Abstract
Background and Objectives
Findings of association between coronavirus disease 2019 (COVID-19) and stroke remain inconsistent, ranging from significant association to absence of association to less than expected ischemic stroke among hospitalized patients with COVID-19. The current study examined the association between COVID-19 and risk of acute ischemic stroke (AIS).
Methods
We included 37,379 Medicare fee-for-service (FFS) beneficiaries aged ≥65 years diagnosed with COVID-19 from April 1, 2020, through February 28, 2021, and AIS hospitalization from January 1, 2019, through February 28, 2021. We used a self-controlled case series design to examine the association between COVID-19 and AIS and estimated the incidence rate ratios (IRRs) by comparing incidence of AIS in risk periods (0–3, 4–7, 8–14, 15–28 days after diagnosis of COVID-19) vs control periods.
Results
Among 37,379 Medicare FFS beneficiaries with COVID-19 and AIS, the median age at diagnosis of COVID-19 was 80.4 (interquartile range 73.5–87.1) years and 56.7% were women. When AIS at day of exposure (day = 0) was included in the risk periods, IRRs at 0–3, 4–7, 8–14, and 15–28 days following COVID-19 diagnosis were 10.3 (95% confidence interval 9.86–10.8), 1.61 (1.44–1.80), 1.44 (1.32–1.57), and 1.09 (1.02–1.18); when AIS at day 0 was excluded in the risk periods, the corresponding IRRs were 1.77 (1.57–2.01) (day 1–3), 1.60 (1.43–1.79), 1.43 (1.31–1.56), and 1.09 (1.01–1.17), respectively. The association appeared to be stronger among younger beneficiaries and among beneficiaries without prior history of stroke but largely consistent across sex and race/ethnicities.
Discussion
Risk of AIS among Medicare FFS beneficiaries was 10 times (day 0 cases in the risk period) as high during the first 3 days after diagnosis of COVID-19 as during the control period and the risk associated with COVID-19 appeared to be stronger among those aged 65–74 years and those without prior history of stroke.
Classification of Evidence
This study provides Class IV evidence that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is associated with increased risk of AIS in the first 3 days after diagnosis in Medicare FFS beneficiaries ≥65 years of age.
Several studies have suggested that infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), has been associated with an increased risk of cerebrovascular events including acute ischemic stroke (AIS).1-6 However, the findings of association between COVID-19 and stroke were not consistent, as studies reported the rates of stroke among patients with COVID-19 ranging from 0.4% to 8.0% with an average of 1.4%.2 Two studies using similar study design suggested that risk of ischemic stroke increased significantly following diagnosis of COVID-19.3,6 One study suggested that the rates of stroke were similar between hospitalized patients with and without COVID-19,7 and another study showed that ischemic stroke occurred less often than expected among hospitalized patients with COVID-19.8 Differences in study designs, countries, inclusion criteria of patients and comparison groups, sample size, and controlling for confounders may contribute to the inconsistent findings. Few studies focused on the older population, where most stroke occurred.9
The objective of the current study is to examine the association between COVID-19 and risk of AIS among Medicare fee- for-service (FFS) beneficiaries aged 65 years or older. We used a self-controlled case series study design that is based on within-person comparisons and implicitly controls for all fixed confounding effects. Our study included more than 37,000 Medicare FFS beneficiaries who were diagnosed with COVID-19 during April 1, 2020–February 28, 2021 and AIS hospitalizations during January 1, 2019–February 28, 2021.
The primary research question of the present study is to determine the association between SARS-CoV-2 infection and risk of AIS among Medicare FFS beneficiaries ≥65 years.
Methods
Study Population
We used the real-time Medicare geographic variation (GV) files to identify the beneficiaries for this study. First, we identified all Medicare beneficiaries who had been diagnosed with COVID-19 from January 1, 2020, through February 28, 2021 from Part A (inpatient claims) and Part B (physician’s office claims). We used ICD-10-CM code U07.1 to identify Medicare beneficiaries diagnosed with COVID-19. If the beneficiaries had more than one date for a COVID-19 diagnosis, the first diagnosed date was chosen. Second, we used the primary diagnosis code for AIS (ICD-10-CM code I63) to identify the beneficiaries with AIS hospitalizations from January 1, 2019, through February 28, 2021. The diagnosis of ischemic stroke in the administrative datasets is valid and the concordance between ICD-10-CM codes in administrative datasets and the clinical diagnosed stroke was shown to be generally high.10,11 If the beneficiaries had more than one date of AIS hospitalization during the study period, the first hospitalization date was chosen. Third, we merged the above 2 datasets using the beneficiaries’ IDs to create the dataset including all Medicare beneficiaries diagnosed with COVID-19 from January 1, 2020, through February 28, 2021, and incident AIS from January 1, 2019, through February 28, 2021. Fourth, we further identified all Medicare FFS beneficiaries with at least 11 months continuous enrollment in Medicare Parts A (hospitalization) and B (office-based care) in 2020 or at least 1 month enrollment in 2021, and those who were diagnosed with COVID-19 from April 1, 2020, through February 28, 2021. For the beneficiaries who died before March 1, 2021, they must have had continuous enrollment in Medicare Parts A and B before death.
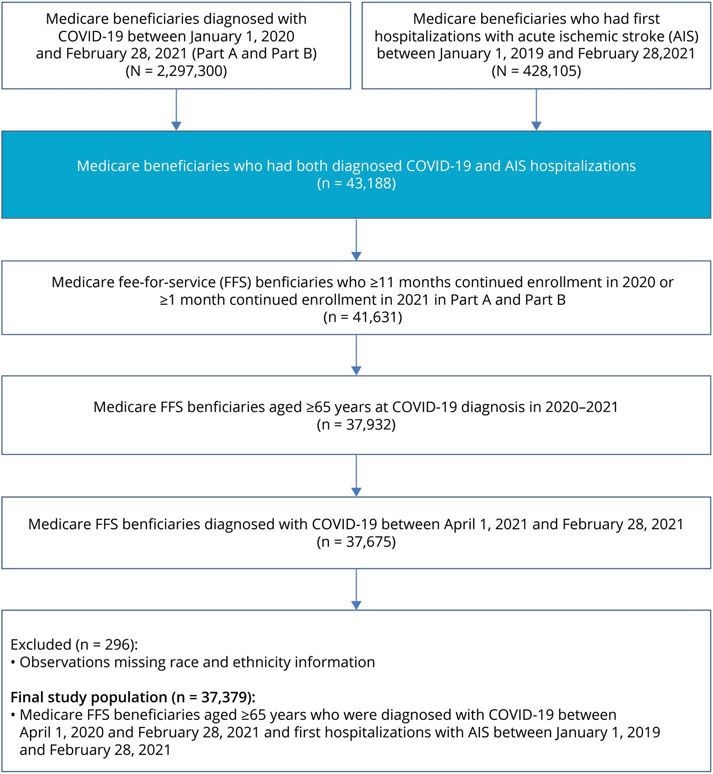
From January 1, 2019, through February 28, 2021, there were 2,297,300 beneficiaries diagnosed with COVID-19, and during January 1, 2019, through February 28, 2021, there were 428,105 beneficiaries hospitalized with incident or recurrent AIS. In the merged dataset, there were 43,188 beneficiaries diagnosed with COVID-19 and incident AIS, among which 41,631 met FFS criteria (≥11 months enrollment in both Part A and B in 2020, or ≥1 month enrollment in both Part A and B in 2021) and of these, 37,932 beneficiaries were aged ≥65 years. Of those Medicare FFS beneficiaries aged ≥65 years, 37,675 were diagnosed with COVID-19 from April 1, 2020, to February 28, 2021. After excluding the beneficiaries with missing information on race/ethnicities, the final analytical cohort had 37,379 Medicare FFS beneficiaries diagnosed with COVID-19 and AIS (Figure 1).
Figure 1.
Flowchart of US Medicare FFS Beneficiaries With Diagnosed COVID-19 and Acute Ischemic Stroke, 2019–2021
Exposure and Outcome
The exposure variable was COVID-19, and the index date of COVID-19 diagnosis for each FFS beneficiary was identified through Medicare real-time GV Part A and Part B claims data for the period between April 1, 2020, and February 28, 2021. The outcome was AIS, and the index date of AIS was identified using primary diagnosis codes in Part A from January 1, 2019, through February 28, 2021. For Medicare FFS beneficiaries with more than one date of being diagnosed with COVID-19 or AIS during the study period, the first occurrence of events was chosen as the index date. If the Medicare FFS beneficiaries had a diagnosis of stroke (any type and including transient ischemic attack) based on the Chronic Conditions Warehouse definition used by Centers for Medicare and Medicaid Services12 that occurred before January 1, 2019, they were classified as having a prior history of stroke.
Statistical Analysis
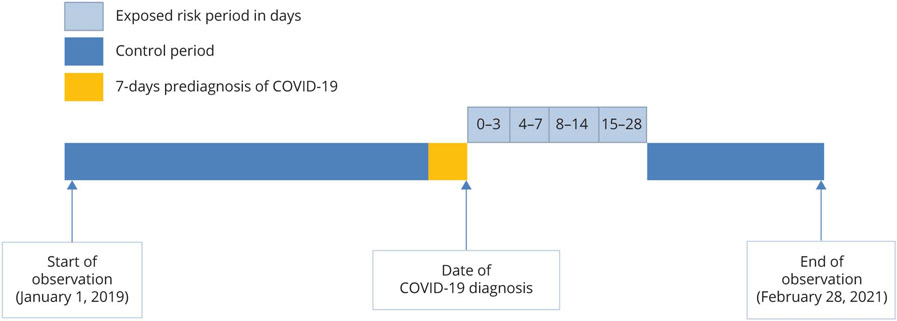
We calculated the median age and interquartile range (IQR) and the distribution of age group, sex, race/ethnicities, AIS with and without prior history of stroke, and death before end of follow-up for all Medicare FFS beneficiaries. We used self-controlled case series study design to estimate incidence rate ratio (IRR) and 95% confidence interval (CI) for risk of AIS between 0 and 28 days following a diagnosis of COVID-19. As shown in Figure 2, the self-controlled case series design is based on within-person comparisons (self-matched) after exposure during an observation period subdivided into risk and control periods, and this method implicitly controls for all fixed confounders during the period of study.13 The null hypothesis, IRR = 1.0, implies that AIS event rates remained constant during the entire observation period and were not affected by having COVID-19. An IRR >1.0 or <1.0 implies an increased or reduced risk of AIS following diagnosis of COVID-19. The study observation period started on January 1, 2019, and ended on February 28, 2021, or the date of death for those who died before the end of follow-up. We categorized the risk periods after diagnosis of COVID-19 into 0–3, 4–7, 8–14, and 15–28 days and the remaining periods served as control periods.14 However, we observed a higher number of AIS at day 0 of COVID-19 (n = 1,924) and more than expected number of AIS cases that occurred 7 days before the COVID-19 index date. We could not determine whether these AIS cases may have occurred after SARS-CoV-2 infection since the incubation period for COVID-19 may extend up to 14 days.15 Therefore, we conducted 2 analyses: one analysis had a preexposure period from day −7 to day −1, and the risk periods of 0–3, 4–7, 8–14, and 15–28 days (day 0 cases in the risk periods) and another analysis with a preexposure period from day −7 to day 0, and the risk periods of 1–3, 4–7, 8–14, and 15–28 days (day 0 cases not in the risk periods) for total AIS analysis.6,16 Other studies observed similar pattern of cases distribution around the COVID-19 index date3,6 and suggested that most patients with the same date of ischemic stroke and COVID-19 were indeed infected with SARS-CoV-2 before stroke event.6 Therefore, we presented results of the risk periods including day 0 cases for the stratified and sensitivity analyses.
Figure 2.
Graphic Representation of Self-Controlled Case Series Study Design
There are 2 key assumptions of the self-controlled case series design: (1) events do not influence subsequent exposures; (2) events do not influence the length of observation periods.17 Among FFS beneficiaries with AIS, 28.5% of them (n = 10,663) died before March 1, 2021, and the assumption of event being independent of observation period was violated (due to increased mortality after AIS hospitalization), therefore we used the modified self-controlled case series method that takes into account the event-dependent observation period of time.17,18 The IRRs were adjusted for age at diagnosis of COVID-19 from 65 to 90 years of age by 2-year age groups.
We conducted stratified analyses by age groups (65–74 years, 75–84 years, and ≥85 years), sex, race/ethnicities (non-Hispanic White, non-Hispanic Black, Hispanic, and all other race/ethnicities), and status of history of prior stroke (first vs recurrent stroke). We tested for interaction to examine whether the IRRs for risk of AIS changed significantly across the subgroups in the stratified analyses based on the likelihood ratio tests and presented adjusted p values by using the Holm method for multiple comparisons.13,17,19
We conducted several sensitivity analyses: (1) we restricted the starting time to January 1, 2020, to reduce the potential temporal changes in AIS before and after the COVID-19 pandemic (n = 21,756); (2) we excluded Medicare beneficiaries with I63.8 and I63.9 (less specific codes for AIS, n = 17,251 after exclusion); (3) we excluded all beneficiaries who died before the end of observation period (n = 26,716 after exclusion) and used the standard self-controlled case series analysis; (4) studies suggested that pneumococcal vaccination was not associated with stroke.20 We examined the association between pneumococcal vaccination and risk of AIS as a negative control to identify potential biases in using self-controlled case series design in Medicare claims data (first date of pneumococcal vaccination and AIS hospitalization, n = 23,651). We changed the starting time to the date of pneumococcal vaccination because patients with heart disease or stroke are advised to get vaccinated against pneumonia,21 which may affect the probability of pneumococcal vaccination.22 We used the standard self-controlled case series analysis among stroke survivors. SAS, version 9.4 (SAS Institute), was used for analysis, and R package SCCS was used for self-controlled case series analyses.17
This activity was reviewed by the Centers for Disease Control and Prevention (CDC) and was conducted consistent with applicable federal law and CDC policy (see for example 45 CFR part 46, 21 CFR part 56; 42 USC §241(d); 5 USC §552a; 44 USC §3,501 et seq.).
Standard Protocol Approvals, Registrations, and Patient Consents
The CDC Human Subjects Coordinator determined that this study did not require review for human subjects protections because the data did not contain personal identifiers and were not originally collected specifically for this study. Therefore, the requirement of informed consent was waived.
Data Availability
Medicare data are available from Centers for Medicare & Medicaid Services, Department of Health and Human Services, for any qualified investigator.
Results
Among 37,379 Medicare FFS beneficiaries diagnosed with COVID-19 and AIS, the median age was 80.4 years (IQR 73.5–87.1 years), 56.7% (95% CI 56.2%–57.2%) were women, and 75.9% (75.4%–76.3%) were non-Hispanic White. Among the beneficiaries, 34.0% (33.5%–34.5%) had prior history of stroke and 28.5% (28.1%–29.0%) died before the end of the observation period (Table 1).
Table 1.
Characteristics of US Medicare Fee-for-Service Beneficiaries With Diagnosed COVID-19 and Acute Ischemic Stroke, Medicare 2019–2021
| Characteristics | Medicare FFS beneficiaries, n |
Median or % (95% CI) |
|---|---|---|
| Age, y, median (IQR) | 37,379 | 80.4 (73.5–87.1) |
| Age group, y | ||
| 65–74 | 11,305 | 30.2 (29.8–30.7) |
| 75–84 | 13,873 | 37.1 (36.6–37.6) |
| ≥85 | 12,201 | 32.6 (32.2–33.1) |
| Sex | ||
| Men | 16,199 | 43.3 (42.8–43.8) |
| Women | 21,180 | 56.7 (56.2–57.2) |
| Race/ethnicities | ||
| Non-Hispanic White | 28,361 | 75.9 (75.4–76.3) |
| Non-Hispanic Black | 5,030 | 13.5 (13.1 −13.8) |
| Hispanic | 2,560 | 6.9 (6.6–7.1) |
| All other race/ethnicities | 1,428 | 3.8 (3.6–4.0) |
| History of stroke a | ||
| Yes | 12,715 | 34.0 (33.5–34.5) |
| No | 24,664 | 66.0 (65.5–66.5) |
| Death before end of follow-up | ||
| Yes | 10,663 | 28.5 (28.1–29.0) |
| No | 26,716 | 71.5 (71.0–71.9) |
Abbreviations: CI = confidence interval; COVID-19 = coronavirus disease 2019; FFS = fee-for-service; IQR = interquartile range.
Medicare FFS beneficiaries who had stroke before January 1, 2019.
When AIS at day of exposure (day 0) was included in the risk period, IRRs at 0–3, 4–7, 8–14, and 15–28 days following COVID-19 diagnosis were 10.3 (9.86–10.8), 1.61 (1.44–1.80), 1.44 (1.32–1.57), and 1.09 (1.02–1.18); when AIS at day 0 was excluded in the risk period, the corresponding IRRs were 1.77 (1.57–2.01) (day 1–3), 1.60 (1.43–1.79), 1.43 (1.31–1.56), and 1.09 (1.01–1.17), respectively (Table 2).
Table 2.
Acute Ischemic Stroke Among US Medicare Fee-for-Service Beneficiaries Diagnosed With COVID-19, 2019–2021
| Risk period | Events, n | Crude IRR (95% CI) | Age-adjusted IRR (95% CI)a |
|---|---|---|---|
| Day 0 cases in the risk period, d | |||
| −7 to −1 | 789 | 1.82 (1.69–1.95) | 1.91 (1.78–2.05) |
| 0–3 | 2,173 | 9.82 (9.40–10.3) | 10.3 (9.86–10.8) |
| 4–7 | 322 | 1.53 (1.37–1.71) | 1.61 (1.44–1.80) |
| 8–14 | 523 | 1.37 (1.25–1.49) | 1.44 (1.32–1.57) |
| 15–28 | 735 | 1.04 (0.96–1.12) | 1.09 (1.02–1.18) |
| Baselineb | 32,837 | 1.0 | 1.0 |
| Day 0 cases not in the risk period, d | |||
| −7 to 0 | 2,713 | 5.34 (5.13–5.55) | 5.60 (5.37–5.83) |
| 1–3 | 249 | 1.69 (1.49–1.91) | 1.77 (1.57–2.01) |
| 4–7 | 322 | 1.52 (1.36–1.70) | 1.60 (1.43–1.79) |
| 8–14 | 523 | 1.36 (1.25–1.49) | 1.43 (1.31–1.56) |
| 15–28 d | 735 | 1.04 (0.96–1.12) | 1.09 (1.01–1.17) |
| Baselineb | 32,837 | 1.0 | 1.0 |
Abbreviations: CI = confidence interval; COVID-19 = coronavirus disease 2019; IRR = incidence rate ratio.
Adjusted by age at diagnosis of COVID-19 of 65–90 years of age by 2-year age group.
All time from observation start on January 1, 2019, to 7 days before date of COVID-19 diagnosis and after 28 days of diagnosis of COVID-19.
The association appeared to be stronger among younger (ages 65–74 years) beneficiaries and among beneficiaries without prior history of stroke. The age-adjusted IRRs at 0–3, 4–7, 8–14, and 15–28 days following the diagnosis of COVID-19 were 14.7 (13.6–15.8), 2.45 (2.02–2.92), 1.70 (1.46–1.98), and 1.06 (0.92–1.22) among those aged 65–74 years compared to 7.04 (6.46–7.66), 1.10 (0.89–1.35), 1.15 (0.98–1.34), and 1.05 (0.93–1.19) among those aged ≥85 years (p < 0.001); the corresponding comparisons for beneficiaries with and without prior history of stroke were 7.92 (7.26–8.63), 1.00 (0.78–1.27), 1.22 (1.03–1.43), and 1.10 (0.96–1.25) vs 14.6 (13.9–15.4), 2.41 (2.13–2.73), 1.88 (1.70–2.08), and 1.24 (1.14–1.36) (p < 0.001). The pattern of association between COVID-19 and risk for AIS were largely consistent across sex and race/ethnicities (Table 3) and for the sensitivity analyses (Table 4). The IRRs between pneumococcal vaccination and AIS were approximately 1.0 across the exposure period, suggesting the validity of using self-controlled case series design in Medicare claims data (Table 4).
Table 3.
Acute Ischemic Stroke Among US Medicare Fee-for-Service Beneficiaries With Diagnosed COVID-19 by Selected Characteristics, 2019–2021
| Characteristics | Events, n | Crude IRR (95% CI) | Age adjusted IRR (95% CI)a | P valueb |
|---|---|---|---|---|
| Age group, y | ||||
| 65–74 | ||||
| −7 to −1 d | 228 | 2.06 (1.80–2.35) | 2.14 (1.88–2.45) | |
| 0–3 d | 794 | 14.1 (13.1–15.1) | 14.7 (13.6–15.8) | |
| 4–7 d | 128 | 2.35 (1.97–2.79) | 2.45 (2.05–2.92) | |
| 8–14 d | 168 | 1.63 (1.40–1.90) | 1.70 (1.46–1.98) | |
| 15–28 d | 204 | 1.02 (0.89–1.17) | 1.06 (0.92–1.22) | |
| Baselinec | 9,783 | 1.0 | 1.0 | |
| 75–84 | ||||
| −7 to −1 d | 277 | 1.80 (1.60–2.03) | 1.87 (1.66–2.11) | |
| 0–3 d | 790 | 10.1 (9.41–10.9) | 10.5 (9.78–11.4) | |
| 4–7 d | 107 | 1.44 (1.19–1.74) | 1.50 (1.24–1.81) | |
| 8–14 d | 196 | 1.43 (1.24–1.65) | 1.49 (1.30–1.72) | |
| 15–28 d | 277 | 1.09 (0.96–1.23) | 1.13 (1.00–1.28) | |
| Baselinec | 12,226 | 1.0 | 1.0 | |
| ≥85 | ||||
| −7 to −1 d | 284 | 1.71 (1.52–1.93) | 1.79 (1.59–2.02) | |
| 0–3 d | 589 | 6.72 (6.18–7.31) | 7.04 (6.46–7.66) | |
| 4–7 d | 87 | 1.05 (0.85–1.29) | 1.10 (0.89–1.35) | |
| 8–14 d | 159 | 1.09 (0.94–1.28) | 1.15 (0.98–1.34) | |
| 15–28 d | 254 | 1.00 (0.88–1.14) | 1.05 (0.93–1.19) | |
| Baselinec | 10,828 | 1.0 | 1.0 | <0.0011 |
| Sex | ||||
| Men | ||||
| −7 to −1 d | 369 | 1.96 (1.77–2.18) | 2.10 (1.89–2.33) | |
| 0–3 d | 990 | 10.4 (9.78–11.1) | 11.2 (10.5–11.9) | |
| 4–7 d | 147 | 1.64 (1.39–1.93) | 1.75 (1.49–2.07) | |
| 8–14 d | 259 | 1.59 (1.40–1.80) | 1.70 (1.50–1.93) | |
| 15–28 d | 285 | 0.95 (0.84–1.07) | 1.02 (0.91–1.15) | |
| Baselinec | 14,149 | 1.0 | 1.0 | |
| Women | ||||
| −7 to −1 d | 420 | 1.77 (1.61–1.95) | 1.83 (1.66–2.02) | |
| 0–3 d | 1,183 | 9.57 (9.01–10.2) | 9.87 (9.29–10.5) | |
| 4–7 d | 175 | 1.47 (1.26–1.70) | 1.52 (1.31–1.76) | |
| 8–14 d | 264 | 1.21 (1.07–1.37) | 1.25 (1.11–1.41) | |
| 15–28 d | 450 | 1.11 (1.01–1.22) | 1.15 (1.04–1.26) | |
| Baselinec | 18,688 | 1.0 | 1.0 | 0.011 |
| Race/ethnicities | ||||
| Non-Hispanic White | ||||
| −7 to −1 d | 601 | 1.82 (1.68–1.97) | 1.90 (1.76–2.07) | |
| 0–3 d | 1,613 | 9.51 (9.04–10.0) | 9.97 (9.46–10.5) | |
| 4–7 d | 239 | 1.48 (1.30–1.68) | 1.55 (1.37–1.76) | |
| 8–14 d | 398 | 1.36 (1.23–1.50) | 1.43 (1.29–1.58) | |
| 15–28 d | 560 | 1.04 (0.96–1.13) | 1.09 (1.00–1.19) | |
| Baselinec | 24,950 | 1.0 | 1.0 | |
| Non-Hispanic Black | ||||
| −7 to −1 day | 99 | 2.01 (1.65–2.46) | 2.11 (1.72–2.57) | |
| 0–3 d | 268 | 11.0 (9.76–12.5) | 11.6 (10.2–13.1) | |
| 4–7 d | 39 | 1.68 (1.23–2.30) | 1.76 (1.28–2.41) | |
| 8–14 d | 62 | 1.42 (1.11–1.83) | 1.49 (1.16–1.91) | |
| 15–28 d | 100 | 1.16 (0.95–1.42) | 1.22 (1.00–1.49) | |
| Baselinec | 4,462 | 1.0 | 1.0 | |
| Hispanic | ||||
| −7 to −1 d | 59 | 2.11 (1.63–2.74) | 2.20 (1.70–2.86) | |
| 0–3 d | 190 | 13.1 (11.2–15.2) | 13.6 (11.7–15.9) | |
| 4–7 d | 30 | 2.12 (1.48–3.04) | 2.22 (1.54–3.19) | |
| 8–14 d | 36 | 1.38 (0.99–1.93) | 1.45 (1.04–2.02) | |
| 15–28 d | 56 | 1.16 (0.89–1.51) | 1.21 (0.93–1.59) | |
| Baselinec | 2,189 | 1.0 | 1.0 | |
| All other race/ethnicities | ||||
| −7 to −1 d | 30 | 1.74 (1.21–2.51) | 1.86 (1.29–2.69) | |
| 0–3 d | 102 | 11.9 (9.71–14.6) | 12.8 (10.4–15.8) | |
| 4–7 d | 14 | 1.74 (1.03–2.96) | 1.87 (1.10–3.18) | |
| 8–14 d | 27 | 1.83 (1.25–2.69) | 1.96 (1.34–2.89) | |
| 15–28 d | 19 | 0.71 (0.45–1.12) | 0.76 (0.48–1.20) | |
| Baselinec | 1,236 | 1.0 | 1.0 | 0.010 |
| History of stroke | ||||
| Yes | ||||
| −7 to −1 d | 238 | 1.52 (1.34–1.73) | 1.69 (1.48–1.92) | |
| 0–3 d | 566 | 7.13 (6.55–7.76) | 7.92 (7.26–8.63) | |
| 4–7 d | 67 | 0.90 (0.70–1.14) | 1.00 (0.78–1.27) | |
| 8–14 d | 147 | 1.09 (0.93–1.28) | 1.22 (1.03–1.43) | |
| 15–28 d | 243 | 0.98 (0.86–1.12) | 1.10 (0.96–1.25) | |
| Baselinec | 11,454 | 1.0 | 1.0 | |
| No | ||||
| −7 to −1 d | 551 | 2.45 (2.25–2.66) | 2.48 (2.28–2.70) | |
| 0–3 d | 1,607 | 14.4 (13.7–15.2) | 14.6 (13.9–15.4) | |
| 4–7 d | 255 | 2.38 (2.10–2.69) | 2.41 (2.13–2.73) | |
| 8–14 d | 376 | 1.85 (1.67–2.05) | 1.88 (1.70–2.08) | |
| 15–28 d | 492 | 1.23 (1.12–1.34) | 1.24 (1.14–1.36) | |
| Baselinec | 21,383 | 1.0 | 1.0 | <0.001 |
Abbreviations: CI = confidence interval; COVID-19 = coronavirus disease 2019; IRR = incidence rate ratio.
Adjusted by age at diagnosis of COVID-19 of 65–90 years by 2-year age group.
p Value for interaction was calculated using likelihood ratio test and adjusted for multiple comparison based on Holm methods.
All times from observation start on January 1, 2019, to 7 days before date of COVID-19 diagnoses and after 28 days of diagnosis of COVID-19.
Table 4.
Sensitivity Analyses of Acute Ischemic Stroke Among US Medicare Fee-for-Service Beneficiaries, Medicare 2019–2021
| Risk period | Number of events |
Crude IRR (95% CI) |
Age-adjusted IRR (95% CI)a |
|---|---|---|---|
| Restricted the starting time of the study period to January 1, 2020 c | |||
| −7 to −1 d | 789 | 1.90 (1.77–2.05) | 1.93 (1.80–2.08) |
| 0–3 d | 2,173 | 10.2 (9.76–10.7) | 10.4 (9.92–10.9) |
| 4–7 d | 322 | 1.58 (1.42–1.77) | 1.61 (1.44–1.80) |
| 8–14 d | 523 | 1.40 (1.28–1.52) | 1.42 (1.30–1.55) |
| 15–28 d | 735 | 1.05 (0.97–1.13) | 1.07 (0.99–1.15) |
| Baselineb | 17,214 | 1.0 | 1.0 |
| Excluding AIS with I63.8 and I63.9 diagnosis codes d | |||
| −7 to −1 d | 403 | 1.95 (1.76–2.15) | 2.00 (1.81–2.21) |
| 0–3 d | 1,108 | 10.3 (9.63–10.9) | 10.5 (9.89–11.2) |
| 4–7 d | 186 | 1.81 (1.57–2.09) | 1.86 (1.61–2.16) |
| 8–14 d | 299 | 1.61 (1.44–1.81) | 1.66 (1.48–1.86) |
| 15–28 d | 392 | 1.15 (1.04–1.27) | 1.18 (1.07–1.31) |
| Baselineb | 14,863 | 1.0 | 1.0 |
| Deleted Medicare FFS beneficiaries who died before the end of observation period e | |||
| −7 to −1 d | 505 | 2.26 (2.17–2.34) | 2.25 (2.16–2.34) |
| 0–3 d | 1,487 | 11.7 (11.6–11.7) | 11.6 (11.6–11.7) |
| 4–7 d | 204 | 1.61 (1.47–1.75) | 1.61 (1.47–1.75) |
| 8–14 d | 353 | 1.61 (1.51–1.72) | 1.61 (1.50–1.71) |
| 15–28 d | 459 | 1.07 (0.98–1.17) | 1.07 (0.98–1.17) |
| Baselineb | 23,708 | 1.0 | 1.0 |
| Medicare FFS beneficiaries who had pneumococcal vaccination and AIS from January 1, 2019, to February 28, 2021 f | |||
| 0–3 d | 256 | 1.13 (0.99–1.25) | 1.13 (0.99–1.28) |
| 4–7 d | 254 | 1.12 (0.99–1.25) | 1.12 (0.98–1.27) |
| 8–14 d | 447 | 1.13 (1.03–1.23) | 1.13 (1.03–1.23) |
| 15–28 d | 841 | 1.07 (0.99–1.14) | 1.07 (0.99–1.14) |
| Baselineb | 21,853 | 1.0 | 1.0 |
Abbreviations: AIS = acute ischemic stroke; CI = confidence interval; COVID-19 = coronavirus disease 2019; FFS = fee-for-service; IRR = incidence rate ratio.
Adjusted by age at diagnosis of COVID-19 of <65–90 years by 2-year age group.
All time from observation start on January 1, 2019, to 7 days before date of COVID-19 diagnoses and after 28 days of diagnosis of COVID-19.
The starting time of the observation period was January 1, 2020, and ended on February 28, 2021 (n = 21,756).
Excluded AIS cases that had I63.8 and I63.9 ICD-10 diagnosis codes (n = 17,251).
Medicare FFS beneficiaries who died before the end of follow-up were excluded (n = 26,716).
Included Medicare FFS beneficiaries who had pneumococcal vaccination and AIS from January 1, 2019, to February 28, 2021. If the Medicare beneficiaries had more than one pneumococcal vaccination or AIS hospitalizations, the first date was chosen for the analysis. The date of pneumococcal vaccination was the starting time of the study period and ended on February 28, 2021. Standard self-controlled case series was used among stroke survivors (n = 23,651).
This study provides Class IV evidence that SARS-CoV-2 infection, the virus that causes COVID-19, is associated with increased risk of AIS in the first 3 days after diagnosis in Medicare FFS beneficiaries ≥65 years of age.
Discussion
We found that the incidence of AIS hospitalizations was 10 times (day 0 cases in the risk periods) as high during the first 3 days after diagnosis of COVID-19 as during the control period among Medicare FFS beneficiaries aged ≥65 years. There was a graded stronger association from older to younger beneficiaries. The association appeared to be stronger among the beneficiaries without prior history of stroke. We observed a higher number of AIS (n = 1,924) at day 0 of COVID-19 index date. A Swedish study using self-controlled case series design also found higher number of ischemic stroke and COVID-19 at day 0. The IRR for ischemic stroke was 6.18 (4.06–9.42) for the first week following diagnosis of COVID-19 (including day 0 cases in the risk periods) and IRR of 2.97 (1.71–5.15) excluding day 0 cases.6 With the mean incubation period for COVID-19 of 5.1 days (97.5% of patients developing symptoms within 12.5 days), the Swedish study suggested that the patients at day 0 were highly likely to be infected with SARS-CoV-2 before their stroke event. Most hospitals screen all patients for COVID-19 on hospital admission in the United States, and some patients with AIS may have had SARS-CoV-2 infection before hospitalizations and continue to have positive testing results after the initial infection. Those patients were likely to have the same date of AIS hospitalizations and COVID-19 diagnosis in the Medicare claims but also to have an AIS precipitated by SARS-CoV-2 infection.
Our results of increased risk for AIS following diagnosis of COVID-19 were consistent with other studies.1,3,5,6 Modin et al.3 used Danish nationwide register data and self-controlled case series design to examine the association between COVID-19 and ischemic stroke and reported an IRR of 12.9 (7.1–23.5) during the first 14 days following diagnosis of COVID-19. However, using data extracted from the electronic medical records of 54 health care facilities in the United States, Qureshi et al.7 documented similar rates of AIS among patients hospitalized with and without COVID-19 (1.3% vs 1.0%). Another retrospective cohort study of hospitalized patients with COVID-19 within a major health system in the New York City metropolitan area reported lower prevalence of imaging-confirmed AIS compared to the contemporary controls (patients without COVID-19) and historical controls (patients in 2019), but suggested that the rate of AIS may be underestimated due to the challenge of diagnosing stroke among critically ill patients with COVID-19.23 A cross-sectional study of the hospital discharge data from a health care system in New York state with data collected from January to April 2020 found that the prevalence of AIS was significantly less frequent among patients with diagnosed COVID-19 compared to those without COVID-19 (adjusted odds ratio 0.29 [0.18–0.48]).8 Residual confounding and uncertainties in coding of COVID-19 during the early phase of the pandemic may contribute to this observed association. The findings of our study support those of previous studies of a significant association between COVID-19 and stroke and provide further evidence of the association from a large Medicare cohort of beneficiaries 65 years or older.
Our results suggest that the risk of AIS following diagnosis of COVID-19 was higher among beneficiaries aged 65–74 years compared to those aged ≥85 years (IRR in 0–3 days 14.7 (13.6–15.8) vs 7.04 (6.46–7.66), p < 0.001). We are not aware of any study that provided age-stratified analysis of risk of COVID-19–associated AIS. The reasons for stronger association among younger Medicare beneficiaries are not clear. Studies have reported increased incidence of large vessel stroke among younger patients with COVID-19.4,24 Other studies observed that patients with COVID-19 and stroke were significantly younger than patients with stroke without COVID-19,23,25-27 with a pooled age difference of 6 years.2 In the general population, the incidence of stroke increases rapidly with age.9,28 If COVID-19 disproportionally affects younger patients with respect to risk of stroke, it might partly explain the stronger association between COVID-19 and AIS among younger Medicare beneficiaries because of relatively lower baseline risk among younger beneficiaries. On the other hand, the higher risk among younger beneficiaries might be due to inclusion bias because older beneficiaries with severe COVID-19 might have not survived to get tested for COVID-19 or evaluated for stroke. Further studies are needed to examine the incidence of stroke subtypes by age group.
The risk of AIS following diagnosis of COVID-19 appeared to be higher among beneficiaries without a history of stroke compared to those with a history of stroke (IRR in 0–3 days 14.6 [13.9–15.4] vs 7.92 [7.26–8.63], p < 0.001). The reasons for the stronger association among the beneficiaries without history of stroke are not clear. Medicare beneficiaries with history of stroke may be more likely to be on medications for secondary prevention of stroke.29,30 The beneficiaries who had a history of stroke were older than the beneficiaries without a history of stroke (median age 82.1 years vs 79.2 years, p < 0.05) and this might partly contribute to the stronger association because of the stronger association between COVID-19 and AIS among younger Medicare beneficiaries. The association between COVID-19 and AIS appeared to be stronger among men compared to women (p = 0.011). The detailed analysis suggested that the difference was mainly driven by the IRRs 8–14 days after diagnosis of COVID-19 (1.70 (1.50–1.93) among men vs 1.25 (1.11–1.41) among women. The association between COVID-19 and AIS appeared to be different across race/ethnic group (p = 0.010). There is no difference between non-Hispanic White and non-Hispanic Black (p = 0.293) and between Mexican American and Other (p = 0.268) but the risk appeared to be stronger among Mexican American and Other compared to non-Hispanic White and non-Hispanic Black. However, the pattern of association remained largely consistent across race/ethnic group, and these statistically significant differences by sex and race/ethnicities may not be clinically significant. Further study is warranted to determine the sex and race/ethnic-specific association between COVID-19 and AIS.
Many studies provided evidence showing the presence of systemic infection, such as influenza, systemic respiratory tract infection, and herpes zoster, as a trigger of stroke.31-36 The mechanisms underlying the association between COVID-19 and risk of stroke are not yet fully understood but are considered complex and may involve multiple pathways. The key proposed mechanisms suggested that SARS-CoV-2 binds to the angiotensin-converting enzyme 2 receptors of epithelial and endothelial cells where the immunologic activation occurs that can lead to development of cytokine storm and hypercoagulability, and an increased tendency of blood clots formation, leading to increased risk of AIS.2,37 SARS-CoV-2 infection may also have direct viral injury causing viral-induced endotheliitis, potentially leading to angiopathic thrombosis.38 The analysis of endothelial cells from the human brain suggested that SARS-CoV-2 can directly affect the brain endothelial cells, triggering a unique gene expression profile in brain endothelia, and increase the risk of stroke.39
Studies suggested that COVID-19–associated ischemic strokes are more severe, with worse functional outcomes and significantly higher mortality, than non–COVID-19–associated ischemic strokes.2,27,40,41 Although the overall incidence of COVID-19–associated AIS is not clear, emerging evidence suggests that the incidence of large vessel stroke may increase in patients with COVID-19 who might not have the typical risk factors for stroke.2,4,24 Timely diagnosis of COVID-19–associated stroke and providing recommended treatment might play important roles in reducing the morbidity and mortality in patients with temporally COVID-19–associated stroke during the pandemic. Further studies are warranted to examine the long-term effects of COVID-19–associated stroke.
The main strength of this study is the use of the self-controlled case series study design. This study design is based on within-person comparisons and implicitly controls for all fixed confounders.42 Also, the self-controlled case series is more efficient than other observational study designs and provides more precise estimates of the exposure effects on risk of outcomes.13
Our study had several limitations. First, we used Medicare real-time GV preliminary data that are updated on a monthly basis. We might have missed some beneficiaries with diagnosed COVID-19 and AIS or have misclassified some beneficiaries who died before end of follow-up as alive because of delayed reporting. However, Centers for Medicare & Medicaid Services indicated that more than 95% of Medicare FFS in-hospital claims were received within 3 months.43 Second, Medicare beneficiaries with COVID-19 were identified through the administrative claims data and might be subject to misclassification. However, one study suggested that the physicians and hospitals were likely to follow the recommendations and guidelines regarding COVID-19 diagnosis because of the seriousness of the COVID-19 pandemic.44 Third, the dates of diagnosed COVID-19 and AIS in Medicare data might be subject to error. Diagnostic testing for COVID-19 in outpatient settings, especially during the early phase of the pandemic, was limited and types of tests used to confirm COVID-19 and turnaround time from the onset of symptoms to return a positive test varied. In addition, the beneficiaries without COVID-19 symptoms were unlikely to get tested in the outpatient settings. We observed higher number of AIS and COVID-19 at day 0 and more than the expected AIS hospitalizations within 7 days before the index date of diagnosed COVID-19. Screening all patients for COVID-19 on hospital admission including some patients who may continue to have positive testing results after the initial infection might contribute to the higher number of AIS and COVID-19 at day 0. These factors may affect the accurate timing of exposure to SARS-CoV-2 among Medicare beneficiaries. Fourth, the self-controlled case series methods assume that the other confounders affecting the outcome remain largely unchanged during the study period. However, the COVID-19 pandemic affected all hospitals and health care systems, including changes in emergency department visits and hospitalizations for stroke or stroke-like symptoms, care of acute stroke, and access to preventive care.5,27,45-47 These changes may affect the assumption of unchanged confounders during the study period. In the sensitivity analyses, we restricted the starting time of the study to January 1, 2020, and pattern of association remained largely consistent. Fifth, we restricted our study to Medicare FFS beneficiaries, which included about 60% of Medicare beneficiaries, thus our findings are not generalizable to non-FFS beneficiaries.
Incidence of AIS hospitalizations was 10 times (including day 0 cases in the risk periods) as high during the 3 days after diagnosis of COVID-19 as during the control periods and risk associated with COVID-19 appeared to be stronger among younger Medicare FFS beneficiaries and among beneficiaries without history of stroke.
Acknowledgment
The authors thank Dr. Heather J. Whitaker, Public Health England, an executive agency of the Department of Health and Social Care in the United Kingdom, for providing help with the self-controlled case series analysis of event-dependent observation periods using R package SCCS; and Eric Hong, Hamilton College, for help with the literature review for this study during his summer student internship in 2020 at Division for Heart Disease and Stroke Prevention, CDC. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Glossary
- AIS
acute ischemic stroke
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- COVID-19
coronavirus disease 2019
- FFS
fee-for-service
- GV
geographic variation
- ICD-10-CM
International Classification of Diseases, 10th revision, Clinical Modification
- IQR
interquartile range
- IRR
incidence rate ratio
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Appendix. Authors
| Name | Location | Contribution |
|---|---|---|
| Quanhe Yang, PhD | Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta, GA | Designed and conceptualized study; conducted statistical analysis; drafted the manuscript for intellectual content |
| Xin Tong, MPH | Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta, GA | Validated and generated analytical datasets; conducted statistical analyses |
| Mary G. George, MD, MSPH | Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta, GA | Interpreted the data; revised the manuscript for intellectual content |
| Anping Chang, MPH | Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta, GA | Generated the analytical datasets |
| Robert Merritt, MS | Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta, GA | Interpreted the data; revised the manuscript for intellectual content |
Footnotes
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020; 77(11):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2021;16(2):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modin D, Claggett B, Sindet-Pedersen C, et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142(21):2080–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382(20):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsanos AH, Palaiodimou L, Zand R, et al. The impact of SARS-Cov-2 on stroke epidemiology and care: a meta-analysis. Ann Neurol. 2021;89(2):380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsoularis I, Fonseca-Rodriguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. 2021;398(10300):599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qureshi AI, Baskett WI, Huang W, et al. Acute ischemic stroke and COVID-19: an analysis of 27 676 patients. Stroke. 2021;52(3):905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekelis K, Missios S, Ahmad J, et al. Ischemic stroke occurs less frequently in patients with COVID-19: a multicenter cross-sectional study. Stroke. 2020;51(12):3570–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics: 2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. [DOI] [PubMed] [Google Scholar]
- 10.McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PLoS One. 2015;10(8):e0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang TE, Tong X, George MG, et al. Trends and factors associated with concordance between International Classification of Diseases, ninth and tenth revision, clinical modification codes and stroke clinical diagnoses. Stroke. 2019;50(8):1959–1967. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Medicare & Medicaid Services. Chronic conditions data warehouse. Available from: 2.ccwdata.org/web/guest/condition-categories. Accessed September 1, 2021.
- 13.Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25(10):1768–1797. [DOI] [PubMed] [Google Scholar]
- 14.Kwong JC, Schwartz KL, Campitelli MA. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378(4):2540–2541. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Available from: cdc.gov/coronavirus/2019-nCoV/hcp/clinical-guidance-management-patients.html. Accessed September 1, 2021.
- 16.Fonseca-Rodriguez O, Fors Connolly AM, Katsoularis I, Lindmark K, Farrington P. Avoiding bias in self-controlled case series studies of coronavirus disease 2019. Stat Med. 2021;40(27):6197–6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrington P, Whitaker H, Ghebremichael Weldeselassie Y. Self-controlled Case Series Studies: A Modelling Guide with R. CRC Press, Taylor & Francis Group; 2018. [Google Scholar]
- 18.Farrington CP, Anaya-Izquierdo K, Whitaker HJ, Hocine MN, Douglas I, Smeeth L. Self-controlled case series analysis with event-dependent observation periods. J Am Stat Assoc. 2011;106:417–426. [Google Scholar]
- 19.Holm SA. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 20.Kaczorowski J Pneumococcal vaccination and cardiovascular events in men. JAMA. 2010;304(7):742. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Heart disease, stroke, or other cardiovascular disease and adult vaccination. Available from: cdc.gov/vaccines/adults/rec-vac/health-conditions/heart-disease.html. Accessed September 1, 2021.
- 22.Weldeselassie YG, Whitaker HJ, Farrington CP. Use of the self-controlled case-series method in vaccine safety studies: review and recommendations for best practice. Epidemiol Infect. 2011;139(12):1805–1817. [DOI] [PubMed] [Google Scholar]
- 23.Yaghi S, Ishida K, Torres J, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51(7):2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fifi JT, Mocco J. Covid-19 related stroke in young individuals. Lancet Neurol. 2020;19(9):713–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majidi S, Fifi JT, Ladner TR, et al. Emergent large vessel occlusion stroke during New York City’s COVID-19 outbreak: clinical characteristics and paraclinical findings. Stroke. 2020;51(9):2656–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sweid A, Hammoud B, Bekelis K, et al. Cerebral ischemic and hemorrhagic complications of coronavirus disease 2019. Int J Stroke. 2020;15(7):733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava PK, Zhang S, Xian Y, et al. Acute ischemic stroke in patients with COVID-19: an analysis from Get With the Guidelines–Stroke. Stroke. 2021;52(5):1826–1829. [DOI] [PubMed] [Google Scholar]
- 28.Kissela BM, Khoury JC, Alwell K, et al. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79(17):1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalli LL, Kim J, Thrift AG, et al. Patterns of use and discontinuation of secondary prevention medications after stroke. Neurology. 2021;96(1):e30–e41. [DOI] [PubMed] [Google Scholar]
- 30.Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–e467. [DOI] [PubMed] [Google Scholar]
- 31.Fugate JE, Lyons JL, Thakur KT, Smith BR, Hedley-Whyte ET, Mateen FJ. Infectious causes of stroke. Lancet Infect Dis. 2014;14:869–880. [DOI] [PubMed] [Google Scholar]
- 32.Elkind MSV, Boehme AK, Smith CJ, Meisel A, Buckwalter MS. Infection as a stroke risk factor and determinant of outcome after stroke. Stroke. 2020;51(10):3156–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(1):2611–2618. [DOI] [PubMed] [Google Scholar]
- 34.Kulick ER, Canning M, Parikh NS, Elkind MSV, Boehme AK. Seasonality of influenza-like-illness and acute cardiovascular events are related regardless of vaccine effectiveness. J Am Heart Assoc. 2020;9(20):e016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langan SM, Minassian C, Smeeth L, Thomas SL. Risk of stroke following herpes zoster: a self-controlled case-series study. Clin Infect Dis. 2014;58(11):1497–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Q, George MG, Chang A, Tong X, Merritt R, Hong Y. Effect of herpes zoster vaccine and antiviral treatment on risk of ischemic stroke. Neurology. 2020;95(6):e708–e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zakeri A, Jadhav AP, Sullenger BA, Nimjee SM. Ischemic stroke in COVID-19-positive patients: an overview of SARS-CoV-2 and thrombotic mechanisms for the neurointerventionalist. J Neurointerv Surg. 2021;13(3):202–206. [DOI] [PubMed] [Google Scholar]
- 38.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaneko N, Satta S, Komuro Y, et al. Flow-mediated susceptibility and molecular response of cerebral endothelia to SARS-CoV-2 infection. Stroke. 2021;52(1):260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain R, Young M, Dogra S, et al. Covid-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ntaios G, Michel P, Georgiopoulos G, et al. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: the global COVID-19 stroke registry. Stroke. 2020;51(9):e254–e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354:i4515. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Medicare & Medicaid Services. Preliminary Medicare Covid-19 Data Snapshot: Medicare Claims and Encounter Data: January 1, 2020 to June 19, 2021, Received by July 16, 2021. Available from: cms.gov/files/document/medicare-COVID-19-data-snapshot-fact-sheet.pdf. Accessed September 1, 2021.
- 44.Izurieta HS, Graham DJ, Jiao Y, et al. Natural history of COVID-19: risk factors for hospitalizations and deaths among >26 million US Medicare beneficiaries. J Infect Dis. 2021;223(6):945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Q, Tong X, Coleman King S, Olivari BS, Merritt RK. Stroke hospitalizations before and during COVID-19 pandemic among Medicare beneficiaries in the United States. Stroke. 2021;52(11):3586–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tong X, King SMC, Asaithambi G, et al. Covid-19 pandemic and quality of care and outcomes of acute stroke hospitalizations: the Paul Coverdell National Acute Stroke Program. Prev Chronic Dis. 2021;18:E82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions: United States, January-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Medicare data are available from Centers for Medicare & Medicaid Services, Department of Health and Human Services, for any qualified investigator.