Abstract
Background and Objectives
To determine how fully automated Elecsys CSF immunoassays for β-amyloid (Aβ) and tau biomarkers and an ultrasensitive Simoa assay for neurofilament light chain (NFL) correlate with neuropathologic changes of Alzheimer disease (AD) and frontotemporal lobar degeneration (FTLD).
Methods
We studied 101 patients with antemortem CSF and neuropathology data. CSF samples were collected a mean of 2.9 years before death (range 0.2–7.5 years). CSF was analyzed for Aβ40, Aβ42, total tau (T-tau), tau phosphorylated at amino acid residue 181 (P-tau), P-tau/Aβ42 and Aβ42/Aβ40 ratios, and NFL. Neuropathology measures included Thal phases, Braak stages, Consortium to Establish a Registry for Alzheimer's Disease (CERAD) scores, AD neuropathologic change (ADNC), and primary and contributory pathologic diagnoses. Associations between CSF biomarkers and neuropathologic features were tested in regression models adjusted for age, sex, and time from sampling to death.
Results
CSF biomarkers were associated with neuropathologic measures of Aβ (Thal, CERAD score), tau (Braak stage), and overall ADNC. The CSF P-tau/Aβ42 and Aβ42/Aβ40 ratios had high sensitivity, specificity, and overall diagnostic performance for intermediate-high ADNC (area under the curve range 0.95–0.96). Distinct biomarker patterns were seen in different FTLD subtypes, with increased NFL and reduced P-tau/T-tau in FTLD–TAR DNA-binding protein 43 and reduced T-tau in progressive supranuclear palsy compared to other FTLD variants.
Discussion
CSF biomarkers, including P-tau, T-tau, Aβ42, Aβ40, and NFL, support in vivo identification of AD neuropathology and correlate with FTLD neuropathology.
Classification of Evidence
This study provides Class II evidence that distinct CSF biomarker patterns, including for P-tau, T-tau, Aβ42, Aβ40, and NFL, are associated with AD and FTLD neuropathology.
Alzheimer disease (AD) and frontotemporal lobar degeneration (FTLD) are common causes of dementia. AD is characterized by β-amyloid (Aβ) and tau aggregation,1 while FTLD is most often associated with tau (FTLD-tau) or TAR DNA-binding protein 43 (TDP-43) (FTLD-TDP) aggregation. Clinical presentations have varying correlations with neuropathology in AD and FTLD.2,3 Biomarkers are therefore needed to assist in diagnosis and to accelerate drug development.
PET and CSF biomarkers are available for neurodegenerative diseases.4 CSF biomarkers include Aβ42 and Aβ40, total tau (T-tau), phosphorylated tau (P-tau), and neurofilament light chain (NFL). PET for Aβ and tau has been validated with neuropathology,5,6 but few studies have reported such validation for CSF AD biomarkers and mainly used older assays.7,8 CSF studies in FTLD focused on differentiation vs AD9-12 or differences between FTLD variants.13-17 Postmortem FTLD studies are rare, with some but not all15 finding reduced P-tau14,16 or P-tau/T-tau17,18 in FTLD-TDP compared to FTLD-tau.
Older CSF assays were hampered by variability, but this has been overcome with novel, fully automated assays.19 We tested associations between Elecsys Aβ42, Aβ40, T-tau and P-tau, and NFL (using Simoa) assays and neuropathology. Our primary research question was whether distinct CSF biomarker patterns, including for P-tau, T-tau, Aβ42, Aβ40, and NFL, were associated with AD and FTLD neuropathology. We hypothesized that AD neuropathologic changes (ADNC) would be associated with reduced Aβ42/Aβ40 and increased P-tau/Aβ42 also when coexisting with other pathologies. We also expected to find reduced tau markers in progressive supranuclear palsy (PSP; a variant of FTLD-tau)13,20 and lower P-tau/T-tau in FTLD-TDP.14,16,17
Methods
Study Group
Patients were recruited from the University of California San Francisco (UCSF) Memory and Aging Center's Alzheimer's Disease Research Center. They met diagnostic criteria for different FTLD syndromes, including behavioral variant frontotemporal dementia (bvFTD),21 corticobasal syndrome (CBS),22 nonfluent variant primary progressive aphasia,23 PSP,24 semantic variant primary progressive aphasia,23 or frontotemporal dementia (FTD)–amyotrophic lateral sclerosis,25 or had probable AD-type dementia.26 Five patients did not meet any research diagnostic criteria (but had syndromes considered most compatible with AD, bvFTD, or PSP). All participants underwent a medical history and physical examination, a structured caregiver interview, lumbar puncture (LP), and neuropsychological testing. We did not have a criterion for maximum time difference between LP and death (mean time 2.9 years [SD 1.8 years, range 0.2–7.5 years]). We included all eligible study participants.
Standard Protocol Approvals, Registrations, and Patient Consents
Written informed consent was obtained from all participants or their assigned surrogate decision makers. The UCSF institutional review board for human research approved the study.
CSF Biomarkers and Cognitive Testing
CSF was obtained following protocols from Alzheimer's Disease Neuroimaging Initiative.27 In short, CSF was sampled in the morning after an overnight fast with a 20- or 24-gauge spinal needle. Samples were transferred from collection tubes into polypropylene tubes and frozen within 1 hour of sampling. Samples were shipped frozen to Lund University and Skåne University Hospital, where they were analyzed for Elecsys (Roche Diagnostics International Ltd, Rotkreuz, Switzerland) CSF AD biomarkers19 and NFL was analyzed by a Simoa method (NF-light Simoa Assay Advantage Kit; Quanterix Inc, Billerica, MA,). Aβ42 and Aβ40 have been associated with Aβ pathology; T-tau has been associated with axonal injury; P-tau has been associated with tau pathology in AD; and NFL has been associated with injury preferentially in large-diameter myelinated axons.28 Cognitive testing with Mini-Mental State Examination (testing overall cognitive status29), Trail Making Test Part B (testing speed of processing and executive function30), and California Verbal Learning Test-II (an episodic memory test31) was done at a median of 2 (interquartile range 1–6) days before LP.
Neuropathology
Comprehensive neuropathologic assessments were performed by investigators at the UCSF Neurodegenerative Disease Brain Bank who were blinded to CSF results, following previously described procedures.32 Classifications of AD,33 FTLD-TDP, and FTLD-tau (including PSP and corticobasal degeneration [CBD]) followed standard neuropathologic criteria.33-35 For each patient, a main (primary) pathology was defined as the pathology that was most likely to explain the clinical syndrome on the basis of its anatomic location and degree of pathologic change. For each patient, other (contributing) pathologies were also defined as the pathologic changes that could explain some of the symptoms in addition to the primary pathology. We also determined the AD Thal amyloid phase,36 indicating topographic extent of Aβ plaque pathology; Braak neurofibrillary tangle stage,37 indicating the topographic extent of tau neurofibrillary pathology; and Consortium to Establish a Registry for Alzheimer's Disease (CERAD) score,38 indicating the density of neocortical neuritic plaques. Thal phase, Braak stage, and CERAD score were aggregated in the ADNC score.32 ADNC has 4 levels: none, low, intermediate, and high. One of our main aims was to test associations between CSF biomarkers and AD pathology. To avoid bias from subjective interpretations, we used intermediate to high ADNC as an indicator of AD pathology39 independently of whether the neuropathologists had identified AD as a primary or contributory pathology to the patient's clinical syndrome (4 patients who presented clinically with PSP, CBS, or bvFTD had intermediate ADNC, although AD changes were not considered primary or contributing).
Statistics
Linear regression models adjusted for age, sex, and time between LP and death were used to test associations between biomarkers (as outcomes) and neuropathologic features (as categorical predictors). The overall effect of each feature (across all levels, e.g., Thal phases 0–5) on biomarkers was tested by likelihood ratio tests comparing nested models with and without neuropathologic score as a predictor. Associations between dichotomous ADNC (none-low vs intermediate-high) and dichotomous biomarker status were tested with the Fisher exact test. Receiver operating characteristics (ROC) analyses were used to test diagnostic performance (by area under the ROC curve, AUC) of different biomarkers for dichotomous ADNC. This used predictions from logistic regression models with ADNC as the outcome and biomarkers as predictors, with CIs generated by a bootstrap procedure, using the pROC package.40 An internal 10-fold cross-validation of AUC estimates and CIs was done using influence curves with the cvAUC package.41 Sensitivity and specificity were tested using a priori cut points. When indicated, we used Akaike information criterion (AIC) for model selection (a difference of >2 favors the model with smaller AIC). Sensitivity analyses were done on subsets of participants. Values of p were considered significant at p < 0.05, 2 tailed. When mentioned, multiplicity correction was done with the Bonferroni method. Statistical analyses were done in R (version 4.0.2, R Foundation for Statistical Computing, Vienna, Austria).
Data Availability
Per the UCSF Memory and Aging Center data-sharing policy, data can be made available on reasonable request via our online portal.42
Results
Group characteristics by primary neuropathologic diagnoses (AD, FTLD-tau [CBD], FTLD-tau [PSP], FTLD-TDP, and others) are shown in Table 1. Frequencies of copathologies are shown in Table 1 (by primary neuropathology) and eTable 1, links.lww.com/WNL/B777 (by clinical diagnosis). Only 3 of 15 patients with a clinical AD diagnosis had a non-AD primary pathologic diagnosis, and 2 of these 3 had AD as a contributing diagnosis (eTable 1). Conversely, AD was the primary underlying pathology in only 4 of 85 with a non-AD diagnosis. Seventy-five patients had a clinical diagnosis of an FTD syndrome (PSP–Richardson syndrome, CBS, bvFTD, semantic variant primary progressive aphasia, nonfluent variant primary progressive aphasia, or amyotrophic lateral sclerosis [most often together with bvFTD]), and all but 3 of these had an FTLD neuropathologic variant as the primary pathologic diagnosis. Most heterogeneity in underlying primary neuropathology was seen in CBS; 10 patients had FTLD-tau (CBD), 8 had FTLD-tau (PSP), 1 had FTLD-tau (Pick disease), 1 had FTLD-TDP, and 1 lacked evidence of neurodegeneration. eFigure 1 shows CSF biomarkers by clinical diagnosis.
Table 1.
Demographics by Primary Neuropathologic Diagnosis
Frequencies of AD-related neuropathologic scores, including Thal phase, Braak stage, CERAD score, and ADNC, are shown in eFigure 2, links.lww.com/WNL/B777. Most patients had either CERAD scores of none or frequent, with few having intermediate CERAD levels. Among patients with a CERAD score of none, most had Braak stage 0 to II and Thal phase 0 to 2. Among patients with a CERAD score of frequent, most had Braak stage IV to VI and Thal phase 4 to 5.
CSF Biomarkers by ADNC Class
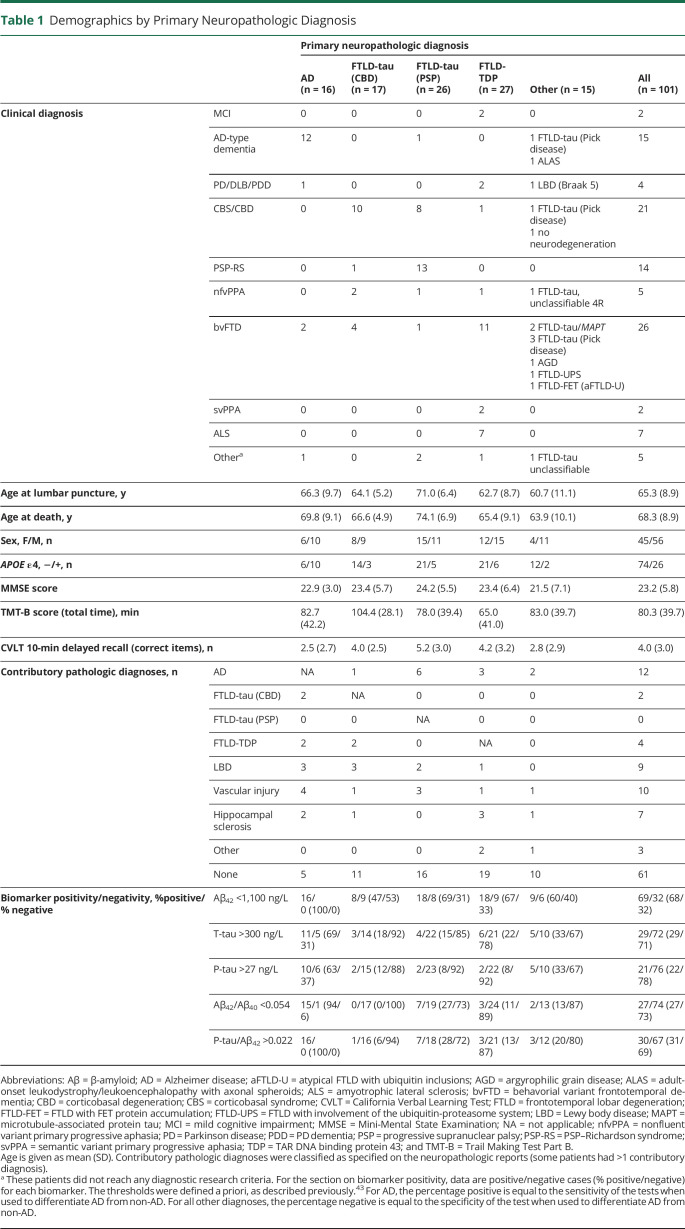
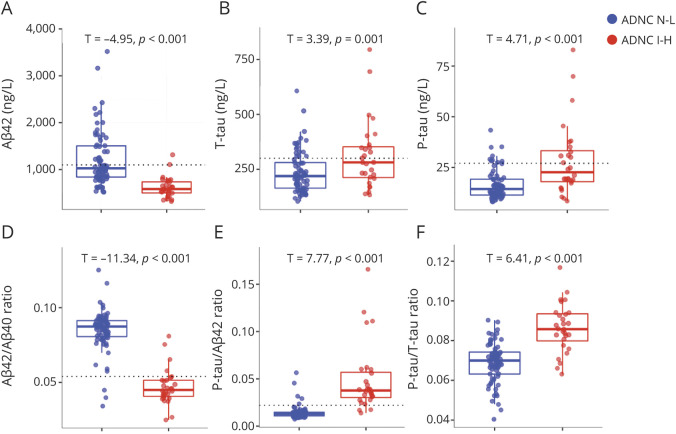
We tested how well biomarkers differentiated between ADNC none-low (n = 70) vs ADNC intermediate-high (n = 28, ADNC data were missing in 3 patients due to missing CERAD score [n = 1] and/or Thal phase [n = 2] data). These comparisons were done regardless of other pathologies, but other pathologies were commonly reported as either primary or contributory in both ADNC none-low and ADNC intermediate-high (eTable 2, links.lww.com/WNL/B777). The ADNC intermediate-high group had significantly lower Aβ42 and Aβ42/Aβ40 and higher T-tau, P-tau, P-tau/Aβ42, and P-tau/T-tau compared to the ADNC none-low group (Figure 1). There were no differences in Aβ40 or NFL (note that all participants had cognitive impairment) (eFigure 3). We also tested the performance of the biomarkers in detecting ADNC intermediate-high in ROC analyses (Figure 2). The best individual biomarker was Aβ42 (AUC 0.886). Both P-tau/Aβ42 and Aβ42/Aβ40 ratios had very high performance (AUC 0.953–0.956) for separating ADNC none-low from ADNC intermediate-high. An internal 10-fold cross-validation of the estimates gave very similar results (eTable 3).
Figure 1. CSF Biomarkers by ADNC N-L vs I-H.
(A–F) Biomarkers are shown as unadjusted raw data in the groups of Alzheimer disease neuropathologic change (ADNC) none-low (N-L; blue) and intermediate-high (I-H; red). T values and p values are shown for group differences, adjusted for age, sex, and lag between lumbar puncture and death. Reference lines are shown for a priori cut points for β-amyloid (Aβ)42, total tau (T-tau), phosphorylated tau (P-tau), Aβ42/Aβ40, and P-tau/Aβ42, as defined previously.43 Aβ40 and neurofilament light biomarkers are shown in eFigure 3, links.lww.com/WNL/B777.
Figure 2. CSF Biomarkers for ADNC Classification.
Performance of logistic regression models for individual biomarkers (A) and biomarker ratios (B), to distinguish between Alzheimer disease neuropathologic change (ADNC) none-low (N-L) and ADNC intermediate-high (I-H). Legends show overall area under the receiver operating curve characteristics (AUC) with 95% CI from a bootstrap procedure. Aβ = β-amyloid; NFL = neurofilament light; P-tau = phosphorylated tau; T-tau = total tau.
Time between LP and death was a possible confounder for associations between biomarkers and neuropathology (participant-level data on LP-to-death time are included in eFigure 4, links.lww.com/WNL/B777). In a sensitivity analysis, the individuals who were correctly classified by Aβ42 (concordance between predicted and observed ADNC class) had slightly longer lag time compared to those who were misclassified (p = 0.04, Mann-Whitney U test). There were no differences in lag time between correctly classified and misclassified groups for other biomarkers/ratios (p = 0.14–0.89).
An a priori defined cut point (derived in the external BioFINDER cohort, manuscript in preparation) for Aβ42/Aβ40 (<0.054) had high specificity for minimal AD pathology, defined as ADNC none-low (high Aβ42/Aβ40 was seen in 67 of 70 [96%] of patients with ADNC none-low), and high sensitivity for significant AD pathology, defined as ADNC intermediate-high (low Aβ42/Aβ40 was seen in 24 of 28 [86%] of patients with ADNC intermediate-high). Similar results were seen for an a priori defined cut point43 for P-tau/Aβ42 (>0.022) with high specificity (61 of 66 ADNC none-low, 92%) and high sensitivity (25 of 28 ADNC intermediate-high, 89%) for AD neuropathology. There were no differences in time from LP to death between correctly classified and misclassified individuals using the a priori cut points (p = 0.57–0.60). In sensitivity analyses, we tested the a priori cut points in subgroups of participants. In patients with a clinical diagnosis of AD (n = 15), both Aβ42/Aβ40 and P-tau/ Aβ42 were positive in 1 of the 2 patients with ADNC none-low. Aβ42/Aβ40 was positive in 12 of the 13 patients with ADNC intermediate-high, and P-tau/Aβ42 was positive in all 13 patients with ADNC intermediate-high. In patients with a non-AD diagnosis and ADNC data (n = 83, P-tau181 data were missing in 4 of these), Aβ42/Aβ40 was positive in 2 of 68 patients with ADNC none-low (97% specificity), and P-tau/Aβ42 was positive in 4 of 64 patients with ADNC none-low (94% specificity). Both Aβ42/Aβ40 and P-tau/Aβ42 were positive in 12 of 15 patients with ADNC intermediate-high (80% sensitivity).
CSF Biomarkers by Thal Phase, Braak Stage, CERAD Score, and ADNC
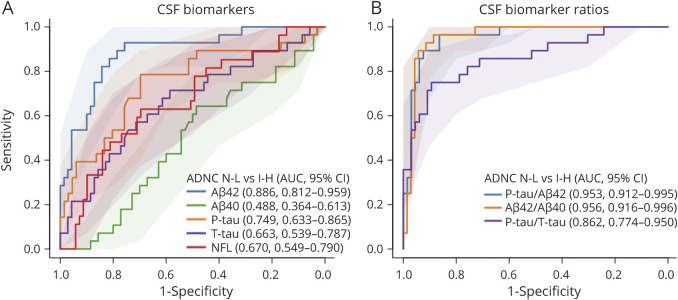
We next tested associations between biomarkers and Thal phase, Braak stage, CERAD score, and all levels of ADNC. Figure 3 shows data for Aβ42/Aβ40 and P-tau/Aβ42 (ADNC data are shown in eFigure 5, links.lww.com/WNL/B777). eFigure 6 (for Thal phase, Braak stage, and CERAD score) and eFigure 7 (for ADNC) show data for Aβ42, Aβ40, P-tau, T-tau, P-tau/T-tau, and NFL. In tests of overall associations (across all levels of the scores), lower levels of Aβ42 and Aβ42/Aβ40 and higher levels of P-tau, T-tau, P-tau/T-tau, and P-tau/Aβ42 were significantly associated with worse pathology. There were no significant associations for Aβ40 or NFL.
Figure 3. CSF Biomarkers by AD Neuropathologic Scores.
Biomarker ratios (β-amyloid [Aβ]42/Aβ40 and phosphorylated tau [P-tau]/Aβ42) are shown as unadjusted raw data by neuropathologic scores for spread of Aβ pathology (Thal phase 0–5), tau pathology (Braak stage 0–VI), and presence/frequency of neuritic plaques (Consortium to Establish a Registry for Alzheimer's Disease [CERAD] score). eFigure 5, links.lww.com/WNL/B777 gives Alzheimer disease (AD) neuropathologic change (ADNC) data. The p values are for the overall associations (from likelihood ratio tests of models with and without including the neuropathologic score as a predictor) between the neuropathologic scores and CSF biomarker levels, adjusted for age, sex, and time between lumbar puncture and death. A reference line is shown for an a priori cut point for Aβ42/Aβ40. Color coding refers to ADNC class (blue = ADNC none-low [N-L], red = ADNC intermediate-high [I-H], gray = missing ADNC data).
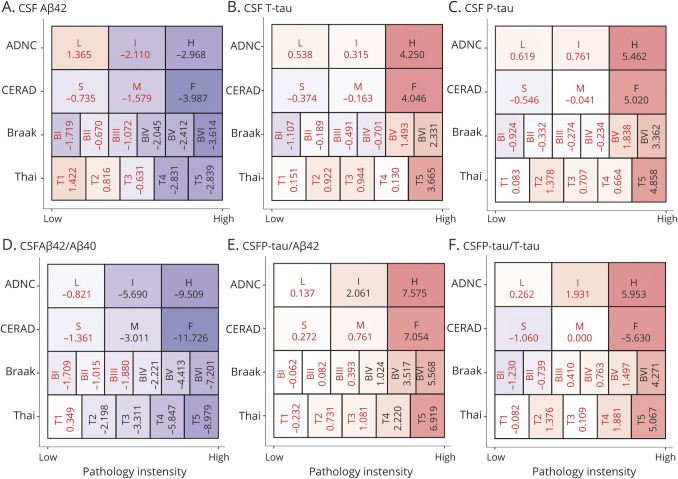
To test the sensitivity of biomarkers for lowest possible level of pathology, we compared biomarkers between every stage of the scores to the respective reference category (i.e., Thal phase 0, Braak stage 0, CERAD score of none, and ADNC none) (Figure 4). These models were corrected for age, sex, and time between LP and death. The first significant reductions in Aβ42 were seen in Thal phase 4, Braak stage IV, CERAD score of frequent, and ADNC intermediate. The first reductions in Aβ42/Aβ40 were seen in Thal phase 2, Braak stage IV, CERAD score of moderate, and ADNC intermediate. The first increases in P-tau/Aβ42 were seen in Thal phase 4, Braak stage V, CERAD score of frequent, and ADNC intermediate. These biomarker changes were always consistent at higher levels of pathology (e.g., Aβ42/Aβ40 remained changed at Thal phase 3–5). Significant increases in P-tau, T-tau, and P-tau/T-tau were seen only at the highest levels of pathology (Thal phase 5, Braak stage VI, CERAD score of frequent, and ADNC high). For Aβ40, there was an increase at Thal phase 2, but this was not seen at greater Thal phases, and there were no significant changes in NFL (eFigure 8, links.lww.com/WNL/B777).
Figure 4. CSF Biomarker Changes at Different Levels of Pathology.
This figure shows how different biomarkers are altered at different levels of pathology compared to the lowest levels of respective pathology. Presented data are T statistics (black = significant at p < 0.05, red = nonsignificant). Box colors are related to the magnitude of the T statistics (red colors = positive, violet = negative). Data are presented for each biomarker (A–F) and the pathologic scores Thal phase (categories range from Thal phase 1–5), Braak stage (categories range from stage I–IV), Consortium to Establish a Registry for Alzheimer's Disease (CERAD) score (sparse [S], medium [M], frequent [F]), and Alzheimer disease neuropathologic change (ADNC) (low [L], intermediate [I], high [H]). For each score, biomarkers were compared between each category and the reference category (Thal phase 0, Braak stage 0, CERAD none, and ADNC no, respectively). The T statistics are from linear regression models, adjusted for age, sex, and time between lumbar puncture and death. For example, CSF β-amyloid (Aβ)42/Aβ40 (D) was significantly reduced at ADNC intermediate, CERAD moderate, Braak stage IV, and Thal phase 2 (and all greater levels of pathology). eFigure 8, links.lww.com/WNL/B777 gives results for Aβ40 and neurofilament light.
We considered the possibility that associations between biomarkers and pathology were driven partly by group differences between AD and FTLD. In a sensitivity analysis, we adjusted all models for AD as a primary pathology. This attenuated associations for Aβ42, P-tau, T-tau, and the P-tau/T-tau ratio but associations for the Aβ42/Aβ40 and P-tau/Aβ42 ratios with neuropathology were robust (eTable 4, links.lww.com/WNL/B777). The lowest pathology levels with significant biomarker changes were slightly attenuated (eFigure 9). Aβ42/Aβ40 was significantly reduced from Thal phase 2, Braak stage V, CERAD score of moderate, and ADNC intermediate, while P-tau/Aβ42 was significantly increased from Thal phase 5, Braak stage V, and CERAD score of frequent and ADNC high.
Although we included time from LP to death as a covariate in the models, we considered the possibility that differences in lag time could still affect the findings. In another sensitivity analysis, we therefore excluded individuals with more than the median lag time (>2.67 years). This did not alter the overall associations between biomarkers and pathology (eTable 4, links.lww.com/WNL/B777). There was no indication that the biomarkers had greater sensitivity for lower grade of pathology in the subset with short time from LP to death (eFigure 10). We also evaluated the interaction between pathology and time from LP to death to predict CSF biomarkers, which was generally not significant (eFigure 11).
We also considered education and level of cognitive impairment as possible confounders (although they were not significantly associated with the biomarkers). We refit the models when also adjusting for years of education and baseline Mini-Mental State Examination score. This did not affect associations between biomarkers and pathology (eTable 4, links.lww.com/WNL/B777).
CSF Biomarkers by Combinations of Thal Phase, Braak Stage, and CERAD Score
To clarify whether biomarkers depended on 1 or several of Thal phase, Braak stage, and CERAD, we compared models with different sets of predictors of CSF biomarkers: a basic model using only age, sex, and time between LP and death (these were also included as covariates in all models below); only Thal phase; only Braak stage; only CERAD score; and combinations of 2 or 3 pathologic features. Models were compared in terms of AIC and R2 (eTable 5, links.lww.com/WNL/B777). For Aβ40 and NFL, no pathology model was better than the basic model. The Thal phase–only model was preferred for Aβ42 (R2 = 0.21, ΔAIC = −18.1 compared to the basic model). The Thal phase and CERAD model was preferred for Aβ42/Aβ40 and P-tau/Aβ42 (R2 = 0.33, ΔAIC = −27.9; R2 = 0.24, ΔAIC = −16.3, respectively). The Braak stage–only model was preferred for P-tau/T-tau (R2 = 0.41, ΔAIC = −34.2).
CSF Biomarkers by Primary Neuropathologic Diagnosis
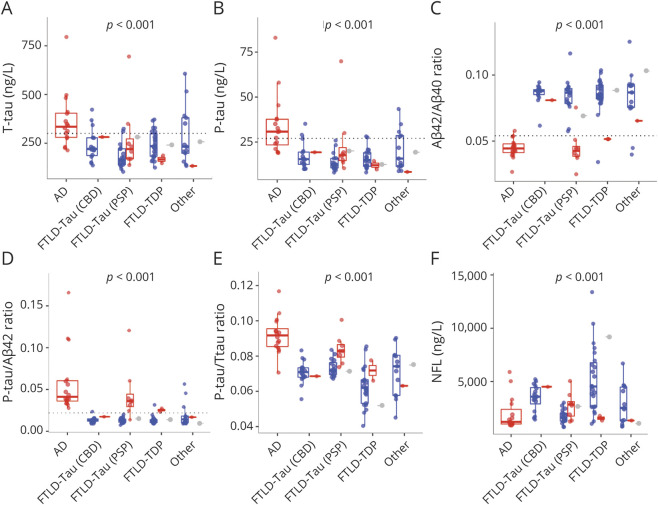
We tested associations between primary neuropathologic diagnosis and biomarkers. All biomarkers except Aβ40 differed across groups (Figure 5 and eFigure 12, links.lww.com/WNL/B777). We also compared biomarkers pairwise between groups (adjusted for multiple comparisons with the Bonferroni method for 10 comparisons). These comparisons are summarized in eTable 6. AD had significantly lower Aβ42/Aβ40 ratio and higher P-tau, P-tau/Aβ42, and P-tau/T-tau than all other groups. AD also had lower Aβ42 than FTLD-tau (CBD), FTLD-TDP, and other and higher T-tau than FTLD-tau (CBD), FTLD-tau (PSP), and FTLD-TDP. Patients with FTLD-tau (PSP) had higher P-tau/T-tau than patients with FTLD-TDP. Patients with FTLD-TDP had higher NFL than those with AD, FTLD-tau (PSP), and other.
Figure 5. CSF Biomarkers by Primary Pathologic Diagnosis.
Biomarkers (A–F) are shown as unadjusted raw data by primary neuropathologic diagnosis. The p values are shown for overall significance of neuropathologic diagnosis, adjusted for age, sex, and time between lumbar puncture and death. eFigure 12, links.lww.com/WNL/B777 gives results for β-amyloid (Aβ)40 and Aβ42. eTable 6 gives pairwise comparisons between different diagnoses. Reference lines are shown for a priori cut points for total tau (T-tau), phosphorylated tau (P-tau181), Aβ42/Aβ40, and P-tau/Aβ42, as defined previously.43 Color coding refers to Alzheimer disease neuropathologic change (ADNC) class (blue = ADNC none-low, red = ADNC intermediate-high, gray = missing ADNC data). AD = Alzheimer disease; CBD = corticobasal degeneration; FTLD = frontotemporal lobar degeneration; PSP = progressive supranuclear palsy; TDP = TAR DNA binding protein 43.
Twelve patients without AD (1 FTLD-tau [CBD], 8 FTLD-tau [PSP], 2 FTLD-TDP, and 1 other) had intermediate-high ADNC (shown in red in Figure 5). When excluding these patients and repeating the pairwise comparisons (eTable 6), we find that patients with FTLD-TDP had higher NFL than FTLD-tau (CBD), in addition to the other groups. The difference in P-tau/T-tau between FTLD-tau (PSP) and FTLD-TDP was attenuated and no longer significant after correction for multiple comparisons (uncorrected p = 0.0065).
A Priori Cut Points for CSF Biomarkers and Primary Neuropathologic Diagnosis
We evaluated a priori cut points for Aβ42, P-tau, T-tau, Aβ42/Aβ40, and P-tau/Aβ42 for primary neuropathologic diagnosis in terms of sensitivity for AD and specificity for non-AD. Aβ42, Aβ42/Aβ40, and P-tau/Aβ42 had 94% to 100% sensitivity for AD (Table 1). Overall, for non-AD, Aβ42 had 38% specificity, T-tau had 79% specificity, P-tau and Aβ42/Aβ40 had 86% specificity, and P-tau/Aβ42 had 83% specificity (Table 1 shows details by individual variants).
For patients without AD, we tested whether biomarker status (positive or negative) varied with comorbid ADNC (none-low vs intermediate-high). These analyses showed that among patients with diseases other than AD as primary pathology (non-AD), positive Aβ42/Aβ40 or P-tau/Aβ42 biomarker ratios (but not individual biomarkers) were significantly associated with AD copathology (eTable 7, links.lww.com/WNL/B777).
CSF Biomarkers by Primary and Contributory Neuropathologic Diagnosis
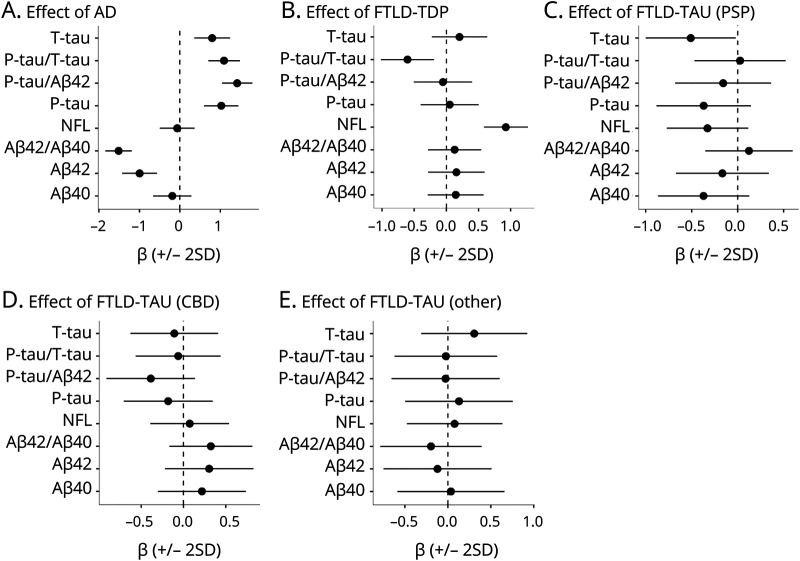
Because the designation of primary neuropathologic diagnosis was not blinded to the clinical syndrome, we also classified each patient as positive or negative for AD (n = 28 positive), FTLD-TDP (n = 32 positive), FTLD-tau (PSP) (n = 26 positive), FTLD-tau (CBD) (n = 19 positive), or FTLD-tau (other) (n = 12 positive) independently of whether the neuropathologic diagnosis was called primary or contributory (meaning that a patient could be positive for >1 of these). Associations were tested between these classifications and biomarkers (Figure 6). AD neuropathologic diagnosis was associated with significantly altered levels (in the expected directions) of all biomarkers except Aβ40 and NFL. FTLD-TDP pathology was associated with reduced P-tau/T-tau and elevated NFL. FTLD-tau (PSP) was associated with reduced T-tau.
Figure 6. CSF Biomarkers vs Primary and Contributory Neuropathology Diagnoses.
Effects are plotted for each neuropathologic class (A–E) from linear regression models adjusted age, sex, and time between lumbar puncture and death. Coefficients represent the average difference in biomarkers between patients who were positive for a neuropathology (e.g., AD in panel A, also if only present as a copathology) compared to the remaining patients. AD = Alzheimer disease; CBD = corticobasal degeneration; FTLD = frontotemporal lobar degeneration; NFL = neurofilament light; P-tau = phosphorylated tau; PSP = progressive supranuclear palsy; T-tau = total tau; TDP = TAR DNA binding protein 43.
Note that these analyses were not adjusted for copathologies. However, we also performed these tests for the FTLD diagnoses after removing patients with intermediate-high ADNC (eFigure 13, links.lww.com/WNL/B777), leaving 24 with FTLD-TDP, 17 with FTLD-tau (PSP), 16 with FTLD-tau (CBD), and 9 with FTLD-tau (other). FTLD-TDP pathology remained associated with reduced P-tau/T-tau and elevated NFL, and FTLD-tau (PSP) remained associated with reduced T-tau. FTLD-tau (other) was associated with elevated P-tau/Aβ42, T-tau, and P-tau.
Genetic Variants, CSF Biomarkers, and Neuropathology
Data on genetic variants were available for APOE ε4 (positive n = 26, negative n = 74, Table 1), C9orf72 variations (positive n = 8 [all FLTD-TDP], negative n = 92), GRN variations (positive n = 5 [1 AD, 4 FTLD-TDP], negative n = 95), and MAPT variations (positive n = 2 [both classified as other, with FTD-PPA syndromes], negative n = 96).
APOE ε4 was associated with lower Aβ42 (p = 0.028) and Aβ42/Aβ40 (β = −0.019, p < 0.001) and higher P-tau/Aβ42 (β = 0.017, p = 0.0056). After adjustment for Thal phase, Braak stage, and CERAD score (used together), these associations were lost (Aβ42 p = 0.91, Aβ42/Aβ40 p = 0.91, P-tau/Aβ42 p = 0.87), supporting that APOE variants affect CSF biomarker levels indirectly through neuropathology.
Among patients with FTLD primary pathologies, there were no associations between C9orf72 variations (n = 8 positive, n = 62 negative) and biomarker levels. The other variations had too few cases for meaningful analyses.
Classification of Evidence
This study provides Class II evidence that distinct CSF biomarker patterns, including for P-tau, T-tau, Aβ42, Aβ40, and NFL, are associated with AD and FTLD neuropathology.
Discussion
This clinicopathologic CSF biomarker study in patients with AD and FTLD, which included Aβ42/Aβ40, P-tau, and P-tau/Aβ42 measured with fully automated Elecsys assays and NFL measured with an ultrasensitive Simoa assay, found that specific biomarkers were strongly correlated with AD, including when AD was present as copathology in patients with another primary pathology. The Aβ42/Aβ40 and P-tau/Aβ42 ratios had very high overall accuracy (AUC 0.95–0.96) to detect significant AD pathology, defined as intermediate-high ADNC. Although most of the biomarker changes were associated with quite advanced neuropathology, group-level changes in CSF Aβ42/Aβ40 appeared already at Thal phase 2, supporting that selected CSF biomarkers may begin to be influenced at low levels of AD pathology. However, the biomarkers did not have sensitivity for single-participant–level prediction at very early stages of Aβ or tau pathologies in this study. Another key finding was that autopsy-confirmed FTLD variants displayed group-level biomarker patterns, including reduced CSF T-tau in FTLD-tau (PSP) and reduced CSF P-tau/T-tau and increased CSF NFL in FTLD-TDP. Taken together, these findings support that CSF biomarkers can be used to identify underlying neuropathologic AD changes, and they are also differentially expressed in different FTLD pathologies. This is one of few studies that combine careful characterization of neuropathologic features with the use of only recently available modern assays for CSF AD biomarkers and NFL. The findings support the use of these biomarkers to characterize the underlying neuropathologic changes in patients with AD and FTLD.
There were important findings for several of the individual biomarkers and ratios. In relation to Aβ pathology, we noted that Aβ42/Aβ40 levels started to change already at Thal phase 2, which may be comparable to what has been described previously for Aβ PET in an overlapping cohort.44 Previous studies directly comparing CSF Aβ and Aβ PET biomarkers have suggested that CSF Aβ measures may be altered before Aβ PET in response to altered Aβ metabolism.45 We note that we did not generally find that CSF biomarkers were altered in very early stages of neuropathology. This was especially true for tau accumulation, for which no biomarker was significantly altered before Braak stage IV. Although this study did not directly compare CSF and PET measures with neuropathology, our findings suggest that the CSF biomarkers studied here were not significantly altered before changes may also be detected by PET imaging. However, a possible caveat for CSF studies of very early changes with regard to neuropathology is the delay between LP and death, which may obscure the exact relationships between subtle biomarker changes and the first neuropathologic changes. Another caveat is that truly quantitative data on Aβ pathology, rather than the semiquantitative data used here, may be necessary to find subtle differences in when CSF and PET biomarkers start to change with respect to pathology. Furthermore, studies that find evidence of early CSF changes before changes in PET measures have typically seen such discrepancy in people without cognitive impairment or only mild cognitive impairment, which is different from the current study population, in which most research participants had dementia. The findings for the P-tau/Aβ42 ratio were similar to those for Aβ42/Aβ40, with high diagnostic accuracy for AD neuropathology. We found pronounced changes in P-tau/Aβ42 also when AD was a copathology in FTLD. Theoretically, a high P-tau/Aβ42 ratio may reflect a combination of Aβ pathology and AD-specific axonal degeneration with tangle material. The ratio correlated with all of Thal, Braak, and CERAD scores, although significant changes seemed to appear later than for Aβ42/Aβ40 (Thal phase 3 instead of Thal phase 2, Braak stage V instead of Braak stage IV).
The individual CSF Aβ42 measure was reduced in patients with AD (as expected) but also in several patients with FTLD, as described before in FTLD cohorts.17 We found that reductions of Aβ42 were seen mainly in patients with FTLD with concomitant AD pathology (defined as intermediate-high ADNC), but several patients with FTLD had low CSF Aβ42 levels despite having none-low ADNC grade (Figure 5B). In contrast, the CSF Aβ42/Aβ40 ratio was very rarely reduced in patients with FTLD without concomitant Aβ pathology (Figure 5E). This supports that CSF Aβ42/Aβ40 can be used a tool to detect concomitant AD pathology in FTLD and illustrates the importance of correcting CSF Aβ42 levels for a shorter Aβ isoform such as Aβ40 or Aβ38 to detect underlying Aβ pathology. Hypothetically, other factors beyond Aβ pathology may have contributed to the reduced levels of Aβ42 (without affecting the Aβ ratio) in patients with FTLD, including white matter disease, neuroinflammation, and neuronal loss leading to reduced overall Aβ release.
CSF T-tau, which is nonspecifically elevated in several conditions with axonal injury, generally did not reach AD levels in the patients with FTLD in this study. Low levels of T-tau were seen in PSP, which is in agreement with previous studies based on clinical diagnosis.13,17 We have no clear explanation for the low tau levels in PSP. Hypothetically, it could be related to involvement of cortical vs subcortical structures (cortical abnormalities may presumably be more readily detected as CSF changes compared to subcortical abnormalities, which dominate in PSP). The low levels of T-tau in PSP may also be related to disease-specific differences in tau fragment confirmations or in neuronal releases of 3R vs 4R tau.13 Hypothetically, CSF tau levels could also be reduced due to sequestration of tau in tangles in tauopathies. We note that the reduced tau levels seem to be more specific for PSP than for CBD, which is interesting given the similarities between these conditions neuropathologically. P-tau, which has been associated with buildup of AD-specific tau aggregates, did not clearly differentiate between Aβ plaques and neurofibrillary tangles in this cohort because associations were seen between high P-tau and neuropathologic measures both of tau and Aβ pathology. Soluble P-tau may be increased in response to Aβ buildup as a first indicator of altered tau metabolism in individuals who are positive on Aβ PET but negative on tau PET.46 However, we did not find that P-tau was altered in response to early Aβ pathology, which may be expected given previous PET studies. The relationship between soluble P-tau and buildup of aggregated Aβ and tau fibrils is complex and warrants further studies. We focused on P-tau181, but other variants of P-tau (including P-tau217) may also be interesting to study in relation to neuropathology. P-tau levels were generally low in FTLD. Combining P-tau and T-tau in a ratio may integrate information about phosphorylation and release of tau proteins. Theoretically, a low P-tau/T-tau ratio may reflect axonal degeneration without AD-type degeneration because T-tau (but not P-tau) is increased nonspecifically due to axonal degeneration. Among the different AD pathology features, the P-tau/T-tau ratio was most closely associated with Braak stage (rather than Thal phase and CERAD score, eTable 6, links.lww.com/WNL/B777). The ratio increased separation between some of the groups compared to the individual tau measures. In particular, P-tau/T-tau was reduced in individuals with FTLD-TDP, which is in line with previous findings based on clinical, neuropathologic, and genetic evidence.17,18 The FTLD-TDP group was further characterized by increased NFL, which is also in line with previous literature.47 One biomarker-pathology study in AD and FTLD that compared CSF T-tau and NFL in a panel of biomarkers found that NFL improved diagnostic accuracy, further supporting that NFL can provide important information.48
One strength of this study is that we had neuropathologic data in all patients, combined with CSF samples, and the sample size was relatively large for an antemortem biomarker vs neuropathology study. A limitation is that the sample size was relatively small for some of the subgroups. A larger population will make it easier to fully disentangle associations between biomarkers and specific pathologic features. Additional weaknesses include the lack of a control population for normal aging, as well as the lack of groups with other dementias such as Lewy body dementia or vascular dementia. Future studies may include these groups to increase the generalizability of the findings. The time interval between LP and death may obscure relationships between CSF biomarkers and neuropathologic features, although we adjusted for this.
CSF Aβ42/Aβ40, T-tau, P-tau, P-tau/Aβ42, and NFL were related to underlying neuropathologic changes of AD and FTLD variants. An AD-like biomarker profile supports AD comorbidity in patients with FTLD. In contrast, a non–AD-like biomarker profile, with reduced P-tau/T-tau and increased NFL, may support FTLD-TDP rather than FTLD-tau lesions in the brain. Low T-tau may support FTLD-tau (PSP) pathology rather than other FTLD pathologies. Further work is needed to assess how these findings can be translated to blood-based biomarkers, which have shown promising results with high diagnostic accuracy for AD and underlying pathologic changes.49,50 Further work is also needed to test how these biomarker profiles perform at the single-participant level, and the findings need to be replicated in an independent sample.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADNC
AD neuropathologic change
- AIC
Akaike information criterion
- AUC
area under the ROC curve
- bvFTD
behavioral variant FTD
- CBD
corticobasal degeneration
- CBS
corticobasal syndrome
- CERAD
Consortium to Establish a Registry for Alzheimer's Disease
- FTD
frontotemporal dementia
- FTLD
frontotemporal lobar degeneration
- LP
lumbar puncture
- NFL
neurofilament light chain
- P-tau
phosphorylated tau
- PSP
progressive supranuclear palsy
- ROC
receiver operating characteristics
- T-tau
total tau
- TDP-43
TAR DNA-binding protein 43
- UCSF
University of California San Francisco
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Podcast: NPub.org/Podcast9811
Study Funding
This work was partially funded by Roche Diagnostics (assay kits, materials, and employee salaries for U.E. and G.K). ELECSYS is a registered trademark of Roche. The Elecsys CSF Aβ42, Aβ40, P-tau, and T-tau immunoassays are not yet cleared or approved for clinical use in the United States. The NeuroToolKit robust prototype assays are for investigational purposes only and are not approved for clinical use. All other product names and trademarks are the property of their respective owners. Work at the authors' laboratory is funded by Knut and Alice Wallenberg Foundation, the Medical Faculty at Lund University, Region Skåne, the European Research Council, the Swedish Research Council, the Strategic Research Area MultiPark at Lund University, the Swedish Alzheimer Foundation, the Swedish Brain Foundation, The Parkinson Foundation of Sweden, The Parkinson Research Foundation, the Skåne University Hospital Foundation, the Swedish federal government under the ALF agreement, and the Bundy Academy. Work at UCSF was supported by NIH/National Institute on Aging grants (P30-AG062422, P01-AG019724, R01-AG038791, K08-AG052648, R01-NS050915, P50 AG023501, R01 AG045611, U19AG063911, K24053435, U54NS092089, R01AG031278, K99AG065501).
Disclosure
O. Hansson has acquired research support (for the institution) from Roche, Pfizer, GE Healthcare, Biogen, Eli Lilly, and AVID Radiopharmaceuticals. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Biogen and Roche. G.D. Rabinovici receives research support from AVID Radiopharmaceuticals, Eli Lilly, Life Molecular Imaging, and GE Healthcare. In the past 2 years, he has received consultancy/speaker from Axon Neurosciences, Eisai, Merck, GE Healthcare, and Johnson & Johnson. He is an associate editor for JAMA Neurology. N. Mattsson-Carlgren is an associate editor for Alzheimer's Dementia: Diagnosis, Assessment & Disease Assessment. A. Ehrenberg is a scientific editor for Alzheimer's & Dementia: The Journal of the Alzheimer's Association and has received compensation for consulting work done with Epiodyne, Inc. A.L. Boxer has been a consultant (past 2 years) for AGTC, Alector, Arkuda, Arvinas, Asceneuron, AZTherapies, Bioage, Humana, Lundbeck, Ono, Roche, Samumed, Sangamo, Stealth, Third Rock, Transposon, UCB, and Wave, and has research support from Biogen, Eli Lilly, and Eisai. L.T. Grinberg has acquired research support (for the institution) from Eli Lilly and AVID Radiopharmaceuticals. She received compensation for consulting work with CuraSen Inc. S. Spina has received compensation for consulting work with Precision Xtract and Acsel Health. W.W. Seeley receive consulting fees from GLG Council, Guidepoint Global LLC, and Corcept Therapeutics. G. Kollmorgen and U. Eichenlaub are employees of Roche Diagnostics. J.C. Rojas is site primary investigator for clinical trials sponsored by Eli Lilly and Eisai. His research is supported by NIH/National Institute on Aging K23AG059888. Dr. Ljubenkov is a clinical site primary investigator for clinical trials sponsored by Alector, AbbVie, and Woolsey. Dr. Ljubenkov serves as an advisor for Retrotrope. Dr. Ljubenkov receives research and salary support from the NIH/National Institute on Aging and the Alzheimer's Association–Part the Cloud Partnership. Howard Rosen has been a consultant for Biogen, Ionis, Alector, Wave, Takeda, and Otsuka. The remaining authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer's disease centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71(4):266-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris JM, Gall C, Thompson JC, et al. Classification and pathology of primary progressive aphasia. Neurology. 2013;81(21):1832-1839. [DOI] [PubMed] [Google Scholar]
- 3.Mackenzie IRA, Neumann M, Baborie A, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122(1):111-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark CM, Pontecorvo MJ, Beach TG, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol. 2012;11:669-678. [DOI] [PubMed] [Google Scholar]
- 6.Soleimani-Meigooni DN, Iaccarino L, La Joie R, et al. 18F-flortaucipir PET to autopsy comparisons in Alzheimer's disease and other neurodegenerative diseases. Brain. 2020;143(11):3477-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bian H, Van Swieten JC, Leight S, et al. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology. 2008;70(19 pt 2):1827-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buerger K, Ewers M, Pirttila T, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035-3041. [DOI] [PubMed] [Google Scholar]
- 9.Ewers M, Mattsson N, Minthon L, et al. CSF biomarkers for the differential diagnosis of Alzheimer's disease: a large-scale international multicenter study. Alzheimers Dement. 2015;11(11):1306-1315. [DOI] [PubMed] [Google Scholar]
- 10.Tang W, Huang Q, Yao Y-Y, et al. Does CSF p-tau181 help to discriminate Alzheimer's disease from other dementias and mild cognitive impairment? A meta-analysis of the literature. J Neural Transm (Vienna). 2014;121(12):1541-1553. [DOI] [PubMed] [Google Scholar]
- 11.Koopman K, Le Bastard N, Martin J-J, et al. Improved discrimination of autopsy-confirmed Alzheimer's disease (AD) from non-AD dementias using CSF P-tau(181P). Neurochem Int. 2009;55(4):214-218. [DOI] [PubMed] [Google Scholar]
- 12.de Souza LC, Lamari F, Belliard S, et al. Cerebrospinal fluid biomarkers in the differential diagnosis of Alzheimer's disease from other cortical dementias. J Neurol Neurosurg Psychiatr. 2011;82(3):240-246. [DOI] [PubMed] [Google Scholar]
- 13.Wagshal D, Sankaranarayanan S, Guss V, et al. Divergent CSF τ alterations in two common tauopathies: Alzheimer's disease and progressive supranuclear palsy. J Neurol Neurosurg Psychiatr. 2015;86(3):244-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lleó A, Irwin DJ, Illán-Gala I, et al. A 2-step cerebrospinal algorithm for the selection of frontotemporal lobar degeneration subtypes. JAMA Neurol 2018;75(6):738-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goossens J, Bjerke M, Van Mossevelde S, et al. Diagnostic value of cerebrospinal fluid tau, neurofilament, and progranulin in definite frontotemporal lobar degeneration. Alzheimers Res Ther. 2018;10(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin DJ, Lleó A, Xie SX, et al. Ante mortem cerebrospinal fluid tau levels correlate with postmortem tau pathology in frontotemporal lobar degeneration. Ann Neurol. 2017;82(2):247-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meeter LHH, Vijverberg EG, Del Campo M, et al. Clinical value of neurofilament and phospho-tau/tau ratio in the frontotemporal dementia spectrum. Neurology. 2018;90(14):e1231-e1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu WT, Watts K, Grossman M, et al. Reduced CSF p-tau181 to tau ratio is a biomarker for FTLD-TDP. Neurology. 2013;81(22):1945-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer's disease concord with amyloid-β PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14(11):1470-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall S, Öhrfelt A, Constantinescu R, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol. 2012;69(11):1445-1452. [DOI] [PubMed] [Google Scholar]
- 21.Rascovsky K, Hodges JR, Kipps CM, et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis Assoc Disord. 2007;21(4):S14-S18. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong MJ. Diagnosis and treatment of corticobasal degeneration. Curr Treat Options Neurol. 2014;16(3):282. [DOI] [PubMed] [Google Scholar]
- 23.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1-9. [DOI] [PubMed] [Google Scholar]
- 25.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546-1554. [DOI] [PubMed] [Google Scholar]
- 26.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939-944. [DOI] [PubMed] [Google Scholar]
- 27.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's Disease Neuroimaging Initiative subjects. Ann Neurol. 2009;65:403-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15(7):673-684. [DOI] [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 30.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. Oxford University Press; 2006. [Google Scholar]
- 31.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-Second Edition (CVLT-II). Psychological Corp. [Google Scholar]
- 32.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute working group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(10):1095-1097. [DOI] [PubMed] [Google Scholar]
- 34.Cairns NJ, Bigio EH, Mackenzie IRA, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114(1):5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010;9(10):995-1007. [DOI] [PubMed] [Google Scholar]
- 36.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791-1800. [DOI] [PubMed] [Google Scholar]
- 37.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239-259. [DOI] [PubMed] [Google Scholar]
- 38.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD), part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479-486. [DOI] [PubMed] [Google Scholar]
- 39.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging–Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LeDell E, Petersen M, van der Laan M. Computationally efficient confidence intervals for cross-validated area under the ROC curve estimates. U.C. Berkeley Division of Biostatistics Working Paper Series 2012. Accessed January 11, 2022. biostats.bepress.com/ucbbiostat/paper304. [DOI] [PMC free article] [PubMed]
- 42.University of California San Francisco. Weill Institute for Neurosciences, Memory and Aging Center. Accessed February 4, 2022. memory.ucsf.edu/research-trials/professional/open-science.
- 43.Blennow K, Shaw LM, Stomrud E, et al. Predicting clinical decline and conversion to Alzheimer's disease or dementia using novel Elecsys Aβ(1-42), pTau and tTau CSF immunoassays. Sci Rep. 2019;9(1):19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.La Joie R, Ayakta N, Seeley WW, et al. Multisite study of the relationships between antemortem [11C]PIB-PET Centiloid values and postmortem measures of Alzheimer's disease neuropathology. Alzheimers Dement. 2019;15(2):205-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palmqvist S, Mattsson N, Hansson O; Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid analysis detects cerebral amyloid-β accumulation earlier than positron emission tomography. Brain. 2016;139(pt 4):1226-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mattsson-Carlgren N, Andersson E, Janelidze S, et al. Aβ deposition is associated with increases in soluble and phosphorylated tau that precede a positive tau PET in Alzheimer's disease. Sci Adv. 2020;6(16):eaaz2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landqvist Waldö M, Frizell Santillo A, Passant U, et al. Cerebrospinal fluid neurofilament light chain protein levels in subtypes of frontotemporal dementia. BMC Neurol. 2013;13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cousins KAQ, Phillips JS, Irwin DJ, et al. ATN incorporating cerebrospinal fluid neurofilament light chain detects frontotemporal lobar degeneration. Alzheimers Dement. 2021;17(5):822-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schindler SE, Bollinger JG, Ovod V, et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93(17):e1647-e1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Per the UCSF Memory and Aging Center data-sharing policy, data can be made available on reasonable request via our online portal.42