Abstract
Aims:
Adequate intake of long-chain (LC) omega-3 polyunsaturated fatty acids (n-3 PUFAs) is considered important for cardiovascular health. However, the effects of LC n-3 PUFAs on the risk of heart failure (HF) remain unclear. This systematic review and meta-analysis aimed to determine the role of LC n-3 PUFAs in the incidence of HF.
Materials and Methods:
Electronic databases were searched for studies up to 31 July 2021. Studies were included for the meta-analysis if they reported the adjusted associations between different dietary intakes or circulating concentrations of LC n-3 PUFAs and the risk of HF. A random-effect model was used to calculate the pooled estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for higher LC n-3 PUFA concentrations.
Results:
Thirteen studies were included in the meta-analysis. Eight studies comprising 316,698 individuals (11,244 incident HF cases), with a median follow-up of 10.7 years, showed that a higher dietary intake of LC n-3 PUFAs was associated with a lower risk of HF (highest versus lowest quintile: HR = 0.84, 95% CI = 0.75–0.94). Six studies, comprising 17,163 participants (2520 HF cases) with a median follow-up of 9.7 years, showed that higher circulating LC n-3 PUFA concentrations were associated with a lower risk of HF (highest versus lowest quintile: HR = 0.59, 95% CI = 0.39–0.91). Higher circulating docosahexaenoic acid concentrations were associated with a decreased risk of HF (top versus bottom quintile: HR = 0.44, 95% CI = 0.26–0.77). The associations between eicosapentaenoic acid (HR = 0.58, 95% CI = 0.26–1.25), docosahexaenoic acid (HR = 0.66, 95% CI = 0.24–1.82), and the risk of HF were not significant.
Conclusion:
High LC n-3 PUFA concentrations measured by dietary intake or circulating biomarkers are associated with a lower risk of developing HF.
Keywords: heart failure, meta-analysis, polyunsaturated fatty acids, risk
Introduction
With an improved life expectancy and an aging population, heart failure (HF) has become a growing global public health burden. More than 5 and 37.7 million patients are estimated to have HF in the United States and worldwide, respectively.1,2 Although there have been remarkable improvements in the management of HF, the 5-year mortality in patients with HF is still as high as 50%.3–5 This finding suggests the importance of identifying novel preventive risk factors and treatment modalities of HF.
Long-chain (LC) omega-3 polyunsaturated fatty acids (n-3 PUFAs), which are obtained in the diet from seafood or in the form of supplements, are considered important regulators of cardiovascular health. Evidence from observational studies has suggested that LC n-3 PUFAs reduce the risk of coronary heart disease, especially sudden cardiac death.6–10 However, the effects of LC n-3 PUFAs on the risk of HF are not well established. Although some studies showed that fish consumption and dietary intake of LC n-3 PUFAs were associated with a protective effect for HF,11–14 other studies did not find a similar association.15–18 In contrast to food questionnaire estimates, circulating LC n-3 PUFA concentrations provide objective measures of exposure, reflecting dietary consumption and related biological processes, such as absorption, incorporation, and metabolism. To overcome inconsistencies related to measurement error in food questionnaire-based studies, some studies evaluated the associations between circulating (serum or plasma) LC n-3 PUFA concentrations and incident HF,15,19–23 although inconsistent results were found. In addition, LC n-3 PUFAs comprise several specific individual types of fatty acids, including eicosapentaenoic acid (EPA, 20:5n-3), docosapentaenoic acid (DPA, 22:5n-3), and docosahexaenoic acid (DHA, 22:6n-3).19,20,22 These specific individual LC n-3 PUFAs may have different biological effects, and whether their effects on HF are similar has not been well evaluated.
To address these issues, we performed a meta-analysis of observational studies to synthesize data on the associations between LC n-3 PUFAs and the risk of incident HF. We aimed to determine (1) whether LC n-3 PUFAs, as evaluated by dietary intake and circulating concentrations, play a role in the risk of HF and (2) whether specific individual LC n-3 PUFAs have different effects on the prevention of HF.
Methods
Search strategy and selection criteria
We performed our study by following the recommendations of the Meta-analysis of Observational Studies in Epidemiology group. 24 Electronic databases (PubMed, Google Scholar, Cochran Library, and EMBASE) were searched for studies from inception until 31 July 2021. We used a combined text and MeSH heading search strategy, with terms related to ‘n-3 PUFAs’ and ‘heart failure’. There were no restrictions on language or publication forms. However, we restricted the literature to human studies. Reference lists of the included studies and the most recent reviews were manually checked to identify potentially related studies. The detailed strategy used for searching PubMed is shown in Supplementary File 1. The strategies for searching other databases were similar, but with adaptations where necessary.
We included studies for analysis if they met the following criteria: (1) prospective cohort studies or nest case–control studies with adult participants (aged ⩾ 18 years); (2) dietary intake or circulating LC n-3 PUFA concentrations and other associated risk factors were detected at baseline; and (3) the risk of HF associated with different LC n-3 PUFA concentrations was reported.
Studies were excluded if they met the following criteria: (1) they were cross-sectional studies; (2) the risk of HF was not adjusted for other confounders; (3) the follow-up duration was <1 year; and (4) duplicated articles were derived from the same participants’ dataset. If multiple reports were derived from the same participants’ cohort, only the latest published data were included in our study.
Data extraction and quality assessment
Two investigators (S.Z. and X.L.) independently conducted article searches and screened the titles and abstracts of the retrieved reports. Full texts of potentially suitable studies were subsequently reviewed. Important information of the included studies, such as the study design, participants’ characteristics, ethnicity, study sample, sex, mean/median age, methods for detecting n-3 PUFAs, and follow-up duration, were recorded. The principal authors were contacted for any additional data if required.
We used the Newcastle–Ottawa Quality Assessment Scale to evaluate the study quality on the basis of the following: selection (3 items, 1 point each), comparability (1 item, up to 2 points), and exposure/outcome (3 items, 1 point each), with a total score ranging from 0 to 9. 25 Included studies were classified by quality as good (⩾7 points), fair (4–6 points), or poor (<4 points).26,27
Data synthesis and analysis
The primary outcome was the risk of incident HF in individuals with different levels of dietary intake or circulating LC n-3 PUFA concentrations. The secondary outcome was the risk of incident HF associated with individual LC n-3 PUFA concentrations (EPA, DHA, and DPA). Outcome data adjusted for the maximal number of confounders were extracted for analysis.
The associations of the HF risk and LC n-3 PUFA concentrations were reported in different ways in the included studies, such as per quartiles or quintiles of LC n-3 PUFA concentrations, or per 1 standard deviation (SD) increment in the continuous trait. Therefore, to perform a consistent approach for analysis and enhance the interpretation of the findings, two comparison methods were performed in our study. We compared the hazard ratios (HRs) of the HF risk in individuals with the highest quintile of LC n-3 PUFAs with those with the lowest quintile. We then calculated the HRs per 1 SD increment in baseline concentrations of LC n-3 PUFAs. If the included studies did not report effect measures per quintile or per 1 SD change, we converted the results according to previously reported methods. 28 Briefly, we assumed that the exposure variable (LC n-3 PUFAs) was normally distributed, and the association with outcome (HF) was log linear. The expected difference in means was 1.96 SDs for the top versus bottom median of the standard normal distribution, 2.18 SDs for the top versus bottom tertiles, 2.54 SDs for the top versus bottom quartiles, and 2.80 SDs for the top versus bottom quintiles. 28 Therefore, HRs reported for the top versus bottom median comparison were converted to per 1 SD change by applying a division conversion factor of 1.96 to the log HRs. Similarly, estimates for comparisons of extreme tertiles, quartiles, and quintiles were divided by 2.18, 2.54, and 2.80, respectively. The HRs reported for comparisons of extreme quartiles or tertiles were converted to comparisons of extreme quintiles by applying a multiplication conversion factor of 2.80/2.54 or 2.80/2.18, respectively, to the log HRs.
We combined the log RRs or log HRs and the corresponding standard errors using the inverse variance approach. 29 We used I2 statistics to test heterogeneity, and an I2 value of >50% was considered to indicate significant heterogeneity. However, even when no statistical heterogeneity was observed, we reported results from the random-effects models as the primary analysis (as opposed to the fixed-effects model), considering the unavoidable clinical and methodological heterogeneity (e.g. baseline characteristics of the patients, methods for detecting the exposure, adjustment of confounders, and follow-up duration). 3 Subgroup analyses of the primary outcome were performed according to region, age, sex, follow-up duration, the measure of exposure (EPA, DHA, or DPA), and the measurement method (gas chromatography or gas-liquid chromatogram) if appropriate. The publication bias was evaluated by inspecting funnel plots for the primary outcome and further tested using Begg’s test and Egger’s test. Sensitivity analyses were conducted by changing the random-effects model to the fixed-effects model for the meta-analysis. We also recalculated the HRs by omitting one study at a time to evaluate the effect of individual studies on the estimated risk.
The analyses were performed using RevMan 5.3 (The Cochrane Collaboration, Copenhagen, Denmark) and Stata 15.0 (StataCorp LP, College Station, TX, USA). All p values are two-tailed, and the statistical significance was set at 0.05.
Results
Retrieved studies
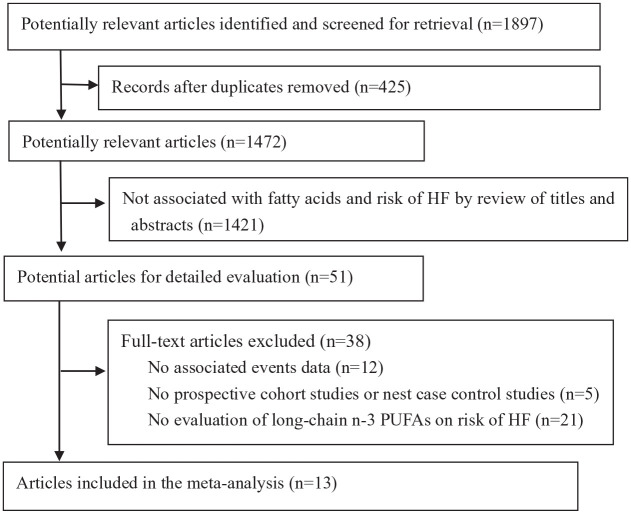
A total of 1897 articles were identified from the searched electronic databases. After duplication records were removed, two authors independently screened the titles and abstracts. The full text from 51 articles was reviewed, and 13 studies were included in the final analysis (Figure 1). In the included studies, seven reported the dietary intake of LC n-3 PUFAs,11–14,16–18 five reported circulating LC n-3 PUFA concentrations,19–23 and only one study report both dietary intake and circulating LC n-3 PUFAs concentrations. 15 Therefore, eight and six studies were included for evaluating incident HF associated with dietary and circulating LC n-3 PUFA concentrations, respectively. According to quality assessment criteria, only two studies were graded as fair, and all other studies were graded as good quality (Supplementary File 2).
Figure 1.
Flowchart of studies included in the review.
CIs, confidence intervals; HF, heart failure; PUFAs, polyunsaturated fatty acids; RRs, relative risks.
Dietary LC n-3 PUFA intake and the risk of HF
The 8 studies on dietary LC n-3 PUFA intake and incident HF included 316,698 individuals for analysis. The main characteristics of the included studies are shown in Table 1. Two studies enrolled only women, two studies enrolled only men, and all others included both sexes. After a median follow-up of 10.7 years (range: 7–12.7 years), 11,244 cases of HF were recorded. The adjusted confounders in the studies are shown in Supplementary File 3.
Table 1.
Characteristics of studies on dietary LC n-3 PUFAs intake and risk of HF.
| Study | Country/region | Cohort characteristics | Dietary data | Measure of exposure | Sample size (% women) | HF case (n) | Age (years), average (range or SD) | Follow-up (years) |
|---|---|---|---|---|---|---|---|---|
| Åkesson et al. 11 | Sweden | Population based | FFQ | EPA + DHA | 69,498 (47.4%) | 5504 | 59.1 (10.0) | 12.0 |
| Wilk et al. 15 | United States | Healthy male physicians | FFQ | EPA + DHA + DPA | 19,097 (0%) | 703 | 58.7 (NA) | 8.4 |
| Belin et al. 16 | United States | Postmenopausal women | FFQ | EPA + DHA | 84,493 (100%) | 1858 | 63.3 (50–79) | 10.0 |
| Levitan et al. 12 | Sweden | Swedish women | FFQ | EPA + DHA | 36,234 (100%) | 651 | 61.6 (48–83) | 9.0 |
| Dijkstra et al. 17 | Netherlands | Population based | FFQ | EPA + DHA | 5299 (59.0%) | 669 | 67.5 (7.7) | 11.4 |
| Levitan et al. 18 | Sweden | Population-based male | FFQ | EPA + DHA | 39,367 (0%) | 597 | 59.3 (45–79) | 7.0 |
| Yamagishi et al. 13 -JACC study | Japan | Population based | FFQ | EPA + DHA + DPA | 57,972 (60.5%) | 307 | 56.1 (40–79) | 12.7 |
| Mozaffarian et al. 14 | United States | Population based | FFQ | EPA + DHA | 4738 (58.1%) | 955 | 73.0 (⩾65) | 12.0 |
DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; FFQ, food frequency questionnaire; HF, heart failure; JACC, Japan Collaborative Cohort; LC, long chain; NA, not available; PUFAs, polyunsaturated fatty acids; SD, standard deviation.
No significant heterogeneity among the included studies was observed for the association of dietary LC n-3 PUFA intake and incident HF, as assessed by quintiles (I2 = 36%, p = 0.14) or per 1 SD increment (I2 = 36%, p = 0.16). A random-effects model analysis showed that a higher intake of LC n-3 PUFAs was associated with a lower risk of HF [highest quintile versus lowest quintile: HR = 0.84, 95% confidence interval (CI) = 0.75–0.94, p = 0.002; per 1 SD increment: HR = 0.94, 95% CI = 0.90–0.98, p = 0.001] (Figure 2). No evidence of publication bias was shown by visual inspection of the funnel plot (Supplementary File 4), or using Egger’s or Begg’s test (both p > 0.10).
Figure 2.
Forest plot of the risk of HF associated with dietary LC n-3 PUFA intake.
CI, confidence interval; HF, heart failure; LC, long chain; PUFAs, polyunsaturated fatty acids.
Circulating LC n-3 PUFA concentrations and the risk of HF
We included six studies comprising 17,163 participants for evaluating the association between circulating LC n-3 PUFA concentrations and the risk of HF. The main characteristics of the included studies are shown in Table 2. One study enrolled only men, and all of the others included women and men. One study enrolled patients with acute myocardial infarction, and all other studies included individuals without baseline cardiovascular disease. After a median follow-up of 9.7 years (range: 3.0–14.3 years), 2520 cases of HF were recorded. The adjusted confounders in the studies are shown in Supplementary File 3.
Table 2.
Characteristics of studies on circulating LC n-3 PUFA and risk of HF.
| Study | Country/region | Cohort characteristics | Measure of exposure | Tissue type (measuring method) | Sample size (% women) | HF case (n) | Age (years), average (range or SD) | Follow-up (years) |
|---|---|---|---|---|---|---|---|---|
| Djousse et al. 19 | United States | Community residents | EPA DHA DPA |
Serum (GC) | 2003 (61.2%) | 655 | 77.7 (4.4) | 9.7 |
| Block et al. 20 | United States | Community residents | EPA DHA DPA EPA + DHA |
Plasma phospholipid (GC-FID) | 6562 (52.0%) | 292 | 63.0 (10.0) | 13 |
| Hara et al. 21 | Japan | AMI patients | EPA DHA |
Serum (GC) | 712 (22.2%) | 35 | 65.0 (NA) | 3.0 |
| Wilk et al. 15 | United States | Healthy male physicians | EPA + DHA + DPA | Plasma phospholipid (GC) | 1576 (0%) | 786 | 58.7 (NA) | 8.4 |
| Mozaffarian et al. 22 | United States | Community residents | EPA DHA DPA EPA + DHA + DPA |
Plasma phospholipid (GC-FID) | 2735 (58.0%) | 555 | 75.2 (NA) | 9.7 |
| Yamagishi et al. 23 -ARIC study | United States | Community residents | EPA DHA EPA + DHA |
Plasma phospholipid (GLC) | 3575 (53.4%) | 197 | 53.7 (5.6) | 14.3 |
AMI, acute myocardial infarction; ARIC, Atherosclerosis Risk in Communities; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; GC, gas chromatography; GC-FID, gas-chromatograph/flame-ionization-detector; GLC, gas-liquid chromatogram; HF, heart failure; LC, long chain; NA, not available; PUFAs, polyunsaturated fatty acids; SD, standard deviation.
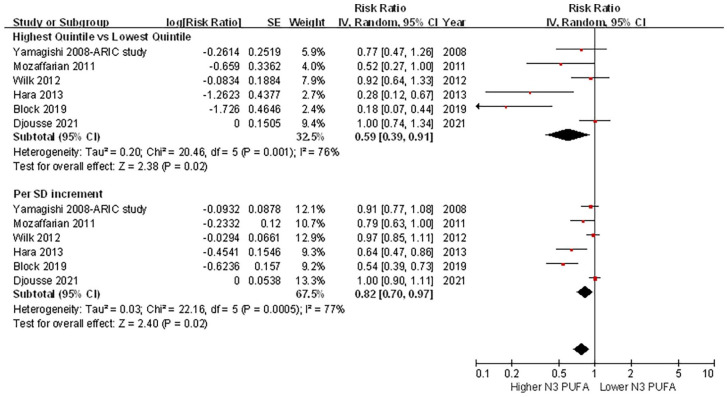
There was significant heterogeneity among the included studies, as assessed by quintiles (I2 = 76%, p = 0.001) or per 1 SD increment (I2 = 77%, p < 0.001). A random-effects model analysis showed that higher circulating LC n-3 PUFA concentrations were associated with a lower risk of HF (highest quintile versus lowest quintile: HR = 0.59, 95% CI = 0.39–0.91, p = 0.02; per 1 SD increment: HR = 0.82, 95% CI = 0.70–0.97, p = 0.02) (Figure 3). No evidence of publication bias was found by visual inspection of the funnel plot (Supplementary File 5), or using Egger’s or Begg’s test (both p > 0.10).
Figure 3.
Forest plot of the risk of HF associated with circulating LC n-3 PUFA concentrations.
CI, confidence interval; HF, heart failure; LC, long chain; PUFAs, polyunsaturated fatty acids.
Individual LC n-3 PUFA concentrations and the risk of HF
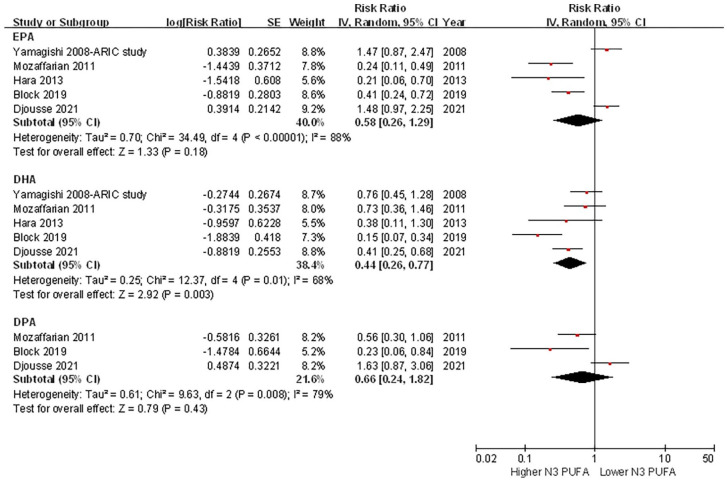
In studies that reported data of individual circulating LC n-3 PUFA concentrations and the risk of HF, pooled analyses showed that a higher circulating DHA concentration was associated with a decreased risk of HF (top quintile versus bottom quintile: HR = 0.44, 95% CI = 0.26–0.77, p = 0.003). The associations of EPA (HR = 0.58, 95% CI = .26–1.25, p = 0.18) and DPA (HR = 0.66, 95% CI = 0.24–1.82, p = 0.43) with the risk of HF were not significant (Figure 4). However, no significant heterogeneity was observed among different individual LC n-3 PUFA concentrations and the risk of HF (I2 = 0%, p = 0.76). Similar results were observed when the risk was evaluated as per 1 SD increment of individual circulating LC n-3 PUFA concentrations (Supplementary File 6).
Figure 4.
Forest plot of the risk of HF associated with individual circulating LC n-3 PUFA concentrations.
CI, confidence interval; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; HF, heart failure; LC, long chain; PUFAs, polyunsaturated fatty acids.
Sensitivity analyses and subgroup analyses
We conducted several sensitivity analyses to confirm that the primary outcomes were not changed by using the fixed-effects model instead of the random-effects model, or recalculating the HRs by omitting one study at a time. No significant heterogeneity was observed among all subgroup comparisons for dietary LC n-3 PUFA intake and the risk of HF (all p ⩾ 0.16, Table 3). The subgroup analyses for the risk of HF and circulating LC n-3 PUFA concentrations are shown in Table 4. There was significant heterogeneity among the subgroups stratified by sex and tissue type (serum versus plasma phospholipids). However, no significant heterogeneity was observed among other subgroup comparisons (age, follow-up duration, region, measurement method, and type of HF).
Table 3.
Subgroup analyses of the association between dietary LC n-3 PUFAs intake and risk of HF.
| Studies (N) | Quintile 5 versus Quintile 1 | Per 1 SD increment | |||
|---|---|---|---|---|---|
| HR (95% CI) | p value a /I2 | HR (95% CI) | p value a /I2 | ||
| Average age (years) | |||||
| <60 | 4 | 0.84 [0.72, 0.97] | 0.98/0% | 0.94 [0.89, 0.99] | 0.98/0% |
| ⩾60 | 4 | 0.84 [0.70, 1.01] | 0.94 [0.88, 1.00] | ||
| Sex | |||||
| Female | 5 | 0.91 [0.81, 1.02] | 0.28/14.2% | 0.98 [0.94, 1.02] | 0.16/49.4% |
| Male | 5 | 0.83 [0.75, 0.93] | 0.94 [0.91, 0.97] | ||
| Follow-up duration (years) | |||||
| <10 | 3 | 0.89 [0.78, 1.03] | 0.45/0% | 0.96 [0.90, 1.02] | 0.36/0% |
| ⩾10 | 5 | 0.83 [0.75, 0.92] | 0.92 [0.88, 0.98] | ||
| Measure of exposure | |||||
| EPA + DHA | 6 | 0.84 [0.75, 0.95] | 0.72/0% | 0.94 [0.90, 0.98] | 0.73/0% |
| EPA + DHA + DPA | 2 | 0.77 [0.49, 1.23] | 0.91 [0.77, 1.08] | ||
| Region | |||||
| Europe | 4 | 0.83 [0.74, 0.93] | 0.16/46.1% | 0.94 [0.89, 0.98] | 0.17/44.2% |
| United States | 3 | 0.90 [0.80, 1.02] | 0.96 [0.90, 1.02] | ||
| Asia | 1 | 0.58 [0.36, 0.93] | 0.82 [0.69, 0.98] | ||
CI, confidence interval; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; HF, heart failure; HR, hazard ratio; LC, long chain; PUFAs, polyunsaturated fatty acids; SD, standard deviation.
For heterogeneity among subgroups.
Table 4.
Subgroup analyses of the association between circulating LC n-3 PUFAs and risk of HF.
| Studies (N) | Quintile 5 versus Quintile 1 | Per 1 SD increment | |||
|---|---|---|---|---|---|
| HR (95% CI) | p value a /I2 | HR (95% CI) | p value a /I2 | ||
| Average age (years) | |||||
| <60 | 2 | 0.86 [0.64, 1.16] | 0.11/59.8% | 0.95 [0.86, 1.05] | 0.11/60.3% |
| ⩾60 | 4 | 0.43 [0.19, 0.97] | 0.74 [0.55, 0.99] | ||
| Sex | |||||
| Female | 1 | 0.37 [0.16, 0.84] | 0.03/78.8% | 0.70 [0.52, 0.95] | 0.04/75.9% |
| Male | 2 | 0.98 [0.71, 1.34] | 0.98 [0.87, 1.11] | ||
| Follow-up duration (years) | |||||
| <10 | 4 | 0.70 [0.45, 1.08] | 0.45/0% | 0.88 [0.75, 1.03] | 0.44/0% |
| ⩾10 | 2 | 0.39 [0.09, 1.63] | 0.71 [0.42, 1.19] | ||
| Region | |||||
| United States | 5 | 0.67 [0.44, 1.02] | 0.08/68.3% | 0.86 [0.74, 1.00] | 0.08/66.7% |
| Asia | 1 | 0.28 [0.12, 0.67] | 0.64 [0.47, 0.86] | ||
| Measure of exposure | |||||
| EPA + DHA | 3 | 0.36 [0.14, 0.92] | 0.08/67.7% | 0.69 [0.49, 0.97] | 0.08/68.1% |
| EPA + DHA + DPA | 3 | 0.87 [0.65, 1.17] | 0.95 [0.86, 1.06] | ||
| Tissue type | |||||
| Serum | 2 | 0.23 [0.12, 0.42] | <0.001/93.6% | 0.58 [0.47, 0.72] | <0.001/94.2% |
| Plasma phospholipid | 4 | 0.87 [0.69, 1.08] | 0.95 [0.88, 1.03] | ||
| Measuring method | |||||
| GC | 3 | 0.75 [0.46, 1.24] | 0.35/17.3% | 0.90 [0.75, 1.08] | 0.30/16.9% |
| GC-FID | 2 | 0.32 [0.11, 0.90] | 0.66 [0.45, 0.97] | ||
| GLC | 1 | 0.77 [0.47, 1.26] | 0.91 [0.77, 1.08] | ||
| Type of HF | |||||
| HFpEF | 2 | 0.27 [0.02, 3.86] | 0.71/0% | 0.63 [0.24, 1.65] | 0.74/0% |
| HFrEF | 2 | 0.49 [0.10, 2.37] | 0.76 [0.43, 1.34] | ||
CI, confidence interval; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; GC, gas chromatography; GC-FID, gas-chromatograph/flame-ionization-detector; GLC, gas-liquid chromatogram; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; LC, long chain; PUFAs, polyunsaturated fatty acids; SD, standard deviation.
For heterogeneity among subgroups.
Discussion
In this comprehensive meta-analysis, we found that a higher dietary LC n-3 PUFA intake was associated with a decreased risk of incident HF. Similarly, higher circulating LC n-3 PUFA concentrations appeared to play a protective role in the risk of HF. This effect was more robust for DHA, although no significant heterogeneity was observed among specific individual LC n-3 PUFAs.
Similar to our study, a previous meta-analysis by Djousse et al. 30 aimed to evaluate the association between LC n-3 PUFAs and the risk of HF. However, they only included seven studies for analysis and found that a higher fish intake was associated with a lower risk of HF (highest versus lowest category of fish intake, RR = 0.85, 95% CI = 0.73–0.99, p = 0.04). However, the corresponding value for n-3 PUFAs was not significant (RR = 0.86, 95% CI = 0.74–1.00, p = 0.05). In this study, we updated the studies published during the past decade and included a much larger number of studies, with a sufficient sample size to separately analyze dietary intake and circulating LC n-3 PUFA concentrations (rather than combining these together as the same exposure). We found that a higher dietary intake or circulating LC n-3 PUFA concentrations were consistently associated with a lower risk of HF. Furthermore, we examined the role of specific individual LC n-3 PUFAs (DHA, EPA, and DPA) in HF. We find that the evidence was more robust for DHA than for the other LC n-3 PUFAs. In addition, no significant association was found for EPA or DPA, although the overall CI was wide, and there was no significant heterogeneity among the three individual LC n-3 PUFAs.
Basic research on individual LC n-3 PUFAs on cardiac function has yielded inconsistent results. In a rat model with cardiac hypertrophy, DHA supplementation decreased mitochondrial membrane viscosity, accelerated Ca2+ uptake, attenuated susceptibility to mitochondrial permeability transition, and prevented the development of cardiac dysfunction. 31 In a mouse model of pressure overload-induced HF, EPA, but not DHA, preserved left ventricular function and prevented interstitial fibrosis.32,33 However, when the same research group used data from the Multi-Ethnic Study of Atherosclerosis, they found that high plasma DHA, DPA, and EPA plus DHA concentrations had similar effects on the risk of HF. 20 This finding indicates that, unlike mice, humans may benefit from LC n-3 PUFAs for protection against HF. Similarly, data from the Cardiovascular Health Study showed that when analyses were limited to 7 years to minimize exposure misclassification over time, all three LC n-3 PUFAs and total LC n-3 PUFA concentrations were inversely associated with incident HF. 22 Taken together, these data suggest that all three LC n-3 PUFAs may have a similar protective effect on cardiac function. However, further human studies are required to confirm this possibility.
Several biological effects of LC n-3 PUFAs, including improvement of the cardiometabolic risk profile, anti-inflammatory and anti-fibrosis effects, improvement of cardiac energy metabolism and the vascular endothelial response, and modification of cardiac ion channels, may explain their protective associations with the incidence of HF.34–38 However, no published randomized controlled trials have assessed the effect of dietary LC n-3 PUFA intake on primary prevention of HF. Therefore, the science advisory from the American Heart Association has not yet made any recommendations on n-3 PUFA intake for the primary prevention of HF. 39 Considering the high and growing disease burden of HF worldwide, our findings underscore the potential importance of LC n-3 PUFAs for preventing HF. Our findings also highlight the urgent need for high-quality and adequately powered randomized controlled trials to test the causal effect of LC n-3 PUFAs in preventing HF. It would be interesting to further explore whether increasing the level of LC n-3 PUFA by nonpharmacological or pharmacological means would confer a superior protection regarding the incidence of HF.
Our study has several major strengths. First, only prospective studies with a multivariable-adjusted estimated risk were included for analysis, and most of them were adjusted for multiple confounders, including traditional cardiovascular risk factors and energy intake. However, residual confounding by unmeasured or imprecisely measured factors cannot be excluded. Second, the association of exposure and outcome was consistently determined by category (top quintile versus bottom quintile) or continuous (per 1 SD) increment of LC n-3 PUFAs. The robustness of the main results was supported by comprehensive subgroup analyses and sensitivity analyses. However, several limitations of the study should be noted. First, most of the included studies were from European countries or the United States, and only two studies were from Japan (one each for dietary intake and circulating concentrations). LC n-3 PUFA concentrations are affected by dietary patterns. Therefore, whether the association of LC n-3 PUFA concentrations with incident HF is consistent among other populations remains unknown, and further studies are required. Second, only a single measurement of LC n-3 PUFAs was performed in the observational studies. The individuals’ dietary patterns and fatty acid metabolism will change over time, and random error caused by a single measurement of LC n-3 PUFA concentrations at baseline may attenuate associations toward the null. However, data from the Physicians’ Health Study showed a modest correlation between measurements of plasma phospholipid fatty acids over 15 years. 40 Importantly, use of a single baseline concentration or the mean LC n-3 PUFA concentration obtained from blood samples collected years apart yielded similar conclusions on their relation with HF. 40 Third, studies that examined the association between individual LC n-3 PUFAs (DHA, EPA, and DPA) and the risk of HF are limited. Therefore, further studies on the role of specific individual LC n-3 PUFAs in HF are required.
Conclusion
Higher LC n-3 PUFA concentrations, as assessed by self-reported intake or circulating biomarkers, are associated with a lower risk of developing HF. Future randomized controlled trials are required to evaluate whether LC n-3 PUFAs are effective in the primary prevention of HF.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223221081616 for Long-chain omega-3 polyunsaturated fatty acids and the risk of heart failure by Sulin Zheng, Min Qiu, Jason H.Y. Wu, Xiong-fei Pan, Xiong Liu, Lichang Sun, Hailan Zhu, Jiandi Wu and Yuli Huang in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-2-taj-10.1177_20406223221081616 for Long-chain omega-3 polyunsaturated fatty acids and the risk of heart failure by Sulin Zheng, Min Qiu, Jason H.Y. Wu, Xiong-fei Pan, Xiong Liu, Lichang Sun, Hailan Zhu, Jiandi Wu and Yuli Huang in Therapeutic Advances in Chronic Disease
Acknowledgments
We thank Ellen Knapp, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn), for editing the English text of a draft of this manuscript.
Footnotes
Author contributions: Sulin Zheng: Conceptualization; Data curation; Formal analysis; Investigation; Project administration; Writing – original draft; Writing – review & editing.
Min Qiu: Conceptualization; Data curation; Formal analysis; Project administration; Validation; Visualization; Writing – original draft; Writing – review & editing.
Jason H.Y. Wu: Conceptualization; Writing – review & editing.
Xiong-fei Pan: Formal analysis; Investigation; Methodology; Writing – review & editing.
Xiong Liu: Data curation; Formal analysis; Investigation; Project administration; Writing – review & editing.
Lichang Sun: Investigation; Project administration; Validation; Writing – review & editing.
Hailan Zhu: Investigation; Methodology; Visualization; Writing – review & editing.
Jiandi Wu: Conceptualization; Funding acquisition; Investigation; Supervision; Writing – review & editing.
Yuli Huang: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Availability of data and materials: The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the Guangdong Basic and Applied Basic Research Fund (Key Project of Guangdong-Foshan Joint Fund, 2019B1515120044), the Science and Technology Innovation Project from Foshan, Guangdong (FS0AA-KJ218-1301-0006), and the Clinical Research Startup Program of Shunde Hospital, Southern Medical University (CRSP2019001).
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Consent for publication: All the contributed authors have agreed to the publication of this manuscript.
ORCID iD: Yuli Huang  https://orcid.org/0000-0001-5423-5487
https://orcid.org/0000-0001-5423-5487
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Sulin Zheng, Department of Cardiology, Shunde Hospital, Southern Medical University (The First People’s Hospital of Shunde), Foshan, China.
Min Qiu, Department of Cardiology, Shunde Hospital, Southern Medical University (The First People’s Hospital of Shunde), Foshan, China.
Jason H.Y. Wu, The George Institute for Global Health, Faculty of Medicine, University of New South Wales Sydney, Sydney, NSW, Australia
Xiong-fei Pan, Laboratory of Molecular Translational Medicine, Center for Translational Medicine, Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, West China Second University Hospital, Sichuan University, Chengdu, China.
Xiong Liu, Department of Cardiology, Shunde Hospital, Southern Medical University (The First People’s Hospital of Shunde), Foshan, China.
Lichang Sun, Department of Cardiology, Shunde Hospital, Southern Medical University (The First People’s Hospital of Shunde), Foshan, China.
Hailan Zhu, Department of Cardiology, Shunde Hospital, Southern Medical University (The First People’s Hospital of Shunde), Foshan, China.
Jiandi Wu, Department of Cardiology, Affiliated Foshan Hospital, Southern Medical University, Foshan, China.
Yuli Huang, Department of Cardiology, Shunde Hospital, Southern Medical University (The First People’s Hospital of Shunde), Jiazhi Road, Lunjiao Town, Shunde District, Foshan 528300, China; The George Institute for Global Health, Faculty of Medicine, University of New South Wales Sydney, Sydney, NSW, Australia Guangdong Provincial Key Laboratory of Shock and Microcirculation, Guangzhou, China.
References
- 1. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020; 141: e139–e596. [DOI] [PubMed] [Google Scholar]
- 2. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol 2016; 13: 368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mai L, Wen W, Qiu M, et al. Association between prediabetes and adverse outcomes in heart failure. Diabetes Obes Metab 2021; 23: 2476–2483. [DOI] [PubMed] [Google Scholar]
- 4. Wu J, Zheng H, Liu X, et al. Prognostic value of secreted frizzled-related protein 5 in heart failure patients with and without type 2 diabetes mellitus. Circ Heart Fail 2020; 13: e007054. [DOI] [PubMed] [Google Scholar]
- 5. Yang S, Chen H, Tan K, et al. Secreted frizzled-related protein 2 and extracellular volume fraction in patients with heart failure. Oxid Med Cell Longev 2020; 2020: 2563508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zelniker TA, Morrow DA, Scirica BM, et al. Plasma omega-3 fatty acids and the risk of cardiovascular events in patients after an acute coronary syndrome in MERLIN-TIMI 36. J Am Heart Assoc 2021; 10: e17401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harris WS, Tintle NL, Imamura F, et al. Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat Commun 2021; 12: 2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonçalinho G, Sampaio GR, Soares-Freitas R, et al. Omega-3 fatty acids in erythrocyte membranes as predictors of lower cardiovascular risk in adults without previous cardiovascular events. Nutrients 2021; 13: 1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Del GL, Imamura F, Aslibekyan S, et al. Omega-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med 2016; 176: 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu J, Micha R, Mozaffarian D. Dietary fats and cardiometabolic disease: mechanisms and effects on risk factors and outcomes. Nat Rev Cardiol 2019; 16: 581–601. [DOI] [PubMed] [Google Scholar]
- 11. Åkesson A, Donat-Vargas C, Berglund M, et al. Dietary exposure to polychlorinated biphenyls and risk of heart failure – a population-based prospective cohort study. Environ Int 2019; 126: 1–6. [DOI] [PubMed] [Google Scholar]
- 12. Levitan EB, Wolk A, Mittleman MA. Fatty fish, marine omega-3 fatty acids and incidence of heart failure. Eur J Clin Nutr 2010; 64: 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamagishi K, Iso H, Date C, et al. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol 2008; 52: 988–996. [DOI] [PubMed] [Google Scholar]
- 14. Mozaffarian D, Bryson CL, Lemaitre RN, et al. Fish intake and risk of incident heart failure. J Am Coll Cardiol 2005; 45: 2015–2021. [DOI] [PubMed] [Google Scholar]
- 15. Wilk JB, Tsai MY, Hanson NQ, et al. Plasma and dietary omega-3 fatty acids, fish intake, and heart failure risk in the Physicians’ Health Study. Am J Clin Nutr 2012; 96: 882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belin RJ, Greenland P, Martin L, et al. Fish intake and the risk of incident heart failure: the Women’s Health Initiative. Circ Heart Fail 2011; 4: 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dijkstra SC, Brouwer IA, van Rooij FJ, et al. Intake of very long chain n-3 fatty acids from fish and the incidence of heart failure: the Rotterdam Study. Eur J Heart Fail 2009; 11: 922–928. [DOI] [PubMed] [Google Scholar]
- 18. Levitan EB, Wolk A, Mittleman MA. Fish consumption, marine omega-3 fatty acids, and incidence of heart failure: a population-based prospective study of middle-aged and elderly men. Eur Heart J 2009; 30: 1495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Djousse L, Biggs ML, Matthan NR, et al. Serum individual nonesterified fatty acids and risk of heart failure in older adults. Cardiology 2021; 146: 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Block RC, Liu L, Herrington DM, et al. Predicting risk for incident heart failure with omega-3 fatty acids: from MESA. JACC Heart Fail 2019; 7: 651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hara M, Sakata Y, Nakatani D, et al. Low levels of serum n-3 polyunsaturated fatty acids are associated with worse heart failure-free survival in patients after acute myocardial infarction. Circ J 2013; 77: 153–162. [DOI] [PubMed] [Google Scholar]
- 22. Mozaffarian D, Lemaitre RN, King IB, et al. Circulating long-chain omega-3 fatty acids and incidence of congestive heart failure in older adults: the cardiovascular health study: a cohort study. Ann Intern Med 2011; 155: 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamagishi K, Nettleton JA, Folsom AR. Plasma fatty acid composition and incident heart failure in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2008; 156: 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 25. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 14 July 2018).
- 26. Cai X, Zhang Y, Li M, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ 2020; 370: m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cai X, Zheng S, Liu Y, et al. Nonalcoholic fatty liver disease is associated with increased risk of atrial fibrillation. Liver Int 2020; 40: 1594–1600. [DOI] [PubMed] [Google Scholar]
- 28. Li W, Huang A, Zhu H, et al. Gut microbiota-derived trimethylamine N-oxide is associated with poor prognosis in patients with heart failure. Med J Aust 2020; 213: 374–379. [DOI] [PubMed] [Google Scholar]
- 29. Yang Y, Li W, Zhu H, et al. Prognosis of unrecognised myocardial infarction determined by electrocardiography or cardiac magnetic resonance imaging: systematic review and meta-analysis. BMJ 2020; 369: m1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Djousse L, Akinkuolie AO, Wu JH, et al. Fish consumption, omega-3 fatty acids and risk of heart failure: a meta-analysis. Clin Nutr 2012; 31: 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dabkowski ER, O’Connell KA, Xu W, et al. Docosahexaenoic acid supplementation alters key properties of cardiac mitochondria and modestly attenuates development of left ventricular dysfunction in pressure overload-induced heart failure. Cardiovasc Drugs Ther 2013; 27: 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eclov JA, Qian Q, Redetzke R, et al. EPA, not DHA, prevents fibrosis in pressure overload-induced heart failure: potential role of free fatty acid receptor 4. J Lipid Res 2015; 56: 2297–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O’Connell TD, Block RC, Huang SP, et al. Omega3-polyunsaturated fatty acids for heart failure: effects of dose on efficacy and novel signaling through free fatty acid receptor 4. J Mol Cell Cardiol 2017; 103: 74–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lechner K, Scherr J, Lorenz E, et al. Omega-3 fatty acid blood levels are inversely associated with cardiometabolic risk factors in HFpEF patients: the Aldo-DHF randomized controlled trial. Clin Res Cardiol. Epub ahead of print 28 August 2021. DOI: 10.1007/s00392-021-01925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toko H, Morita H, Katakura M, et al. Omega-3 fatty acid prevents the development of heart failure by changing fatty acid composition in the heart. Sci Rep 2020; 10: 15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sakamoto A, Saotome M, Iguchi K, et al. Marine-derived omega-3 polyunsaturated fatty acids and heart failure: current understanding for basic to clinical relevance. Int J Mol Sci 2019; 20: 4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oikonomou E, Vogiatzi G, Karlis D, et al. Effects of omega-3 polyunsaturated fatty acids on fibrosis, endothelial function and myocardial performance, in ischemic heart failure patients. Clin Nutr 2019; 38: 1188–1197. [DOI] [PubMed] [Google Scholar]
- 38. Heydari B, Abdullah S, Shah R, et al. Omega-3 fatty acids effect on post-myocardial infarction ST2 levels for heart failure and myocardial fibrosis. J Am Coll Cardiol 2018; 72: 953–955. [DOI] [PubMed] [Google Scholar]
- 39. Rimm EB, Appel LJ, Chiuve SE, et al. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation 2018; 138: e35–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Djousse L, Petrone AB, Weir NL, et al. Repeated versus single measurement of plasma omega-3 fatty acids and risk of heart failure. Eur J Nutr 2014; 53: 1403–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223221081616 for Long-chain omega-3 polyunsaturated fatty acids and the risk of heart failure by Sulin Zheng, Min Qiu, Jason H.Y. Wu, Xiong-fei Pan, Xiong Liu, Lichang Sun, Hailan Zhu, Jiandi Wu and Yuli Huang in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-2-taj-10.1177_20406223221081616 for Long-chain omega-3 polyunsaturated fatty acids and the risk of heart failure by Sulin Zheng, Min Qiu, Jason H.Y. Wu, Xiong-fei Pan, Xiong Liu, Lichang Sun, Hailan Zhu, Jiandi Wu and Yuli Huang in Therapeutic Advances in Chronic Disease