Abstract
It has been suggested that class C β-lactamases have evolved to carry out a metabolic reaction other than hydrolysis of β-lactam antibiotics. It is demonstrated in the present study that the class C β-lactamase from Enterobacter cloacae P99 has reached the diffusion limit in its ability to hydrolyze its preferred cephalosporin substrates. The increase in the solution viscosity by addition of a microviscogen (sucrose) caused the decline in the parameter kcat/Km for hydrolysis of cephaloridine and cephalosporin C (approximately 2.5-fold at a relative viscosity of 2.9). A similar increase in viscosity has no effect on the turnover rate of the poorer substrates cefepime and penicillin G. Addition of a macroviscogen (polyethylene glycol) to the reaction mixture did not change the rate of turnover for any of the substrates tested because in this case the viscogen would not interfere with the motion of small molecules, as was expected. Therefore, it would appear that the driving force behind the evolution of this class C β-lactamase and, in principle, other enzymes of this class is indeed the functional reaction of this enzyme as a drug resistance factor.
β-Lactamases are the primary cause of bacterial resistance to β-lactam antibiotics. These enzymes hydrolyze the β-lactam bonds of these antibacterial agents, whereby the activity of the drug is lost and the phenotypic expression of resistance is manifested. There are four classes of β-lactamases, of which class A enzymes are the most common group and class C enzymes are the second most common group (7, 8).
Literature from the late 1960s had suggested that certain β-lactamases might have additional metabolic functions besides hydrolysis of β-lactams (32, 34). The recent disclosure of an elaborate system for regulation and recycling of the peptidoglycan has revealed metabolic ties to induction of the class C β-lactamases from gram-negative organisms (19, 29, 30). These observations have prompted the assertion that, indeed, for the case of the class C β-lactamases an alternative metabolic function may have been at the roots of the evolution of these enzymes (26). We disclose herein evidence that evolution of class C β-lactamases has been driven solely by the need of the organisms that harbor them as a protective means against cephalosporin antibiotics.
Enzymes as biocatalysts evolve to perform the metabolic task for which they specialize. A measure of the catalytic competence of any enzyme is the kinetic parameters (kcat, Km, and kcat/Km) for the given reaction performed by the enzyme. The kcat/Km ratio has acquired a special place in these analyses since it can be considered a “bimolecular rate constant” for the reaction between the enzyme and the substrate, permitting direct comparison of different catalysts to one another. It has been noted that there exists an upper limit for this ratio in enzymatic reactions. According to theory, for the reaction of a large molecule (i.e., an enzyme) and a small molecule (a typical nonpolymeric substrate) this value approaches 108 to 109 M−1 s−1 (33, 35, 36). Once this limiting level for catalysis is reached for any enzyme, the actual chemical steps in the catalytic processes, that is, bond making and bond breaking, are considered to have reached “catalytic perfection” (1). That is, the steps that require covalent bond making and bond breaking, which typically are slow processes, are no longer limiting for such a perfect catalyst. On the contrary, diffusional steps, which are rapid, become the limiting steps in catalysis by such an enzyme. To put this differently, travel (diffusion) of the substrate into the active site of the enzyme or movement of the product away from the active site becomes the slow step in catalysis. Such a “perfect” enzyme can no longer improve its catalytic ability in the course of evolution from that point on and is said to be “diffusion controlled.” The chances are that many critical metabolic enzymes have reached such a diffusion-controlled state, because the advantage that the rapid reaction provides for the organisms is selected in the course of evolution. However, few enzymes have specifically been shown to operate at such a level. The following are a few examples: triosephosphate isomerase (20), phosphorylase b (11), horseradish peroxidase (14), chymotrypsin (6), carbonic anhydrase (17, 31), invertase (27), acetylcholinesterase (2), adenosine deaminase (22), class A β-lactamase (16), and aminoglycoside 3′-phosphotransferase type III (25).
Of the four classes of β-lactamases (7, 8, 23), the class A β-lactamases (penicillinases) are the most common, and it is widely accepted that they have evolved to hydrolyze penicillins (10). This matter was put on firm ground by the demonstration of Hardy and Kirsch (16) that indeed the class A β-lactamase from Bacillus cereus (β-lactamase I) operates at the diffusion-controlled limit. As discussed earlier, it has been suggested in the literature that the chromosomal class C β-lactamases may have evolved to catalyze a reaction other than hydrolysis of β-lactam (4, 26, 32, 34, 38). This assertion can be tested, even if one does not know the nature of the alternative reaction. The rationale is as follows. These enzymes are known as cephalosporinases, and if one demonstrates that they catalyze hydrolysis of cephalosporins at the diffusion limit, then it is unlikely that their evolution may have been driven by a different reaction. Indeed, we have performed such an analysis, and it is clear that these enzymes have evolved to “perfection” for their reaction in hydrolysis of their preferred cephalosporin substrates, as will be detailed below.
MATERIALS AND METHODS
Cephaloridine, cephalosporin C, penicillin G, sucrose and polyethylene glycol (PEG) 8000 were purchased from Sigma Chemical Co. (St. Louis, Mo.). Cefepime was a gift from Bristol-Myers Squibb (Princeton, N.J.). Spectrophotometric studies were performed on a Hewlett-Packard 8453 diode array instrument. Nonlinear regression analysis was performed by the use of the program SigmaPlot (Jandel Scientific). Other calculations were performed with the Microsoft Excel software. The class C β-lactamase was purified from Enterobacter cloacae P99 by affinity chromatography (9). The purified enzyme was homogeneous, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
The kinetic parameters for turnover (Km and kcat) of substrates were determined either from the Lineweaver-Burk plot or by nonlinear regression of the equation for Michaelis-Menten kinetics. Six to seven substrate concentrations were used for each kinetic determination, and the reported parameters were the averages for at least three independent measurements. All the experiments were carried out in 100 mM sodium phosphate (pH 7.0) at 20°C with the corresponding amount of viscogen added. The typical assay volume was 1.0 ml. The concentration ranges for various substrates were as follows: cephaloridine, 200 to 600 μM; cephalosporin C, 200 to 800 μM; penicillin G, 100 to 700 μM; cefepime, 10 to 150 μM. A portion of the enzyme was added to a solution of substrate to give a final enzyme concentration of 15 nM. Substrate hydrolysis was monitored at 290 nm for cephaloridine (Δɛ290 = 2,070 M−1 cm−1), 280 nm for cephalosporin C (Δɛ280 = 2,390 M−1 cm−1), 240 nm for penicillin G (Δɛ240 = 560 M−1 cm−1), and 260 nm for cefepime (Δɛ260 = 750 M−1 cm−1). The viscosity of the solution was controlled by the addition of the appropriate amounts of sucrose or PEG 8000 to the buffer. The relative viscosities (ηrel) of the solutions were determined from the reference data (37).
RESULTS AND DISCUSSION
The way to demonstrate that the rate of enzymatic reaction is controlled by the diffusion-controlled limit is to probe for the change in the rate of the reaction as a function of the viscosity of the solution. The more viscous the solution, the more difficult will be the diffusion of the molecules in and out of the active site of the enzyme, resulting in a decrease in the value of kcat/Km. Moreover, the Km component should be influenced more than the kcat component. We hasten to add that the decrease in the second-order rate constant on an increase in solution viscosity does not necessarily mean that the reaction is under diffusion control. The decrease in the rate could also be attributed to the decrease in the free energy of the unbound substrate. To prove that the reaction is indeed under the diffusion limit, a control experiment should be performed. In a control experiment one can use either a poor substrate for the given enzyme (16) or, if no poor substrate is available for the system, a sluggish mutant variant of the enzyme (5). In either case the rate for hydrolysis of a sluggish enzyme-substrate system should not undergo change upon the increase in solution viscosity.
The viscosity of the solution is commonly altered by the addition of viscogens such as sucrose, glycerol, Ficoll, or PEG. Although the presence of any of the four compounds in solution would increase the macroscopic viscosity of the solution, at the microscopic level their behaviors are quite different. According to theory, polymers such as Ficoll and PEG do not influence the rates of diffusion of small molecules (3, 28). On the other hand, small-molecule viscogens such as sucrose and glycerol not only will increase the macroscopic viscosity of the solution but also will slow down the diffusion of molecular particles in solution (18, 21). For this reason, sucrose and glycerol are called microviscogens, in contrast to macroviscogens, such as Ficoll and PEG.
β-Lactamases are typically efficient catalysts in hydrolysis of the β-lactam bonds of their preferred substrates. In many cases the kcat/Km values for the β-lactamase hydrolysis of a good substrate is in the range of 107 to 108 M−1 s−1. That is also true for the AmpC family of β-lactamases, for which the kcat/Km for turnover of cephaloridine by several of the members is in the range of 107 to 108 M−1 s−1 (13).
We have investigated the hydrolysis rates for four selected β-lactam substrates for the E. cloacae P99 β-lactamase in the presence of viscogens. The substrate selection was made such that both good and poor substrates would be represented. Two cephalosporins, cephaloridine and cephalosporin C, are exceptionally good substrates for this β-lactamase. Penicillin G, which was used as a representative penicillin substrate, is not preferred by class C β-lactamases. Finally, cefepime is one of the worst cephalosporin substrates for the enzyme.
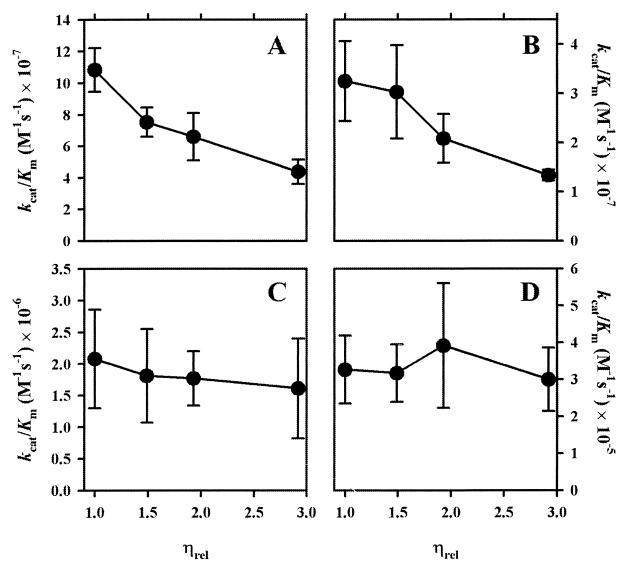
Analysis of the kinetic parameters (kcat, Km, and kcat/Km) revealed the following trends (Tables 1 to 4 and Fig. 1). As expected, addition of PEG (macroviscogen) did not influence appreciably the kinetic parameters for any of the substrates tested. However, in a manner similar to that for other enzymes that operate at the diffusion-controlled limit, the rate of hydrolysis of the good substrates for the E. cloacae P99 β-lactamase decreased proportionally with the increase in the relative viscosity in the presence of sucrose (microviscogen). The kcat/Km value decreased 2.5-fold in the case of cephaloridine and cephalosporin C (Fig. 1A and B, respectively). An important factor that affected the ratio kcat/Km for these substrates was the increase in Km (Tables 1 and 2). The effect of viscosity on kcat values was very small throughout the viscosity range, giving no trends as a function of increasing viscosity, as would be expected.
TABLE 1.
Kinetic parameters for turnover of cephaloridine by the class C β-lactamase from E. cloacae P99
| ηrel (% viscogen) | kcat (s−1) | Km (μM) | kcat/Km (M−1 s−1) |
|---|---|---|---|
| 1 (0) | 230 ± 20 | 2.1 ± 0.2 | (1.08 ± 0.14) × 108 |
| 1.49 (13% sucrose) | 310 ± 25 | 4.1 ± 0.4 | (7.5 ± 0.9) × 107 |
| 1.93 (21.6% sucrose) | 280 ± 40 | 4.3 ± 0.7 | (6.60 ± 0.15) × 107 |
| 2.92 (28% sucrose) | 345 ± 50 | 7.9 ± 0.9 | (4.4 ± 0.7) × 107 |
| 2 (6.7% PEG) | 285 ± 15 | 3.4 ± 0.2 | (8.3 ± 0.7) × 107 |
TABLE 4.
Kinetic parameters for turnover of cefepime by the class C β-lactamase from E. cloacae P99
| ηrel (% viscogen) | kcat (s−1) | Km (μM) | kcat/Km (M−1 s−1) |
|---|---|---|---|
| 1 (0) | 3.7 ± 0.2 | 11 ± 3 | (3.3 ± 0.9) × 105 |
| 1.49 (13% sucrose) | 8.4 ± 0.6 | 27 ± 6 | (3.2 ± 0.7) × 105 |
| 1.93 (21.6% sucrose) | 7.4 ± 1.3 | 19 ± 7 | (3.9 ± 1.7) × 105 |
| 2.92 (28% sucrose) | 3.3 ± 0.2 | 11 ± 3 | (3.0 ± 0.8) × 105 |
| 2 (6.7% PEG) | 3.1 ± 0.5 | 10 ± 3 | (3.1 ± 1.0) × 105 |
FIG. 1.
Dependence of kcat/Km on relative viscosity (ηrel) of the solution for hydrolysis of cephaloridine (A), cephalosporin C (B), penicillin G (C), and cefepime (D) by the class C β-lactamase from E. cloacae P99.
TABLE 2.
Kinetic parameters for turnover of cephalosporin C by the class C β-lactamase from E. cloacae P99
| ηrel (% viscogen) | kcat (s−1) | Km (μM) | kcat/Km (M−1 s−1) |
|---|---|---|---|
| 1 (0) | 460 ± 15 | 14 ± 3 | (3.25 ± 0.80) × 107 |
| 1.49 (13% sucrose) | 790 ± 70 | 26 ± 8 | (3.0 ± 0.9) × 107 |
| 1.93 (21.6% sucrose) | 600 ± 35 | 29 ± 7 | (2.1 ± 0.5) × 107 |
| 2.92 (28% sucrose) | 670 ± 12 | 50 ± 4 | (1.3 ± 0.1) × 107 |
| 2 (6.7% PEG) | 420 ± 20 | 16 ± 4 | (2.6 ± 0.7) × 107 |
The situation is quite different for the poorer substrates. The kcat/Km value virtually did not change, when one considers the calculated standard deviations in each case for penicillin G (Fig. 1C; Table 3) and cefepime (Fig. 1D; Table 4), as would be expected. The effects on other kinetic parameters were also negligible, with no trends for the fluctuation being observed.
TABLE 3.
Kinetic parameters for turnover of penicillin G by the class C β-lactamase from E. cloacae P99
| ηrel (% viscogen) | kcat (s−1) | Km (μM) | kcat/Km (M−1 s−1) |
|---|---|---|---|
| 1 (0) | 22 ± 5 | 10 ± 3 | (2.1 ± 0.8) × 106 |
| 1.49 (13% sucrose) | 21 ± 4 | 11 ± 4 | (1.8 ± 0.7) × 106 |
| 1.93 (21.6% sucrose) | 21 ± 2 | 12 ± 3 | (1.8 ± 0.4) × 106 |
| 2.92 (28% sucrose) | 22 ± 6 | 14 ± 6 | (1.6 ± 0.8) × 106 |
These observations confirm the hypothesis that the hydrolytic process for the good substrates for the E. cloacae P99 β-lactamase is diffusion controlled. In the case of moderate to poor substrates, the slow steps are at the bond-making and bond-breaking levels. Therefore, the diffusional ability in the presence of the microviscogen has minimal to no effect on the overall rate of turnover of the poorer substrates by the E. cloacae P99 class C β-lactamase.
The processing of murein (peptidoglycan) in gram-negative bacteria is elaborate, and it involves several gene products (19). The presence of some of the intermediates in this process induces the expression of the AmpC gene product, which encodes the class C β-lactamase of gram-negative bacteria. It is likely that the presence of such intermediates is a signal for expression of the resistance enzyme because, indeed, such degradation of peptidoglycan takes place as a consequence of the action of β-lactam drugs on the organism. Therefore, this may serve as a signal to upregulate the expression of the resistance enzyme to come to the rescue of the organism in distress.
It is actually tantalizing that it has been demonstrated that the AmpC gene products do perform other reactions such as hydrolysis of depsipeptides and amides (12, 15). These are taken as “vestigial reactions” for these enzymes, suggestive of their relationships to other proteins such as certain penicillin-binding proteins (PBPs). However, it is evident that true to the term “vestigial reaction,” these atypical transformations for the AmpC enzyme are carried out at rates that approach those for some of the poorer β-lactam substrates for the class C β-lactamases (12, 15, 39, 40).
An enzyme would reach catalytic “perfection” only for the reaction that drives its evolution. On the basis of the results presented here, it would appear that that reaction for class C β-lactamases is hydrolysis of their preferred cephalosporin substrates. Previous findings argued the same for the evolution of class A β-lactamases in response to the challenge by penicillins (16). It would appear to be intuitive, in retrospect, that these enzymes should be chemically perfect for their resistance function, since this matter has a direct bearing on the ability of the bacteria to survive in the presence of the antibacterial agent.
Structural and kinetic considerations led Matagné et al. (24) to suggest recently that class C enzymes are “primitive” forms of β-lactamases. The results presented in this report are inconsistent with this characterization of class C enzymes. We have argued recently that the diversification of the two lines of PBPs that ultimately gave rise to classes A and C of β-lactamases was an early event in the evolution of PBPs (23). Furthermore, the details of the mechanisms for the catalytic processes of the two classes of β-lactamases argue for independent and perhaps parallel evolutions for the two classes of enzymes. The results presented in this report shed further light on this process by showing that the evolutionary developments of both classes A and C of β-lactamases have been driven to catalytic perfection. Since class A β-lactamases prefer penicillins as substrates, whereas class C enzymes show better competence in turnover of cephalosporins, it is clear that evolution of each class of enzymes was advanced by those respective substrates. The structures of penicillins and cephalosporins are different, and they each provided a differential selection pressure for evolution of β-lactamases. This differential selection pressure is at the roots of the differences in evolution of classes A and C of β-lactamases, but what is significant in our opinion is the fact that each selection pressure was sufficient individually to drive the evolution of their respective enzymes to catalytic perfection.
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health.
REFERENCES
- 1.Albery W J, Knowles J R. Evolution of enzyme function and the development of catalytic efficiency. Biochemistry. 1976;15:5631–5640. doi: 10.1021/bi00670a032. [DOI] [PubMed] [Google Scholar]
- 2.Bazelyansky M, Robey E, Kirsch J F. Fractional diffusion-limited component of reactions catalyzed by acetylcholiesterase. Biochemistry. 1986;25:125–130. doi: 10.1021/bi00349a019. [DOI] [PubMed] [Google Scholar]
- 3.Biancheria A, Kegeles G J. Diffusion measurements in aqueous solutions of different viscosity. J Am Chem Soc. 1957;79:5908–5912. [Google Scholar]
- 4.Bishop R E, Wiener J H. Coordinate regulation of murein peptidase activity and AmpC β-lactamase synthesis in Escherichia coli. FEBS Lett. 1992;304:103–108. doi: 10.1016/0014-5793(92)80598-b. [DOI] [PubMed] [Google Scholar]
- 5.Blacklow S C, Raines R T, Lim W A, Zamore P D, Knowles J R. Triosephosphate isomerase catalysis is diffusion controlled. Biochemistry. 1988;27:1158–1167. doi: 10.1021/bi00404a013. [DOI] [PubMed] [Google Scholar]
- 6.Brouwer A C, Kirsch J F. Investigation of diffusion-limited rates of chymotrypsin reactions by viscosity variation. Biochemistry. 1982;21:1302–1307. doi: 10.1021/bi00535a030. [DOI] [PubMed] [Google Scholar]
- 7.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bush K, Mobashery S. How β-lactamases have driven pharmaceutical discovery: from mechanistic knowledge to classical circumvention. In: Rosen B P, Mobashery S, editors. Resolving the antibiotic paradox: progress in understanding drug resistance and development of new antibiotics. New York, N.Y: Plenum Press; 1998. pp. 71–98. [Google Scholar]
- 9.Cartwright S J, Waley S G. Purification of β-lactamases by affinity chromatography on phenylboronic acid-agarose. Biochem J. 1984;221:505–512. doi: 10.1042/bj2210505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen H, Martin M T, Waley S G. Beta-lactamases as fully efficient enzymes. Determination of all the rate constants in the acyl-enzyme mechanism. Biochem J. 1990;266:853–861. [PMC free article] [PubMed] [Google Scholar]
- 11.Damjanovich S, Bot J, Somogyi B, Sumegi J. Effect of glycerol on some kinetic parameters of phosphorylase b. Biochim Biophys Acta. 1972;284:345–348. doi: 10.1016/0005-2744(72)90072-1. [DOI] [PubMed] [Google Scholar]
- 12.Dryjanski M, Pratt R F. Steady-state kinetics of the binding of β-lactams and penicilloates to the second binding site of the Enterobacter cloacae P99 β-lactamase. Biochemistry. 1995;34:3561–3568. doi: 10.1021/bi00011a010. [DOI] [PubMed] [Google Scholar]
- 13.Dubus A, Ledent P, Lamotte-Brasseur J, Frère J-M. The roles of residues Tyr150, Glu272, and His314 in class C beta-lactamases. Proteins. 1996;25:473–485. doi: 10.1002/prot.7. [DOI] [PubMed] [Google Scholar]
- 14.Dunford B H, Hewson W D. Effect of mixed solvents on the formation of horseradish peroxidase compound I. The importance of diffusion-controlled reactions. Biochemistry. 1977;16:2949–2957. doi: 10.1021/bi00632a023. [DOI] [PubMed] [Google Scholar]
- 15.Govardhan C P, Pratt R F. Kinetics and mechanism of the serine β-lactamase catalyzed hydrolysis of depsipeptides. Biochemistry. 1987;26:3385–3395. doi: 10.1021/bi00386a021. [DOI] [PubMed] [Google Scholar]
- 16.Hardy L W, Kirsch J F. Diffusion-limited component of reactions catalyzed by Bacillus cereus β-lactamase I. Biochemistry. 1984;23:1275–1282. doi: 10.1021/bi00301a040. [DOI] [PubMed] [Google Scholar]
- 17.Hasinoff B B. Kinetics of carbonic anhydrase catalysis in solvents of increased viscosity: a partially diffusion-controlled reaction. Arch Biochem Biophys. 1984;233:676–681. doi: 10.1016/0003-9861(84)90494-6. [DOI] [PubMed] [Google Scholar]
- 18.Hasinoff B B, Chisthi S B. Viscosity dependence of the kinetics of the diffusion-controlled reaction of carbon monoxide and myoglobin. Biochemistry. 1982;21:4275–4278. doi: 10.1021/bi00261a015. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs C, Frère J-M, Normark S. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible β-lactam resistance in gram-negative bacteria. Cell. 1997;88:823–832. doi: 10.1016/s0092-8674(00)81928-5. [DOI] [PubMed] [Google Scholar]
- 20.Knowles J R, Albery W J. Perfection in enzyme catalysis: the energetics of triosephosphate isomerase. Acc Chem Res. 1977;10:105–111. [Google Scholar]
- 21.Kramers H A. Brownian motion in a field of force and the diffusion model of chemical reactions. Physica (Amsterdam) 1940;7:284–304. [Google Scholar]
- 22.Kurz L C, Weitkamp E, Frieden C. Adenosine deaminase: viscosity studies and the mechanism of binding of substrate and of ground- and transition-state analogue inhibitors. Biochemistry. 1987;26:3027–3032. doi: 10.1021/bi00385a012. [DOI] [PubMed] [Google Scholar]
- 23.Massova I, Mobashery S. Kinship and diversification of bacterial penicillin-binding proteins and β-lactamases. Antimicrob Agents Chemother. 1998;42:1–17. doi: 10.1128/aac.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matagné A, Dubus A, Galleni M, Frère J-M. The beta-lactamase cycle: a tale of selective pressure and bacterial ingenuity. Nat Prod Rep. 1999;16:1–19. doi: 10.1039/a705983c. [DOI] [PubMed] [Google Scholar]
- 25.McKay G A, Wright G D. Catalytic mechanism of enteroccoccal kanamycin kinase (APH(3′)-IIIa): viscosity, thio, and solvent isotope effects support a Theorell-Chance mechanism. Biochemistry. 1996;35:8680–8685. doi: 10.1021/bi9603884. [DOI] [PubMed] [Google Scholar]
- 26.Medeiros A A. Evolution and dissemination of β-lactamases accelerated by generation of β-lactam antibiotics. Clin Infect Dis. 1997;24(Suppl. 1):S19–S45. doi: 10.1093/clinids/24.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 27.Monsan P, Combes D. Effect of water activity on enzyme action and stability. Ann N Y Acad Sci. 1984;434:48–60. [Google Scholar]
- 28.Muhr A H, Blanshard J M V. Diffusion in gels. Polymer. 1982;23:1012–1026. [Google Scholar]
- 29.Normark S. β-Lactamase induction in gram-negative bacteria is intimately linked to peptidoglycan recycling. Microb Drug Res. 1995;1:111–114. doi: 10.1089/mdr.1995.1.111. [DOI] [PubMed] [Google Scholar]
- 30.Olson O, Bergstrom S, Normark S. Identification of a novel ampC β-lactamase promoter in a clinical isolate of Escherichia coli. EMBO J. 1982;1:1411–1416. doi: 10.1002/j.1460-2075.1982.tb01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pocker Y, Yanjic N. Enzyme kinetics in solvents of increased viscosity. Dynamic aspects of carbonic anhydrase catalysis. Biochemistry. 1987;26:2597–2606. doi: 10.1021/bi00383a028. [DOI] [PubMed] [Google Scholar]
- 32.Pollock M R. Origin and function of penicillinase: a problem in biochemical evolution. Br Med J. 1967;4:71–77. doi: 10.1136/bmj.4.5571.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samson R, Deutch J M. Diffusion-controlled reaction rate to a buried active site. J Chem Phys. 1978;68:285–290. [Google Scholar]
- 34.Saz A K. An introspective view of penicillinase. J Cell Physiol. 1970;76:397–404. doi: 10.1002/jcp.1040760318. [DOI] [PubMed] [Google Scholar]
- 35.Schurr J H, Schmitz J. Orientation constrains and rotational diffusion in bimolecular solution kinetics. Simplification. J Phys Chem. 1976;80:1934–1936. [Google Scholar]
- 36.Solc K, Stockmayer W H. Kinetics of diffusion-controlled reaction between chemically asymmetric molecules. II. Approximate steady-state solution. Int J Chem Kinet. 1973;5:733–752. [Google Scholar]
- 37.Weast R C, editor. Handbook of chemistry and physics. 61st ed. Boca Raton, Fla: CRC Press, Inc.; 1981. pp. D–270. [Google Scholar]
- 38.Wise E M, Park J T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci USA. 1965;54:75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Pratt R F. β-Lactam-recognizing enzymes exhibit different structural specificity in acyclic amide and ester substrates: a starting point in β-lactamase evolution? Bioorg Med Chem Lett. 1994;4:2291–2296. [Google Scholar]
- 40.Xu Y, Soto G, Adachi H, van der Linden M P G, Keck W, Pratt R F. Relative specificities of a series of β-lactam-recognizing enzymes towards side-chain of penicillins and of acyclic thioldepsipeptides. Biochem J. 1994;302:851–856. doi: 10.1042/bj3020851. [DOI] [PMC free article] [PubMed] [Google Scholar]