Abstract
Tuberculosis (TB) is the leading cause of death in people living with HIV (PLHIV) globally, causing 208,000 deaths in PLHIV in 2019. PLHIV have an 18-fold higher risk of TB, and HIV/TB mortality is highest in inpatient facilities, compared with primary care and community settings. Here we discuss challenges and potential mitigating solutions to address TB-related mortality in adults with HIV. Key factors that affect healthcare engagement are stigma, knowledge, and socioeconomic constraints, which are compounded in people with HIV/TB co-infection. Innovative approaches to improve healthcare engagement include optimizing HIV/TB care integration and interventions to reduce stigma. While early diagnosis of both HIV and TB can reduce mortality, barriers to early diagnosis of TB in PLHIV include difficulty producing sputum specimens, lower sensitivity of TB diagnostic tests in PLHIV, and higher rates of extra pulmonary TB. There is an urgent need to develop higher sensitivity biomarker-based tests that can be used for point-of-care diagnosis. Nonetheless, the implementation and scale-up of existing tests including molecular World Health Organization (WHO)-recommended diagnostic tests and urine lipoarabinomannan (LAM) should be optimized along with expanded TB screening with tools such as C-reactive protein and digital chest radiography. Decreased survival of PLHIV with TB disease is more likely with late HIV diagnosis and delayed start of antiretroviral (ART) treatment. The WHO now recommends starting ART within 2 weeks of initiating TB treatment in the majority of PLHIV, aside from those with TB meningitis. Dedicated TB treatment trials focused on PLHIV are needed, including interventions to improve TB meningitis outcomes given its high mortality, such as the use of intensified regimens using high-dose rifampin, new and repurposed drugs such as linezolid, and immunomodulatory therapy. Ultimately holistic, high-quality, person-centered care is needed for PLHIV with TB throughout the cascade of care, which should address biomedical, socioeconomic, and psychological barriers.
Keywords: PLHIV, HIV/TB co-infection, TB-related mortality, TB IRIS, TB meningitis
Introduction
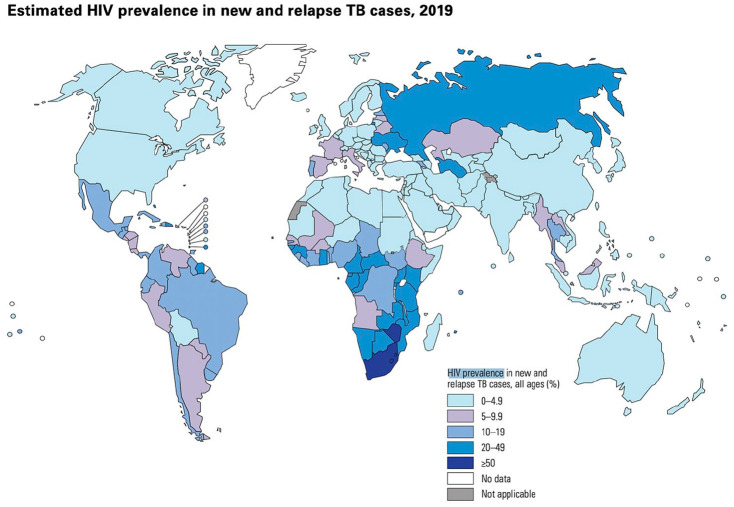
Tuberculosis (TB) is the leading cause of death in people living with HIV (PLHIV) globally, causing 208,000 deaths in PLHIV in 2019. 1 The World Health Organization (WHO) estimates that in 2019, 456,426 PLHIV were diagnosed with TB (see Figure 1 for global HIV TB prevalence estimates); yet 44% of TB disease goes undiagnosed in PLHIV. 1 This is a major concern as PLHIV are 18 times as likely to develop TB, compared with those without HIV. 1 TB risk increases in PLHIV as CD4 T cell count decreases, with data from a systematic review and meta-analysis of 164 full-text articles reporting that the odds of getting TB in PLHIV was 1.43 times higher [95% confidence interval (CI): 1.16–1.88] for every 100 cells per mm3 decrease in CD4 cell count. 2 Post-mortem studies demonstrate that TB is the cause of approximately 40% of facility-based deaths in PLHIV, 3 and that TB is undiagnosed at the time of death in almost half of these decedents. 4 TB prevalence and mortality are considerably higher for PLHIV seen in inpatient facilities (36.4% and 22.6%, respectively) and in primary care settings (20.5% and 3.1%, respectively) compared with community settings (6.9% and 1.6%, respectively). 5 Table 1 compares the differences in TB mortality between PLHIV and people without HIV, demonstrating a marked increase in TB mortality in PLHIV in countries with a high HIV prevalence such as Lesotho and South Africa. The WHO’s End TB Strategy aims to reduce all TB deaths by 95% by 2035, compared to 2015. 6 There is an urgent need to understand the drivers of TB mortality in PLHIV and develop interventions to decrease these largely preventable deaths.
Figure 1.
Global estimation of HIV prevalence in new and relapsed TB cases in percentage, 2019. Figure credit: WHO Global Tuberculosis Report 2020, WHO.
Table 1.
Estimated global burden of TB and comparison of TB mortality in people with and without HIV co-infection globally and in high TB burden countries with differing HIV prevalence a , 2019.
| Population, per 1000 | Total TB incidence, per 100,000 | HIV prevalence among incident TB cases (%) | HIV-negative TB mortality, per 100,000 | HIV-positive TB mortality, per 100,000 | |
|---|---|---|---|---|---|
| Global | 7,690,000 | 130 | 8.2 | 16 | 2.7 |
| Lower HIV prevalence | |||||
| Angola | 31,800 | 351 | 7.6 | 53 | 8.2 |
| China | 1,430,000 | 58 | 1.6 | 2.2 | 0.15 |
| Ethiopia | 112,000 | 140 | 6.5 | 19 | 2.5 |
| Indonesia | 271,000 | 312 | 2.2 | 34 | 1.7 |
| Papua New Guinea | 8780 | 432 | 3.8 | 47 | 3.5 |
| Higher HIV prevalence | |||||
| Lesotho | 2130 | 654 | 62 | 57 | 168 |
| Mozambique | 30,400 | 361 | 34 | 19 | 18 |
| South Africa | 58,600 | 615 | 58 | 38 | 62 |
| Zambia | 17,900 | 333 | 46 | 33 | 53 |
| Zimbabwe | 14,600 | 199 | 60 | 11 | 31 |
TB, tuberculosis; HIV, human immunodeficiency virus.
Per the Global Tuberculosis Report 2020, World Health Organization (WHO).
Based on the 2020 estimated list of top 30 high-burden HIV/TB countries.
High TB case fatality in PLHIV has been attributed to the combination of the following biological factors: rapid progression of disease due to failure of the immune system to control Mycobacterium tuberculosis (Mtb) in PLHIV because of CD4+ T-cell depletion, 7 delayed diagnosis and treatment of TB due to more variable clinical presentations (for example, due to higher rates of extrapulmonary TB seen in PLHIV), 8 lower rates of sputum smear and molecular diagnostic test positivity, 8 and higher rates of MDR-TB in PLHIV (24% higher than people who tested negative for HIV), 9 which result in delayed initiation of effective TB therapy and requires a regimen with additional anti-TB drugs with overlapping toxicities.8,9
The cascade of care, which maps the stages and gaps in HIV care delivery, has become a useful model to evaluate the quality of HIV care and has driven policy guidance on targets for HIV diagnosis, treatment, and viral suppression. 10 More recently, this model has been developed in the context of TB and is helpful to understand the extent of different gaps within local and national TB care cascades, with a view to designing targeted interventions that may vary by setting. 11 This narrative review seeks to explore how different aspects of TB care delivery, organized according to the stages of the TB cascade of care, impact the risk of mortality due to drug-susceptible TB disease in adults with HIV, and consider potential interventions that may mitigate this risk.
Challenges that drive TB-related mortality in PLHIV
Epidemiological risk factors for HIV/TB infection and mortality
Several studies have investigated epidemiological risk factors for increased TB mortality in PLHIV. Delayed initiation of ART is a critical risk factor for TB-related mortality in PLHIV (see section on Timing of treatment). 12 Other risk factors reported to be associated with HIV/TB mortality include low CD4 count, advanced HIV, not receiving ART, not being on co-trimoxazole preventive therapy, older age, male sex, being a female sex worker, incarceration, use of intravenous drugs, low weight, and being bed-ridden.13–17 Several studies have demonstrated that socioeconomic factors such as being illiterate, 18 and having a poor nutritional status 19 also increase the risk of mortality in people with HIV/TB co-infection.
Challenges impacting healthcare engagement
Barriers to healthcare engagement at each stage of the HIV/TB cascade of care impact the quality of care and can adversely affect all outcomes, including mortality. 20 A systematic review found that the main factors that affected initial engagement by Indian patients with TB care were not recognizing symptoms as severe enough to seek medical attention, work pressure, dissatisfaction with nearby health facility, lack of proximity to a healthcare facility, financial constraints, alcohol dependence, and inconvenient working hours. 21 A systematic review reported that barriers to healthcare engagement among Ethiopian presumptive TB patients included short duration of cough, absence of previous TB treatment history, lack of money, old age, low education level, lack of jobs, perceived wellness, and use of traditional healers. 22 A cross-sectional study in the Democratic Republic of Congo found that fear of discrimination and stigmatization, health worker attitudes, availability of both TB and HIV drugs, affected healthcare engagement in people with HIV/TB co-infection. 23 Barriers to healthcare engagement such as stigma are often intersectional and compounded in PLHIV compared with people without HIV (dual stigma for people with HIV/TB co-infection), and also highlight broader health system weaknesses, such as inefficient care delivery processes, gaps in data communication, and infrastructure. 20
TB preventive therapy
Given their increased risk of TB infection, TB preventive therapy (TPT) is recommended for PLHIV to prevent TB infection and reduce mortality. 24 The Temprano randomized controlled trial(RCT) from Côte d’Ivoire found that 6 months of TPT reduced TB mortality by in PLHIV 39%. 25 However, implementation of TPT remains weak with only 21% of eligible PLHIV initiated on TPT globally in 2021. 1 A systematic review and meta-analysis evaluating the latent TB cascade of care found that people with an immune-compromising condition like HIV were more likely to complete screening and receive a result (86%) compared with the general population (62%) and that people with medical conditions including HIV were more likely to complete treatment (50%) than the general population (10%); yet HIV infection was also cited as a risk factor associated with not starting treatment in one of the three included studies that evaluated PLHIV. 26 A retrospective study from Namibia evaluating TPT uptake in PLHIV found that only 20% of eligible patients completed the TPT cascade of care. 27 A cross-sectional study from Zambia also found low TPT completion rates with only 38% of the 25% of eligible PLHIV who initiated TPT completing treatment. 28
Challenges with the diagnosis of TB in people living with HIV
Interventions to increase the likelihood of earlier diagnoses for both HIV and TB reduce mortality in adults with HIV/TB co-infection, since factors such as having a higher CD4 level and being on ART are associated with lower mortality. 29 However, sputum, which remains the mainstay of TB diagnostic testing, is more difficult to obtain in the adults with HIV/TB co-infection, 30 due to paucibacillary disease and the lack of cavitation, which are associated with a higher rate of smear negative disease. 31 In addition, the accuracy of many TB diagnostic tests is lower in PLHIV, 32 who are also more likely to have extra pulmonary TB. 33
Screening and triage
There is an urgent need for improved TB screening or triage tests, due to their potential impact on facilitating earlier diagnosis and reducing the number of confirmatory molecular tests needed. 34 Chest radiography (CXR) has a sensitivity of 89–96% compared to 82–93% for symptom-based screening. 35 The use of computer-aided detection (CAD) software to enable automated CXR analysis is now conditionally recommended by WHO as an alternative to human readers. 35 However, a recent study of PLHIV with presumptive TB with negative smear microscopy in Uganda demonstrated that while CXR interpretation in conjunction with Xpert MTB/RIF testing increased the overall sensitivity for TB diagnosis (88%), specificity was decreased (52%) compared to Xpert MTB/RIF alone (96%), highlighting the diagnostic limitations of CXR in this patient population. 36
A prospective cohort study from 2019 conducted in PLHIV with a cell count of ⩽350 cells/μL from two clinics in Kampala, Uganda found that those with elevated C-reactive protein (CRP) levels (⩾8 mg/L) were 1.95 times as likely to develop an opportunistic infection like TB over the course of 3 months as compared to those with lower CRP levels (⩽8 mg/L).34,37 A prospective study of PLHIV in Uganda found that the sensitivity and specificity of CRP as a screening test were 89% and 72%, respectively. 38 A cross-sectional study from South Africa in PLHIV found that compared with TB symptom screening, CRP screening had the same sensitivity (90.5%) but a higher specificity (58.5% versus 37.1%). 39 A screening test with a higher specificity than symptom screening reduces the number of false positive results (for TB disease) in people with TB symptoms and may thus improve uptake of TPT in PLHIV (in whom it is essential to exclude active TB disease prior to treatment of latent TB infection). 38 The WHO now recommends that CRP, using a cut-off of >5 mg/L, may be used to screen PLHIV for TB disease. 35 Yet, although CRP is a commercially available validated test that can cost as little as US$1,35,40 implementation remains limited.
Diagnosis – molecular tests
Molecular WHO-recommended rapid diagnostic tests (mWRDs) should be used, whenever possible, as the initial diagnostic test for pulmonary TB and certain forms of extrapulmonary TB such as TB meningitis since they not only detect Mtb but also enable analysis of rifampin (RIF) resistance. 32 Xpert MTB/RIF, Xpert Ultra, and TrueNat MTB are the mWRDs currently recommended by WHO for adults and children undergoing initial TB diagnostic evaluation. 32 Xpert Ultra has an increased pooled sensitivity in PLHIV compared to Xpert MTB/RIF (87.6% versus 74.9%) although the pooled specificity was decreased (92.8% versus 99.7%). 41
Diagnosis – microbiological tests
Smear microscopy. In many high TB incidence countries, including those with a high prevalence of HIV/TB co-infection, smear microscopy remains the most widely used and available diagnostic test,42,43 as it is both inexpensive and rapid. 30 However, smear microscopy has a low, and often variable sensitivity ranging from 20% to 60% overall and 38% to 69% in PLHIV.30,44 In addition, smear microscopy cannot differentiate TB from non-tuberculosis mycobacteria. 33
Culture. While mycobacterial culture testing remains the primary reference standard for TB diagnosis, the sensitivity of culture in PLHIV can vary, particularly in those with advanced HIV who have a greater likelihood of extra pulmonary and disseminated TB, which are often culture negative. 45 However, since the risk of smear- and Xpert-negative TB is also increased in advanced HIV, culture plays an important role in confirming TB in PLHIV, and it has been reported that mortality is higher in patients without culture-confirmed TB.45,46
Diagnosis – biomarker-based tests
LAM. Despite much interest in biomarkers for TB diagnosis, 47 only one such test is currently recommended by WHO: the lateral flow lipoarabinomannan (LF-LAM) assay. It tests for the presence of the LAM antigen, a lipopolysaccharide present in mycobacterial cells that can be detected in the urine of TB patients.33,48 Compared with sputum-based testing, LF-LAM is relatively easy to perform and has minimal biosafety requirements. 33 A Cochrane review evaluating 15 studies on LF-LAM concluded that the pooled sensitivity and specificity of this test in PLHIV was 42% and 91%, respectively. 49 LF-LAM had a sensitivity of 42% in PLHIV who presented with TB symptoms and 35% in those who were not assessed for TB symptoms. 49 In inpatients versus outpatients, the pooled sensitivity was 62% versus 31%. 49 WHO recommends that LF-LAM be used to assist in active TB diagnosis in HIV-positive adults, adolescents and children who show signs and symptoms of TB, or who are seriously ill in inpatient and outpatient settings, and irrespective of signs and symptoms for PLHIV with a CD4 count of less than 200 in inpatient or less than 100 cells/mm3 in outpatient settings. 32 Yet only 13/37 (35%) countries with a high HIV/TB prevalence have policies that are aligned with this recommendation. 50
Other challenges associated with diagnosis in high HIV/TB incidence settings
Access to currently WHO-recommended diagnostic tests remains a major limitation for PLHIV undergoing TB evaluation in high TB incidence settings. 30 The relatively high cost and necessary infrastructure associated with mWRDs prohibit wider scale-up. 51 Optimizing implementation of mWRDs like Xpert also requires consideration of multiple factors beyond diagnostic accuracy alone, including comprehensive training, quality assurance, ensuring adequate supply chains, maintenance, and technical support. 52
Timing of TB and HIV treatment and treatment adherence on HIV/TB mortality
Decreased survival of people with HIV/TB co-infection is more likely with late HIV diagnosis and delayed start of ART treatment, with lower mortality risks reported in PLHIV diagnosed at CD4 > 350 cells/mm3 compared to PLHIV diagnosed at CD4 < 350 cells/mm3. 53 A systematic review and meta-analysis from 2020 evaluating the effect and timing of ART initiation in eight RCTs found that early ART initiation (1–4 weeks following TB treatment initiation) was associated with a lower overall mortality rate as compared to those with later ART initiation [8 weeks to 6 months following TB treatment initiation; risk ratio (RR) = 0.81; 95% CI: 0.66–0.99, p = 0.04]. 12 Nonetheless, major concerns encountered when treating HIV/TB co-infection are drug–drug interactions and immune reconstitution following the initiation of ART, which can lead to TB-immune reconstitution inflammatory syndrome (TB-IRIS). 54
Challenges with TB and HIV treatment adherence and completion
Following diagnosis and treatment initiation, ensuring adherence to and completion of TB treatment remain critical challenges to preventing TB mortality in PLHIV. A systematic review from 2015 evaluating TB treatment non-adherence and loss to follow-up found that the main factors associated with these outcomes were socio-economic factors (lack of food, transportation costs, unemployment, etc.), individual behavior (lack of knowledge, medication side effects, etc.), healthcare workers (poor communication, stigma, etc.), and HIV status. 55 The increased financial burden of paying for both TB and HIV treatment is also a recognized barrier, along with the lack of social support and dual disease stigma. 55 A review article from 2019 looked at the gaps in the cascade of TB care for PLHIV and found that healthcare system level gaps pertaining to TB treatment completion included interrupted supply chain management of TB drugs and diagnostics, poor implementation of TB treatment guidelines, and poor quality of care. 20 Virological failure, either due to poor adherence to ART or the development of HIV drug resistance, is also a risk factor for mortality in PLHIV. A nested observational cohort study evaluating unselected hospitalized PLHIV in Malawi found that 32% of those on ART for at least 6 months had virological failure, the majority of whom (83%) had resistance to at least two ART drugs, which was associated with increased mortality (adjusted hazard ratio 1.7, 95% CI: 1.2 to 2.4; p = 0.0042).20,56
TB treatment in special situations – TB meningitis
TB meningitis is a form of central nervous system (CNS) TB that accounts for 5% of all extrapulmonary TB. 57 This form of TB is typically the most severe, as up to 50% of people die or suffer neurological complications. 57 In PLHIV, in whom 40% of TB is extrapulmonary (compared to the 10% in HIV-negative patients), there is a fivefold increase of the likelihood of CNS involvement. 58 PLHIV who get TB meningitis will commonly present with extrameningeal involvement and are more likely to suffer from altered mental status. 58 Diagnosis of TB meningitis is particularly challenging as the clinical manifestations may be nonspecific 59 and the accuracy of TB diagnostic tests (including smear, microscopy, culture, and NAAT) is lower on cerebrospinal fluid samples, compared with sputum.32,59,60 Treatment of TB meningitis is challenging because mortality is high at both 6 and 9 months into treatment (27.9% and 22.4%, respectively), with 53.4% of co-infected TB meningitis patients dying during treatment compared to 17.4% of HIV-negative patients. 54 Furthermore, mortality risk after relapse of TB meningitis in PLHIV is higher than in those without HIV (83% versus 51%). 58 Current guidelines from the WHO recommend 9–12 months of treatment on RIF and isoniazid (compared to 6 months for pulmonary TB), with the inclusion of pyrazinamide and ethambutol in the first 2 months, 54 with adjunctive steroid treatment from the time of treatment initiation that should be slowly tapered. 61
TB treatment in special situations – TB-IRIS
TB-IRIS is a severe inflammatory syndrome associated with HIV/TB co-infection and occurs following the rapid reconstitution of the immune system after ART initiation. 62 The likelihood of TB-IRIS increases with the severity of CD4+ T-cell deficiency prior to ART initiation, with data from China demonstrating that patients with initial CD4 cell counts less than 50 cells/mm3 were at a 4.6-fold increased likelihood of getting TB-IRIS as compared to those with CD4 cell counts greater than 100 cells/mm3 [odds ratio (OR): 4.6, 95% CI (1.033–20.238)].62,63 After ART initiation, there was a 2.3-fold increased likelihood of getting TB-IRIS among patients who had a fourfold or greater increased in CD4 cell count as compared with those who had less than fourfold increase in CD4 cell count. 63 There are three types of TB-IRIS: paradoxical, unmasking, and CNS. 62 Both paradoxical and unmasking TB-IRIS typically occur within 3 months of ART initiation. Paradoxical TB-IRIS occurs when patients with known TB experience worse symptoms post-ART initiation while unmasking TB-IRIS occurs in patients without a prior TB diagnosis and experience marked inflammatory symptoms. 62 CNS TB-IRIS occurs in patients with TB meningitis 62 and is a contributor to poor outcomes including mortality in this high-risk population. 64
High rates of recurrent TB and post-TB disease sequelae
A recent systematic review reports higher rates of recurrent TB in high HIV prevalence areas, 65 consistent with a prior systematic review that reported a TB recurrence rate of 4.5 (3.2–5.8) in PLHIV compared to 1.9 (1.2–2.7) in people without HIV per 100 person-years. 66 Post-TB lung disease following TB completion is an additional concern. A prospective cohort study in Malawi found that after successful treatment of pulmonary TB, one-third of the cohort had abnormal spirometry and 40% had bronchiectasis. Although PLHIV were less likely to experience an acute respiratory event 1 year post-TB treatment (12.9% versus 21.9%), rates of post-TB disease were also high in this population. 67 The burden of life post-TB successful treatment extends to social, economic, and psychological factors, since treatment can be financially burdensome, stigma from TB can affect social status, and there may be long-term mental health impacts. 68
Potential solutions to mitigate TB-related mortality in PLHIV
Addressing challenges with healthcare engagement
Addressing challenges with healthcare engagement in people with HIV/TB co-infection requires a multifaceted approach. Health workers in Namibia who received training in TB infection control, and patients’ rights and confidentiality, were more willing to report discrimination in the healthcare setting [adjusted odds ratio (aOR) 2.1 with 95% CI: 1.03–4.39% and 2.2 with 95% CI: 1.11–4.47]. 69 In addition, since many HIV patients avoid local ART centers due to anticipated stigma, including fear of recognition, ensuring anonymity is essential, along with reassurance from doctors and patient support networks that could encourage patients to seek care. 70 Internal stigma is also common, with patients expressing embarrassment and shame regarding both HIV and TB diagnoses. 71 Given that stigma is associated with a lack of knowledge about TB, 72 community-informed education campaigns may help reduce stigma on a larger scale. Financial support can reduce the socioeconomic barriers that impact engagement in care,73,74 although data on strategies such as financial incentives for populations that include PLHIV are limited. 75 There is an urgent need to increase health system resources to improve healthcare infrastructure, integration of TB and HIV healthcare, and enable person-centered care that addresses socioeconomic constraints such as food insecurity and lack of transportation. 20
Optimizing HIV/TB integrated care
A descriptive cross-sectional study conducted at Mulago National Referral Hospital in Kampala, Uganda investigating the hospital’s efforts to integrate TB and HIV care found that the main challenges were lack of program coordination (providers being late for appointments and poor instruction provided to patients) and lack of treatment for other opportunistic infections. 76 A retrospective cohort study in Nigeria found that the difficulties in running an HIV/TB integrated healthcare facility included missing data due to poor documentation, which led to miscommunication and lost referrals from the clinic to other care facilities (X-ray/lab units) that limited the ability of patients to complete treatment. 77 Given the importance of integrated HIV/TB care, we sought examples of changes in healthcare systems and policies to facilitate its implementation. A programmatic evaluation of almost 120,000 patients treated by the ART program in South Africa from 2009 to 2013 demonstrated increased uptake of ART in ART-naïve patients from 37.0% to 77.7% and reduced TB case fatality from 7.4% to 5.2% (p < 0.001), and reported that ART initiation during TB treatment was associated with lower mortality [adjusted hazard ratio (aHR): 0.39; 95% CI: 0.35–0.42; p < 0.001]. 78 A cross-sectional study evaluating the implementation of the updated HIV Standard of Care (SOC) 79 to match the WHO standards within the TB detection and treatment program in Nigeria showed that the number of PLHIV screened for TB increased from 14,530 to 29,467 people in 12 months and there was a correlational increase in the number of persons diagnosed and treated for TB, as a result of the implementation of the HIV SOC. 80 The integration of HIV/TB care in medical facilities can decrease socioeconomic barriers by preventing patients having to go to multiple facilities for treatment. 81 Improving the quality of HIV/TB care delivery using person-centered approaches to address these individual and healthcare system level factors is essential to ensure HIV and TB treatment adherence and TB treatment completion. 20
Implementing TB preventive therapy
Alternative shorter TPT regimens (instead of 9 months of isoniazid) are now recommended by WHO 24 based on several recent trials. An RCT that enrolled patients from the United States, Spain, Canada, and Brazil found that the use of rifapentine plus isoniazid for 3 months was as effective at preventing TB as the standard 9 months of isoniazid, with higher completion rates (82.1% versus 69.0%). 82 Another RCT conducted in nine countries found that a 4-month regimen of RIF was non-inferior to the standard 9-month isoniazid treatment at preventing active TB (difference <0.01 cases per 100 person-years). 83 A recent RCT looking at TB prevention in PLHIV found that a 1-month regimen of rifapentine plus isoniazid was non-inferior to the standard 9 months of isoniazid at preventing TB (incidence rate of 0.65 per 100 person-years versus 0.67 per 100 person-years). 84 Improving the efficiency of healthcare systems and effective training on TPT may also allow for better implementation of TPT and reduced TB mortality in PLHIV.27,28
Improving TB screening and diagnosis
Wider implementation of TB screening in PLHIV is essential to reducing TB-related mortality. The WHO consolidated guidelines on tuberculosis recommend that PLHIV should be screened systematically for TB at every visit to a healthcare facility. 35 An RCT to be completed in 2023 will be conducted in PLHIV in Ugandan clinics to test the effect of CRP results on uptake of TPT. 85 The WHO also recommends that further clinical trials should be undertaken to evaluate other screening tools including CXR with CAD in PLHIV. 35
There is an urgent need for rapid, accurate biomarker-based tests for TB diagnosis in PLHIV. A diagnostic accuracy study from 2019 using frozen urine PLHIV samples from FIND Specimen Bank and the University of Cape Town Biobank found that the sensitivity of Fujifilm SILVAMP TB LAM (FujiLAM), a next-generation LAM assay, was 70.4% (95% CI: 53.0, 83.1) compared to 42.3% (95% CI: 31.7, 51.8) for AlereLAM in PLHIV, without a statistically significant difference in specificity between the two tests (FujiLAM: 90.8%, 95% CI: 86.0–94.4; AlereLAM: 95.0%, 95% CI: 87.7–98.8) 86 Trials evaluating the impact of this higher sensitivity next-generation LAM assay are underway. 87
Given the higher risk of mixed-strain infections and drug-resistant TB in PLHIV, improving access to whole-genome sequencing and next-generation sequencing, as strategies that include drug resistance profiling and enable the identification of multiple strains will also improve TB care and public health surveillance efforts.30,44 Such initiatives will require greater funding to be dedicated TB diagnostics research and development, as well as building laboratory capacity in low- and middle-income countries. 88
Impact of TB diagnostic tests on HIV/TB patient important outcomes including mortality
Xpert and mortality
A recent Cochrane Review from 2021 analyzing data from five RCTs with 9932 participants judged to represent moderate-certainty evidence reported that it was not possible to determine the impact of the use of Xpert MTB/RIF compared with smear microscopy on all-cause mortality (RR: 0.9, 95% CI: 0.8–1.1). 61 They did, however, report that there was probably a reduction in mortality in PLHIV (OR: 0.8, 95% CI: 0.67–0.96), which emphasizes the importance of access to Xpert and other mWRDs in PLHIV being evaluated for TB. 61 Given the increased sensitivity of Xpert Ultra, including on CSF samples, wider scale-up of Xpert Ultra can facilitate TB diagnosis in PLHIV. 41
LAM and mortality
Urine LAM is the only currently recommended biomarker-based TB diagnostic test and has been demonstrated to have an impact on mortality. 89 A Cochrane review analyzing two RCTs in inpatient settings and one RCT in outpatient settings, all in sub-Saharan Africa, reported that the use of LAM as part of a TB diagnostic strategy likely reduces mortality at 8 weeks and probably results in a slight increase in TB treatment initiation in PLHIV in inpatient settings, and that the use of LAM as part of a TB diagnostic strategy may reduce mortality at 6 months and may result in a large increase in TB treatment initiation in PLHIV in outpatient settings. 90 LAM is hypothesized to indirectly lead to increased mortality risk in people with HIV/TB co-infection, as increased LAM levels were found to contribute to the survival of M. tuberculosis in macrophages, 91 and PLHIV with a positive LAM result account for a disproportionate proportion of those who die, 92 which may reflect the association between detection of LAM and a lower CD4 count. 90 A cost-effectiveness analysis demonstrated that urine-based TB screening using LAM in hospitalized patients with HIV could increase life expectancy and be cost-effective in resource-limited settings. 93
Improving the treatment of HIV/TB co-infection
Timing of ART in adults with HIV/TB co-infection
In 2021, the WHO updated their guidelines to recommend that ART should be started within 2 weeks regardless of CD4 cell count following initiation of antituberculosis treatment in people with HIV/TB co-infection. 94 A systematic review and meta-analysis from 2021 looked into nine clinical trials from over 2000 screened articles to determine the best timeline for starting ART in people with HIV/TB co-infection. 95 While there was no difference in mortality in earlier (⩽4 weeks) ART initiation versus later (>4 weeks) ART initiation regardless of CD4 count [risk difference (RD) 0%, 95% CI: −2% to +1%], those with CD4 count ⩽50 cells/mm3 had a decrease in likelihood of death of 6% (1–10%) when ART was initiated earlier. 95 In all patients regardless of CD4 count, earlier ART initiation was associated with increased risk of TB-IRIS (RD: +6%, 95% CI: +2% to +10%) and decreased risk of AIDS-defining events (Kaposi’s sarcoma, etc.; RD: −2%, 95% CI: −4% to 0%). 95 The overall conclusions of the systematic review were that earlier ART initiation did not adversely affect mortality in people with HIV/TB co-infection and thus earlier ART initiation may be preferred from both a health system logistical and patient preference standpoint. 95 In contrast to other forms of TB, the WHO recommend that ART should be delayed at least 4 weeks (and initiated within 8 weeks) after treatment for TB meningitis is initiated. 94
While implementation outside of clinical trials is often more challenging, a number of studies have looked at the implementation of strategies to improve time to ART initiation in real-world situations. A prospective cohort study in South Africa looked at the implementation of a collaborative learning network, where representatives from each sector of healthcare meet to learn about quality improvement methods and to discuss methods of improvement in HIV healthcare, to HIV healthcare and found that monthly ART initiation increased from 179 patients per month to 511 patients per month following the intervention. 96 A prospective cohort in Siberia looked at ART initiation in a TB referral clinic following the implementation of increased education, HIV testing, and prioritization of HIV referrals and found that ART initiation rates increased from 17% to 54%. 97
Duration of TB treatment
There is considerable interest in shortening TB treatment regimens to minimize toxicity, drug interactions, impact on patient quality of life, and facilitating TB care from a programmatic standpoint. Typically, longer durations of treatment may be recommended in PLHIV, as the Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines states that an additional 3 months can be added on to the standard 6 months of TB in PLHIV due to their increased risk of TB relapse. 98 Observational data has demonstrated higher risk of unsuccessful TB treatment outcomes in PLHIV treated for less than 6 months. A recent RCT compared the standard 6-month TB treatment to two different 4-month regimens, one where RIF was replaced with rifapentine and the other where RIF was replaced with rifapentine and ethambutol with moxifloxacin in 2343 patients with culture-positive pulmonary TB in 13 countries. 99 The 4-month regimen containing rifapentine and moxifloxacin was noninferior to the standard regimen (15.5% versus 14.6% had an unfavorable outcome of death; adjusted absolute difference of 1.0% point; 95% CI: −2.6 to 4.5). 99 Although this trial’s sensitivity analyses in PLHIV suggested that the shorter regimen was also non-inferior in this subgroup, since only 8% of the trial population were co-infected with HIV, there was limited power to compare the regimens in this population. 99 We note that PLHIV, particularly those with CD4+ T-cell counts below 100 cells/mm3, are underrepresented in TB treatment trials, thus dedicated treatment trials are needed to understand nuances such as treatment timing, duration, medication dosages, and drug interactions in PLHIV. 100
Role of empiric TB treatment in PLHIV
Empiric treatment of TB without definitive microbiological confirmation of disease is often pursued given the challenges of TB diagnosis. 101 A prospective cohort study conducted in Kenya found that there was no difference in risk of mortality for PLHIV who were treated for TB empirically compared with those treated based on microbiological confirmation. 102 Similarly, an RCT conducted in the Ivory Coast, Uganda, Vietnam, and Cambodia that compared the risk of mortality at 48 months for empiric TB treatment with test-guided TB treatment in HIV-infected adults who had not previously received ART and had CD4+ T-cell counts below 100 cells/mm3 found that empiric TB treatment did not reduce the risk of mortality (aHR: 0.97; 95% CI: 0.67–1.40). 101 However, an observational cohort study of TB/HIV co-infected patients conducted in South Africa found that in-hospital mortality was lower in patients with microbiologically confirmed TB, compared with patients treated empirically (HR: 0.5; 95% CI: 0.3–0.9), emphasizing the importance of trying to obtain a microbiological diagnosis of TB in PLHIV. 46
Optimal treatment for TB meningitis
There is increasing evidence that we are underdosing RIF in many people with TB and that higher RIF doses are safe and well tolerated. 103 A pharmacokinetic analysis of a prior placebo-controlled trial comparing immediate versus deferred ART in PLHIV with TB meningitis in Vietnam suggested that current dosing regimens put patients at risk of treatment failure due to suboptimal RIF dosing. An open-label phase 2 RCT in Indonesia randomized hospitalized patients with TB meningitis to standard dose RIF (450 mg, ~10 mg/kg) orally versus high-dose RIF (600 mg, ~13 mg/kg) intravenously, with either oral moxifloxacin 400 mg, moxifloxacin 800 mg, or ethambutol 750 mg once daily and found that 6-month mortality was substantially lower in patients given high-dose rifampicin intravenously [10 (35%) versus 20 (65%) aHR: 0.42; 95% CI: 0.20–0.91; p = 0.03]. 104 In contrast, an RCT from Vietnam compared a standard 9-month TB regimen with oral RIF at 10 mg/kg dosing per day versus the experimental group of oral RIF at 15 mg/kg per day plus 20 mg of levofloxacin per day and found no difference in the death outcome in TB meningitis patients (HR: 0.94; 95% CI: 0.73, 1.22; p = 0.66). 105 Nonetheless expert consensus, based on data from preclinical, clinical, and modeling studies, supports the use of high-dose RIF, at a dose of at least 30 mg/kg/day, in patients with TB meningitis. 106 A more recent double-blind placebo-controlled Phase II dose-finding RCT in Indonesia compared 10-, 20-, and 30-mg/kg doses of oral RIF per day in TB meningitis patients and found that 6-month mortality was 35%, 45%, and 15%, respectively, demonstrating a trend toward decreased mortality with higher doses, and with a demonstrable large increase in RIF plasma and CSF exposure without safety concerns. 107 Small non-randomized observational studies suggest the addition of linezolid to TB treatment regimens may improve clinical outcomes, including Glasgow Coma Scale scores and resolution of fever, in patients with TB meningitis.108,109 There is increasing interest in the potential role of aspirin to improve TB meningitis outcomes due to its anti-thrombotic and anti-inflammatory properties. An RCT in 120 HIV-uninfected adults with TB meningitis in Vietnam comparing daily aspirin 81 or 1000 mg to placebo found that the observed risk of death or new brain infarction by day 60 was lower in the aspirin arms (22% in the aspirin 81 mg group and 16% in the aspirin 1000 mg group) compared with placebo (29%), although this was not statistically significant (p = 0.4). 110 Two trials evaluating the effect of intensified TB meningitis treatment including high-dose RIF and linezolid with and without aspirin in PLHIV (LASER-TB) 111 and in people with and without HIV (INTENSE-TB) 112 are underway.
Reducing the risk of TB-IRIS
Current recommendations for treatment of TB-IRIS depend on the type of TB-IRIS presentation but most commonly will include corticosteroid therapy to reduce the aberrant inflammation that drives TB-IRIS. 62 An RCT demonstrating PLHIV with TB who received prednisone during the first 4 weeks after ART initiation had a reduced TB-IRIS incidence of 32.5% compared with 45.7% who received placebo (RR = 0.7, 95% CI: 0.51, 0.96; p = 0.03). 113 Steroids also remain the mainstay of management for CNS-TB-IRIS and unmasking TB-IRIS. 62 Other immunomodulatory agents such as NSAIDs, TNF-α inhibitors, thalidomide, montelukast, pentoxifylline, and VEGF inhibitors have all been studied for symptom control in people with paradoxical IRIS, but data to support these treatments is typically from case reports or case series. 62
Ensuring that care does not end at TB treatment completion
A recent modeling analysis estimated that 155 million TB survivors were alive in 2020, of whom 8% were estimated to have HIV at the time they were diagnosed, highlighting the importance of interventions to prevent ongoing morbidity, recurrent TB, and reduce the impact of ongoing stigma. 114 This call has been echoed by TB survivors, who emphasize the importance of mental health support and services to mitigate the long-term negative impacts of TB. 115 Secondary preventive therapy has been shown to reduce the risk of recurrent TB in PLHIV. 116 Currently there are no evidence-based guidelines for the management of post-TB lung disease or other complications of TB. 68 The scope of TB care delivery needs to be expanded such that we can ensure patients will have a stable and successful life post-TB treatment and address adverse health effects like post-TB lung disease, along with the socioeconomic and psychological burden.
Conclusion
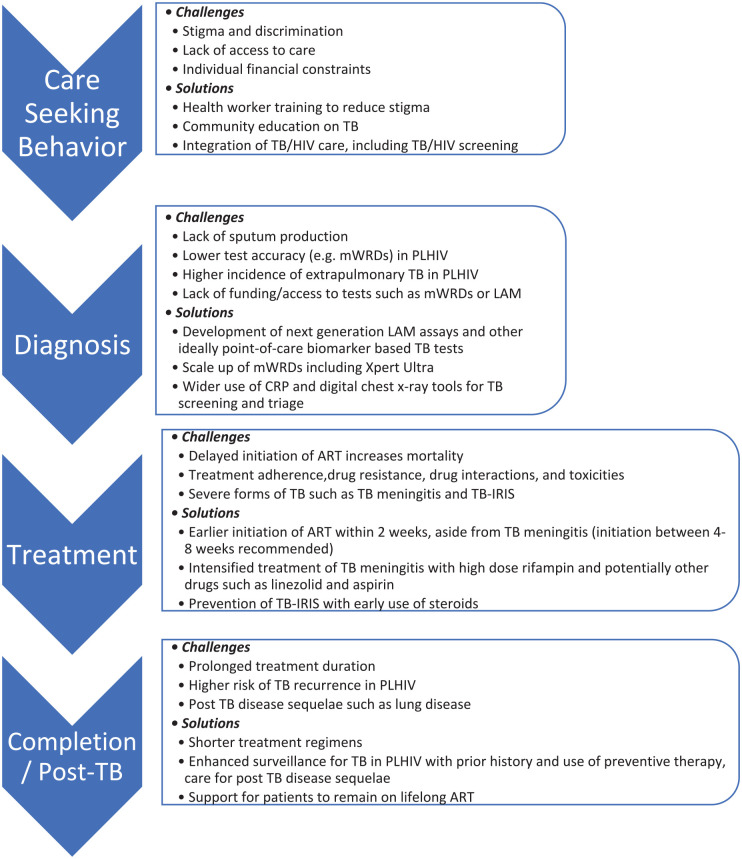
Addressing the high mortality faced by PLHIV with TB is an urgent priority. Although many of the drivers of this increased mortality require further research, such as optimizing treatment regimens, including the use of adjunctive immunomodulatory therapy beyond steroids alone, to reduce morbidity and mortality from TB meningitis, there is clearly much more that can be done with existing tools. Improving the delivery of integrated person-centered HIV/TB care should incorporate innovative approaches to reduce stigma and improve healthcare engagement, as well as ensure access to quality-assured WHO-recommended screening and diagnostic tests including CRP, CXR, mWRDs, and urine LAM, with prompt linkage to care and close follow-up to ensure initiation of effective treatment and management of drug interactions and side effects. Clinicians should consider the possibility of TB-IRIS and whether steroids should be used pre-emptively to reduce this risk. Table 2 provides a summary of evidence-based interventions that can decrease HIV/TB mortality and provides an overview of ongoing challenges and research gaps. High-quality implementation research is urgently needed to evaluate how best to implement evidence-based TB interventions using contextually acceptable strategies, 20 which should include key populations such as PLHIV who have histor-ically often been excluded. 100 Implementers and clinicians need to ensure the holistic care of people with HIV/TB co-infection throughout the cascade of care (Figure 2) to ensure prompt diagnosis and treatment of both infections, with comprehensive and supportive care that does not end at treatment completion and that addresses the biomedical, socioeconomic and psychosocial barriers faced by affected individuals and communities.
Table 2.
Interventions to reduce HIV/TB co-infection mortality: current evidence-based approaches and research gaps.
| Intervention | Mechanism: how it reduces mortality | Supporting evidence, for example, RCTs/systematic reviews | Challenges and/or research gaps |
|---|---|---|---|
| Diagnostics | |||
| mWRD | • Molecular test, most commonly performed on sputum to identify MtbTB and rifampin resistance, results can be available in 90 min, which can facilitate earlier diagnosis and initiation of effective treatment | • Cochrane review: Xpert MTB/RIF pooled sensitivity 81.8%, specificity 97.4% in PLHIV.
41
• Cochrane review: reduction in all-cause mortality in PLHIV; OR 0.80 (95% CI: 0.67–0.96) 61 |
• Remains expensive, requires infrastructure that typically preclude these tests from being used at the point of care, training, and maintenance. • Often reliant on obtaining sputum specimens, lower sensitivity in extra pulmonary specimens. • Implementation research needed (and underway) on the use of mobile mWRD testing platforms that can be used for testing in community settings |
| Culture | • Typically most sensitive microbiologic test for TB, which can help to avoid missed diagnoses. • Enables both phenotypic and genotypic testing for drug resistance to ensure effective treatment |
• Sensitivity: 86.9%, specificity: 92% 117 | • Needs extensive laboratory infrastructure and training. • Results may take between 2 and 8 weeks, which precludes prompt clinical decisions being made |
| LAM | • Detects mycobacterial antigen in urine. • Enables rapid diagnosis (point of care test) and may provide information on prognosis (levels are correlated with disease severity) |
• Cochrane review: pooled sensitivity 42%, specificity 91% (in PLHIV with TB symptoms).
49
• Cochrane review: reduction in all-cause mortality in PLHIV with the addition of LF-LAM to diagnosis: pooled 0.85 (95% CI: 0.76, 0.94) 90 |
• Need for newer generation LAM assays with higher sensitivity in both PLHIV and ideally general population being evaluated for TB. • Implementation research to understand how tests are acted upon to optimize cascade of care |
| Screening tests such as chest X-ray (including digital chest X-ray with automated detection) and CRP | • Use for screening and triage of populations at risk of TB or with TB symptoms to determine need for confirmatory test | • Sensitivity of 89–96% for digital CXR.
35
• Sensitivity of 89% in PLHIV outpatient population for CRP 39 |
• Requires infrastructure and resources for radiography. • Implementation barriers for CRP not well established, trials are underway. 85 • Optimal frequency for community screenings is unknown. • Requires linkage to care for confirmatory testing/follow-up |
| Treatment | |||
| Timing of ART in people with TB co-infection | • Delayed ART initiation is associated with higher mortality | A systematic review demonstrated there was no difference in mortality in those started on ART at ⩽ 4 weeks versus > 4 weeks [risk difference (RD) 0%, 95% confidence interval • CI: −2% to +1%] 95 |
• Requires better integration of HIV and TB care to ensure linkage to care and support that enables assessment of drug interactions and monitoring for complications such as TB-IRIS |
| Intensified TB meningitis treatment | • Evidence that rifampin is being underdosed in most forms of TB including CNS disease. • Intensified treatment that includes higher doses and new or repurposed antibiotics with better CSF penetration may decrease high mortality and morbidity |
RCT in Indonesia demonstrated lower mortality in patients receiving regimen with high-dose intravenous rifampin: 35% versus 65% (adjusted HR: 0.42; 95% CI: 0.20–0.91; p = 0.03).
104
Follow-up RCT in Indonesia found dose response in TB meningitis patients given higher dose aspirin with trend toward decrease in mortality although not statistically significant 107 |
• Trials evaluating role of high-dose rifampin in conjunction with other new or repurposed drugs such as linezolid are underway and should be evaluated in both PLHIV and HIV-uninfected populations in different clinical settings. • Aspirin (and other immune-modulatory therapies) may reduce the risk of stroke and mortality in patients with TB meningitis 110 and trials are underway to evaluate use and optimal dosing for aspirin in conjunction with intensified treatment111,112 |
| Steroids | • Immunomodulatory action improves mortality in certain forms of TB such as TB mortality and can reduce the risk of TB-IRIS | RCT: steroids have shown some promise for decreasing risk for adverse events.
54
RCT: reduced development of TB-IRIS in patients with a CD4 count < 100 cells/mm3 by 30% 30 |
• Additional studies needed to optimize steroid dosing and use in conjunction with other immunomodulatory therapies |
ART, antiretroviral; CI, confidence interval; CNS, central nervous system; CRP, C-reactive protein; CSF, cerebrospinal fluid; CXR, chest radiography; HR, hazard ratio; LAM, lipoarabinomannan; LF-LAM, lateral flow lipoarabinomannan; MtbTB, Mycobacterium tuberculosis; mWRD, molecular WHO-recommended rapid diagnostic tests; OR, odds ratio; PLHIV, people living with HIV; RCT, randomized controlled trial; RD, risk difference; RIF, rifampin; TB, tuberculosis; TB-IRIS, TB-immune reconstitution inflammatory syndrome.
Figure 2.
Challenges and potential solutions to improve TB HIV mortality organized according to the stages of the cascade of TB care for PLHIV.
Author contributions: Amanda Sullivan: Conceptualization; Data curation; Writing – original draft; Writing – review & editing.
Ruvandhi R. Nathavitharana: Conceptualization; Data curation; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: RRN is supported by a National Institutes of Health Career Development Award (NIAID K23 AI132648-04) and an American Society of Tropical Medicine and Hygiene Burroughs Wellcome Fellowship.
ORCID iD: Ruvandhi R. Nathavitharana  https://orcid.org/0000-0002-3544-5021
https://orcid.org/0000-0002-3544-5021
Contributor Information
Amanda Sullivan, Division of Infectious Diseases, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA.
Ruvandhi R. Nathavitharana, Division of Infectious Diseases, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA 02215, USA.
References
- 1. WHO. Global tuberculosis report 2020, 2020, https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2020
- 2. Ellis PK, Martin WJ, Dodd PJ. CD4 count and tuberculosis risk in HIV-positive adults not on ART: a systematic review and meta-analysis. PeerJ 2017; 5: e4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ford N, Matteelli A, Shubber Z, et al. TB as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: a systematic review and meta-analysis. J Int AIDS Soc 2016; 19: 20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta RK, Lucas SB, Fielding KL, et al. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS 2015; 29: 1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nliwasa M, MacPherson P, Gupta-Wright A, et al. High HIV and active tuberculosis prevalence and increased mortality risk in adults with symptoms of TB: a systematic review and meta-analyses. J Int AIDS Soc 2018; 21: e25162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO. The end TB strategy, 2015, https://www.who.int/tb/strategy/End_TB_Strategy.pdf
- 7. Chang CC, Crane M, Zhou J, et al. HIV and co-infections. Immunol Rev 2013; 254: 114–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev 2011; 24: 351–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh A, Prasad R, Balasubramanian V, et al. Drug-resistant tuberculosis and HIV infection: current perspectives. HIV AIDS 2020; 1212: 99–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52: 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Subbaraman R, Nathavitharana RR, Mayer KH, et al. Constructing care cascades for active tuberculosis: a strategy for program monitoring and identifying gaps in quality of care. PLoS Med 2019; 16: e1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chelkeba L, Fekadu G, Tesfaye G, et al. Effects of time of initiation of antiretroviral therapy in the treatment of patients with HIV/TB co-infection: a systemic review and meta-analysis. Ann Med Surg 2020; 55: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mollel EW, Todd J, Mahande MJ, et al. Effect of tuberculosis infection on mortality of HIV-infected patients in Northern Tanzania. Trop Med Health 2020; 48: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Birtukan Tsehayneh HA. Survival experience and its predictors among TB/HIV co-infected patients in southwest Ethiopia. Epidemiol Open Access 2015; 5: 191. [Google Scholar]
- 15. Sileshi B, Deyessa N, Girma B, et al. Predictors of mortality among TB-HIV Co-infected patients being treated for tuberculosis in Northwest Ethiopia: a retrospective cohort study. BMC Infect Dis 2013; 13: 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teklu AM, Nega A, Mamuye AT, et al. Factors associated with mortality of TB/HIV co-infected patients in Ethiopia. Ethiop J Health Sci 2017; 27(Suppl. 1): 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tabarsi P, Chitsaz E, Moradi A, et al. Treatment outcome and mortality: their predictors among HIV/TB co-infected patients from Iran. Int J Mycobacteriol 2012; 1: 82–86. [DOI] [PubMed] [Google Scholar]
- 18. Aung ZZ, Saw YM, Saw TN, et al. Survival rate and mortality risk factors among TB–HIV co-infected patients at an HIV-specialist hospital in Myanmar: a 12-year retrospective follow-up study. Int J Infect Dis 2019; 80: 10–15. [DOI] [PubMed] [Google Scholar]
- 19. Mukuku O, Mutombo AM, Kakisingi CN, et al. Tuberculosis and HIV co-infection in Congolese children: risk factors of death. Pan Afr Med J 2019; 33: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naidoo K, Gengiah S, Singh S, et al. Quality of TB care among people living with HIV: gaps and solutions. J Clin Tuberc Other Mycobact Dis 2019; 17: 100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Samal J. Health seeking behaviour among tuberculosis patients in India: a systematic review. J Clin Diagn Res 2016; 10: LE01–LE06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gamtesa DF, Tola HH, Mehamed Z, et al. Health care seeking behavior among presumptive tuberculosis patients in Ethiopia: a systematic review and meta-analysis. BMC Health Serv Res 2020; 20: 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaboru BB. Geographical, health systems’ and sociocultural patterns of TB/HIV co-infected patients’ health seeking behavior in a conflict affected setting: the case of Eastern Democratic Republic of Congo. J Community Med Health Educ 2013; 4: 263. [Google Scholar]
- 24. World Health Organization. WHO consolidated guidelines on tuberculosis. Module 1: prevention. Tuberculosis preventive treatment. Tuberc Lung Dis HIV Infect 2021; 2: 86–92. [Google Scholar]
- 25. Badje A, Moh R, Gabillard D, et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the Temprano ANRS 12136 trial. Lancet Glob Health 2017; 5: e1080–e1089. [DOI] [PubMed] [Google Scholar]
- 26. Alsdurf H, Hill PC, Matteelli A, et al. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16: 1269–1278. [DOI] [PubMed] [Google Scholar]
- 27. Roscoe C, Lockhart C, de Klerk M, et al. Evaluation of the uptake of tuberculosis preventative therapy for people living with HIV in Namibia: a multiple methods analysis. BMC Public Health 2020; 20: 1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Melgar M, Shiraishi RW, Tende C, et al. Assessment of the tuberculosis case-finding and prevention cascade among people living with HIV in Zambia – 2018: a cross-sectional cluster survey. BMC Public Health 2021; 21: 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Humphrey JM, Mpofu P, Pettit AC, et al. Mortality among people with HIV treated for tuberculosis based on positive, negative, or no bacteriologic test results for tuberculosis: the IeDEA Consortium. Open Forum Infect Dis 2020; 7: ofaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Méndez-Samperio P. Diagnosis of tuberculosis in HIV co-infected individuals: current status, challenges and opportunities for the future. Scand J Immunol 2017; 86: 76–82. [DOI] [PubMed] [Google Scholar]
- 31. Sabur NF, Esmail A, Brar MS, et al. Diagnosing tuberculosis in hospitalized HIV-infected individuals who cannot produce sputum: is urine lipoarabinomannan testing the answer? BMC Infect Dis 2017; 17: 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. WHO. WHO consolidated guidelines on tuberculosis, module 3 diagnosis, rapid diagnostics for tuberculosis detection, 2021, https://www.who.int/publications/i/item/9789240029415
- 33. Torpey K, Agyei-Nkansah A, Ogyiri L, et al. Management of TB/HIV co-infection: the state of the evidence. Ghana Med J 2020; 54: 186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nathavitharana RR, Yoon C, Macpherson P, et al. Guidance for studies evaluating the accuracy of tuberculosis triage tests. J Infect Dis 2019; 220(Suppl. 3): S116–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. WHO. WHO consolidated guidelines on tuberculosis, module 2: screening, systematic screening for tuberculosis disease, 2021, https://reliefweb.int/report/world/who-consolidated-guidelines-tuberculosis-module-2-screening-systematic-screening [PubMed]
- 36. Nakiyingi L, Bwanika JM, Ssengooba W, et al. Chest X-ray interpretation does not complement Xpert MTB/RIF in diagnosis of smear-negative pulmonary tuberculosis among TB-HIV co-infected adults in a resource-limited setting. BMC Infect Dis 2021; 21: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chaisson LH, Semitala FC, Asege L, et al. Point-of-care C-reactive protein and risk of early mortality among adults initiating antiretroviral therapy. AIDS 2019; 33: 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoon C, Semitala FC, Atuhumuza E, et al. Point-of-care C-reactive protein-based tuberculosis screening for people living with HIV: a diagnostic accuracy study. Lancet Infect Dis 2017; 17: 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shapiro AE, Hong T, Govere S, et al. C-reactive protein as a screening test for HIV-associated pulmonary tuberculosis prior to antiretroviral therapy in South Africa. AIDS 2018; 32: 1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Samuels THA, Wyss R, Ongarello S, et al. Evaluation of the diagnostic performance of laboratory-based C-reactive protein as a triage test for active pulmonary tuberculosis. PLoS ONE 2021; 16: e0254002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zifodya JS, Kreniske JS, Schiller I, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst Rev 2021; 2: CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harries A, Kumar A. Challenges and progress with diagnosing pulmonary tuberculosis in low- and middle-income countries. Diagnostics 2018; 8: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cazabon D, Pande T, Kik S, et al. Market penetration of Xpert MTB/RIF in high tuberculosis burden countries: a trend analysis from 2014 - 2016. Gates Open Res 2018; 2: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scott L, da Silva P, Boehme CC, et al. Diagnosis of opportunistic infections: HIV co-infections – tuberculosis. Curr Opin HIV AIDS 2017; 12: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Crabtree-Ramırez B, Jenkins C, Jayathilake K, et al. HIV-related tuberculosis: mortality risk in persons without vs. with culture-confirmed disease. Int J Tuberc Lung Dis 2019; 23: 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bresges C, Wilson D, Fielding K, et al. Early empirical tuberculosis treatment in HIV-positive patients admitted to hospital in South Africa: an observational cohort study. Open Forum Infect Dis 2021; 8: ofab162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. MacLean E. A systematic review of biomarkers to detect active tuberculosis. Nat Microbiol 2019; 4: 748–758. [DOI] [PubMed] [Google Scholar]
- 48. WHO. Chest radiography in tuberculosis detection, 2016, https://apps.who.int/iris/bitstream/handle/10665/252424/9789241511506-eng.pdf
- 49. Bjerrum S, Schiller I, Dendukuri N, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst Rev 2019; 10: CD011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Step Up for TB 2020. Step up for TB 2020, 2020, https://www.msf.org/step-tb-report-2020
- 51. Nakiyingi L, Nakanwagi P, Briggs J, et al. Performance of loop-mediated isothermal amplification assay in the diagnosis of pulmonary tuberculosis in a high prevalence TB/HIV rural setting in Uganda. BMC Infect Dis 2018; 18: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Albert H, Nathavitharana RR, Isaacs C, et al. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? Eur Respir J 2016; 48: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zenner D, Abubakar I, Conti S, et al. Impact of TB on the survival of people living with HIV infection in England, Wales and Northern Ireland. Thorax 2015; 70: 566–573. [DOI] [PubMed] [Google Scholar]
- 54. Wang MG, Luo L, Zhang Y, et al. Treatment outcomes of tuberculous meningitis in adults: a systematic review and meta-analysis. BMC Pulm Med 2019; 19: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tola HH, Tol A, Shojaeizadeh D, et al. Tuberculosis treatment non-adherence and lost to follow up among TB patients with or without HIV in developing countries: a systematic review. Iran J Public Health 2015; 44: 1–11. [PMC free article] [PubMed] [Google Scholar]
- 56. Gupta-Wright A, Fielding K, van Oosterhout JJ, et al. Virological failure, HIV-1 drug resistance, and early mortality in adults admitted to hospital in Malawi: an observational cohort study. Lancet HIV 2020; 7: e620–e628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Manyelo CM, Solomons RS, Walzl G, et al. Tuberculous meningitis: pathogenesis, immune responses, diagnostic challenges, and the potential of biomarker-based approaches. J Clin Microbiol 2021; 59: e01771-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bourgi K, Fiske C, Sterling TR. Tuberculosis meningitis. Curr Infect Dis Rep 2017; 19: 39. [DOI] [PubMed] [Google Scholar]
- 59. Foppiano Palacios C, Saleeb PG. Challenges in the diagnosis of tuberculous meningitis. J Clin Tuberc Mycobact Dis 2020; 20: 100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kohli M, Schiller I, Dendukuri N, et al. Xpert MTB/RIF ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2021; 1: CD012768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Haraka F, Kakolwa M, Schumacher SG, et al. Impact of the diagnostic test Xpert MTB/RIF on patient outcomes for tuberculosis. Cochrane Database Syst Rev 2021; 5: CD012972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Quinn CM, Poplin V, Kasibante J, et al. Tuberculosis IRIS: pathogenesis, presentation, and management across the spectrum of disease. Life 2020; 10: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xue M, Xie R, Pang Y, et al. Prevalence and risk factors of paradoxical tuberculosis associated immune reconstitution inflammatory syndrome among HIV-infected patients in Beijing, China. BMC Infect Dis 2020; 20: 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lanzafame M, Vento S. Tuberculosis-immune reconstitution inflammatory syndrome. J Clin Tuberc Mycobact Dis 2016; 3: 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vega V, Rodríguez S, Van der Stuyft P, et al. Recurrent TB: a systematic review and meta-analysis of the incidence rates and the proportions of relapses and reinfections. Thorax 2021; 76: 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Korenromp EL, Scano F, Williams BG, et al. Effects of human immunodeficiency virus infection on recurrence of tuberculosis after rifampin-based treatment: an analytical review. Clin Infect Dis 2003; 37: 101–112. [DOI] [PubMed] [Google Scholar]
- 67. Meghji J, Lesosky M, Joekes E, et al. Patient outcomes associated with post-tuberculosis lung damage in Malawi: a prospective cohort study. Thorax 2020; 75: 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Allwood BW, Byrne A, Meghji J, et al. Post-tuberculosis lung disease: clinical review of an under-recognised global challenge. Respiration 2021; 100: 751–763. [DOI] [PubMed] [Google Scholar]
- 69. Straetemans M, Bakker MI, Mitchell EMH. Correlates of observing and willingness to report stigma towards HIV clients by (TB) health workers in Africa. Int J Tuberc Lung Dis 2017; 21: 6–18. [DOI] [PubMed] [Google Scholar]
- 70. Balasundaram A, Sarkar S, Hamide A, et al. Socioepidemiologic profile and treatment-seeking behaviour of HIV/AIDS patients in a tertiary-care hospital in South India. J Health Popul Nutr 2014; 32: 587–594. [PMC free article] [PubMed] [Google Scholar]
- 71. Wouters E, Sommerland N, Masquillier C, et al. Unpacking the dynamics of double stigma: how the HIV-TB co-epidemic alters TB stigma and its management among healthcare workers. BMC Infect Dis 2020; 20: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Datiko DG, Jerene D, Suarez P. Stigma matters in ending tuberculosis: nationwide survey of stigma in Ethiopia. BMC Public Health 2020; 20: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Anku PJ, Amo-Adjei J, Doku D, et al. Challenges of scaling-up of TB-HIV integrated service delivery in Ghana. PLoS ONE 2020; 15: e0235843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Herce ME, Morse J, Luhanga D, et al. Integrating HIV care and treatment into tuberculosis clinics in Lusaka, Zambia: results from a before-after quasi-experimental study. BMC Infect Dis 2018; 18: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lutge EE, Wiysonge CS, Knight SE, et al. Incentives and enablers to improve adherence in tuberculosis. Cochrane Database Syst Rev 2015; 9: CD007952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Katende JN, Omona K. User – provider perspectives to overcome the challenges of TB/HIV service integration at Mulago National Referral Hospital _ Kampala. Afr Health Sci 2021; 21: 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Odo M, Ochei KC, Obeagu EI, et al. Cascade variabilities in TB case finding among people living with HIV and the use of IPT: assessment in three levels of care in cross River State, Nigeria. J Pharm Res Int 2020; 32: 9–18. [Google Scholar]
- 78. Kaplan R, Hermans S, Caldwell J, et al. HIV and TB co-infection in the ART era: CD4 count distributions and TB case fatality in Cape Town. BMC Infect Dis 2018; 18: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. United States Department of State. FY 2015 Nigeria Country Operational Plan (COP), 2015, www.PEPFAR.org
- 80. Meribe SC, Harausz E, Lawal I, et al. Improving indicators of tuberculosis program cascades by leveraging HIV program strategies. Public Health Action 2019; 9: 191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lessells RJ, Swaminathan S, Godfrey-Faussett P. HIV treatment cascade in tuberculosis patients:. Curr Opin HIV AIDS 2015; 10: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011; 365: 2155–2166. [DOI] [PubMed] [Google Scholar]
- 83. Menzies D, Adjobimey M, Ruslami R, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med 2018; 379: 440–453. [DOI] [PubMed] [Google Scholar]
- 84. Swindells S, Ramchandani R, Gupta A, et al. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med 2019; 380: 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yoon C. TB screening improves preventive therapy uptake (TB SCRIPT), 2021, https://clinicaltrials.gov/ct2/show/NCT04557176?term=CRP&cond=tuberculosis&draw=3&rank=4 [DOI] [PMC free article] [PubMed]
- 86. Broger T, Sossen B, du Toit E, et al. Novel lipoarabinomannan point-of-care tuberculosis test for people with HIV: a diagnostic accuracy study. Lancet Infect Dis 2019; 19: 852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ruhwald M. FujiLAM prospective evaluation trial, 2021, https://clinicaltrials.gov/ct2/show/NCT04089423?term=FujiLAM&cond=tuberculosis&draw=2&rank=1
- 88. Parsons LM, Somoskövi Á, Gutierrez C, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev 2011; 24: 314–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Peter JG, Zijenah LS, Chanda D, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016; 387: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 90. Nathavitharana R. Impact of diagnostic strategies for tuberculosis using lateral flow urine lipoarabinomannan assay in people living with HIV. Cochrane Database Syst Rev 2021: 8: CD014641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gupta-Wright A, Peters JA, Flach C, et al. Detection of lipoarabinomannan (LAM) in urine is an independent predictor of mortality risk in patients receiving treatment for HIV-associated tuberculosis in sub-Saharan Africa: a systematic review and meta-analysis. BMC Med 2016; 14: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kerkhoff AD, Longley N, Kelly N, et al. Determine TB-LAM point-of-care tuberculosis assay predicts poor outcomes in outpatients during their first year of antiretroviral therapy in South Africa. BMC Infect Dis 2020; 20: 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Reddy KP, Gupta-Wright A, Fielding KL, et al. Cost-effectiveness of urine-based tuberculosis screening in hospitalised patients with HIV in Africa: a microsimulation modelling study. Lancet Glob Health 2019; 7: e200–e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. WHO. WHO consolidated guidelines on tuberculosis, module 4 treatment, drug-resistant tuberculosis treatment, 2021, https://www.who.int/publications/i/item/9789240007048 [PubMed]
- 95. Burke RM, Rickman HM, Singh V, et al. What is the optimum time to start antiretroviral therapy in people with HIV and tuberculosis coinfection? A systematic review and meta-analysis. J Int AIDS Soc 2021; 24: e25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Webster PD, Sibanyoni M, Malekutu D, et al. Using quality improvement to accelerate highly active antiretroviral treatment coverage in South Africa. BMJ Qual Saf 2012; 21: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ogarkov OB, Ebers A, Zhdanova S, et al. Administrative interventions associated with increased initiation on antiretroviral therapy in Irkutsk, Siberia. Public Health Action 2016; 6: 252–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 2016; 63: e147–e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dorman SE, Nahid P, Kurbatova EV, et al. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N Engl J Med 2021; 384: 1705–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gupta A, Hughes MD, Garcia-Prats AJ, et al. Inclusion of key populations in clinical trials of new antituberculosis treatments: current barriers and recommendations for pregnant and lactating women, children, and HIV-infected persons. PLoS MED 2019; 16: e1002882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Blanc FX, Badje AD, Bonnet M, et al. Systematic or test-guided treatment for tuberculosis in HIV-infected adults. N Engl J Med 2020; 382: 2397–2410. [DOI] [PubMed] [Google Scholar]
- 102. Huerga H, Ferlazzo G, Wanjala S, et al. Mortality in the first six months among HIV-positive and HIV-negative patients empirically treated for tuberculosis. BMC Infect Dis 2019; 19: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dooley KE. High-dose rifampin: shall we be bolder? Am J Respir Crit Care Med 2018; 198: 558–560. [DOI] [PubMed] [Google Scholar]
- 104. Ruslami R, Ganiem AR, Aarnoutse RE, et al. Rifampicin and moxifloxacin for tuberculous meningitis – authors’ reply. Lancet Infect Dis 2013; 13: 570. [DOI] [PubMed] [Google Scholar]
- 105. Heemskerk AD, Bang ND, Mai NTH, et al. Intensified antituberculosis therapy in adults with tuberculous meningitis. N Engl J Med 2016; 374: 124–134. [DOI] [PubMed] [Google Scholar]
- 106. Cresswell FV, Te Brake L, Atherton R, et al. Intensified antibiotic treatment of tuberculosis meningitis. Expert Rev Clin Pharmacol 2019; 12: 267–288. [DOI] [PubMed] [Google Scholar]
- 107. Dian S, Yunivita V, Ganiem AR, et al. Double-blind, randomized, placebo-controlled phase II dose-finding study to evaluate high-dose rifampin for tuberculous meningitis. Antimicrob Agents Chemother 2018; 62: e01014-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Li H, Lu J, Liu J, et al. Linezolid is associated with improved early outcomes of childhood tuberculous meningitis. Pediatr Infect Dis J 2016; 35: 607–610. [DOI] [PubMed] [Google Scholar]
- 109. Sun F, Ruan Q, Wang J, et al. Linezolid manifests a rapid and dramatic therapeutic effect for patients with life-threatening tuberculous meningitis. Antimicrob Agents Chemother 2014; 58: 6297–6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mai NT, Dobbs N, Phu NH, et al. A randomised double blind placebo controlled phase 2 trial of adjunctive aspirin for tuberculous meningitis in HIV-uninfected adults. eLife 2018; 7: e33478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wilkinson RJ. Linezolid, aspirin and enhanced dose rifampicin in HIV-TBM (LASER-TBM), 2020, https://clinicaltrials.gov/ct2/show/NCT03927313?term=aspirin&cond=Tuberculosis%2C+Meningeal&draw=2&rank=2
- 112. Bonnet F, Anglaret X. Intensified tuberculosis treatment to reduce the mortality of patients with tuberculous meningitis (INTENSE-TBM), 2020, https://clinicaltrials.gov/ct2/show/NCT04145258 [DOI] [PMC free article] [PubMed]
- 113. Meintjes G, Stek C, Blumenthal L, et al. Prednisone for the prevention of paradoxical tuberculosis-associated IRIS. N Engl J Med 2018; 379: 1915–1925. [DOI] [PubMed] [Google Scholar]
- 114. Dodd PJ, Yuen CM, Jayasooriya SM, et al. Quantifying the global number of tuberculosis survivors: a modelling study. Lancet Infect Dis 2021; 21: 984–992. [DOI] [PubMed] [Google Scholar]
- 115. Schoeman I, Sifumba Z. Tuberculosis care does not end at treatment completion – a perspective from tuberculosis survivors. Lancet Infect Dis; 21: 896–897. [DOI] [PubMed] [Google Scholar]
- 116. Bruins WS, van Leth F. Effect of secondary preventive therapy on recurrence of tuberculosis in HIV-infected individuals: a systematic review. Infect Dis 2017; 49: 161–169. [DOI] [PubMed] [Google Scholar]
- 117. Wang WH, Takeuchi R, Jain SH, et al. A novel, rapid (within hours) culture-free diagnostic method for detecting live Mycobacterium tuberculosis with high sensitivity. EBioMedicine 2020; 60: 103007. [DOI] [PMC free article] [PubMed] [Google Scholar]