, autosomal dominant, autosomal recessive and sporadic types restricted to individual families have been reported.
Advances in molecular genetics encouraged Aldred 2008 to suggest a modification of the previous AI classification by considering the knowledge of the molecular basis of each case of AI (chromosomal/location/locus mutation when known). It has been demonstrated that enamel formation requires the expression of multiple genes that transcribe matrix proteins and proteinases necessary to control crystal growth and mineralisation of forming enamel (Hart 2003). Currently, there are seven candidate genes for AI including amelogenin, enamelin, ameloblastin, tuftelin, distal less homobox 3, enamelysin (MMP20) and kallikrein (KLK) (Kim 2006).
Genetic studies have shown that not all forms of AI respond favourably to enamel bonding. An association between successful bonding and specific affected gene has been suggested. For instance, a mutation in the KLK4 gene, but not in the enamelin or ameloblastin genes, resulted in enamel malformations that do not respond to etching and bonding agents (Simmer 2001).
Clinically, AI can be classified into four categories: hypoplastic (type I), hypomaturation (type II), hypocalcified (type III), and hypomature hypoplastic enamel with taurodontism (type IV) (Fonseca 2006).
Description of the intervention
Several treatment options have been described for each category of AI ranging from prevention to orthognathic surgery. Sari 2003 has noted that age, socioeconomic status of the patient, the type and severity of AI, and the intraoral situation at the time of treatment planning are all factors which affect the selection of restorative treatment. New restorative materials and bonding techniques have provided less invasive and more promising treatment options (Yamaguti 2006). Microabrasion with 18% hydrochloric acid and pumice has been found, in a 4‐year follow‐up study, to be an effective treatment for generalised defects resembling hypomaturation AI, to improve aesthetics and decrease discolouration without increasing dental sensitivity or staining (Ashkenazi 2000). However, it was found that microabrasion is not the best option when enamel is soft and is easily penetrated by an explorer (Fonseca 2006).
In hypomaturation, where enamel tends to chip from the underlying dentine, it was recommended to remove the enamel and to apply bonding directly to dentine (Fonseca 2006). Aldred 2008 has recommended using acid‐etch composite resin to hypoplastic rather than to hypocalcified enamel whilst Venezie 1994 has shown that sodium hypochlorite can improve bond strength to hypocalcified teeth. When there is a greater loss of tooth structure such as in hypoplastic teeth in which loss of enamel exposes the dentine to the oral environment, direct resin restorations or porcelain veneers have been the best option (Fonseca 2006).
Similarly, after clinical examination and scanning with electron microscope, Vitkov 2006 has shown that composite crowns and veneers luted adhesively by a total bonding technique and low viscosity resin composite, are the best management for treating severely affected AI primary teeth.
Why it is important to do this review
Given a wide range of available alternatives for restoring teeth with AI, difficulty of providing restorative care, and effects of the compromised appearance of the teeth on children and their parents, it is important for clinicians to have an evidence‐based approach for selecting an effective, acceptable and cost‐effective restoration. Therefore, this systematic review would gather evidence to support which of the available treatments for the restorative care of AI are more effective.
Objectives
To compare the success rates of different restorative materials and techniques used for the restoration of anterior teeth with AI in terms of patient satisfaction (aesthetics and sensitivity) and function.
To compare the success rates of different restorative materials and techniques used for the restoration of posterior teeth with AI in terms of patient satisfaction (aesthetics and sensitivity) and function.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) in which restorations of teeth affected by AI were compared with regard to patient satisfaction (aesthetics and sensitivity) and function.
Types of participants
All children and adolescents under 18, who had AI and required restorative care, were eligible for inclusion irrespective of their nationality.
Types of interventions
Any study comparing permanent restorative materials and techniques used for restoring teeth affected by AI. Interventions may include metal restorations (amalgam); all types of glass ionomers (type I, II, III and IV) and composite resins (macrofilled, microfilled, hybrid and nanofilled); resin‐modified glass ionomers (compomers, giomers); cement; stainless steel/nickel‐chrome crowns and pre‐fabricated resin/porcelain veneer facings.
All studies comparing microabrasion with dental restorations for treating AI would have been included. Dental restorations versus overdenture or extraction were excluded as this systematic review is only intended to cover all RCTs which have tested restorative interventions.
Types of outcome measures
Primary outcome
Patient satisfaction due to reduced dental sensitivity and improved aesthetics.
Secondary outcomes
Improved aesthetics (self esteem, comfort, positive psychological impact).
Improved masticatory function.
Longevity of restoration.
Adverse events would also have been documented.
Search methods for identification of studies
Electronic searches
The searches of the electronic databases were not restricted by language of publication. Detailed search strategies were developed for each database using a combination of controlled vocabulary and free text terms. The MEDLINE search combined the subject search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) (Higgins 2011). The EMBASE and CINAHL searches were combined with sensitive search strategies developed by the Cochrane Oral Health Group for identifying RCTs.
The following electronic databases were searched.
Cochrane Oral Health Group's Trials Register (to 18 April 2013) (Appendix 1).
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 3) (Appendix 2).
MEDLINE via OVID (1946 to 18 April 2013) (Appendix 3).
EMBASE via OVID (1980 to 18 April 2013) (Appendix 4).
CINAHL via EBSCO (1980 to 18 April 2013) (Appendix 5).
Abstracts of the Conference Proceedings of the International Association for Dental Research (2001 to 18 April 2013) (Appendix 6).
Searching other resources
Only handsearching done as part of the Cochrane Worldwide Handsearching Programme and uploaded to CENTRAL was included (see the Cochrane Masterlist for details of journal issues searched to date).
The metaRegister of Controlled Trials was also searched (30 April 2013) to identify ongoing and completed trials (Appendix 7).
The reference list of related review articles and all articles obtained were checked for further trials. Contact with investigators of included and ongoing studies was attempted by electronic mail to ask for details of additional published and unpublished trials.
Data collection and analysis
Selection of studies
The titles and abstracts (when available) of all reports identified through the electronic searches were scanned independently by two review authors. For studies appearing to meet the inclusion criteria, or for which there were insufficient data in the title and abstract to make a clear decision, the full report was obtained. The full reports obtained from all the electronic and other methods of searching were assessed independently and in duplicate by two review authors with expertise in this content area, to establish whether the studies met the inclusion criteria or not. Disagreements were resolved by discussion. Where resolution was not possible, a third review author was consulted. Studies rejected at this or subsequent stages were recorded in the Characteristics of excluded studies table, and reasons for exclusion recorded.
Data extraction and management
Data were extracted by two review authors independently and in duplicate using specially designed data extraction forms. Disagreement between the two review authors was resolved by consulting a third review author. Some of the study authors were contacted for clarification or for further information.
In case that ongoing and future studies are included in updates, the characteristics of the trial participants, interventions and outcomes, will be presented in the 'Characteristics of included studies' table. If stated, sample size calculation and sources of funding will also be recorded.
Assessment of risk of bias in included studies
We did not identify any relevant RCT. However, if further studies are identified in future updates, the risk of bias assessment in the included studies will be undertaken independently and in duplicate by two review authors. Disagreements will be resolved by discussion. This will be carried out using The Cochrane Collaboration's tool for assessing risk of bias and a 'Risk of bias' table will be constructed for each study as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (updated March 2011) (Higgins 2011).
The following domains will be assessed as 'low risk' of bias, 'high risk' of bias, or 'unclear risk' of bias.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective reporting.
Other bias.
Further quality assessment will be carried out to assess definition of inclusion/exclusion criteria, adequate definition of success criteria, and comparability of control and treatment groups at the start of the trial. These assessments will be reported for each individual study in the 'Risk of bias' table under 'Characteristics of included studies'.
Overall risk of bias will be categorised according to the following.
Low risk of bias (plausible bias unlikely to seriously alter the results) if all key domains were assessed as at low risk of bias.
Unclear risk of bias (plausible bias that raises some doubt about the results) if one or more key domains were assessed as at unclear of bias.
High risk of bias (plausible bias that seriously weakens confidence in the results) if one or more key domains were assessed as at high risk of bias.
Measures of treatment effect
For dichotomous outcomes, the estimate of effect of an intervention was to be expressed as a risk ratio together with 95% confidence interval. For continuous outcomes, it was planned to use means and standard deviations to summarise the data for each group.
Assessment of heterogeneity
Clinical heterogeneity would be assessed by testing the types of participants, interventions and outcomes in each study. The significance of any discrepancies in the estimates of the treatment effects from the different trials would be assessed by means of Cochran's test for heterogeneity and I2 statistic. Any identified significant statistical heterogeneity (P < 0.1) detected would be explored (Higgins 2011).
Data synthesis
If there were studies of similar comparisons reporting the same outcome measures, a meta‐analysis would be undertaken. Risks ratios would be combined for dichotomous data, and either weighted or standardised mean differences for continuous data, using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
A planned subgroup analysis would be utilised to investigate some potential factors for heterogeneity, which may affect outcomes.
Age of participants.
Location of restoration (anterior or posterior).
Type of AI (hypoplastic, hypomaturation, hypocalcified, and hypomature hypoplastic enamel with taurodontism).
Difference in techniques applied before restorations (acid‐etch technique, dentine adhesive systems).
Sensitivity analysis
Sensitivity analysis was planned to be undertaken to examine the effect of randomisation, allocation concealment and blinded outcome assessment on the overall estimates of effect if sufficient number of trials had been included in the review. In addition, the effect of including unpublished literature on the review's findings was also to be examined if data allowed.
Presentation of main results
In future updates if sufficient studies are eligible for inclusion, the main findings of the review will be presented in a 'Summary of findings' table according to Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (Higgins 2011). The quality of evidence will be graded using the GRADE system.
Results
Description of studies
Results of the search
After all the searches and de‐duplication, 224 studies were identified of which 204 were excluded after reviewing the titles and abstracts. Full texts were obtained of the remaining 20 studies. After screening the full articles, 19 were excluded and reasons for exclusion presented in the Characteristics of excluded studies table. No study was included. One ongoing study with 1‐year results (ISRCTN70438627) was identified as potentially meeting the inclusion criteria. The process of study identification is presented in Figure 1.
1.
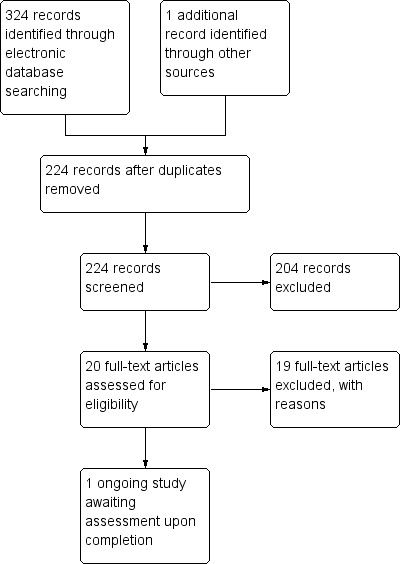
Study flow diagram.
Included studies
No studies met the inclusion criteria.
Excluded studies
SeeCharacteristics of excluded studies table for further details.
Studies were excluded due to the lack of a comparison group (Walls 1988) or randomisation (Sönmez 2009), or due to different study designs such as case series (Markovic 2010) or different outcomes (Sönmez 2009). Studies undertaken on teeth affected by non‐carious lesions rather than AI (erosion, abfraction, attrition, white spot lesions, molar‐incisor hypomineralisation (MIH)) were also excluded (Coll 1991; Gladys 1998; Marta 2000; Folwaczny 2001; Baratieri 2003; Cheng 2004; Kramer 2005; Peumans 2005; Van Meerbeek 2005; Deliperi 2006; Van Landuyt 2008; Lygidakis 2009; Peumans 2010; Fron 2011; Kim 2011). Zagdwon 2003 included both AI and participants with other severe enamel defects. Further correspondence with the study authors is needed to identify the results of the five patients who specifically suffered from AI.
Ongoing studies
SeeCharacteristics of ongoing studies table for further details.
ISRCTN70438627 was identified through searching the metaRegister of Controlled Trials and potentially met the review's inclusion criteria. The trial is being undertaken in a special care department for children and adolescents in Falun, Dalarna, Sweden. The start date was 2009 and it will last 3 years. One‐year results of this trial were presented at two congresses in 2012. The trial will be followed for further assessment after completion for possible inclusion in future updates of this review.
Risk of bias in included studies
No studies met the inclusion criteria.
Effects of interventions
No studies met the inclusion criteria.
Discussion
Summary of main results
Given a wide range of available alternatives for restoring teeth with AI, and the difficulty of providing restorative care for patients with this disorder, it is important for clinicians to have an evidence‐based approach for selecting an effective, acceptable and cost‐effective restoration regardless of the age, socioeconomic status, type and severity of the disorder, and oral situation at the time of examination.
This systematic review has not been able to answer questions asked in the protocol on improved aesthetics (positive psychological impact), improved masticatory function, and longevity of restoration and the presence of adverse events or complications. Many RCTs found, which were identified in our search, investigated the clinical success of restorative materials in non‐carious lesions and did not investigate the outcome in teeth affected by AI. Other investigations which assessed restorative materials and techniques in AI teeth focused on case reports or case series.
The Zagdwon 2003 trial, which compared stainless steel crowns (SSCs) with cast adhesive coping for restoring 42 first permanent molars affected by AI or severe enamel defects, was excluded since the investigators did not provide information about the number of patients with AI or other enamel defects, and obtaining data for the five AI patients was not successful.
There is an ongoing study (ISRCTN70438627) which potentially met the review's inclusion criteria and was identified through searching the metaRegister of Controlled Trials. The trial is being undertaken in a special care department for children and adolescents in Falun, Dalarna, Sweden to compare treatment outcomes in patients with AI treated with ceramic crowns of IPS E‐max (n = 112) compared to Procera with Zirconia inner copings (n = 118). AI type was recorded as either hypoplastic type or hypomineralised/hypomaturation type. The start date was 2009 and it will last 3 years. One‐year results of this trial were presented at two congresses in 2012. Follow‐up clinical and radiographic examinations will be made after 1 and 2 years to assess caries, gingivitis, plaque, periodontitis, apical status, quality of restorations, sensitivity, and complications. The review authors will assess the trial after its completion to consider its inclusion in future updates of this review.
Further well defined RCTs which focus on the age of the patient together with the type and severity of the disorder should be undertaken to determine the best intervention for restoring teeth affected by AI.
Agreements and disagreements with other studies or reviews
Enamel defects have been an area for active research in the last few years. Many studies and reviews have been published about treatment modalities in children and adolescents affected by enamel defects such as molar‐incisor hypomineralisation (MIH) (Lygidakis 2010). However, such studies and reviews were so limited for AI. To some extent, MIH and AI show similar serious clinical management problems in dental settings that could face the clinician. Children, in both cases, may have behavioural management problems, dental fear and anxiety due to the appearance of their teeth, and bad experience they can face during multiple and unsuccessful dental treatments. A very useful six‐step management approach for MIH has been proposed for restorative care of MIH starting from risk identification, early diagnosis, remineralisation, prevention of dental caries and post‐eruptive enamel breakdown, restoration and maintenance (William 2006). Well designed trials supported by laboratory studies should be undertaken to determine the clinical approach and set guidelines for treating AI.
Authors' conclusions
Implications for practice.
The introduction of new restorative materials, in the last few decades, such as glass ionomer cements, resin‐modified glass ionomer cements, polyacid‐modified resin composites, resin composites, and indirect adhesive or cast onlays or crowns, holds promising outcomes in clinical settings for patients with AI. However, assessment of clinical performance is still based on case reports or case series and there are no RCTs to provide high quality evidence to set guidelines for clinical practice.
It is important for clinicians to have an evidence‐based approach for selecting effective, acceptable and cost‐effective restorations. Our systematic review could not specify the best restoration. It is difficult to draw sound conclusions and to generalise results to all defective teeth.
Implications for research.
There are currently no RCTs comparing outcomes for different restorative materials available for AI.
After improving the chemical, physical and biological properties of dental restorative materials, there is an increasing emphasis on achieving clinical success in dental settings for normal and defective teeth. The type of AI should be considered in any treatment. More studies should be undertaken among different age groups and should include different ethnic groups. Existing and new bonding and aesthetic materials which have promising results in normal teeth, should evaluated for teeth affected by AI by comparing them with techniques and bonding materials which have been established in the laboratory and clinical practice.
Well designed RCTs should be undertaken to determine the best effective, acceptable and cost‐effective restoration. RCTs should adhere to the CONSORT statement in terms of both design and reporting. Clear inclusion and exclusion criteria should be identified. Sample size should be sufficiently large to show clinically important differences in important outcomes such as aesthetics and function. Objective outcome measures which have been demonstrated to be valid and reliable, should be used in future studies.
What's new
| Date | Event | Description |
|---|---|---|
| 11 March 2014 | Review declared as stable | This empty review will not be updated until a substantial body of evidence on the topic becomes available. If trials are conducted and found eligible for inclusion in the future, the review would then be updated accordingly. |
Acknowledgements
We would like to thank Ms Anne Littlewood at the Cochrane Oral Health Group in Manchester, UK for her expert help in searching the literature, Ms Joanne Leese for her help in providing full text articles necessary for this review, Mrs Luisa Fernandez Mauleffinch, and Dr Assem Sbentai for his previous contribution in the protocol. The authors also would like to thank Professor Goran Dahllöf, Professor Matthias Folwaczny, Professor Shin Kim, Dr Gunilla Pousette Lundgren, Professor Ulla Pallesen and Professor Marleen Peumans for their kind response and for providing information about their publications.
Appendices
Appendix 1. Cochrane Oral Health Group's Trials Register search strategy
An updated search of the Cochrane Oral Health Group's Trials Register was conducted in April 2013 using the Cochrane Register of Studies and the search strategy below:
#1 ((enamel or dental or tooth or teeth):ti,ab) AND (INREGISTER) #2 ((hypocalcif* or hypominerali* or hypomatur* or hypoplas*):ti,ab) AND (INREGISTER) #3 (#1 and #2) AND (INREGISTER) #4 ("amelogenesis imperfecta":ti,ab) AND (INREGISTER) #5 (#3 or #4) AND (INREGISTER)
A previous search was conducted in June 2012 using the Procite software and the search strategy below:
(((enamel or dental or tooth or teeth) AND (hypocalcif* or hypominerali* or hypomatur* or hypoplas*)) or "amelogenesis imperfecta")
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor Amelogenesis imperfecta explode all trees
#2 "amelogenesis imperfecta" in Title, Abstract or Keywords
#3 ((enamel in Title, Abstract or Keywords near/6 defect* in Title, Abstract or Keywords) or (enamel in Title, Abstract or Keywords near/6 abnormalit* in Title, Abstract or Keywords) or (enamel in Title, Abstract or Keywords near/6 hypocalcif* in Title, Abstract or Keywords) or (enamel in Title, Abstract or Keywords near/6 hypominerali* in Title, Abstract or Keywords) or (enamel in Title, Abstract or Keywords near/6 hypomatur* in Title, Abstract or Keywords) or (enamel in Title, Abstract or Keywords near/6 hypoplas* in Title, Abstract or Keywords))
#4 ((dental in Title, Abstract or Keywords near/6 defect* in Title, Abstract or Keywords) or (dental in Title, Abstract or Keywords near/6 abnormalit* in Title, Abstract or Keywords) or (dental in Title, Abstract or Keywords near/6 hypocalcif* in Title, Abstract or Keywords) or (dental in Title, Abstract or Keywords near/6 hypominerali* in Title, Abstract or Keywords) or (dental in Title, Abstract or Keywords near/6 hypomatur* in Title, Abstract or Keywords) or (dental in Title, Abstract or Keywords near/6 hypoplas* in Title, Abstract or Keywords))
#5 ((tooth in Title, Abstract or Keywords near/6 defect* in Title, Abstract or Keywords) or (tooth in Title, Abstract or Keywords near/6 abnormalit* in Title, Abstract or Keywords) or (tooth in Title, Abstract or Keywords near/6 hypocalcif* in Title, Abstract or Keywords) or (tooth in Title, Abstract or Keywords near/6 hypominerali* in Title, Abstract or Keywords) or (tooth in Title, Abstract or Keywords near/6 hypomatur* in Title, Abstract or Keywords) or (tooth in Title, Abstract or Keywords near/6 hypoplas* in Title, Abstract or Keywords))
#6 ((teeth in Title, Abstract or Keywords near/6 defect* in Title, Abstract or Keywords) or (teeth in Title, Abstract or Keywords near/6 abnormalit* in Title, Abstract or Keywords) or (teeth in Title, Abstract or Keywords near/6 hypocalcif* in Title, Abstract or Keywords) or (teeth in Title, Abstract or Keywords near/6 hypominerali* in Title, Abstract or Keywords) or (teeth in Title, Abstract or Keywords near/6 hypomatur* in Title, Abstract or Keywords) or (teeth in Title, Abstract or Keywords near/6 hypoplas* in Title, Abstract or Keywords))
#7 (#1 or #2 or #3 or #4 or #5 or #6)
#8 MeSH descriptor Dental Restoration, Permanent this term only
#9 MeSH descriptor Dental veneers this term only
#10 MeSH descriptor Crowns this term only
#11 MeSH descriptor Glass ionomer cements this term only
#12 MeSH descriptor Composite resins explode all trees
#13 MeSH descriptor Dental amalgam this term only
#14 ((dental in Title, Abstract or Keywords near/3 restor* in Title, Abstract or Keywords) or (tooth in Title, Abstract or Keywords near/3 restor* in Title, Abstract or Keywords) or (teeth in Title, Abstract or Keywords near/3 restor* in Title, Abstract or Keywords) or (resin* in Title, Abstract or Keywords near/6 restor* in Title, Abstract or Keywords) or (glass in Title, Abstract or Keywords near/6 restor* in Title, Abstract or Keywords))
#15 ((dental in Title, Abstract or Keywords or tooth in Title, Abstract or Keywords or teeth in Title, Abstract or Keywords) and fill* in Title, Abstract or Keywords)
#16 crown* in Title, Abstract or Keywords
#17 giomer* in Title, Abstract or Keywords
#18 ((composite* in Title, Abstract or Keywords near/6 restor* in Title, Abstract or Keywords) or (composite* in Title, Abstract or Keywords near/6 fill* in Title, Abstract or Keywords))
#19 ((dental in Title, Abstract or Keywords or tooth in Title, Abstract or Keywords or teeth in Title, Abstract or Keywords) and veneer* in Title, Abstract or Keywords)
#20 MeSH descriptor Enamel microabrasion this term only
#21 microabras* in Title, Abstract or Keywords
#22 (#8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21)
#23 (#7 and #22)
Appendix 3. MEDLINE via OVID search strategy
1. Amelogenesis Imperfecta/
2. "amelogenesis imperfecta".ti,ab.
3. (enamel adj6 (defect$ or abnormalit$ or hypocalcif$ or hypomineraliz$ or hypomineralis$ or hypomatur$ or hypoplas$)).ti,ab.
4. (dental adj6 (defect$ or abnormalit$ or hypocalcif$ or hypomineraliz$ or hypomineralis$ or hypomatur$ or hypoplas$)).ti,ab.
5. (tooth adj6 (defect$ or abnormalit$ or hypocalcif$ or hypomineraliz$ or hypomineralis$ or hypomatur$ or hypoplas$)).ti,ab.
6. (teeth adj6 (defect$ or abnormalit$ or hypocalcif$ or hypomineraliz$ or hypomineralis$ or hypomatur$ or hypoplas$)).ti,ab.
7. or/1‐6
8. Dental restoration permanent/
9. Dental veneers/
10. Crowns/
11. Glass ionomer cements/
12. exp Composite resins/
13. Dental amalgam/
14. ((dental adj3 restor$) or (tooth adj3 restor$) or (teeth adj3 restor$) or (resin$ adj6 restor$) or (glass$ adj6 restor$)).ti,ab.
15. ((dental or tooth or teeth) and fill$).ti,ab.
16. crown$.ti,ab.
17. giomer$.ti,ab.
18. (composite$ adj6 (restor or fill$)).ti,ab.
19. ((dental or tooth or teeth) and veneer$).ti,ab.
20. Enamel microabrasion/
21. microabras$.ti,ab.
22. or/8‐21
23. 7 and 22
Cochrane search filter for MEDLINE via OVID
Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. EMBASE via OVID search strategy
1. Amelogenesis Imperfecta/
2. "amelogenesis imperfecta".ti,ab.
3. (enamel adj6 (defect$ or abnormalit$ or hypocalcif$ or hypomineraliz$ or hypomineralis$ or hypomatur$ or hypoplas$)).ti,ab.
4. (dental adj6 (defect$ or abnormalit$ or hypocalcif$ or hypomineraliz$ or hypomineralis$ or hypomatur$ or hypoplas$)).ti,ab.
5. (tooth adj6 (defect$ or abnormalit$ or hypocalcif$ or hypomineraliz$ or hypomineralis$ or hypomatur$ or hypoplas$)).ti,ab.
6. (teeth adj6 (defect$ or abnormalit$ or hypocalcif$ or hypomineraliz$ or hypomineralis$ or hypomatur$ or hypoplas$)).ti,ab.
7. or/1‐6
8. Tooth filling/
9. Tooth crown/
10. Glass ionomer/
11. exp Resin/
12. Amalgam/
13. ((dental adj3 restor$) or (tooth adj3 restor$) or (teeth adj3 restor$) or (resin$ adj6 restor$) or (glass$ adj6 restor$)).ti,ab.
14. ((dental or tooth or teeth) and fill$).ti,ab.
15. crown$.ti,ab.
16. giomer$.ti,ab.
17. (composite$ adj6 (restor or fill$)).ti,ab.
18. ((dental or tooth or teeth) and veneer$).ti,ab.
19. microabras$.ti,ab.
20. or/8‐19
21. 7 and 20
The above subject search was linked to the Cochrane Oral Health Group filter for identifying RCTs in EMBASE via OVID:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 16. HUMAN/ 17. 16 and 15 18. 15 not 17 19. 14 not 18
Appendix 5. CINAHL via EBSCO search strategy
S1 MH Amelogenesis imperfecta
S2 "amelogenesis imperfecta"
S3 ((enamel N6 defect*) or (enamel N6 abnormal*) or (enamel N6 hypocalcif*) or (enamel N6 hypominerali*) or (enamel N6 hypomatur*) or (enamel N6 hypoplas*))
S4 ((dental N6 defect*) or (dental N6 abnormal*) or (dental N6 hypocalcif*) or (dental N6 hypominerali*) or (dental N6 hypomatur*) or (dental N6 hypoplas*))
S5 ((tooth N6 defect*) or (tooth N6 abnormal*) or (tooth N6 hypocalcif*) or (tooth N6 hypominerali*) or (tooth N6 hypomatur*) or (tooth N6 hypoplas*))
S6 ((teeth N6 defect*) or (teeth N6 abnormal*) or (teeth N6 hypocalcif*) or (teeth N6 hypominerali*) or (teeth N6 hypomatur*) or (teeth N6 hypoplas*))
S7 S1 or S2 or S3 or S4 or S5 or S6
S8 MH "Dental restoration, permanent"
S9 MH "Dental veneers"
S10 MH "Crowns"
S11 MH "Glass ionomer cements"
S12 MH "Composite resins+"
S13 MH "Dental amalgam"
S14 ((dental N3 restor*) or (tooth N3 restor*) or (teeth N3 restor*) or (resin* N6 restor*) or (glass* N6 restor*))
S15 ((dental or tooth or teeth) and fill*)
S16 crown*
S17 giomer*
S18 (composite* N6 restor*) or (composite* N6 fill*)
S19 ((dental or tooth or teeth) and veneer*)
S20 microabras*
S21 S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20
S22 S7 and S21
The above subject search was linked to the Cochrane Oral Health Group filter for identifying RCTs in CINAHL via EBSCO:
S1 MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design
S2 TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study")
S3 TI random* or AB random*
S4 AB "latin square" or TI "latin square"
S5 TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over)
S6 MH Placebos
S7 AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*)
S8 TI blind* or AB mask* or AB blind* or TI mask*
S9 S7 and S8
S10 TI Placebo* or AB Placebo* or SU Placebo*
S11 MH Clinical Trials
S12 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial)
S13 S1 or S2 or S3 or S4 or S5 or S6 or S9 or S10 or S11 or S12
Appendix 6. Abstracts of the Conference Proceedings of the International Association for Dental Research search strategy
amelogenesis AND imperfecta AND trial
Appendix 7. metaRegister of Controlled Trials search strategy
amelogenesis AND imperfecta
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baratieri 2003 | Study included teeth affected by non‐carious lesions rather than amelogenesis imperfecta (AI) |
| Cheng 2004 | Study included teeth affected by non‐carious lesions rather than AI |
| Coll 1991 | Study included incisors with enamel surface defects rather than AI |
| Deliperi 2006 | Study included teeth affected by non‐carious lesions rather than AI |
| Folwaczny 2001 | Study included teeth affected by non‐carious lesions rather than AI |
| Fron 2011 | Study included teeth affected by non‐carious lesions rather than AI |
| Gladys 1998 | Study included teeth affected by non‐carious lesions rather than AI |
| Kim 2011 | Study included teeth affected by molar‐incisor hypomineralisation (MIH) only |
| Kramer 2005 | Study included teeth affected by non‐carious lesions rather than AI |
| Lygidakis 2009 | Not a randomised controlled trial, MIH |
| Markovic 2010 | Case series |
| Marta 2000 | Study included teeth affected by non‐carious lesions rather than AI |
| Peumans 2005 | Study included teeth affected by non‐carious lesions rather than AI |
| Peumans 2010 | Study included teeth affected by non‐carious lesions rather than AI |
| Sönmez 2009 | Not a randomised controlled trial, different outcomes |
| Van Landuyt 2008 | Study included teeth affected by non‐carious lesions rather than AI |
| Van Meerbeek 2005 | Study included teeth affected by non‐carious lesions rather than AI |
| Walls 1988 | No AI, no controls |
| Zagdwon 2003 | Study includes both AI and participants with other severe enamel defects. Further correspondence with study authors needed to identify the results of the five patients who specifically suffered from AI |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN70438627.
| Trial name or title | Early restorative crown therapy in children and adolescents with amelogenesis imperfecta (AI): a prospective double‐blind randomised controlled trial |
| Methods | Single centre double‐blind randomised controlled trial, split‐mouth design |
| Participants | Setting: Centre for Oral Rehabilitation, special care department for children and adolescents, Falun, Dalarna, Sweden Inclusion criteria: Children and adolescents with AI, 6 to 25 years of age, referred for oral rehabilitation Exclusion criteria: AI in combination with syndromes including mental retardation Randomised: 28 (15 boys and 13 girls). 12 to 23 years of age, all of Caucasian origin AI type was recorded as either hypoplastic type (14) or hypomineralised or hypomaturation type (14) |
| Interventions | Treatment with Procera crown therapy with Zirconia inner coping cemented with Rely X ARC cement or E‐MAX crowns with Zirconia inner coping cemented with Rely X ARC cement Follow‐up examinations: 1 month, 1 year and 2 years |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | 1 January 2009 |
| Contact information | Professor Goran Dahllöf Karolinska Institutet Department of Dental Medicine Division of Orthodontics and Pediatric Dentistry POB 4064 Huddinge SE‐14104 Sweden |
| Funding | Centre for Clinical Research, Public Dental Service Dalarna (Sweden) |
| Notes | 1‐year results were published as an abstract in 2012 |
Contributions of authors
Search and retrieval of papers: Mayssoon Dashash (MD), C Albert Yeung (AY), Issam Jamous (IJ). Screening articles and handsearching if needed: MD, IJ. Accepting articles for inclusion: MD, AY, IJ, Anthony Blinkhorn (ASB). Data extraction: MD, IJ. Obtaining and screening data on unpublished studies: MD. Entering data into RevMan: MD, AY. Statistical and methodological analysis: MD. Data analysis: MD, AY. Writing the review: MD, AY, IJ, ASB.
Sources of support
Internal sources
Damascus University, Syrian Arab Republic.
External sources
-
Cochrane Oral Health Group Global Alliance, UK.
All reviews in the Cochrane Oral Health Group are supported by Global Alliance member organisations (British Orthodontic Society, UK; British Society of Paediatric Dentistry, UK; Canadian Dental Hygienists Association, Canada; National Center for Dental Hygiene Research & Practice, USA and New York University College of Dentistry, USA) providing funding for the editorial process (http://ohg.cochrane.org).
-
National Institute for Health Research (NIHR), UK.
CRG funding acknowledgement: The NIHR is the largest single funder of the Cochrane Oral Health Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies excluded from this review
Baratieri 2003 {published data only}
- Baratieri LN, Canabarro S, Lopes GC, Ritter AV. Effect of resin viscosity and enamel beveling on the clinical performance of Class V composite restorations: three‐year results. Operative Dentistry 2003;28(5):482‐7. [PubMed] [Google Scholar]
Cheng 2004 {published data only}
- Cheng PH, He GR. Observation of the clinical effects of two kinds of resin materials for restoring the defects of maxillary incisor. Shanghai Journal of Stomatology 2004;13(4):353‐4. [PubMed] [Google Scholar]
Coll 1991 {published data only}
- Coll JA, Jackson P, Strassler HE. Comparison of enamel microabrasion techniques: Prema Compound versus a 12‐fluted finishing bur. Journal of Esthetic Dentistry 1991;3(5):180‐6. [DOI] [PubMed] [Google Scholar]
Deliperi 2006 {published data only}
- Deliperi S, Bardwell DN. Clinical evaluation of direct cuspal coverage with posterior composite resin restorations. Journal of Esthetic and Restorative Dentistry 2006;18(5):256‐65; discussion 266‐7. [DOI] [PubMed] [Google Scholar]
Folwaczny 2001 {published and unpublished data}
- Folwaczny M, Loher C, Mehl A, Kunzelmann KH, Hickel R. Class V lesions restored with four different tooth‐colored materials – 3‐year results. Clinical Oral Investigations 2001;5(1):31‐9. [DOI] [PubMed] [Google Scholar]
Fron 2011 {published data only}
- Fron H, Vergnes JN, Moussally C, Cazier S, Simon AL, Chieze JB, et al. Effectiveness of a new one‐step self‐etch adhesive in the restoration of non‐carious cervical lesions: 2‐year results of a randomized controlled practice‐based study. Dental Materials 2011;27(3):304‐12. [DOI] [PubMed] [Google Scholar]
Gladys 1998 {published data only}
- Gladys S, Meerbeek B, Lambrechts P, Vanherle G. Marginal adaptation and retention of a glass‐ionomer, resin‐modified glass‐ionomers and a polyacid‐modified resin composite in cervical Class‐V lesions. Dental Materials 1998;14(4):294‐306. [DOI] [PubMed] [Google Scholar]
Kim 2011 {published and unpublished data}
- Kim S, Kim EY, Jeong TS, Kim JW. The evaluation of resin infiltration for masking labial enamel white spot lesions. International Journal of Paediatric Dentistry 2011;21(4):241‐8. [DOI] [PubMed] [Google Scholar]
Kramer 2005 {published data only}
- Kramer N, Frankenberger R. Clinical performance of bonded leucite‐reinforced glass ceramic inlays and onlays after eight years. Dental Materials 2005;21(3):262‐71. [DOI] [PubMed] [Google Scholar]
Lygidakis 2009 {published data only}
- Lygidakis NA, Dimou G, Stamataki E. Retention of fissure sealants using two different methods of application in teeth with hypomineralised molars (MIH): a 4 year clinical study. European Archives of Paediatric Dentistry 2009;10(4):223‐6. [DOI] [PubMed] [Google Scholar]
Markovic 2010 {published data only}
- Markovic D, Petrovic B, Peric T. Case series: clinical findings and oral rehabilitation of patients with amelogenesis imperfecta. European Archives of Paediatric Dentistry 2010;11(4):201‐8. [DOI] [PubMed] [Google Scholar]
Marta 2000 {published data only}
- Marta SN, Oliveira FS, Silva SMB, Lanza CRM, Machado MAAM. Microbrasion clinical comparison: PREMA compound X phosphoric acid. Journal of Dental Research 2000;79(5):1040 (Abs No 284). [Google Scholar]
Peumans 2005 {published and unpublished data}
- Peumans M, Munck J, Landuyt K, Lambrechts P, Meerbeek B. Three‐year clinical effectiveness of a two‐step self‐etch adhesive in cervical lesions. European Journal of Oral Sciences 2005;113(6):512‐8. [DOI] [PubMed] [Google Scholar]
Peumans 2010 {published and unpublished data}
- Peumans M, Munck J, Landuyt KL, Poitevin A, Lambrechts P, Meerbeek B. Eight‐year clinical evaluation of a 2‐step self‐etch adhesive with and without selective enamel etching. Dental Materials 2010;26(12):1176‐84. [DOI] [PubMed] [Google Scholar]
Sönmez 2009 {published data only}
- Sِönmez IS, Aras S, Tunç ES, Küçükeşmen C. Clinical success of deproteinization in hypocalcified amelogenesis imperfecta. Quintessence International 2009;40(2):113‐8. [PubMed] [Google Scholar]
Van Landuyt 2008 {published data only}
- Landuyt KL, Peumans M, Fieuws S, Munck J, Cardoso MV, Ermis RB, et al. A randomized controlled clinical trial of a HEMA‐free all‐in‐one adhesive in non‐carious cervical lesions at 1 year. Journal of Dentistry 2008;36(10):847‐55. [DOI] [PubMed] [Google Scholar]
Van Meerbeek 2005 {published data only}
- Meerbeek B, Kanumilli P, Munck J, Landuyt K, Lambrechts P, Peumans M. A randomized controlled study evaluating the effectiveness of a two‐step self‐etch adhesive with and without selective phosphoric‐acid etching of enamel. Dental Materials 2005;21(4):375‐83. [DOI] [PubMed] [Google Scholar]
Walls 1988 {published data only}
- Walls AW, Murray JJ, McCabe JF. Composite laminate veneers: a clinical study. Journal of Oral Rehabilitation 1988;15(5):439‐54. [DOI] [PubMed] [Google Scholar]
Zagdwon 2003 {published data only}
- Zagdwon AM, Fayle SA, Pollard MA. A prospective clinical trial comparing preformed metal crowns and cast restorations for defective first permanent molars. European Journal of Paediatric Dentistry 2003;4(3):138‐42. [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN70438627 {published and unpublished data}
- ISRCTN70438627. Early restorative crown therapy in children and adolescents with Amelogenesis Imperfecta: a prospective double‐blind randomised controlled trial. www.controlled‐trials.com/ISRCTN70438627 (accessed 30 April 2013). [DOI: 10.1186/ISRCTN70438627] [DOI]
- Pousette Lundgren G, Trulsson M, Dahllöf G. One year results of a RCT of prosthetic therapy in patients with amelogenesis imperfecta. In: Abstract Book of the 11th Congress of the European Academy of Paediatric Dentistry; 24–27 May 2012; Strasbourg; page 26 (Abs No O55). Available from http://eapd2012.eu/en/Abstracts‐book‐191.html.
- Pousette Lundgren G, Trulsson M, Dahllöf G. RCT of prosthetic therapy in young patients with amelogenesis imperfecta ‐ one year results. Swedish Dental Journal 2012;36(4):219 (Abs No 21). [Google Scholar]
Additional references
Aldred 2008
- Aldred M, Crawford PJM, Cameron A, King NM, Widmer R. Dental anomalies. In: Cameron AC, Widmer RP editor(s). Handbook of Pediatric Dentistry. 3rd Edition. Edinburgh: Mosby Elsevier, 2008:217‐77. [Google Scholar]
Ashkenazi 2000
- Ashkenazi M, Sarnat H. Microabrasion of teeth with discoloration resembling hypomaturation enamel defects: four‐year follow up. Journal of Clinical Pediatric Dentistry 2000;25(1):29‐34. [DOI] [PubMed] [Google Scholar]
Ayers 2004
- Ayers KM, Drummond BK, Harding WJ, Salis SG, Liston PN. Amelogenesis imperfecta – multidisciplinary management from eruption to adulthood. Review and case report. The New Zealand Dental Journal 2004;100(4):101‐4. [PubMed] [Google Scholar]
Bäckman 1988
- Bäckman B, Holmgren G. Amelogenesis imperfecta: a genetic study. Human Heredity 1988;38(4):189‐206. [DOI] [PubMed] [Google Scholar]
Coffield 2005
- Coffield KD, Phillips C, Brady M, Roberts MW, Strauss RP, Wright JT. The psychosocial impact of developmental dental defects in people with hereditary amelogenesis imperfecta. Journal of the American Dental Association 2005;136(5):620‐30. [DOI] [PubMed] [Google Scholar]
Fonseca 2006
- Fonseca RB, Correr Sobrinho L, Fernandes Neto AJ, Mota AS, Soares CJ. Enamel hypoplasia or amelogenesis imperfecta – a restorative approach. Brazilian Journal of Oral Sciences 2006;5(16):941‐3. [Google Scholar]
Hart 2003
- Hart PS, Wright JT, Savage M, Kang G, Bensen JT, Gorry MC, et al. Exclusion of candidate genes in two families with autosomal dominant hypocalcified amelogenesis imperfecta. European Journal of Oral Sciences 2003;111(4):326‐31. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoppenreijs 1998
- Hoppenreijs TJ, Voorsmit RA, Freihofer HP. Open bite deformity in amelogenesis imperfecta. Part 1: An analysis of contributory factors and implications for treatment. Journal of Cranio‐Maxillo‐Facial Surgery 1998;26(4):260‐6. [DOI] [PubMed] [Google Scholar]
Kida 2002
- Kida M, Ariga T, Shirakawa T, Oguchi H, Sakiyama Y. Autosomal‐dominant hypoplastic form of amelogenesis imperfecta caused by an enamelin gene mutation at the exon‐intron boundary. Journal of Dental Research 2002;81(11):738‐42. [DOI] [PubMed] [Google Scholar]
Kim 2006
- Kim JW, Simmer JP, Lin BP, Seymen F, Bartlett JD, Hu JC. Mutational analysis of candidate genes in 24 amelogenesis imperfecta families. European Journal of Oral Sciences 2006;114 Suppl 1:3‐12; discussion 39‐41, 379. [DOI] [PubMed] [Google Scholar]
Lygidakis 2010
- Lygidakis NA. Treatment modalities in children with teeth affected by molar‐incisor enamel hypomineralisation (MIH): A systematic review. European Archives of Paediatric Dentistry 2010;11(2):65‐74. [DOI] [PubMed] [Google Scholar]
Mackie 1991
- Mackie IC, Blinkhorn AS. Amelogenesis imperfecta: early interception to prevent attrition. Dental Update 1991;18(2):79‐80. [PubMed] [Google Scholar]
Sari 2003
- Sari T, Usumez A. Restoring function and esthetics in a patient with amelogenesis imperfecta: a clinical report. The Journal of Prosthetic Dentistry 2003;90(6):522‐5. [DOI] [PubMed] [Google Scholar]
Simmer 2001
- Simmer JP, Hu JC. Dental enamel formation and its impact on clinical dentistry. Journal of Dental Education 2001;65(9):896‐905. [PubMed] [Google Scholar]
Venezie 1994
- Venezie RD, Vadiakas G, Christensen JR, Wright JT. Enamel pretreatment with sodium hypochlorite to enhance bonding in hypocalcified amelogenesis imperfecta: case report and SEM analysis. Pediatric Dentistry 1994;16(6):433‐6. [PubMed] [Google Scholar]
Vitkov 2006
- Vitkov L, Hannig M, Krautgartner WD. Restorative therapy of primary teeth severely affected by amelogenesis imperfecta. Quintessence International 2006;37(3):219‐24. [PubMed] [Google Scholar]
William 2006
- William V, Messer LB, Burrow MF. Molar incisor hypomineralization: review and recommendations for clinical management. Pediatric Dentistry 2006;28(3):224‐32. [PubMed] [Google Scholar]
Witkop 1988
- Witkop CJ Jr. Amelogenesis imperfecta, dentinogenesis imperfecta and dentin dysplasia revisited: problems in classification. Journal of Oral Pathology 1988;17(9–10):547‐53. [DOI] [PubMed] [Google Scholar]
Yamaguti 2006
- Yamaguti PM, Acevedo AC, Paula LM. Rehabilitation of an adolescent with autosomal dominant amelogenesis imperfecta: case report. Operative Dentistry 2006;31(2):266‐72. [DOI] [PubMed] [Google Scholar]


