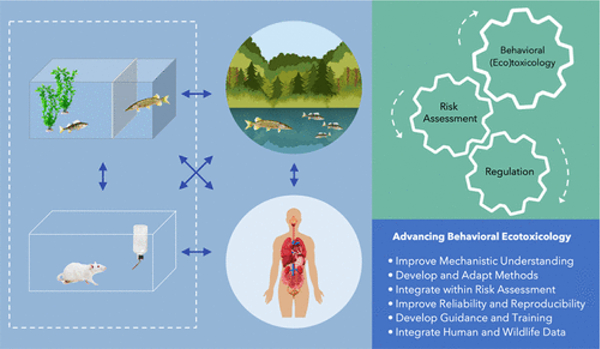
Abstract
For decades, we have known that chemicals affect human and wildlife behavior. Moreover, due to recent technological and computational advances, scientists are now increasingly aware that a wide variety of contaminants and other environmental stressors adversely affect organismal behavior and subsequent ecological outcomes in terrestrial and aquatic ecosystems. There is also a groundswell of concern that regulatory ecotoxicology does not adequately consider behavior, primarily due to a lack of standardized toxicity methods. This has, in turn, led to the exclusion of many behavioral ecotoxicology studies from chemical risk assessments. To improve understanding of the challenges and opportunities for behavioral ecotoxicology within regulatory toxicology/risk assessment, a unique workshop with international representatives from the fields of behavioral ecology, ecotoxicology, regulatory (eco)toxicology, neurotoxicology, test standardization, and risk assessment resulted in the formation of consensus perspectives and recommendations, which promise to serve as a roadmap to advance interfaces among the basic and translational sciences, and regulatory practices.
Graphical Abstract
Introduction
Behavioral ecotoxicology is the study of behavioral responses to determine the potential effects of toxicants and other stressors on individuals, populations, and communities.(1) It is the combination of an understanding of adaptive responses of individuals to stressors present in their environment, particularly chemical contaminants, and the resulting impacts of these behavioral changes at the individual, population, and community levels of ecological organization.
The field of behavioral toxicology can be traced back to the 1960s(2–4) with behavioral responses in historic environmental legislation (Box 1). However, there is concern that regulatory ecotoxicology does not adequately consider behavior, primarily due to a lack of standardized toxicity methods. More recently, technological advances are allowing for the detection and quantification of subtle changes in the behaviors of animals and the increasing environmental occurrence of neuroactive compounds (e.g., neuroendocrine disruptors, pharmaceuticals), which has led to growing concerns surrounding the disruption of wildlife behavior by chemical contaminants.(5,6) Moreover, there is mounting evidence that environmental risk assessment of chemicals does not adequately incorporate or consider behavioral data (discussed in ref (7)). With growing concerns over human behavioral health and contaminants,(8,9) there may be a need for greater cross-disciplinary integration of environmental and human health risk assessments. Indeed, in the 1990s, it was highlighted that the general public is largely unaware of the potential effects of toxicants on behavior.(10) However, despite recent studies suggesting growing public interest and understanding of environmental issues,(11) awareness that contaminants might be affecting wildlife and human behavior is still, despite decades of research, broadly lacking. This reality exists despite well-known cases of behavioral toxicants leading to changes in legislation, such as the removal of lead from fuels,(12) limitations or prohibitions placed on alcohol consumption and the operation of motor vehicles.(13) The objectives of this paper are, therefore, to examine, through development of consensus perspectives, interfaces among behavioral ecology, ecotoxicology, and chemical risk assessment and to provide recommendations to improve integration of behavioral end points in risk assessment, criteria development, and the regulation of chemical contaminants.
Box 1. Behavioral (eco)toxicology in historical environmental litigation.
Behavioral (eco)toxicology emerged during the 1960s, driven by the merging of behavioral pharmacology and neurotoxicology. What followed was a string of litigation regarding workforce exposure to a wide range of toxicants. Weiss 10, reflecting on the development of behavioral toxicology, highlighted that during the 1970s legislators in the U.S. were already drafting requirements that behavioral disturbances could be included among the criteria of adverse effects (p403–404) in what later became the Toxic Substances Control Act. In 1986, the U.S. EPA’s Guidelines for Deriving Ambient Water Quality Criteria for Protection of Aquatic Life stated, ‘Pertinent information that could not be used in earlier sections might be available concerning adverse effects on aquatic organisms and their uses. The most important of these are data on cumulative and delayed toxicity, flavour impairment, reduction in survival, growth, or reproduction, or any other adverse effect that has been shown to be biologically important. Especially important are data for species for which no other data are available. Data from behavioral, biochemical, physiological, microcosm, and field studies might also be available.’ A number of chemical regulations now include behavior internationally with respect to risk assessment. Furthermore, inclusion of neurobehavioral endpoints in the Organisation for Economic Co-operation and Development (OECD) test guidelines (TG) (e.g. 14–16) mean that they can be used in European Union (EU) regulatory frameworks such as regulation for medicinal products (27/2004), REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals (EC) No. 1907/2006), Plant Protection Products Regulation ((EC) No. 11)07/2009), and Biocidal Products Regulation (528/2012).
While environmental risk assessment does not preclude behavioral toxicity test results, such tests are seldom included.(7) There have also been perceptions that behavioral ecotoxicology test end points are less reliable and not repeatable or that behavioral responses are hard to extrapolate to the population level.(1,6,17)()() As noted in Box 2, specific (eco)toxicological considerations for data reliability and relevance are defined in the EU. For convenience, we used the term (eco)toxicology when referring to both human toxicology and ecotoxicology. In behavioral studies, it may not be the fundamental findings of the study that are perceived as unreliable but rather the interpretation of relevance and the fact that the experiments are not standardized and thus aligned to national or international accepted and validated guidelines, as required in regulatory human toxicology and ecotoxicology. These concerns are certainly not new. For example, a workshop held in 1976, entitled “Behavioral Toxicology: An emerging discipline,” brought together experts in toxicology, pharmacology, psychology, physiology, and veterinary health.(3) The published proceedings highlight that researchers were acutely aware of the importance of tackling the effects of environmental contaminants on the health of humans and wildlife. It was also evident that the researchers were mindful of the challenges faced by behavioral toxicology in relation to reliability and reproducibility of the experimental systems used.
Box 2. Reliability and relevance of (eco)toxicology data in the EU.
The European Chemicals Agency (ECHA) defines reliability as ‘the inherent quality of a test report or publication relating to preferably standardized methodology and the way the experimental procedure and results are described to give evidence of the clarity and plausibility of the findings’ 18. Relevance is ‘the extent to which data and tests are appropriate for a particular hazard identification or risk characterization’. ECHA has strict guidance on the assessment of relevance and reliability of (eco)toxicity studies used for human and environmental risk assessment 18. Reliability of the information in (eco)toxicological studies is categorized as 1) reliable without restrictions, 2) reliable with restrictions, 3) not reliable, or 4) not assignable 19.
Despite the legacy of behavioral toxicology dating back over half a century, there continues to be very little of this information used in environmental protection. Therefore, judgements cannot currently be made that might otherwise result in improved environmental quality standards, alternative assessments of environment risks, or just fundamental improvements in our understanding of contaminant-induced behavioral effects. To increase understanding of behavioral ecotoxicology and advance its integration into regulatory environmental risk assessments of chemicals, a workshop was organized at the German Environment Agency in Dessau, Germany. International representatives from the fields of behavioral ecology, ecotoxicology, regulatory (eco)toxicology, neurotoxicology, test standardization, and risk assessment were invited to provide contemporary synthesis. Here, we provide consensus perspectives and recommendations to improve use of behavioral end points. We specifically examined a key question: What should be the future role of behavioral ecotoxicology in environmental protection? Therefore, we aimed to address whether there are methodological approaches that need to be improved/developed and/or if regulatory authorities need more confidence in current approaches.
Consensus Perspectives
1. Chemical Contaminants Affect Wildlife and Human Behavior
Considerable evidence exists demonstrating that chemical contaminants can impact both wildlife and human behavior. Research from the early 1900s first described altered swimming behaviors in fish exposed to various chemicals,(20,21) with numerous studies reporting similar effects having emerged over the past century.(5,22) Examples of behavioral responses reported in fish include effects on learning and memory, reproduction, sociality, aggression, and predator avoidance, as well as a multitude of others (reviewed in ref (23)). Importantly, such effects are not limited to aquatic vertebrates, with a growing number of studies describing similar responses in invertebrates(24) and diverse terrestrial species.(25) That effects are seen across animal taxa is not surprising given the highly conserved nature of biochemical pathways and processes in all living organisms, including those in the brain that ultimately control behavior.
Humans share many biochemical pathways with other species. Indeed, pharmaceutical development commonly incorporates screening of potential novel neuroactive chemicals for effects on animal behaviors.(26) When considered in this context, it is not surprising that many bioactive chemicals designed for humans use also influence wildlife behavior. Recent studies have linked chemical contaminants with effects on human behavior, cognition, and brain development.(27,28) For example, early exposure to many industrial chemicals has been identified as contributing to an increasing frequency of neurobehavioral disabilities in humans.(29,30) Furthermore, besides direct toxicity, the knowledge that contaminant levels are rising and can cause harmful effects on human health has itself been linked to increased psychological distress in the general population.(31)
2. Behavior Is Connected to Fundamental Ecological Processes (e.g., Population Health and Fitness)
Behavior can profoundly impact individual fitness, with consequences for population dynamics, species interactions, and ecosystem function (reviewed in refs (22) and (32)). Behavioral responses can affect individual fitness by influencing reproductive success, feeding, growth, and survival. For example, selection of a mating partner or location of nest sites can have a direct bearing on the quality and quantity of offspring produced,(33) while behavioral responses to predators can be a matter of life or death.(34,35) These types of individual-level behavioral responses can, in turn, have population-level consequences by altering demographic parameters, such as birth, death, and migration rates.
The interplay between individual behavior and population dynamics is complex. In seeking to maximize fitness, individuals can engage in behaviors that are beneficial to themselves but are potentially detrimental to the population.(36) Conversely, changes to population dynamics can also affect individual behavior, for example, through density-dependent effects on foraging, mate searching, aggression, and competition.(37,38)
Due to the complex interactions that exist among different organisms within their environment, behaviors that lead to population changes in one species can affect the strength and nature of its interactions with others.(39–41) The structure and complexity of these interactions can have important community- and ecosystem-level effects.(42) This is because changes in one part of the system can drive changes throughout, with consequences for biodiversity, ecosystem function, and stability. For instance, the abundance and behavior of apex predators can induce top-down effects,(43,44) while the complexity of the networks connecting different species can act as a potential safeguard, buffering species against the risk of extinction.(45)
3. Laboratory-Derived Behavioral Data Can Be Linked to Individual, Population, and/or Ecosystem Processes in the Field
Laboratory-based research is widely employed to investigate the causes and consequences of animal behavior.(46) Well-designed experimental studies in the laboratory allow researchers to explicitly control for myriad interacting variables present in characteristically complex and dynamic natural systems. In turn, these also yield insights that can be directly linked to individual, population, and ecosystem processes. There is strong evidence, for instance, that data collected in the laboratory can predict variation in ecologically important behaviors in the field, including activity (e.g., (47) and (48)), boldness (e.g., (49) and (50)), exploration (e.g., (48) and (51)), and aggression (e.g., (47) and (50)). Further, research has demonstrated that variation in behavioral traits measured in the laboratory can predict the fitness of individuals in the wild (reviewed in ref (52)). For example, in great tits (Parus major), the degree of exploratory behavior exhibited in the laboratory is related to annual adult survival, as well as offspring survival to breeding age, measured in the field.(53) Moreover, behavioral data derived from laboratory assays can be linked to fundamental ecosystem and evolutionary processes at the population and community levels (reviewed in ref (41)). For instance, in Trinidadian guppies (Poecilia reticulata), female preference for male nuptial coloration, which is readily quantifiable in the laboratory (e.g., ref (54)), shapes evolutionary trajectories in the wild due to a trade-off in males between attractiveness to females and vulnerability to predation.(55)
In ecotoxicology, laboratory-based studies are fundamentally important in achieving a mechanistic understanding of contaminant-induced behavioral changes under controlled experimental conditions and have revealed that a wide variety of fitness-related behaviors are vulnerable to disruption (reviewed in refs (5), (22), (25), (56), and (57)). Where field studies have been carried out to validate behavioral effects of contaminant exposure observed in the laboratory, a variety of effects observed in the laboratory have been shown to accurately predict those seen in the field. For example, Klaminder et al.(58) reported that European perch (Perca fluviatilis) exposed to the antianxiety medication oxazepam displayed increased boldness and activity both when measured in the laboratory and in an experimental lake system. Furthermore, Hellström et al.(59) found that exposure to oxazepam resulted in Atlantic salmon (Salmo salar) smolts migrating faster both in large-scale laboratory pools and in a natural river system.
Effects seen in the laboratory and the field are not always in alignment, which can be due to incompatibility between behaviors measured in either setting (i.e., activity within a restricted area versus large-scale dispersal). A mismatch between laboratory and field findings is evident, for instance, in the reporting of inconsistencies in neonicotinoid-induced behavioral changes in honeybees.(60–62) However, such gaps may be remedied by taking a more integrative, multipronged approach involving laboratory, semifield, and field studies.(63) For example, contaminant-induced effects on behavioral end points measured in semifield and field studies—and/or their consequences at the individual and population levels—can be used to inform the design of laboratory-based studies to elucidate mechanisms of toxic action. Information derived from these mechanistic investigations will be important for establishing adverse outcome pathways from molecular initiating events to population-, community-, and ecosystem-level effects.(64)
4. Behavioral Data Can Be Environmentally Relevant but Are Rarely Used in an Environmental Regulatory Context
Behavioral (eco)toxicological data are often considered of “low relevance” and do not adhere to the existing paradigm of what constitutes relevant data for regulatory decisions. A recent study identified only six examples of behavioral ecotoxicological data being considered in environmental risk assessment in the EU,(7) and these examples vary in the use of behavioral data within the assessment. Differences in the level of inclusion span the specific end points and test design, regulatory framework, and the weight given to behavioral responses. Given the relevance of behavioral ecotoxicological data to environmental risk assessment, why is its use so sparse?
One possible reason for the low regulatory use of behavioral studies is the lack of promotion of behavioral end points in legislation and guidance documents. In addition, there have been very few side-by-side comparisons between behavioral tests and (eco)toxicity standard tests. Environmental risk assessments have traditionally been based on standard studies measuring growth, mortality, and fecundity as the key end points linked to population-level effects (ECHA, 2011). There is also a lack of understanding as to how behavioral effects relate to population fitness and ecosystem-level impacts, and which effects should be considered. As such, behavioral studies have been disregarded because often the end point is not considered to be linked to population effects and, hence, is regarded as being of “low relevance.”
Although not explicitly mentioned, several regulatory frameworks do allow for the incorporation of nonstandard end points such as behavior (Box 1). For example, different chemical legislations are in place in the EU, covering industrial chemicals, pesticides, biocides, and pharmaceuticals. Some of them require experimental data performed according to the OECD Test Guidelines (TGs). Behavioral end points are used for validity criteria (e.g., burrowing behavior of worms in ref (65)), selection of test species (e.g., fish in ref (66)), indication of water quality, status of the experimental animals, and the continuation of the experiment. These TGs require reporting behavioral observations, but in the case of ecotoxicological studies, they are seldom used for making regulatory decisions because these observations are not consistently perceived as relevant at the population level. However, regulatory risk assessment and regulatory bodies are flexible enough to integrate these end points, if the requirements mentioned above are fulfilled (see statements 5 and 6).
Quite contrarily, behavioral end points are included in several OECD rat studies recommended for use in human health assessment (e.g., refs (14–16)). Specifically, reproductive and neurobehavioral testing is included in the Extended One-Generation Reproductive Toxicity Study, OECD TG 443,(16) and in the Developmental Neurotoxicity Study, OECD TG 426.(14) Through these studies, behavioral end points have gained regulatory acceptance and consistent use in human health assessments.(67)
5. For Use in a Regulatory Context, Behavioral Studies Should Incorporate the Elements of Standardized Studies
Behavioral ecotoxicity experiments are primarily carried out as nonstandard studies performed by academic researchers and published in the peer-reviewed literature.(7) Nonstandard ecotoxicity studies include a wide range of test designs, which reflect differences in available equipment between laboratories but also highlight the diversity of behavioral responses. However, the lack of standardization, such as the lack of analytical verification of nominal treatment levels, the use of too few replicates, or missing information needed for a full evaluation of the reliability of the study, has resulted in nonstandard studies being disregarded for regulatory use.(68)
Most test guidelines are written with the understanding that the results are used in a regulatory context and will need to stand up to challenges under various legal systems. Therefore, standard studies share common characteristics, which have been identified through practice to increase replicability across time and laboratories. Typically, they are designed for use with a single species at a specific life stage(s), a single end point class, and require reporting of a base set of experimental and quality assurance data necessary to demonstrate reliability of results. The use of standardized methods ensures that risk assessors have appropriate information required for data reliability assessment and use in a risk assessment.
These specific requirements are not always documented in peer-reviewed published studies and sometimes not even integrated into the planning of the studies.(7) Many behavioral studies have been motivated by fundamental (rather than applied) research questions and, therefore, may have been carried out as a proof of principle study or may have been performed as part of a small project on a limited budget that precluded comprehensive chemical analyses. These studies were never designed and never intended to be used in regulation and therefore often do not fulfill the acceptance criteria. More generally, the authors of such studies also may not be aware of the acceptance criteria nor the significance of guideline studies for regulation. In fact, such a spectrum of fundamental to applied/translational scientific inquiry is not uncommon among disciplines. Therefore, an acceptance for regulatory purposes is often difficult. Results from many behavioral toxicology studies can therefore be used as background information only. Background information in regulation means that this information is used in a weight of evidence approach to explain certain effects or support the identification of a hazard concern but cannot be used for deriving effect levels for a quantitative risk assessment. This could be partly solved with more effective communication and guidance between regulators, industry, and researchers.(7) Often, the study design could be quite easily adapted, and sometimes the missing information has been generated but not published. In many cases, Supporting Information from older studies will be inevitably lost.
In a recent review on “An Ecotoxicological View on Neurotoxicity Assessment,” Legradi et al.(17) presented a comprehensive analysis of how behavioral data can be used in a regulatory context. The authors concluded that considering the increasing numbers of environmental contaminants with potential neurotoxic potency, eco-neurotoxicity should be considered in risk assessment. In order to do so, novel test systems are needed that can cope with species differences within ecosystems. For eco-neurotoxicity, methods need to focus on potentially sensitive species in an ecosystem. A test battery using species from different trophic levels might be the best approach and, importantly, using different timing of exposure (e.g., gestation vs adult). To implement eco-neurotoxicity and behavioral assessment into EU risk assessment, cheminformatics and in vitro screening tests could be used as a first approach to identify eco-neurotoxic pollutants. In a second step, a small species test battery could be applied to assess the risks to ecosystems.(17)
6. Standardized Ecotoxicity Studies Can Include and Quantify Behavioral End Points
The OECD guidelines for testing the effects of chemicals on biotic systems include about 50 ecotoxicity TGs with aquatic and terrestrial animals. In response to legal data requirements and guidance documents, most use mortality, growth, and reproductive outcomes for the derivation of (no) effect concentrations, but almost all TGs reference abnormal behavior as a potential effect caused by exposure to the test chemical. In most cases, the reference to behavior is limited to a note that behavioral changes must be recorded in the study report. No information is provided on what is considered as (ab)normal behavior or the types of behavioral changes that are considered important. Exceptions are TGs for fish and amphibians and for bees and bumblebees, some of which give a description of behavioral changes that may occur. For example, TG 231 on amphibian metamorphosis(69) states that abnormal behavior would include floating on the surface, lying on the bottom of the tank, inverted or irregular swimming, lack of surfacing activity, and being unresponsive to a stimulus. Test guideline 203 on acute fish mortality(70) includes an extensive description of clinical signs and a scoring sheet to record these abnormal behaviors.
However, the TGs also state that the purpose of recording behavioral changes is not to include them as regulatory apical end points. Instead, behavioral changes are incorporated in the TGs to optimize test design, to facilitate interpretation of data, or from the perspective of monitoring animal welfare during testing. For example, three fish toxicity TGs (TG 215, 229, and 230(71–73)) provide recommendations to adjust the number of test organisms because territorial behavior may induce stress responses, which could in turn influence the test end points, in this case growth and endocrine effects. Test guidelines 229(72) and 230(73) require that signs of toxicity should be considered carefully for data interpretation, because they may indicate concentrations at which biomarkers of endocrine activity are not reliable. Behavior observations can aid in the interpretation of data, such as providing information on the potential mode of action (MOA) of a chemical. For example, in zebrafish (Danio rerio), the characteristic mating and spawning behavior after morning onset of light is reduced or hindered by exposure to estrogenic or antiandrogenic compounds. Animal welfare is the reason to include abnormal behavior in TG 203(74) and 210.(75) These TGs specify that fish should be euthanized and treated as mortalities for subsequent data analysis when abnormal behavior is considered so severe that there is considerable suffering to the organism and the organism has reached a point beyond which it will not recover. Similar considerations are made for cladoceran immobility as a surrogate for mortality when using EPA methods in the U.S.
Almost all ecotoxicity TGs for fish and mammals require that behavioral changes are recorded. This suggests regulators recognize that chemicals affect behavior and that these altered behaviors should be considered when evaluating test results. Therefore, it would be beneficial to review all TGs so that behavioral end points could be incorporated as quantitative measures of toxicity in addition to their use to optimize test design or as markers of animal welfare.
Recommendations
Evident from the workshop was a consistent shared perspective that considerable data exist highlighting that chemical pollutants can impact the behavior of humans and wildlife. Similarly, experts agreed that behavior is a sensitive indicator of disturbance and is linked to fundamental processes that influence individual fitness and can lead to population- and ecosystem-level adverse outcomes. There was also agreement that the field is still evolving and that the current body of research has limitations that will need to be overcome in terms of design, intraspecies variability, cross-species extrapolation, repeatability, and confirming laboratory responses with field collected data. It was also evident that behavior was already incorporated into international regulatory (eco)toxicology either through recorded end points, observational end points, or important factors of consideration within the study design. Further, the commercial sector (e.g., pharmaceutical industry) is making use of behavioral tests during drug development and is increasingly looking toward model aquatic species to replace mammalian species, for reasons of costs and ethics. Hence, based on the consensus perspectives outlined above, the team generated the following recommendations for the improved use of behavioral end points in environmental risk assessment of chemicals (Figure 1).
Figure 1.
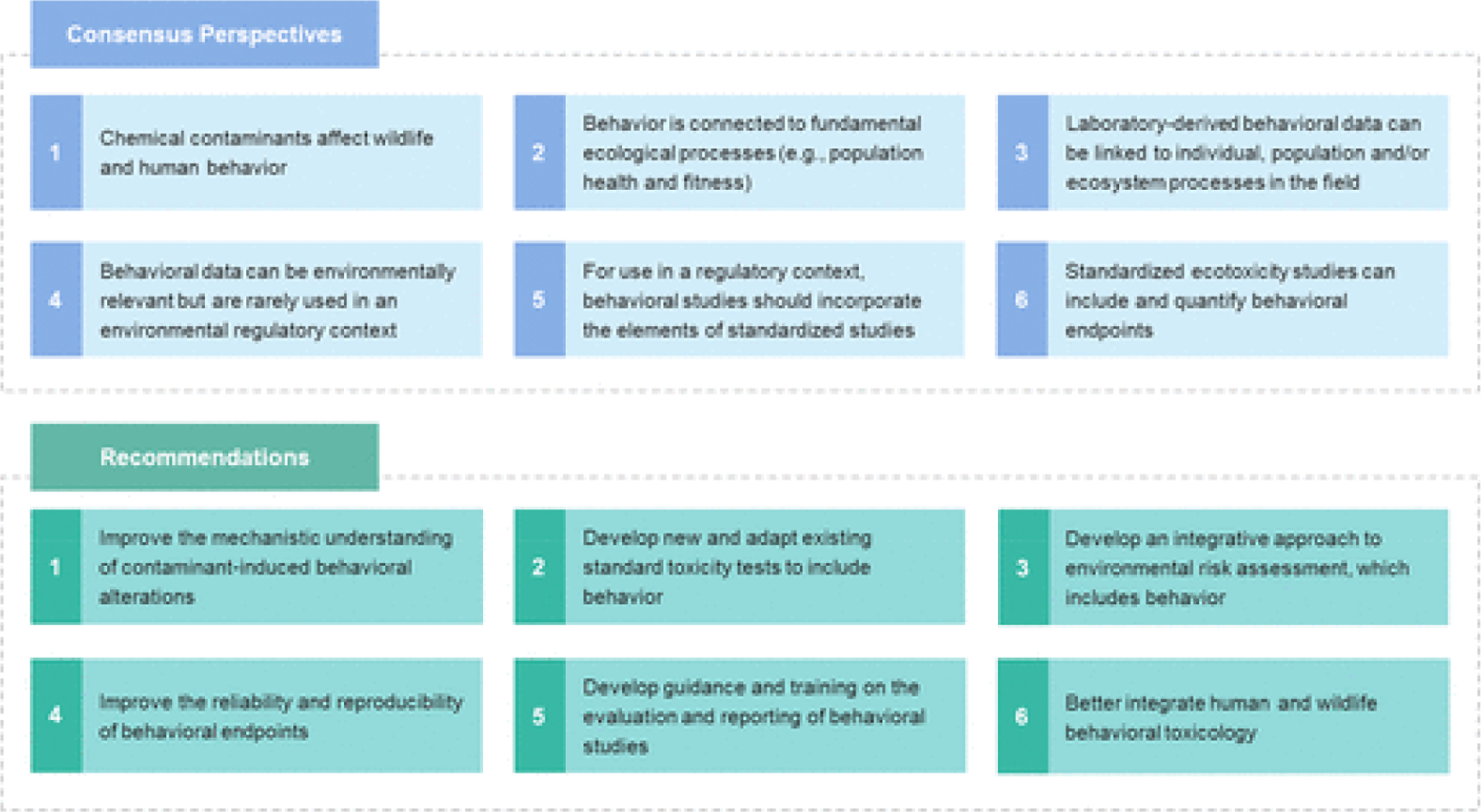
Consensus perspectives and recommendations for the advancement of behavioral ecotoxicology for environmental protection.
1. Improve the Mechanistic Understanding of Contaminant-Induced Behavioral Alterations
In order to obtain a more hypothesis-driven approach to behavioral ecotoxicology, research based on mechanistic (laboratory-based) studies should be promoted. Studies are required that are designed to link behavior to mode of action, so that generalizations can be made on the impact of behavioral change. Furthermore, behavioral ecotoxicologists should explain and emphasize, where possible, the ecological relevance of their recorded end points. A better understanding of the underpinning mechanisms of behavioral ecotoxicology will lead to a more accurate and reliable risk assessment. By providing such robust information on the causality and reliability (quantification, repeatability, etc.), it will also lead to the development and improvements of guidance documents and a better framework for risk assessors.
2. Develop New and Adapt Existing Standard Toxicity Tests to Include Behavior
Environmental risk assessments are often based on the use of standard studies. There is a need to develop standardized behavioral assays, or to add behavioral end points to already established standard methods. An inventory of established methods in behavioral ecology and ecotoxicology could therefore be used to identify representative, reliable, and sensitive combinations of taxa (model species) and related behaviors (end points). New behavioral end points should be developed for use in risk assessment that are indicative of specific MOAs. Such end points are dependent on improving the mechanistic understanding of behavioral toxicants in wildlife (see recommendation 1). These efforts should initially focus on representative substances with conserved MOAs to develop a reference data system, which can aid studying relationships among behavioral end points, population-relevant effects, and MOA-related end points.(17)
3. Develop an Integrative Approach to Environmental Risk Assessment, Which Includes Behavior
Currently, environmental risk assessment is fixed around a series of laboratory-based evidence with a limited number of model species and end points. In conjunction with the development of new behavioral end points in standard ecotoxicity tests (see recommendation 2), an integrative approach to risk assessment is required, where impacts of chemicals are assessed using information from laboratory (controlled), mesocosm (semirealistic), and field (realistic) settings, thus providing more environmentally realistic decision support using multiple lines of evidence. Here, modeling approaches can serve as a cost-effective complementary approach to mesocosm and field studies or monitoring.
4. Improve the Reliability and Reproducibility of Behavioral End Points
In order to use behavioral end points in a regulatory framework, there is a need to ensure reliable and reproducible behavioral methods. Each end point will need to establish its single laboratory and multilaboratory variability, and side-by-side testing will need to be conducted with behavioral methods and standard ecotoxicity methods to illustrate how behavioral methods compare with end points assessed within the existing guidelines. Side-by-side method comparisons could begin with chemicals that have a significant ecotoxicological database such as metals and legacy organics and some contaminants of emerging concern. Improved minimum reporting standards (see recommendation 5) would also improve the amount of research available to risk assessment.
The use of computer-based analysis has removed questions of subjectivity often impacting confidence in past behavioral studies. The use of “big data” generated from automated recording devices has inevitably come with benefits to both logistics (in experimental design) and accuracy (in terms of eliminating unconscious biases). In time, the use of artificial intelligence (AI) and machine learning will further advance this field to generate new ways of recording and interpreting behavioral data. When working with big data sets and new types of data, there is also a necessity to understand the limitations in the statistical analysis conducted by the scientists. Melvin et al.(76) highlight the importance of acclimation and recording times in the potential for false positives and negatives in statistical analysis of behavioral data. Therefore, there is a need for scientists to understand their study model organisms and the limitations of their experimental designs and to improve their statistical approaches.
5. Develop Guidance and Training on the Evaluation and Reporting of Behavioral Studies
Evaluating the reliability and relevance of behavioral end points can be difficult for novel test designs, end points, test species, and technologies. Therefore, guidance and training on evaluation of behavioral studies for regulatory use is needed. In addition, improving minimum reporting standards in behavioral ecotoxicology would increase the throughput of behavioral studies available for evaluation in risk assessment. Such reporting recommendations are already available for ecotoxicity studies in general(68) and could be adopted for behavioral studies. Providing guidance and training both for risk assessors and researchers within this field could potentially increase data quality and the use of behavioral studies in environmental risk assessment of chemicals.
6. Better Integrate Human and Wildlife Behavioral Toxicology
The field of ecotoxicology has always benefited from knowledge transfer from human toxicology aligned to safeguarding public health and the environment. Many of the underlying mechanisms of toxicology have been developed through human toxicology and medicine, which have led to a better understanding of toxicological modes of action in wildlife. Furthermore, the financial investments supporting medical research have resulted in techniques and technologies that are now commonplace in ecotoxicology. Diverse developments in the field of neurobiology, neuropharmacology, and neurotoxicology over many decades have led to the application of standard toxicity testing (e.g., anxiolytic assays) from rodents to aquatic models. Ecotoxicologists on the other hand have complemented the field of human toxicology by providing sentinel species information and real-world examples of chemical exposures. For example, evidence provided on feminization and reproductive disorders in wildlife complemented the science being generated by human toxicologists and led to decade-long studies of endocrine disruption and subsequent regulatory safeguards for human health and environmental quality. To continue to develop synergies in methodologies and to develop the “one health” initiative, communication among different fields, particularly behavioral ecology, comparative physiology, environmental chemistry, and human toxicology and ecotoxicology, should be encouraged and strengthened further. In doing so, we may capture currently unknown impacts on behavior for both human health and the environment.
Conclusions
Chemical contaminants can impact the behavior of humans and wildlife. However, our ability to regulate chemicals for these risks, and thus safeguard the environment, is rarely used and is hampered by a lack of understanding and alignment with more traditional end points. Therefore, we must expand the toolbox of behavioral markers and embrace the reliability and robustness of these novel end points. It is evident from human toxicology and pharmaceutical drug development that regulatory authorities and industry have advanced with confidence in the application of behavioral end points for related fields of study and translational applications within regulatory practice. Thus, the perceived hurdles held by some are not insurmountable within behavioral ecotoxicology. In addition, whereas behavioral links with reproduction and growth might seem self-evident within behavioral ecology, there needs to be alignment with standard toxicity methods so that side-by-side comparisons can be made. This progress will allow for robust assessment of their utility as part of the tool kit for ecological risk assessment and regulatory ecotoxicology practice.
Acknowledgments
This workshop was organized by the German Environment Agency (UBA) and financed by UBA and by Stockholm University (Sweden). The authors would also like to thank the following agencies for funding support: the Australian Research Council (DP190100642 and FT190100014 to BBMW), the Swedish Research Council Formas (2018–00828 to TB), the Kempe Foundations (SMK-1954), and the Marie-Claire Cronstedt Foundation (to M.G.B.); the Excellence Initiative of the German Federal and State Governments and BMBF (EXC 2186 and FKZ 02WRS1419C to H.H.); and the U.S. Geological Survey Environmental Health Mission Area. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. EPA or any agencies/organizations. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Biography
Alex T. Ford

Professor Alex Ford is a Professor of Biology and Deputy Director for the Centre of Blue Governance at the University of Portsmouth (U.K.). His interests and expertise lie within invertebrate biology, parasitology, and environmental toxicology. He is a long serving member of the Society of Environmental Toxicology and Chemistry (SETAC) and has a passion for cross-disciplinary research and scientific communication.
Footnotes
The authors declare no competing financial interest.
References
- 1.Peterson EK; Buchwalter DB; Kerby JL; LeFauve MK; Varian-Ramos CW; Swaddle JP Integrative behavioral ecotoxicology: bringing together fields to establish new insight to behavioral ecology, toxicology, and conservation. Curr. Zool 2017, 63, 185–194, DOI: 10.1093/cz/zox010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruffin JB Functional testing for behavioral toxicity: a missing dimension in experimental environmental toxicology. J. Occup. Med 1963, 5, 117–121 [PubMed] [Google Scholar]
- 3.Zenick H; Reiter LW Behavioral Toxicology: An Emerging Discipline Proceedings of the Southwest Psychological Association Annual Meeting; April 30, 1976, Albuquerque, New Mexico; PsycEXTRA Dataset, 1977. DOI: 10.1037/e516362012-001. [DOI] [Google Scholar]
- 4.Dell’Omo G Behavioral Ecotoxicology; John Wiley & Sons, 2002. [Google Scholar]
- 5.Melvin SD; Wilson SP The utility of behavioral studies for aquatic toxicology testing: A meta-analysis. Chemosphere 2013, 93, 2217–2223, DOI: 10.1016/j.chemosphere.2013.07.036 [DOI] [PubMed] [Google Scholar]
- 6.Pyle G; Ford AT Behavior revised: Contaminant effects on aquatic animal behavior. Aquat. Toxicol 2017, 182, 226–228, DOI: 10.1016/j.aquatox.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 7.Ågerstrand M; Arnold K; Balshine S; Brodin T; Brooks BW; Maack G; McCallum ES; Pyle G; Saaristo M; Ford AT Emerging investigator series: use of behavioral endpoints in the regulation of chemicals. Environ. Sci. Process. Impacts 2020, 22, 49–65, DOI: 10.1039/C9EM00463G [DOI] [PubMed] [Google Scholar]
- 8.Tyler CR; Allan AM The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr. Environ. Health Rep 2014, 1, 132–147, DOI: 10.1007/s40572-014-0012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders AP; Claus Henn B; Wright RO Perinatal and Childhood Exposure to Cadmium, Manganese, and Metal Mixtures and Effects on Cognition and Behavior: A Review of Recent Literature. Curr. Environ. Health Rep 2015, 2, 284–294, DOI: 10.1007/s40572-015-0058-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss B The scope and promise of behavioral toxicology. in Behavioral measures of neurotoxicity Report of a Symposium; National Academy Press: Washington, DC, 1990; pp 395–413. [Google Scholar]
- 11.Niankara I; Zoungrana DT Interest in the biosphere and students environmental awareness and optimizm: A global perspective. Global Ecology and Conservation 2018, 16, e00489 DOI: 10.1016/j.gecco.2018.e00489 [DOI] [Google Scholar]
- 12.Benson SM; Talbott EO; Brink LL; Wu C; Sharma RK; Marsh GM Environmental lead and childhood blood lead levels in US children: NHANES, 1999–2006. Arch. Environ. Occup. Health 2017, 72 (2), 70–78, DOI: 10.1080/19338244.2016.1157454 [DOI] [PubMed] [Google Scholar]
- 13.Martin TL; Solbeck PA; Mayers DJ; Langille RM; Buczek Y; Pelletier MR A review of alcohol-impaired driving: the role of blood alcohol concentration and complexity of the driving task. J. Forensic Sci 2013, 58, 1238–1250, DOI: 10.1111/1556-4029.12227 [DOI] [PubMed] [Google Scholar]
- 14.OECD Guidelines for the Testing of Chemicals, Section 4 Test No. 426: Developmental Neurotoxicity Study; OECD Publishing, 2007. [Google Scholar]
- 15.OECD Guidelines for the Testing of Chemicals, Section 4 Test No. 424: Neurotoxicity Study in Rodents; OECD Publishing, 1997. [Google Scholar]
- 16.OECD Guidelines for the Testing of Chemicals, Section 4 Test No. 443: Extended One-Generation Reproductive Toxicity Study; OECD Publishing, 2018. [Google Scholar]
- 17.Legradi JB; Di Paolo C; Kraak MHS; Van der Geest HG; Schymanski EL; Williams AJ; Dingemans MML; Massei R; Brack W; Cousin X; Begout ML An ecotoxicological view on neurotoxicity assessment. Environ. Sci. Eur 2018, 30, 46, DOI: 10.1186/s12302-018-0173-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ECHA. European Chemicals Agency. Guidance on information requirements and chemical safety assessment. Chapter R. 4: Evaluation of available information in 2011 https://echa.europa.eu/documents/10162/13643/information_requirements_r4_en.pdf/d6395ad2-1596-4708-ba86-0136686d205e.
- 19.Klimisch HJ; Andreae M; Tillmann U A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul. Toxicol. Pharmacol 1997, 25, 1–5, DOI: 10.1006/rtph.1996.1076 [DOI] [PubMed] [Google Scholar]
- 20.Sollmann T The effects of a series of poisons on adult and embryonic Funduli. Am. J. Physiol 1906, 16, 1–46, DOI: 10.1152/ajplegacy.1906.16.1.1 [DOI] [Google Scholar]
- 21.Shelford VE An experimental study of the effects of gas waste upon fishes with especial reference to stream pollution. Illinois Natural History Survey Bulletin 1918, 11 (4), 381, DOI: 10.21900/j.inhs.v11.363 [DOI] [Google Scholar]
- 22.Saaristo M; Brodin T; Balshine S; Bertram MG; Brooks BW; Ehlman SM; McCallum ES; Sih A; Sundin J; Wong BB; Arnold KE Direct and indirect effects of chemical contaminants on the behavior, ecology and evolution of wildlife. Proc. Biol. Sci 2018, 285. [DOI] [PMC free article] [PubMed]
- 23.Scott GR; Sloman KA The effects of environmental pollutants on complex fish behavior: integrating behavioral and physiological indicators of toxicity. Aquat. Toxicol 2004, 68, 369–392, DOI: 10.1016/j.aquatox.2004.03.016 [DOI] [PubMed] [Google Scholar]
- 24.Boyd WA; Brewer SK; Williams PL Altered behavior of invertebrates living in polluted environments. Behavioral Ecotoxicology 2002, 293–336
- 25.Zala SM; Penn DJ Abnormal behaviors induced by chemical pollution: a review of the evidence and new challenges. Anim. Behav 2004, 68, 649–664, DOI: 10.1016/j.anbehav.2004.01.005 [DOI] [Google Scholar]
- 26.Bruni G; Lakhani P; Kokel D Discovering novel neuroactive drugs through high-throughput behavior-based chemical screening in the zebrafish. Front. Pharmacol 2014, 5, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calderón-Garcidueñas L; Torres-Jardón R; Kulesza RJ; Park S-B; D’Angiulli A Air pollution and detrimental effects on children’s brain. Need for a multidisciplinary approach to the issue complexity and challenges. Front. Hum. Neurosci 2014, 8, 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu JG; Lee JJ; Gino F; Galinsky AD Polluted Morality: Air Pollution Predicts Criminal Activity and Unethical Behavior. Psychol. Sci 2018, 29, 340–355, DOI: 10.1177/0956797617735807 [DOI] [PubMed] [Google Scholar]
- 29.Grandjean P; Landrigan PJ Neurobehavioral effects of developmental toxicity. Lancet Neurol 2014, 13, 330–338, DOI: 10.1016/S1474-4422(13)70278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett D; Bellinger DC; Birnbaum LS; Dabt ATS; Bradman A; Chen A; Cory-Slechta DA; Engel SM; Fallin MD; Halladay A Project TENDR: Targeting Environmental Neuro-Developmental Risks The TENDR Consensus Statement. Environ. Health Perspect 2016, 124, A118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans GW Projected Behavioral Impacts of Global Climate Change. Annu. Rev. Psychol 2019, 70, 449–474, DOI: 10.1146/annurev-psych-010418-103023 [DOI] [PubMed] [Google Scholar]
- 32.Candolin U; Wong BBM Mate choice in a polluted world: consequences for individuals, populations and communities. Philos. Trans. R. Soc., B 2019, 374, 20180055, DOI: 10.1098/rstb.2018.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jennions MD; Petrie M Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. Camb. Philos. Soc 1997, 72, 283–327, DOI: 10.1111/j.1469-185X.1997.tb00015.x [DOI] [PubMed] [Google Scholar]
- 34.Sih A The Behavioral Response Race Between Predator and Prey. Am. Nat 1984, 123, 143–150, DOI: 10.1086/284193 [DOI] [Google Scholar]
- 35.Sih A; Englund G; Wooster D Emergent impacts of multiple predators on prey. Trends Ecol. Evol 1998, 13, 350–355, DOI: 10.1016/S0169-5347(98)01437-2 [DOI] [PubMed] [Google Scholar]
- 36.Haldane J The Causes of Evolution; Harper’s’s, New York, 1932. [Google Scholar]
- 37.Kokko H; López-Sepulcre A The ecogenetic link between demography and evolution: can we bridge the gap between theory and data?. Ecol. Lett 2007, 10, 773–782, DOI: 10.1111/j.1461-0248.2007.01086.x [DOI] [PubMed] [Google Scholar]
- 38.Robert K; Garant D; Pelletier F Keep in touch: does spatial overlap correlate with contact rate frequency?. J. Wildl. Manage 2012, 76, 1670–1675, DOI: 10.1002/jwmg.435 [DOI] [Google Scholar]
- 39.Hoover SER; Tylianakis JM Species interactions. Behavioral Responses to a Changing World 2012, 129–142, DOI: 10.1093/acprof:osobl/9780199602568.003.0010 [DOI]
- 40.Sih A Understanding variation in behavioral responses to human-induced rapid environmental change: a conceptual overview. Anim. Behav 2013, 85, 1077–1088, DOI: 10.1016/j.anbehav.2013.02.017 [DOI] [Google Scholar]
- 41.Wong BBM; Candolin U Behavioral responses to changing environments. Behav. Ecol 2015, 26, 665–673, DOI: 10.1093/beheco/aru183 [DOI] [Google Scholar]
- 42.Janetski DJ; Chaloner DT; Tiegs SD; Lamberti GA Pacific salmon effects on stream ecosystems: a quantitative synthesis. Oecologia 2009, 159, 583–595, DOI: 10.1007/s00442-008-1249-x [DOI] [PubMed] [Google Scholar]
- 43.Estes JA; Tinker MT; Williams TM; Doak DF Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 1998, 282, 473–476, DOI: 10.1126/science.282.5388.473 [DOI] [PubMed] [Google Scholar]
- 44.Eriksson BK; Ljunggren L; Sandström A; Johansson G; Mattila J; Rubach A; Råberg S; Snickars M Declines in predatory fish promote bloom-forming macroalgae. Ecol. Appl 2009, 19, 1975–1988, DOI: 10.1890/08-0964.1 [DOI] [PubMed] [Google Scholar]
- 45.Traveset A; Heleno R; Chamorro S; Vargas P; McMullen CK; Castro-Urgal R; Nogales M; Herrera HW; Olesen JM Invaders of pollination networks in the Galápagos Islands: emergence of novel communities. Proc. R. Soc. London, Ser. B 2013, 280, 20123040, DOI: 10.1098/rspb.2012.3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell DLM; Weiner SA; Starks PT; Hauber ME Context and Control: Behavioral Ecology Experiments in the Laboratory. Ann. Zool. Fenn 2009, 46, 112–123, DOI: 10.5735/086.046.0204 [DOI] [Google Scholar]
- 47.Boon AK; Réale D; Boutin S Personality, habitat use, and their consequences for survival in North American red squirrels Tamiasciurus hudsonicus. Oikos 2008, 117, 1321–1328, DOI: 10.1111/j.0030-1299.2008.16567.x [DOI] [Google Scholar]
- 48.Fisher DN; James A; Rodríguez-Muñoz R; Tregenza T Behavior in captivity predicts some aspects of natural behavior, but not others, in a wild cricket population. Proc. Biol. Sci 2015, 282, 20150708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fraser DF; Gilliam JF; Daley MJ; Le AN; Skalski GT Explaining Leptokurtic Movement Distributions: Intrapopulation Variation in Boldness and Exploration. Am. Nat 2001, 158, 124, DOI: 10.1086/321307 [DOI] [PubMed] [Google Scholar]
- 50.Yuen CH; Pillay N; Heinrichs M; Schoepf I; Schradin C Personality traits are consistent when measured in the field and in the laboratory in African striped mice (Rhabdomys pumilio). Behavioral Ecology and Sociobiology 2016, 70, 1235–1246, DOI: 10.1007/s00265-016-2131-1 [DOI] [Google Scholar]
- 51.Herborn KA; Macleod R; Miles WT; Schofield AN; Alexander L; Arnold KE Personality in captivity reflects personality in the wild. Anim. Behav 2010, 79, 835–843, DOI: 10.1016/j.anbehav.2009.12.026 [DOI] [Google Scholar]
- 52.Moiron M; Laskowski KL; Niemelä PT Individual differences in behavior explain variation in survival: a meta-analysis. Ecol. Lett 2020, 23, 399–408, DOI: 10.1111/ele.13438 [DOI] [PubMed] [Google Scholar]
- 53.Dingemanse NJ; Both C; Drent PJ; Tinbergen JM Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. London, Ser. B 2004, 271, 847–852, DOI: 10.1098/rspb.2004.2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Houde AE Mate choice based upon naturally occurring color-pattern variation in a guppy population. Evolution 1987, 41, 1–10, DOI: 10.1111/j.1558-5646.1987.tb05766.x [DOI] [PubMed] [Google Scholar]
- 55.Reznick DA; Bryga H; Endler JA Experimentally induced life-history evolution in a natural population. Nature 1990, 346, 357–359, DOI: 10.1038/346357a0 [DOI] [Google Scholar]
- 56.Brodin T; Piovano S; Fick J; Klaminder J; Heynen M; Jonsson M Ecological effects of pharmaceuticals in aquatic systems—impacts through behavioral alterations. Philos. Trans. R. Soc., B 2014, 369, 20130580, DOI: 10.1098/rstb.2013.0580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aulsebrook LC; Bertram MG; Martin JM; Aulsebrook AE; Brodin T; Evans JP; Hall MD; O’Bryan MK; Pask AJ; Tyler CR; Wong BB Reproduction in a polluted world: implications for wildlife. Reproduction 2020, 160, R13–R23, DOI: 10.1530/REP-20-0154 [DOI] [PubMed] [Google Scholar]
- 58.Klaminder J; Hellström G; Fahlman J; Jonsson M; Fick J; Lagesson A; Bergman E; Brodin T Drug-Induced Behavioral Changes: Using Laboratory Observations to Predict Field Observations. Front. Environ. Sci. Eng. China 2016, 4, 81 [Google Scholar]
- 59.Hellström G; Klaminder J; Finn F; Persson L; Alanärä A; Jonsson M; Fick J; Brodin T GABAergic anxiolytic drug in water increases migration behaviour in salmon. Nat. Commun 2016, 7, 13460–389, DOI: 10.1038/ncomms13460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Godfray HCJ; Blacquiere T; Field LM; Hails RS; Petrokofsky G; Potts SG; Raine NE; Vanbergen AJ; McLean AR A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. Biol. Sci 2014, 281. [DOI] [PMC free article] [PubMed]
- 61.Sanchez-Bayo F; Goka K Pesticide residues and bees--a risk assessment. PLoS One 2014, 9, e94482 DOI: 10.1371/journal.pone.0094482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goulson D; Nicholls E; Botías C; Rotheray EL Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957, DOI: 10.1126/science.1255957 [DOI] [PubMed] [Google Scholar]
- 63.Henry M; Cerrutti N; Aupinel P; Decourtye A; Gayrard M; Odoux JF; Pissard A; Rüger C; Bretagnolle V Reconciling laboratory and field assessments of neonicotinoid toxicity to honeybees. Proc. Biol. Sci 2015, 282. [DOI] [PMC free article] [PubMed]
- 64.Ankley GT; Bennett RS; Erickson RJ; Hoff DJ; Hornung MW; Johnson RD; Mount DR; Nichols JW; Russom CL; Schmieder PK; Serrrano JA; Tietge JE; Villeneuve DL Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem 2010, 29, 730–741, DOI: 10.1002/etc.34 [DOI] [PubMed] [Google Scholar]
- 65.OECD Guidelines for the Testing of Chemicals, Section 2 Test No. 222: Earthworm Reproduction Test (Eisenia fetida/Eisenia andrei); OECD Publishing, 2016. [Google Scholar]
- 66.OECD Guidelines for the Testing of Chemicals, Section 3 Test No. 305: Bioaccumulation in Fish: Aqueous and Dietary Exposure; OECD Publishing, 2012. [Google Scholar]
- 67.Makris SL; Raffaele K; Allen S; Bowers WJ; Hass U; Alleva E; Calamandrei G; Sheets L; Amcoff P; Delrue N; Crofton KM A retrospective performance assessment of the developmental neurotoxicity study in support of OECD test guideline 426. Environ. Health Perspect 2009, 117, 17–25, DOI: 10.1289/ehp.11447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moermond CTA; Kase R; Korkaric M; Ågerstrand M CRED: Criteria for reporting and evaluating ecotoxicity data. Environ. Toxicol. Chem 2016, 35, 1297–1309, DOI: 10.1002/etc.3259 [DOI] [PubMed] [Google Scholar]
- 69.OECD Guidelines for the Testing of Chemicals, Section 2 Test No. 231: Amphibian Metamorphosis Assay; OECD Publishing, 2009. [Google Scholar]
- 70.Test No. 203: Fish, Acute Toxicity Test. OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing, 2019. DOI: 10.1787/9789264069961-en. [DOI] [Google Scholar]
- 71.OECD Guidelines for the Testing of Chemicals, Section 2 Test No. 215: Fish, Juvenile Growth Test; OECD Publishing, 2000. [Google Scholar]
- 72.Test No. 229: Fish Short Term Reproduction Assay; OECD Publishing, 2009. [Google Scholar]
- 73.OECD Guidelines for the Testing of Chemicals, Section 2: Effects on Biotic Systems Test No. 230: 21-day Fish Assay A Short-Term Screening for Oestrogenic and Androgenic Activity, and Aromatase Inhibition: A Short-Term Screening for Oestrogenic and Androgenic Activity, and Aromatase Inhibition; OECD Publishing, 2009. [Google Scholar]
- 74.OECD Guidelines for the Testing of Chemicals, Section 2 Test No. 203: Fish, Acute Toxicity Test; OECD Publishing, 2019. [Google Scholar]
- 75.OECD Guidelines for the Testing of Chemicals, Section 2 Test No. 210: Fish, Early-life Stage Toxicity Test; OECD Publishing, 2013. [Google Scholar]
- 76.Melvin SD Effect of antidepressants on circadian rhythms in fish: Insights and implications regarding the design of behavioral toxicity tests. Aquat. Toxicol 2017, 182, 20–30, DOI: 10.1016/j.aquatox.2016.11.007 [DOI] [PubMed] [Google Scholar]


