Abstract
Introduction:
Alkalising agents have the potential to enhance the efficacy of many antimicrobial agents used in the treatment of Urinary Tract Infections; they also have the potential to cause significant patient harm if used incorrectly. This work seeks to illustrate and quantify these risks and synergies by modelling drug solubility and supersaturation against pharmacokinetic data for commonly used antibiotic agents.
Methods:
Solubility-pH relationships are employed to quantify the crystalluria risk for compounds which may be reasonably expected to be co-prescribed—or co-administered—with urinary alkalisers (amoxicillin, nitrofurantoin, trimethoprim, sulfamethoxazole and ciprofloxacin). These results are correlated against reports of crystalluria in the literature and in the EU Adverse Drug Reaction database.
Results and Discussion:
We find a correlation between the maximum theoretical supersaturation attainable and crystalluria reports for sulfamethoxazole, amoxicillin and ciprofloxacin. Shifts in urine pH which can be induced by alkalising agents may produce supersaturated states (and thus induce crystalluria) and may also affect antimicrobial efficacy. The importance of employing biorelevant media to improve predictive capacity of this analysis is also discussed.
Conclusion:
Despite their widespread use, alkalising agents have significant effects on the pharmacokinetics of the most common drugs used to treat UTIs. With self-care set to increase, all OTC products should be critically re-evaluated to ensure patient safety, particularly within contexts where healthcare professionals are not involved in treatment selection. This analysis suggests a need for consistency across patient and healthcare professional documents to improve clarity.
Plain Language Summary
OTC Alkalising agents need additional warning information
Alkalising agents (e.g., sodium and potassium citrate) can be purchased in many locations without the supervision of a healthcare professional.
Although they are thought as innocuous agents, alkalisers can greatly influence the way some antibiotics behave in the body and this can potentially cause patient harm.
This work illustrates these risks and synergies by modelling drug solubility and supersaturation against pharmacokinetic data for commonly used antibiotic agents.
Manufacturers and patients should be aware that the use of alkalising agents with these drugs (and potentially many others) may cause unintended consequences.
Keywords: antimicrobial, excretion, pH efficacy, supersaturation, urinary alkalisation, urinary pH, urinary tract infection, clinical pharmaceutics
Introduction
Urinary alkalising agents are classified as General Sale List products in the United Kingdom and Ireland and as such, they are widely available in supermarkets where they can be sold without the supervision of a healthcare professional. The market for Over-The-counter (OTC) medicines is worth $34 billion in the United States and $3.54 billion in the United Kingdom 1 and advocates for the increased use of OTC products predict dramatic healthcare savings in these continents.2,3 This analysis suggests that OTC products will play a new role in future healthcare, and as such, they need to be critically evaluated within this new context to ensure patient safety.
Dysuria is one of the most common conditions encountered in primary care and can be treated by urinary alkalisers alone, or as adjuvants to antibiotics (e.g. trimethoprim, nitrofurantoin or ciprofloxacin). Some advocate for alkalization of the urine to improve antibiotic efficacy, 4 however, there are number of factors to consider, discussed herein. Although alkalisation of the urine has been shown to decrease pain and urinary frequency associated with urinary tract infections (UTIs), 5 systematic analysis indicates that evidence of their effectiveness is lacking. 6 In a wider context, clinicians have utilised urinary alkalisation to reduce the systemic toxicity of acidic drugs, 7 prevent crystalluria,8,9 and treat acid-base imbalances. The effect of alkalising agents on urinary pH is determined by the patient biochemistry at the point of administration, which is known to change dramatically throughout the day. This can result in minimal (or no effect) in some patients and dramatic shifts in others.
Urine alkalisation can be achieved with citrate or bicarbonate salts, which act through their own distinct mechanism. 10 If bicarbonate ions reach the renal tubules intact, they will cause increases in urinary pH. Urine alkalisation by citrate has been suggested to arise from its metabolism via the citric acid cycle to release CO2 which is then catalysed to bicarbonate by carbonic anhydrase. However, the precise mechanism is likely to be more complex and renal handling of citrate has been discussed extensively by Simpson 11 and later by Hamm. 12 Oral alkalisers can cause a pH shift of > 1 unit in less than 1 hour (mean 12 h). 7 Alkalisation can also occur as an unintended side-effect of other drugs such as antiacids, which can also raise urinary pH. One study illustrated that this effect is more pronounced when urine pH is approximately 5, where pH can increase up to 1 pH unit. 13 A follow-up study by the same authors determined that it takes approximately 24 h for these changes to occur and they remain until approximately 24 h after cessation of treatment. 14
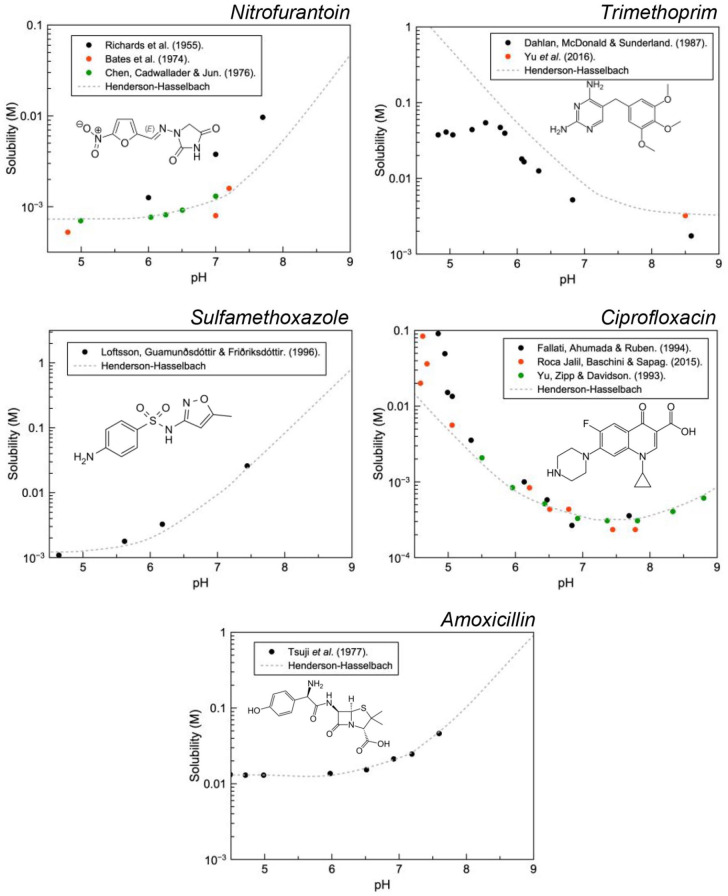
Solution pH has a strong effect on the solubility of endogenous and exogenous species and rapid changes, therefore, may result in supersaturation and ultimately their precipitation. In the context of pharmaceutical care, this is referred to as drug induced crystalluria. 9 Figure 1 illustrates that pH has a dramatic effect on the solubility of ionisable species. This becomes more important as pH approaches the pKa of the drug.
Figure 1.
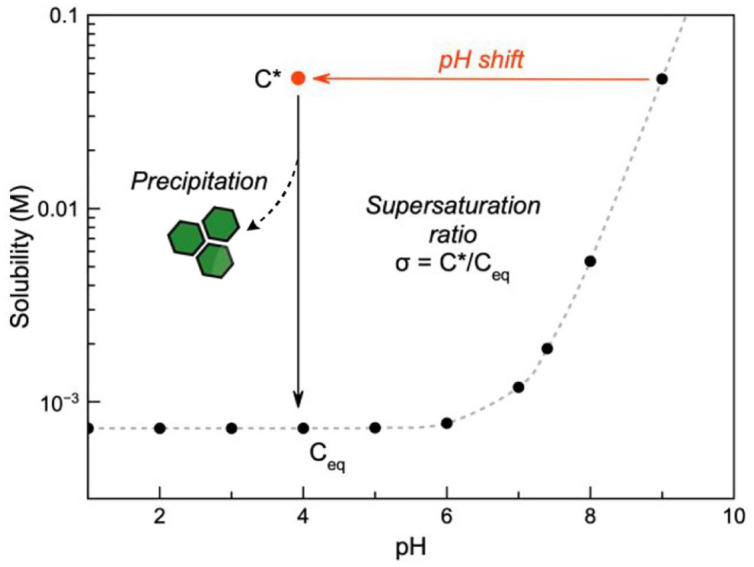
When the pH of a saturated solution of a weak acid (pKa = 7.2) at pH 9 is rapidly decreased to pH 4, the drug will eventually precipitate out of solution to reach the new solubility equilibrium; precipitation is not always instant and the rate is proportional to the supersaturation ratio (i.e., the difference between the momentary solubility, C* and the equilibrium solubility, Ceq).
This pH effect on solubility also manifests itself in vivo in any anatomical site which can dramatically change the solution pH. Unsurprisingly, drug crystals have been observed in the gastrointestinal tract, 15 macrophages (specifically their lysosomal compartment), 16 and the renal system. 8 The function of the kidneys is such that there are marked changes in filtrate composition (and pH) along the renal tubules as substances are excreted, metabolised and reabsorbed. Urine pH fluctuates over 24 h, usually within 5 to 8.5 but this range can extend in pathological situations (4.5–9).
Large shifts in urinary pH from alkalisers may cause renal damage resulting from the induction of crystalluria or alter the pharmacokinetic and pharmacodynamic activity of various drugs, including those used to treat UTIs. Although thought of as innocuous agent, like any drug product, its use has its associated risks. The aim of this work is to provide an analysis of these risks to minimise patient harm.
Methodology
Analysis of products available in United Kingdom, United States, and Irish markets
The Food and Drug Administration (FDA) Drugs.com (USA), the electronic medicines compendium (UK) and the Health Products Regulatory Authority (HPRA) databases were searched for ‘alkaliser’, ‘urinary alkaliser’, ‘alkalizer’, ‘urinary alkalizer’, ‘bicarbonate’ and ‘citrate’. These searches were manually screened, and alkalising agents were added to the list compiled in Table 3.
Table 3.
Selection of products available in United States, United Kingdom, and Irish markets.
| Drug name | Manufacturer(s) | Active ingredient(s) | Region |
|---|---|---|---|
| Poly-Citra K | Janssen Pharmaceuticals | Citric acid and potassium citrate | USA |
| Potassium citrate | Generic (Rising, Zydus Pharma, Strides Pharma, Teva Pharms, Ani Pharms, Biopharma inc., Ascent pharms, Eywa). | Potassium citrate | USA, IRL and UK |
| UROCIT-K | Mission Pharma | Potassium citrate | USA |
| Cytra-K | Cypress Pharma | Citric acid and potassium citrate | USA |
| Virtrate-3 | Virtus Pharmaceuticals, LLC | Citric acid, sodium and potassium citrates | USA |
| Oracit | CMP Pharma | Citric acid and sodium citrate | USA |
| Bicitra | Janssen Pharmaceuticals | Citric acid and sodium citrate | USA |
| Cystopurin | Pharmapac Ltd. | Potassium citrate | UK and IRL |
| Potassium citrate | Available generically | Potassium citrate | UK |
| Effercitrate | Cambridge Healthcare Supplies Ltd | Potassium citrate and citric acid | UK |
| Cymaclear | Teva Pharma | Potassium citrate | UK |
| Cymalon | Actavis Group | Sodium citrate | UK |
| Cystitis relief | The Boots Company | Sodium citrate | UK and IRL |
Product information obtained from Drugs@FDA, Drugs.com (USA), the electronic medicines compendium (UK) and the Health Products Regulatory Authority (Ireland).
Literature survey of drug induced crystalluria
The Scopus database (Search preformed on December 2020) was searched for ‘ciprofloxacin’, ‘trimethoprim’, ‘sulfamethoxazole OR co-trimoxazole’, ‘amoxicillin’, ‘nitrofurantoin’ AND ‘crystalluria’. Article abstracts and titles were then manually screened and articles that discussed new cases which identified drug induced crystalluria were used in the analysis (Figure S1).
Analysis of EU database for Suspected Adverse Reactions (EUADR)
Employing methodology published previously, 17 The EUADR database was searched for products containing ciprofloxacin, trimethoprim, sulfamethoxazole (co-trimoxazole), amoxicillin, and nitrofurantoin. Results were screened to those reported by healthcare professionals only and refined by renal. The author recognises the limitations of this database, namely: the number of reports may be related to the publicity of the drug and or its adverse reactions, no information regarding how often the drug is prescribed accompanies the reports and, in many cases, it cannot be definitively proven that the drug is the cause of the observed event.
pH solubility curves
Equilibrium solubility studies were selected which allowed the drug to equilibrate for 24 h in 37 ºC, in aqueous solvents with ionic strength > 0.1 M across the range of pH found in the urine where possible. Studies that employed biorelevant media were rare but used when available. Data were extracted using WebPlotDigitiser (v. 4.4) and modelled using the Henderson-Hasselbalch model which has been derived previously 18 (Table 1). The pKa for each molecule was determined using the pKa calculator implemented in Marvin which makes a prediction based on calculations of partial charges of atoms in the molecules.
Table 1.
Summary of data used in calculations.
| Drug | pKa a | Solubility (mM) b | Maximum urinary concentrations (mg/L) [mM] |
Maximum Supersaturation ratio c |
|---|---|---|---|---|
| Amoxicillin d | 7.16 | 41-2,877 19 | ~12,500,000 [29,832] 23 | 70 (727) |
| Ciprofloxacin | 6.18; 8.73 | 0.23-9124–27 | 1981 [5.98] 28 | 395 (26) |
| Nitrofurantoin | 7.2 | 1.68-47 22 ,29 | 320 [1.34] 30 | 28 (<1) |
| Sulfamethoxazole | 6.16 | 1.1-817 31 | 1016 [3.2] 32 | 743 (2.9) |
| Trimethoprim | 7.2 | 3.2-40 33 ,34 | 720 [2.48] 35 | 12 (<1) |
The pKa calculator implemented in Marvin predicts pKa data based on calculations of partial charges of atoms in the molecules.
Minimum-maximum solubility in biorelevant media when available.
Supersaturation attainable from maximum reported urinary concentrations in parenthesis.
Amoxicillin trihydrate.
Solubility data from Yu et al. was combined with that of Dahlan, McDonald, and Sunderland as they measured equilibrium solubility of trimethoprim closer to physiological temperatures of 37ºC, whereas the latter tested solubility at 32ºC. Preforming supersaturation analysis using data obtained from Dahlan, McDonald, and Sunderland predicted a supersaturation was greater than 1. The absence of literature reports regarding trimethoprim induced crystalluria prompted reanalysis with data closer to physiological temperatures.
One study evaluated the solubility of amoxicillin in the urine, 19 although it was used in this analysis there are no details of the specificity of the quantification method for other penicillin derivatives or breakdown products and no details of the phase remaining (or its purity) after 24 h equilibrium studies. This is an important consideration as amoxicillin is known to degrade > 70% in aqueous solutions buffered to pH 7 within 24 h. 20
Another study suggested that complexation with urea and creatinine (found in the urine at approximately 250 mM and 1.5 mM respectively) will contribute to increased nitrofurantoin solubility and suggested that the shape of a solubility curve using urine may deviate from that obtained using pH buffers.21,22 As such, the concentration of nitrofurantoin in these solutions was taken as the intrinsic solubility to increase the accuracy of the supersaturation model.
Pharmacokinetic data
Pharmacokinetic data for amoxicillin,23,36,37 nitrofurantoin,38–40 ciprofloxacin,28,41,42 co-trimoxazole32,43 (trimethoprim Cmax) 35 was extracted from the literature as before with WebPlotDigitiser (v. 4.4). The highest observed datapoint is highlighted in the figure, was used to calculate the supersaturation that could have been generated in that patient. When available, the data was inserted into an excel sheet to obtain the standard deviation and mean, as this data was not available for sulfamethoxazole, the range was used as a surrogate for standard deviation.
Results and discussion
With relevance to the clinical context of dysuria, equilibrium solubility data has been obtained from the literature for ciprofloxacin, trimethoprim, nitrofurantoin, amoxicillin and sulfamethoxazole and modelled using Henderson-Hasselbalch to aid illustration (Figure 2). Importantly all of these drugs have a pKa within the range of physiological urinary pH, therefore, rapid changes to the pH brought about by urinary alkalisers can present a risk for precipitation. Conversely, the opposite can be applied if crystalluria is determined to be caused by these drugs, patients could be treated with the acid or base to increase drug solubility and potentially redissolve the crystals.
Figure 2.
Equilibrium solubility data as a function of solution pH obtained from the literature for nitrofurantoin, trimethoprim, ciprofloxacin, sulfathiazole and amoxicillin.
Theoretically, precipitation is possible for all of these compounds as they all exhibit pH dependant solubility in the range of urinary pH. Critically, sulfamethoxazole, ciprofloxacin and amoxicillin have been identified in clinical isolates using FTIR, 44 MS 45 and XPS. 46 In an attempt to quantify the risk of precipitation (i.e., crystalluria) solubility data must be placed in the context of supersaturation. Supersaturation describes the driving force for crystallisation where increased supersaturation reduces the critical cluster size and the nucleation rate. Equation 1 is a practical expression of the relationship between supersaturation, σ and nucleation rate, dN/dt where k and m denote the nucleation rate constant and nucleation order respectively.47,48
| (1) |
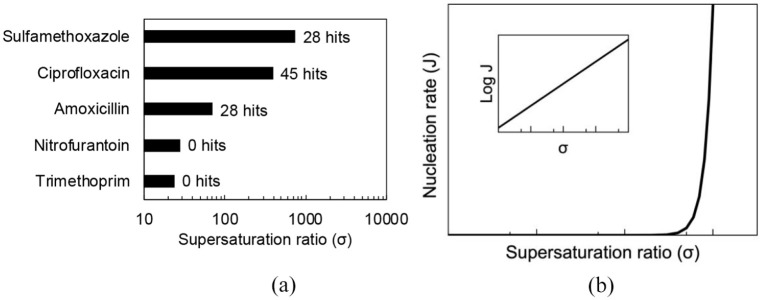
Figure 3 reveals the maximum supersaturation attainable (calculated from the maximum and minimum solubility values across the pH range) for each agent alongside the number of hits from a literature analysis. Interestingly it highlights the significant risk of crystalluria with compounds that can attain large supersaturation indexes ⩾ 70.
Figure 3.
(a) Maximum supersaturation ratio of UTI anti-infectives (nitrofurantoin, σ ≈ 3; ciprofloxacin, σ ≈ 303; trimethoprim, σ ≈ 24; amoxicillin, σ ≈ 70 and sulfamethoxazole, σ ≈ 1345) labelled with number of hits from literature analysis. (b) Supersaturation on crystal nucleation rate.
Crystalluria has been observed in trimethoprim products, but only as trimethoprim/sulfamethoxazole. 49 This is unsurprising, as crystalluria is a common feature of sulfonamide antibiotics and is associated with urine pH < 7.9,50–52 Amoxicillin induced crystalluria is also well characterised and has been widely reported,8,23,45 it is associated with acidic urine around pH 5. 23
Ciprofloxacin crystalluria may be induced by increasing urine pH from 4.5 to 7. 53 Although it has been postulated (manufacture information) that this is because ciprofloxacin solubility is decreased in alkaline environments, this is not strictly correct. 46 From the pH solubility diagram in Figure 2 it is apparent that ciprofloxacin solubility increases above pH 7.5. This is due to the formation of a low solubility complex which precipitates from solution,54–56 these results could be extrapolated to other quinolones which are known to precipitate in alkaline solutions in the presence of certain metal cations. 27 Alarmingly, crystalluria was observed in half (n = 3/6) of the participants administered 500 mg ciprofloxacin alongside sodium bicarbonate (42 mEq, three times daily for four doses) with additional fluid intake (not quantified). 46
While nitrofurantoin associated crystalluria is not widely described in the literature, one report was identified 57 and due to the acidity of the patients urine reported, and the solubility data above, nitrofurantoin crystalluria is plausible. However, it is possible that the authors have misidentified the, ‘small purple crystals’ observed. Purple crystals in urine catheter bags are commonly due to the precipitation of indirubin and indigo crystals, known as ‘Purple bag syndrome’. 58 This has been documented since 1857 59 and was described by their contemporaries as a well-known event in geriatric patients. 60 Although they report disappearance of the crystals after cessation of nitrofurantoin, presumably the authors changed the catheter, the nidus of the infection which may have contained the bacteria that catalysed the conversion of indole sulphate to indirubin and indigo.
The EU database of Suspected Adverse Drug Reactions reveals that—although rare—crystalluria has been suspected in all of the drugs used in UTIs, in descending order: co-trimoxazole (n = 87/2,330,256), ciprofloxacin (n = 52/25,853), amoxicillin (n = 25/30,683), nitrofurantoin (n = 7/6,325), trimethoprim (n = 2/2,891) and sulfamethoxazole (n = 0/611). The implication of ciprofloxacin, amoxicillin and co-trimoxazole in drug-induced crystalluria can be rationalised from the analysis above, and the absence of sulfamethoxazole reports is probably because it is almost exclusively prescribed as co-trimoxazole. A deeper analysis of nitrofurantoin hits revealed other drugs which may be responsible; separate reports contained methotrexate, amoxicillin, ciprofloxacin and piperacillin-tazobactam. One report may have been mischaracterised as it is associated with a publication that does not mention nitrofurantoin crystalluria (in fact the paper describes nitrofurantoin induced pneumonitis) 61 which leaves two remaining reports. The two reports involving trimethoprim do not appear to have been mischaracterised.
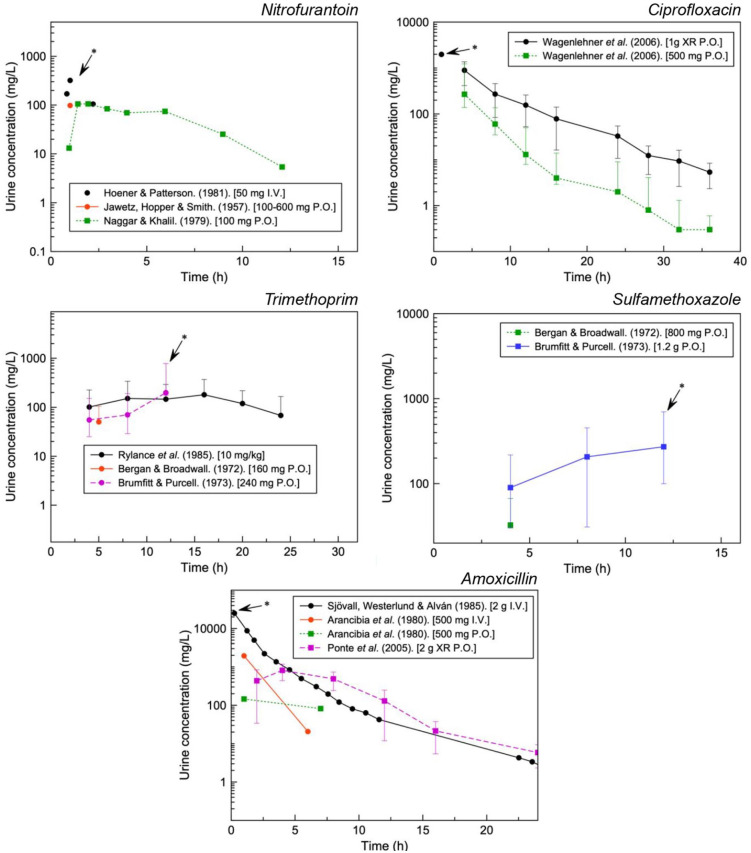
Further context can be provided with the concentrations of drug that have been observed in the urine (Figure 4). With the exception of sulfamethoxazole, urinary concentrations can reach approximately 102 to 103 the respective plasma concentrations for the drugs analysed (Cmax as per manufacturer information: trimethoprim, 1–2 mg/L; amoxicillin, ~6 mg/L; ciprofloxacin, 0.56–3.7 mg/L; nitrofurantoin, ~1 mg/L and sulfamethoxazole, 57.4 mg/L).
Figure 4.
Urinary concentrations for various formulations of UTI antibiotics. Maximum urinary concentrations used for further analysis indicated with an arrow.
Figure 5 aims to highlight the risk supersaturation with clinically relevant concentrations. This may account for the quantity of reports for ciprofloxacin, amoxicillin and sulfamethoxazole with nitrofurantoin and trimethoprim illustrating undersaturation even at the highest concentrations available in the literature.
Figure 5.
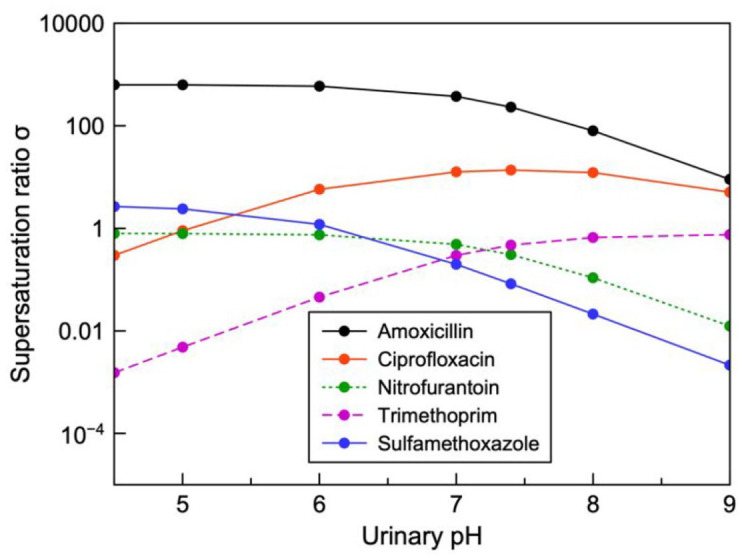
Supersaturation ratio plotted as a function of urinary pH for observed maximum urinary concentrations of various antibiotics used in the treatment of UTIs. When supersaturation exceeds 1 the drug is prone to precipitation.
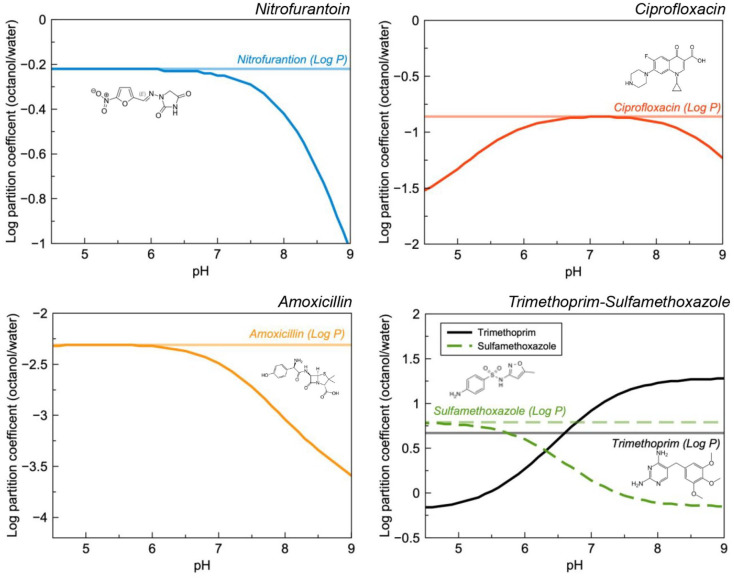
Changing solution pH (i.e. renal filtrate pH) can also alter the excretion of some drugs which is explained—in part—by their increased solubility in the medium. This may be rationalised by the influences of the drugs pKa and its dramatic effect on the pH solubility curve and log D (Figure 6). This is generally illustrated using the classic examples of aspirin, 62 methamphetamine 63 and methotrexate. 64 In the context of dysuria, key pharmaceuticals which are likely to be co-prescribed include nitrofurantoin, trimethoprim and ciprofloxacin and amoxicillin. Although this author could find no evidence to suggest that amoxicillin exhibits pH dependant excretion and clinical studies suggest that pH does not affect excretion of ciprofloxacin, 65 it is possible that increased excretion could be seen at the extremes of urinary pH for each of these drugs. Alkaline urine is known to increase the excretion rate of nitrofurantion 29 and the pH effect on trimethoprim and sulfamethoxazole is well characterised. 66
Figure 6.
Calculated log P and D as a function of pH for nitrofurantoin, ciprofloxacin, trimethoprim-sulfamethoxazole and amoxicillin. A decreasing log D value is correlated with increased solubility in water.
Notwithstanding active excretion/reabsorption, the propensity for the drug to penetrate into tissues can be estimated using log P and log D. Log D has a higher predictive capacity as it describes the partition coefficient—between octanol and water—of a drug molecule across the pH scale; this becomes particularly important for ionisable drugs as the unionised form has higher solubility in the organic phase (i.e. unionised molecules are expected to penetrate across biological membranes to a greater degree than their ionised counterparts). It could be hypothesised therefore, that increased ionisation could result in increased solubility in the urine, reduced reabsorption in the kidney, and subsequently increased clearance.
Evidence suggests that many antibiotics used in the treatment of UTIs demonstrate pH dependant synergy, 67 which could be exploited to reduce the treatment failure rate (Table 2). Although this is not the first time hypothesis has appeared in the literature, this author could find no randomised controlled trials to demonstrate that in vitro pH synergy is clinically translatable. With co-trimoxazole, alkalisation demonstrated increased efficacy against a variety of clinically relevant strains,67,68 in E.coli (a common UTI pathogen), these effects become pronounced particularly at pH 7. 69 One study suggested that urinary pH > 6.5 is likely to decrease nitrofurantoin activity, 70 while acidic conditions increased activity. 67 Ciprofloxacin activity is also positively influenced by alkaline pH 67 whereas amoxicillin activity does not change. 4 In the context of Log D, it could be postulated that this pH efficacy is related to the propensity of membrane penetration into the bacterial cytoplasm.
Table 2.
Effect of alkalisation on UTI antibiotics..
| Drug | Maximum ΔSolubility [M] a |
Maximum Δlog P b |
Pharmacokinetics | Pharmacodynamic effects |
|---|---|---|---|---|
| Sulfamethoxazole | 0.816 | -0.93 | Increased excretion | in vitro synergy |
| Trimethoprim | -1.613 | 1.46 | Decreased excretion | in vitro synergy |
| Nitrofurantoin | 0.046 | -1.56 | Increased excretion | in vitro interaction |
| Ciprofloxacin | 0.084 | -0.94 | No effect | in vitro synergy |
| Amoxicillin | 0.899 | -1.28 | - | - |
UTI: urinary tract infection.
Solubility shift as calculated from the Henderson-Hasselbach except in the case of trimethoprim which reaches pHmax when solution pH = 6.
The log D calculator implemented in Marvin predicts Log D of a molecule based on the atomic log P increment of its constituent atoms as per Viswanadhan et al. 71
An analysis of products available on the British, American and Irish markets (Table 3) reveals that while sodium bicarbonate has similar effectiveness, 72 citrate salts are used almost exclusively in OTC products. All products surveyed have a statement which reminds patients to speak to their doctor or pharmacist if they are on other medicines, this advice might be unclear for many patients; when specific drug related advice does appear, it is inconsistent. Some products have a statement which draws attention to interactions with other drugs, but these generally focus on the role of potassium and sodium as they are the predominant counter-ions to citrate (e.g., they recommend care with potassium sparing diuretics). Oracit®, Cytra-2®, Bicitra® expand this list to include salicylates and other agents such as antiacids and Cymaclear, effercitrate and Potassium citrate (Thornton & Ross), note the potential increased clearance of drugs and specifically mention nitrofurantoin. The British National Formulary monograph on citric acid and potassium citrate does not identify these risks highlighted by the manufacturers.
With self-care set to increase, all OTC products should be critically re-evaluated to ensure patient safety, particularly within contexts where healthcare professionals are not involved in treatment selection. This analysis suggests a need for consistency across patient and healthcare professional documents to improve clarity.
Conclusion
This work has illustrated how solubility-pH relationships can enable an analysis of crystalluria risk for compounds which may be reasonably expected to be co-prescribed—or co-administered—with urinary alkalisers, namely: ciprofloxacin, amoxicillin and sulfamethoxazole. These empirical relationships have been validated with systematic literature analysis and further explored with the EUADR database. The importance of employing biorelevant media to improve predictive capacity is also discussed particularly for nitrofurantoin and amoxicillin.
Future studies may expand on the in vitro results in the literature which suggest increased antimicrobial efficacy for trimethoprim (the most widely prescribed UTI antibiotic in the United Kingdom),73,74 sulfamethoxazole and ciprofloxacin, at increased pH, which could be achieved with alkalisers and vice versa for nitrofurantoin. Greater depth is needed to quantify the relationship between plasma and urinary concentrations and to relate urine pH to its the pharmacokinetic effects and to explore if there are other common drugs taken by patients that may be associated with crystalluria or changes in pharmacokinetics, this is a particular concern for drugs with a pKa in the range of urinary pH.
Ethics
Our study did not require an ethical board approval because it does not contain identifiable patient information. All information is also available on the public domain.
Supplemental Material
Supplemental material, sj-docx-1-taw-10.1177_20420986221080794 for Alkalising agents in urinary tract infections: theoretical contraindications, interactions and synergy by Oisín N. Kavanagh in Therapeutic Advances in Drug Safety
Footnotes
Author contributions: Oisín N. Kavanagh: Conceptualisation; Data curation; Formal analysis; Investigation; Methodology; Visualisation; Writing—original draft; Writing——review & editing.
Conflict of interest statement: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Oisín N. Kavanagh  https://orcid.org/0000-0001-7369-4611
https://orcid.org/0000-0001-7369-4611
Supplemental material: Supplemental material for this article is available online.
References
- 1. Connelly D. A breakdown of the over-the-counter medicines market in Britain in 2016. Pharm J 2017; 298: 7900. [Google Scholar]
- 2. Noone J, Blanchette CM. The value of self-medication: summary of existing evidence. J Med Econ 2018; 21: 201–211. [DOI] [PubMed] [Google Scholar]
- 3. Consumer Healthcare Products Association. Value of OTC medicines to the US healthcare system. Consumer Healthcare Products Association, https://www.chpa.org/sites/default/files/media/docs/2020-10/Value-of-OTC-Medicines-to-the-US-Healthcare-System-03012019.pdf
- 4. Cunha BA. An infectious disease and pharmacokinetic perspective on oral antibiotic treatment of uncomplicated urinary tract infections due to multidrug-resistant Gram-negative uropathogens: the importance of urinary antibiotic concentrations and urinary pH. Eur J Clin Microbiol Infect Dis 2016; 35: 521–526. [DOI] [PubMed] [Google Scholar]
- 5. Ueda T, Yoshida T, Tanoue H, et al. Urine alkalization improves the problems of pain and sleep in hypersensitive bladder syndrome. Int J Urol 2014; 21: 512–517. [DOI] [PubMed] [Google Scholar]
- 6. O’Kane DB, Dave SK, Gore N, et al. Urinary alkalisation for symptomatic uncomplicated urinary tract infection in women. Cochrane Database Syst Rev 2016; 4: CD010745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rouch JA, Burton B, Dabb A, et al. Comparison of enteral and parenteral methods of urine alkalinization in patients receiving high-dose methotrexate. J Oncol Pharm Pract 2017; 23: 3–9. [DOI] [PubMed] [Google Scholar]
- 8. Werness PG, Bergert JH, Smith LH. Crystalluria. J Cryst Growth 1981; 53: 166–181. [Google Scholar]
- 9. Perazella MA. Crystal-induced acute renal failure. Am J Med 1999; 106: 459–465. [DOI] [PubMed] [Google Scholar]
- 10. Kamphuis GM, Wouter Van Hattum J, De Bie P, et al. Method of alkalization and monitoring of urinary pH for prevention of recurrent uric acid urolithiasis: a systematic review. Transl Androl Urol 2019; 8(Suppl. 4): S448–S456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simpson DP. Citrate excretion: a window on renal metabolism. Am J Physiol 1983; 244: F223–F234. [DOI] [PubMed] [Google Scholar]
- 12. Hamm LL. Renal handling of citrate. Kidney Int 1990; 38: 728–735. [DOI] [PubMed] [Google Scholar]
- 13. Gibaldi M, Grundhofer B, Levy G. Effect of antacids on pH of urine. Clin Pharmacol Ther 1974; 16: 520–525. [DOI] [PubMed] [Google Scholar]
- 14. Gibaldi M, Grundhofer B, Levy G. Time course and dose dependence of antacid effect on urine pH. J Pharm Sci 1975; 64: 2003–2004. [DOI] [PubMed] [Google Scholar]
- 15. Sassene PJ, Michaelsen MH, Mosgaard MD, et al. In vivo precipitation of poorly soluble drugs from lipid-based drug delivery systems. Mol Pharm 2016; 13: 3417–3426. [DOI] [PubMed] [Google Scholar]
- 16. Baik J, Rosania GR. Macrophages sequester clofazimine in an intracellular liquid crystal-like supramolecular organization. PLoS One 2012; 7: e47494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. García M, Lertxundi U, Aguirre C. Tramadol-induced hiccups: a case-noncase study in the European pharmacovigilance database. Ther Adv Drug Saf 2021; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kramer SF, Flynn GL. Solubility of organic hydrochlorides. J Pharm Sci 1972; 61: 1896–1904. [DOI] [PubMed] [Google Scholar]
- 19. Tsuji A, Nakashima E, Hamano S, et al. Physicochemical properties of amphoteric β-lactam antibiotics I: stability, solubility, and dissolution behavior of amino penicillins as a function of pH. J Pharm Sci 1978; 67: 1059–1066. [DOI] [PubMed] [Google Scholar]
- 20. Jerzsele Á, Nagy G. The stability of amoxicillin trihydrate and potassium clavulanate combination in aqueous solutions. Acta Vet Hung 2009; 57: 485–493. [DOI] [PubMed] [Google Scholar]
- 21. Chen L-K, Cadwallader DE, Jun HW. Nitrofurantoin solubility in aqueous urea and creatinine solutions. J Pharm Sci 1976; 65: 868–872. [DOI] [PubMed] [Google Scholar]
- 22. Paul MF, Harrington C, Bender RC, et al. Effect of pH and of urea on nitrofurantoin activity. Proc Soc Exp Biol Med 1967; 125: 941–947. [DOI] [PubMed] [Google Scholar]
- 23. Sjövall J, Westerlund D, Alván G. Renal excretion of intravenously infused amoxycillin and ampicillin. Br J Clin Pharmacol 1985; 19: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun J, Sakai S, Tauchi Y, et al. Determination of lipophilicity of two quinolone antibacterials, ciprofloxacin and grepafloxacin, in the protonation equilibrium. Eur J Pharm Biopharm 2002; 54: 51–58. [DOI] [PubMed] [Google Scholar]
- 25. Fallati CS, Ahumada A, Manzo RH. El perfil de solubilidad de la Ciprofloxacina en función del pH. Acta Farm Bonaer 1994; 13: 73–77. [Google Scholar]
- 26. Roca Jalil ME, Baschini M, Sapag K. Influence of pH and antibiotic solubility on the removal of ciprofloxacin from aqueous media using montmorillonite. Appl Clay Sci 2015; 114: 69–76. [Google Scholar]
- 27. Yu X, Zipp GL, Davidson GW. The effect of temperature and pH on the solubility of quinolone compounds: estimation of heat of fusion. Pharm Res 1994; 11: 522–527. [DOI] [PubMed] [Google Scholar]
- 28. Wagenlehner FME, Kinzig-Schippers M, Sörgel F, et al. Concentrations in plasma, urinary excretion and bactericidal activity of levofloxacin (500 mg) versus ciprofloxacin (500 mg) in healthy volunteers receiving a single oral dose. Int J Antimicrob Agents 2006; 28: 551–559. [DOI] [PubMed] [Google Scholar]
- 29. Woodruff MW, Malvin RL, Thompson IM. The renal transport of nitrofurantoin: effect of acid-base balance upon its excretion. J Am Med Assoc 1961; 175: 1132–1135. [DOI] [PubMed] [Google Scholar]
- 30. Bach MC, Gold O, Finland M. Absorption and urinary execretion of trimethoprim, sulfamethoxazole, and trimethoprim-sulfamethoxazole: results with single doses in normal young adults and preliminary observations during therapy with trimethoprim-sulfamethoxazole. J Infect Dis 1973; 128(Suppl.): 584–599p. [DOI] [PubMed] [Google Scholar]
- 31. Loftsson T, Guoˇmundsdóttir TK, Frioˇriksdóttir H. The influence of water-soluble polymers and pH on hydroxypropyl-β-cyclodextrin complexation of drugs. Drug Dev Ind Pharm 1996; 22: 401–405. [Google Scholar]
- 32. Bergan T, Brodwall EK. Human pharmacokinetics of a sulfamethoxazole-trimethoprim combination. Acta Med Scand 1972; 192: 483–492. [DOI] [PubMed] [Google Scholar]
- 33. Dahlan R, McDonald C, Sunderland VB. Solubilities and intrinsic dissolution rates of sulphamethoxazole and trimethoprim. J Pharm Pharmacol 1987; 39: 246–251. [DOI] [PubMed] [Google Scholar]
- 34. Yin D-P, Liu M-X, Fu H-L, et al. Solubility of trimethoprim in selected pure solvents and (water + ethanol/2-propanol) mixed-solvent systems. J Chem Eng Data 2016; 61: 404–411. [Google Scholar]
- 35. Rylance GW, George RH, Healing DE, et al. Single dose pharmacokinetics of trimethoprim. Arch Dis Child 1985; 60: 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arancibia A, Guttmann J, González G, et al. Absorption and disposition kinetics of amoxicillin in normal human subjects. Antimicrob Agents Chemother 1980; 17: 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ponte C, Gracia M, Giménez MJ, et al. Urinary concentrations and bactericidal activity against amoxicillin-nonsusceptible strains of Escherichia coli with single-dose, oral, sustained-release amoxicillin/clavulanic acid: a phase I, open-label, noncomparative clinical trial in healthy volunteers. Clin Ther 2005; 27: 1043–1049. [DOI] [PubMed] [Google Scholar]
- 38. Hoener B, Patterson SE. Nitrofurantoin disposition. Clin Pharmacol Ther 1981; 29: 808–816. [DOI] [PubMed] [Google Scholar]
- 39. Jawetz E, Hopper J, Smith DR, et al. Nitrofurantoin in chronic urinary tract infection. AMA Arch Intern Med 1957; 100: 549–557. [DOI] [PubMed] [Google Scholar]
- 40. Naggar VF, Khalil SA. Effect of magnesium trisilicate on nitrofurantoin absorption. Clin Pharmacol Ther 1979; 25: 857–863. [DOI] [PubMed] [Google Scholar]
- 41. Borner K, Höffken G, Lode H, et al. Pharmacokinetics of ciprofloxacin in healthy volunteers after oral and intravenous administration. Eur J Clin Microbiol 1986; 5: 179–186. [DOI] [PubMed] [Google Scholar]
- 42. Wagenlehner FME, Kinzig-Schippers M, Tischmeyer U, et al. Pharmacokinetics of ciprofloxacin XR (1000 mg) versus levofloxacin (500 mg) in plasma and urine of male and female healthy volunteers receiving a single oral dose. Int J Antimicrob Agents 2006; 27: 7–14. [DOI] [PubMed] [Google Scholar]
- 43. Brumfitt W, Pursell R. Trimethoprim-sulfamethoxazole in the treatment of bacteriuria in women. J Infect Dis 1973; 128(Suppl.): 657–665p. [DOI] [PubMed] [Google Scholar]
- 44. De Liso F, Garigali G, Ferraris Fusarini C, et al. How to identify sulfamethoxazole crystals in the urine. Clinica Chimica Acta 2016; 452: 106–108. [DOI] [PubMed] [Google Scholar]
- 45. Barceló B, Rodriguez A, Lopez MO, et al. OrbitrapTM high-resolution mass spectrometry for the identification of amoxicillin crystalluria. Clin Chem Lab Med 2018; 56: 268–271. [DOI] [PubMed] [Google Scholar]
- 46. Thorsteinsson SB, Bergan T, Oddsdottir S, et al. Crystalluria and ciprofloxacin, influence of urinary pH and hydration. Chemotherapy 1986; 32: 408–417. [DOI] [PubMed] [Google Scholar]
- 47. Nývlt J. Kinetics of nucleation in solutions. J Cryst Growth 1968; 3–4: 377–383. [Google Scholar]
- 48. Richardson JF, Harker JH, Backhurst JR. Crystallisation. In: Coulson JM, Richardson JF. (eds) Chemical engineering. Oxford: Elsevier, 2002, pp. 827–900. [Google Scholar]
- 49. Fraser TN, Avellaneda AA, Graviss EA, et al. Acute kidney injury associated with trimethoprim/sulfamethoxazole. J Antimicrob Chemother 2012; 67: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 50. Schwartz L, Flippin HF, Reinhold JG, et al. The effect of alkali on crystalluria from sulfathiazole and sulfadiazine. J Am Med Assoc 1941; 117: 514–515. [Google Scholar]
- 51. Simon DI. Sulfadiazine crystalluria revisited. Arch Intern Med 1990; 150: 2379–2384. [DOI] [PubMed] [Google Scholar]
- 52. Dorfman LE, Smith JP. Sulfonamide crystalluria: a forgotten disease. J Urol 1970; 104: 482–483. [DOI] [PubMed] [Google Scholar]
- 53. Khan M, Ortega LM, Bagwan N, et al. Crystal-induced acute kidney injury due to ciprofloxacin. J Nephropathol 2015; 4: 29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Turel I, Živec P, Pevec A, et al. Compounds of antibacterial agent ciprofloxacin and magnesium – crystal structures and molecular modeling calculations. Eur J Inorg Chem 2008; 2008: 3718–3727. [Google Scholar]
- 55. Wallis SC, Charles BG, Gahan LR, et al. Interaction of norfloxacin with divalent and trivalent pharmaceutical cations. In vitro complexation and in vivo pharmacokinetic studies in the dog. J Pharm Sci 1996; 85: 803–809. [DOI] [PubMed] [Google Scholar]
- 56. Urbaniak B, Kokot ZJ. Analysis of the factors that significantly influence the stability of fluoroquinolone-metal complexes. Anal Chim Acta 2009; 647: 54–59. [DOI] [PubMed] [Google Scholar]
- 57. Macdonald JB, Macdonald ET. Nitrofurantoin crystalluria. Br Med J 1976; 2: 1044–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kalsi DS, Ward J, Lee R, et al. Purple urine bag syndrome: a rare spot diagnosis. Dis Markers 2017; 2017: 9131872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Golding B. Urinary deposits, their diagnosis, pathology, and therapeutical indications. London: John Churchill, 1857. [Google Scholar]
- 60. Barlow GB, Dickson JAS. Purple urine bags. Lancet 1978; 311: 220–221. [Google Scholar]
- 61. Capaccione KM, Tran CV, Leb JS, et al. Acute pulmonary function decline and radiographic abnormalities: chronic cause? Breathe 2021; 17: 200286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cham EB, Dykman J, Bochner F. Urinary excretion of aspirin. Br J Clin Pharmacol 1982; 14: 562–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Beckett AH, Rowland M. Urinary excretion kinetics of amphetamine in man. J Pharm Pharmacol 1965; 17: 628–639. [DOI] [PubMed] [Google Scholar]
- 64. Sand TE, Jacobsen S. Effect of urine pH and flow on renal clearance of methotrexate. Eur J Clin Pharmacol 1981; 19: 453–456. [DOI] [PubMed] [Google Scholar]
- 65. Kamberi M, Tsutsumi K, Kotegawa T, et al. Influences of urinary pH on ciprofloxacin pharmacokinetics in humans and antimicrobial activity in vitro versus those of sparfloxacin. Antimicrob Agents Chemother 1999; 43: 525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Craig WA, Kunin CM. Trimethoprim-sulfamethoxazole: pharmacodynamic effects of urinary pH and impaired renal function. Studies in humans. Ann Intern Med 1973; 78: 491–497. [DOI] [PubMed] [Google Scholar]
- 67. Yang L, Wang K, Li H, et al. The influence of urinary pH on antibiotic efficacy against bacterial uropathogens. Urology 2014; 84: 731.e1–731.e7. [DOI] [PubMed] [Google Scholar]
- 68. Burian A, Erdogan Z, Jandrisits C, et al. Impact of pH on activity of trimethoprim, fosfomycin, amikacin, colistin and ertapenem in human urine. Pharmacology 2012; 90: 281–287. [DOI] [PubMed] [Google Scholar]
- 69. AlRabiah H, Allwood JW, Correa E, et al. pH plays a role in the mode of action of trimethoprim on Escherichia coli. PLoS One 2018; 13: e0200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fransen F, Melchers MJB, Lagarde CMC, et al. Pharmacodynamics of nitrofurantoin at different pH levels against pathogens involved in urinary tract infections. J Antimicrob Chemother 2017; 72: 3366–3373. [DOI] [PubMed] [Google Scholar]
- 71. Viswanadhan VN, Ghose AK, Revankar GR, et al. Atomic physicochemical parameters for three dimensional structure directed quantitative structure-activity relationships. 4. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain. J Chem Inf Comput Sci 1989; 29: 163–172. [Google Scholar]
- 72. Sakhaee K, Nicar M, Hill K, et al. Contrasting effects of potassium citrate and sodium citrate therapies on urinary chemistries and crystallization of stone-forming salts. Kidney Int 1983; 24: 348–352. [DOI] [PubMed] [Google Scholar]
- 73. Pujades-Rodriguez M, West RM, Wilcox MH, et al. Lower urinary tract infections: management, outcomes and risk factors for antibiotic re-prescription in primary care. EClinicalMedicine 2019; 14: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ahmed H, Farewell D, Jones HM, et al. Incidence and antibiotic prescribing for clinically diagnosed urinary tract infection in older adults in UK primary care, 2004-2014. PLoS One 2018; 13: e0190521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taw-10.1177_20420986221080794 for Alkalising agents in urinary tract infections: theoretical contraindications, interactions and synergy by Oisín N. Kavanagh in Therapeutic Advances in Drug Safety