Abstract
Background and Objectives
The care of patients with large vessel occlusion (LVO) stroke has been revolutionized by endovascular thrombectomy (EVT). While EVT has a large effect size, most patients treated with EVT remain disabled or die within 90 days. A better understanding of outcomes may influence EVT selection criteria, novel therapies, and prognostication. We sought to identify associations between outcomes and brain regions involved in ischemic lesions.
Methods
For this cohort study, consecutive patients with LVO who were treated with EVT and underwent post-EVT MRI were identified from a tertiary referral center (2011–2019). Acute ischemic lesions were manually segmented from diffusion-weighted imaging and spatially normalized. Individual lesions were parcellated (atlas-defined 94 cortical regions, 14 subcortical nuclei, 20 white matter tracts) and reduced to 10 essential lesion patterns with the use of unsupervised dimensionality reduction techniques. Ninety-day modified Rankin Scale (mRS) score (>2) was modeled via bayesian regression, taking the 10 lesion patterns as inputs and controlling for lesion size, age, sex, acute NIH Stroke Scale (NIHSS) score, alteplase, prior stroke, intracerebral hemorrhage, and good reperfusion (Thrombolysis in Cerebral Infarction 2b–3). In comparative analyses, 90-day mRS score was modeled considering covariates only, and compartment-wise relevances for acute stroke severity and 90-day mRS score were evaluated.
Results
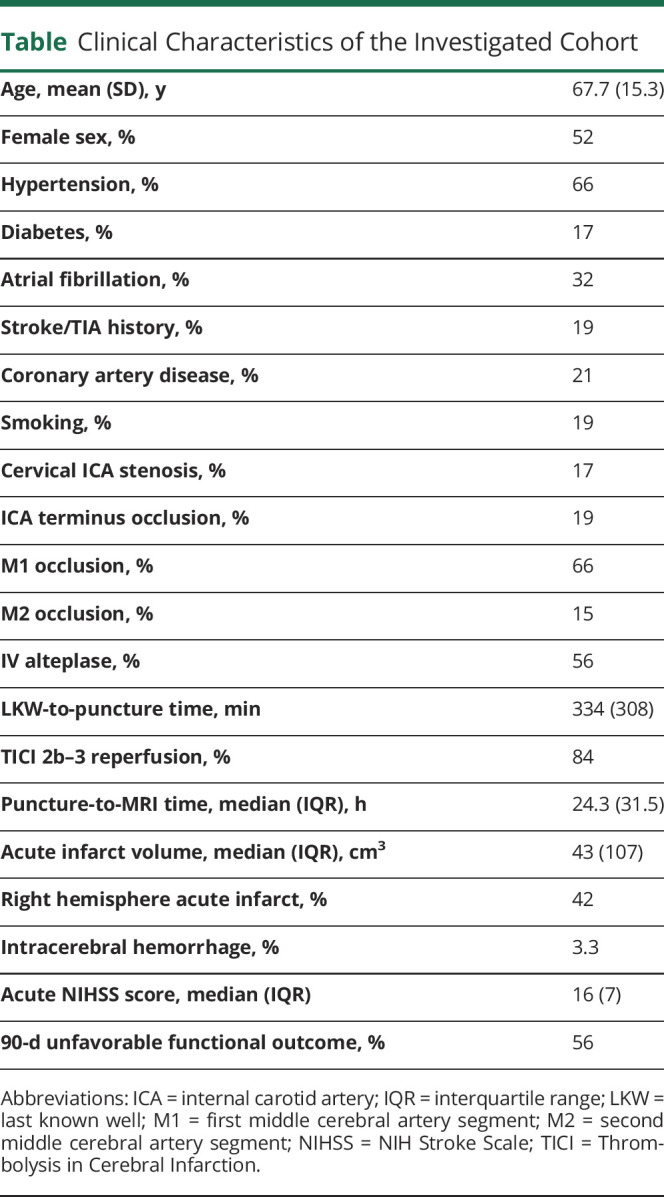
There were 151 patients with LVO identified (age 68 ± 15 years, 52% female). The median NIHSS score was 16 (interquartile range 13–20); 56% had mRS score >2. Lesion locations predictive of 90-day mRS score involved bilateral but left hemispherically more pronounced precentral and postcentral gyri, insular and opercular cortex, and left putamen and caudate (area under the curve 0.91, highest probability density interval [HPDI] covering 90% certainty 0.90–0.92). The lesion location model outperformed the simpler model relying on covariates only (bayesian model comparison of 97% weight to the model with vs 3% weight to the model without lesion location). While lesions affecting subcortical nuclei had the highest relevance for stroke severity (posterior distribution mean 0.75, 90% HPDI 0.256–1.31), lesions affecting white matter tracts had the highest relevance for 90-day mRS score (0.656, 90% HPDI 0.0864–1.12).
Discussion
These data describe the significance for outcomes of specific brain regions involved in ischemic lesions on MRI after EVT. Future work in additional datasets is needed to confirm these granular findings.
Among all stroke subtypes, large vessel occlusion (LVO) stroke is associated with the worst outcomes.1 While endovascular thrombectomy (EVT) has revolutionized its management with a large treatment effect,2 more than half of those treated are functionally disabled or die within 90 days.3 It is therefore essential to understand and improve outcomes for patients with LVO stroke. Furthermore, the focused study of a stroke subtype such as LVO may be advantageous given the heterogeneous pathophysiology of stroke in general. Outcomes after stroke are difficult to predict,4,5 with different factors more relevant to different subtypes.6 Neuroimaging provides a window into the mechanisms of stroke. Beyond routine clinical imaging assessments, infarcts can be mapped 3-dimensionally to gain insight into affected brain parenchyma. Region-specific and tissue-specific infarct topography may better explain variability in outcomes, have implications for EVT treatment selection, and aid in advances of novel therapeutic agents.1,4
Independently of stroke subtype, several previous studies have highlighted the importance of both the size and location of infarcts.7-9 In the EVT era, the Alberta Stroke Program Early Computed Tomography Score (ASPECTS) gained popularity; one analysis compared its prescribed regions and their relevance for outcomes.10 However, ASPECTS has limitations given that its regions often comprise several different brain structures that are not assessed individually, and white matter (WM) is not well represented. Furthermore, ASPECTS has substantial interobserver variability.11 In addition, CT has poor sensitivity for acute infarction compared to MRI, especially within WM. More recently, MRI-defined infarct topography after EVT has been described.12 Cortex comprised 42%, WM comprised 39%, and basal ganglia comprised 7% of total infarct volume after EVT; WM infarct volume was particularly associated with outcomes.13
In the present study, we sought to use bayesian regression modeling to better understand the relationships of lesions to individual cortical and subcortical gray matter (GM) regions and WM tracts involved in acute infarcts after EVT and long-term outcomes. In ancillary analyses, we strove to disentangle the overall cortical, subcortical, and WM effects on short- and long-term outcomes and to qualitatively investigate the impact of lesion disconnection, that is, indirect lesion effects, in LVO stroke via lesion network mapping.14,15 We hypothesized that a pronounced involvement of bilateral motor and left-lateralized language areas would predict long-term functional outcomes and a more widespread functional disconnection in cases with less favorable EVT results.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The Massachusetts General Hospital institutional review board approved this study. Because there was minimal patient risk, informed consent was waived for inclusion in the research database by the institutional review board.
Patients and Clinical Features
Patients with anterior circulation LVO stroke who were treated with EVT were retrospectively identified at a single tertiary referral center from January 2011 to September 2019. Data for consecutive patients were recorded prospectively in a local EVT database.13 This database includes demographic information, medical history, presentation details, treatments, and outcomes.16 Acute stroke severity was measured by presenting NIH Stroke Scale (NIHSS) score.17 A vascular neurologist made alteplase treatment decisions on the basis of guidelines.17 A neurointerventionalist and vascular neurologist made EVT treatment decisions. LVO was defined as occlusion of the internal carotid artery terminus, first segment of the middle cerebral artery, or proximal second segment of the middle cerebral artery.18 Cervical internal carotid artery stenosis was defined as severe stenosis (>70% by North American Symptomatic Carotid Endarterectomy Trial [NASCET] criteria) or occlusion related to atherosclerosis or dissection.19
A neurointerventionalist determined the Thrombolysis in Cerebral Infarction (TICI) scores using the modified scale: 2a = partial filling <50%, 2b = partial filling ≥50%, and 3 = complete perfusion. TICI 2b to 3 was considered good reperfusion.12 Intracerebral hemorrhage was defined as PH1 or PH2 by European Cooperative Acute Stroke Study (ECASS) criteria.20 The modified Rankin Scale (mRS) score at 90 days was obtained by clinical follow-up visit or telephone call; it was available for 87% of patients. Unfavorable long-term outcome was defined as 90-day mRS score >2.21,22
Imaging Acquisition and Processing
A Siemens (Munich, Germany) 3T MRI or a General Electric (Fairfield, CT) 1.5T MRI was used to obtain post-EVT diffusion-weighted imaging (DWI). The echo time was 60 to 120 milliseconds; repetition time was 5,300–5,600 milliseconds; and slice thickness was 5 mm with a 1-mm gap. A vascular neurologist blinded to clinical data used Slicer 4.8.1 (Brigham and Women's Hospital, Boston, MA) to manually trace infarct lesion masks. If hemorrhage was present, it was included in the infarct lesion mask. Subsequently, the DWI sequences and lesion masks were registered to Montreal Neurological Institute 152 space with RegLSM (University of Calgary, Alberta, Canada).12 With the use of the Elastix toolbox, linear registration was followed by nonlinear registration. A vascular neurologist visually inspected for appropriate registration all individual DWI sequences and DWI lesion masks. If there were significant artifacts or poor registration, cases were excluded (n = 23).
Deriving a Low-Dimensional Lesion Representation
Our first aim was to derive a compact, low-dimensional representation of individual lesions that could serve as feasible input to our regression model of functional outcomes. To this end, the number of voxels was counted involving the lesion within 94 cortical and 14 subcortical regions as defined by the Harvard-Oxford atlas. Similarly, the lesion load was computed within 20 Johns Hopkins University atlas–based WM tracts. In cases of overlapping voxels between the atlases, these voxels were considered for the cortical/subcortical region only. By these means, the binary lesion information (i.e., lesioned vs not lesioned) that was available for every voxel in the whole brain (≈7,000,000 voxels) was transformed into volume-defined brain region and tract information (i.e., the number of lesioned voxels for each of the 128 cortical or subcortical regions or WM tracts). Each patient's lesion was hence expressed by 128 values, each stating the number of voxels that were affected within each atlas region or tract. For example, a patient with a subcortical lesion in the putamen and adjacent corticospinal tract would be represented by a vector of zeros for all regions and tracts but the putamen and corticospinal tract. To reduce the dimensionality even further, nonnegative matrix factorization was used to reduce the 128-dimension region-wise lesion space and to obtain 10 unique lesion patterns.23 Each lesion pattern combined various regions and tracts, and individual lesions could then be reconstructed by a weighted combination these 10 lesion patterns. eFigure 1 (links.lww.com/WNL/B775) shows a visual overview of our lesion representation derivation workflow.
Modeling Region-Specific Contributions to 90-Day Unfavorable Outcome
In the main analysis, unfavorable functional outcome at 90 days was modeled via bayesian hierarchical logistic regression. The 10 lesion patterns were entered as the input variables of interest. Further integrated covariates were age, sex, prior stroke or TIA, acute NIHSS score, IV thrombolysis, TICI 2b to 3 good reperfusion, intracerebral hemorrhage, and total lesion volume (GM and WM; full model specifications in supplementary eAppendices 1 and 2 [links.lww.com/WNL/B775]; additional methodologic details as previously described9,24). In a control analysis, unfavorable outcome was modeled with the covariates only (i.e., we did not include the lesion patterns). The models were compared to determine whether either (with or without inclusion of lesion patterns) performed more favorably. Model performance was investigated on the basis of the area under the curve (AUC), which takes into account the true-positive rate (i.e., sensitivity) and false-positive rate (i.e., 1 − specificity).25
Determining Overall Compartment-Specific Contributions to Acute Stroke Severity and 90-Day Unfavorable Outcomes
In a second analysis, our aim was to quantify the overall contributions of (1) cortical lesions, (2) subcortical GM lesions, and (3) WM tract lesions to acute stroke severity and 90-day unfavorable outcomes. Our analysis pipeline was modified in the following way. Instead of dimensionality-reducing the 128-dimensional region-wise lesion space at once, nonnegative matrix factorization was applied separately to the 94 cortical regions, 14 subcortical regions, and 20 WM tract regions. Each compartment-specific representation was reduced to 6 components. These 18 components in total were then adjusted for total lesion volume and entered into a bayesian regression model as input variables of interest. The effects of all 6 components per compartment were captured through a joint hyperprior (full model specifications in supplementary eAppendices 1 and 2 [links.lww.com/WNL/B775]). Age and sex were integrated as covariates. Acute NIHSS score–based stroke severity was modeled via linear regression, while unfavorable outcome was modeled via logistic regression.
Investigating Indirect Lesion Effects: Functional Lesion Connectivity
To complement the first 2 analyses that were focused on direct lesion effects, our third analysis investigated indirect lesion effects, i.e., connectivity of the lesion to other brain regions. To this end, lesion network maps were computed for all 151 patients by a previously validated technique.14,15 In brief, each individual lesion was used as a seed region to estimate the functional connectivity to all voxels in the brain by relying on a connectome dataset from 1,000 healthy controls (43% male, mean age 21 years, range 18–35 years).26,27 Resting-state fMRI data were preprocessed as previously described with global signal regression included.28,29 Both positive and negative correlations were retained. For each patient, the Fisher z-transformed voxel-wise lesion connectivity within 100 Yeo-Schaefer atlas–defined cortical parcels was summarized.30 For this analysis, an atlas template based on fMRI data was used instead of structural MRI data as used in the previous 2 analyses. Parcels that included >1% of lesioned tissue were excluded to ensure that indirect lesion effects were evaluated rather than direct ones caused by the infarcted tissue itself. The parcel-wise lesion connectivity was averaged across all participants to determine regions of maximum connectivity to the lesion. Because reperfusion status after EVT is an important determinant of outcome after stroke,12 network-wise lesion connectivity of patients with and without TICI 2b to 3 reperfusion was compared by independent 2-sample t tests (level of significance p < 0.05, false discovery rate corrected for multiple comparisons). Spearman correlation analyses between mean absolute whole-brain lesion connectivity and 90-day mRS score were completed (level of significance p < 0.05). Sensitivity analyses were completed to test whether there were differences with respect to lesion volumes between the groups of patients with and without reperfusion (independent 2-sample t test, level of significance p < 0.05) and to adjust for lesion volume.
Data Availability
We will make available the data that support the findings of this study on reasonable request pending local institutional review board approval.
Results
Patient Demographic, Presentation, Treatment, and Outcome Characteristics
We studied 151 patients with LVO with mean ± SD age of 68 ± 15 years; 52% were female. Median acute NIHSS score was 16 (interquartile range [IQR] 7), and 56% experienced an unfavorable functional outcome (mRS score >2) at 90 days after stroke (the table gives further clinical characteristics). There was a slight imbalance with respect to the lesioned hemisphere in that 58% of our patients experienced a left hemispheric stroke.
Table.
Clinical Characteristics of the Investigated Cohort
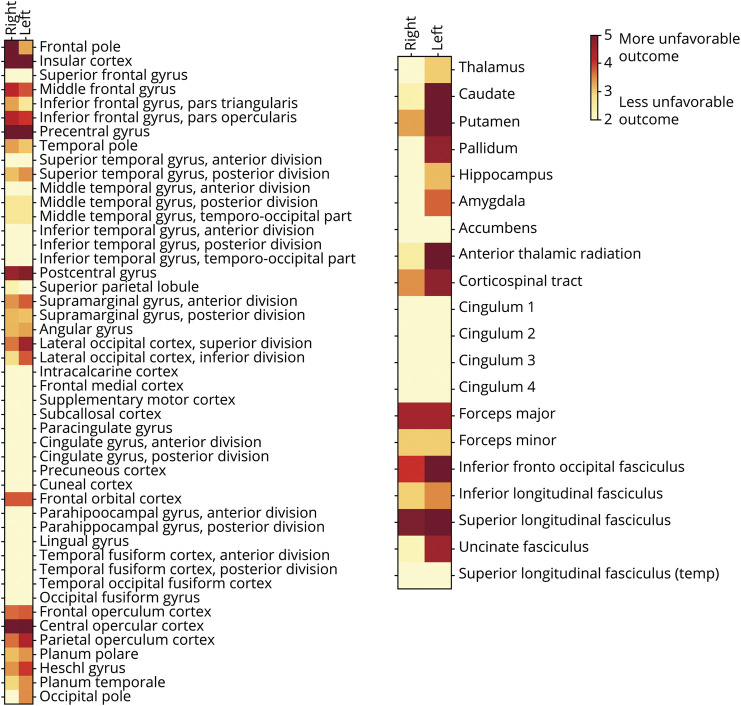
Lesion Representation and Region-wise Explanation of Unfavorable 90-Day Functional Outcomes
A low-dimensional representation of individual lesions was derived by the use of unsupervised learning algorithms. Our representation consisted of 10 unique lesion patterns that each represented an anatomically plausible combination of lesioned regions (Figure 1, outer circle). Lesion topographies were found to be more variable in the left hemisphere in our sample; we uncovered 6 distinct patterns for the left hemisphere yet only 3 for the right. One lesion topography captured anterior cerebral artery strokes of both hemispheres.
Figure 1. Low-Dimensional Lesion Representation (Outer Circle) and Region-wise Relevance for Explaining Unfavorable 90-Day Functional Outcomes.
Six unique lesion patterns, each combining various lesion locations, represented left hemispheric stroke lesions, with varying focuses from anterior to posterior brain regions. Similar anterior to posterior dimensions were covered by right-sided lesion patterns. However, the overall spatial resolution was slightly lower for right-sided lesions, given that they were captured by only 3 individual lesion patterns. Evaluated on a region-wise basis, stroke lesions relating to bilateral precentral and postcentral gyri, insular and opercular cortex, and left putamen, caudate nucleus, and multiple white matter tracts had the highest relevance in explaining unfavorable functional outcome at 90-day (mRS score >2). Left side of images represents the left hemisphere.
Lesion locations relevant for unfavorable long-term outcome were particularly focused on bilateral, but left hemispherically more pronounced, precentral and postcentral gyri, insular and opercular cortex, and left putamen and caudate (Figure 1, inner circle, and Figure 2). Model performance totaled an area under the curve (AUClesion location) of 0.91 (highest probability density interval [HPDI] covering 90% certainty 0.90–0.92). Indeed, the model incorporating region-wise lesion information outperformed the covariate model that did not incorporate granular lesion location information (AUCcovariate 0.87, 90% HPDI 0.86–0.89). Bayesian model comparison showed 97% weight to the model with and 3% weight to the model without lesion location information.
Figure 2. Region-wise Relevance for Unfavorable 90-Day Outcomes Across All 151 Patients With Large Vessel Occlusion Stroke.
Bilateral precentral and postcentral gyri, insula, and operculum were the most relevant regions in the cortex. Subcortically, the highest relevance was assigned to left putamen, pallidum, and caudate nucleus, as well as the left hemispheric white matter tracts: anterior thalamic radiation, corticospinal tract, and inferior fronto-occipital and superior longitudinal fasciculus. Color map: darker red corresponds to a higher relevance in explaining unfavorable 90-day outcomes.
Overall Effects of Lesioned Cortical and Subcortical GM and WM Tracts on Acute Stroke Severity and 90-Day Unfavorable Outcomes
When modeling overarching joint effects of all cortical regions, subcortical GM regions, and WM tracts, we found diverging effects for acute vs chronic stroke outcomes. For acute stroke severity, the joint effect of all subcortical GM lesions was the only one substantially different from zero (mean of the bayesian posterior distribution 0.75, 90% HPDI 0.256–1.31, Figure 3A). In contrast, WM tract lesions had the only posterior distribution not overlapping with zero and thus the highest relevance in explaining 90-day functional outcomes (mean of the bayesian posterior distribution 0.656, 90% HPDI 0.0864–1.12, Figure 3B). These findings, i.e., the relevance of subcortical GM lesions for acute outcomes and WM tract lesions for chronic outcomes, remained the same when we separately fitted models for cortical/subcortical GM and WM lesions to exclude biasing effects due to potential collinearity.
Figure 3. Joint Effects of All Cortical and Subcortical GM Regions and WM Tracts.

(A) Acute stroke severity (NIH Stroke Scale [NIHSS] score). The posterior distribution of the hyperprior capturing the effect of all subcortical gray matter (GM) regions did not substantially overlap with zero (i.e., the highest probability density interval covering 90% certainty did not include the zero, highlighted by the red frame). (B) Ninety-day unfavorable outcome (modified Rankin Scale score >2). Conversely, the contribution of white matter (WM) tract lesions was the most meaningful for long-term outcomes, while posterior distributions for GM lesion hyperpriors did not substantially differ from zero.
Investigating Indirect Lesion Effects: Functional Lesion Connectivity
Across all patients, insular, opercular, and precentral and postcentral regions were the ones maximally functionally connected to the acute ischemic lesions (Figure 4A, regions in red). Posterior and medial frontal regions, on the other hand, were predominantly functionally negatively connected to the acute ischemic lesions; i.e., we observed anticorrelations between the lesions and respective brain regions (Figure 4A, regions in blue). It is important to note that these lesion connectivity patterns describe indirect lesion effects because connectivity to any parcels that were directly affected by ischemia were not included. Patients without good reperfusion after EVT featured more pronounced lesion connectivity in the positive and negative directions. In particular, precentral and postcentral regions in the right hemisphere were more positively and left occipital regions more negatively connected to lesioned tissue (Figure 4B). Whole-brain lesion connectivity (i.e., averaged absolute connectivity within all 100 Yeo-Schaefer atlas–defined parcels) correlated positively with 90-day mRS scores (r = 0.32, p = 0.00005; for comparison, Spearman correlation between acute NIHSS score and 90-day mRS score: r = 0.23, p = 0.005). Therefore, the higher lesion connectivity in the absence of good reperfusion after EVT may indicate worse functional outcomes. While patients with and those without good reperfusion differed significantly with respect to lesion volume (median 34.1 cm3 [IQR 96.9 cm3] vs 132.5 cm3 [IQR 148.4 cm3], p = 0.002), these results remained broadly the same after adjustment for lesion volume (eFigure 2, links.lww.com/WNL/B775).
Figure 4. Indirect Lesion Effects as Evaluated Through Functional Lesion Connectivity.
(A) Lesion connectivity of the entire stroke sample. Lesions were positively connected to insular, opercular, and precentral and postcentral regions that were mostly part of the sensorimotor network. Negative lesion connectivity could be observed to posterior and medial frontal regions. (B) Significantly different lesion connectivity between patients without and with good reperfusion after endovascular thrombectomy. Generally, patients without good reperfusion experienced more pronounced positive lesion connectivity to right precentral and postcentral regions.
Discussion
Among this large cohort of patients with LVO, MRI-defined region-specific infarct topography after EVT is associated with both acute stroke severity and long-term functional outcomes. Lesion locations predictive of 90-day mRS involved bilateral precentral and postcentral gyri, insula and operculum, and left putamen and caudate nucleus. There was a more pronounced effect within the left hemisphere. While lesions affecting subcortical nuclei had the highest relevance in explaining acute stroke severity, lesions affecting WM tracts had the highest relevance for 90-day mRS scores.
The findings from this large LVO cohort are likely generalizable because the cohort has clinical features similar to those described in other EVT studies.3 The mean age was 68 years; 52% were female; median NIHSS score was 16; and 84% achieved TICI 2b to 3 reperfusion. It is surprising that lesion topographies were found to be more variable in the left hemisphere; 6 distinct patterns were uncovered for the left hemisphere yet only 3 for the right. This is not easily explained by differences in vascular anatomy but may have arisen due to slightly higher numbers of patients with left hemispheric stroke.
Regarding 90-day functional outcomes, several regions were identified as particularly important. The results of our bayesian model of region-specific infarct topography are particularly robust because it outperformed a model excluding the regions. Several regions identified in our model affirm what is believed in clinical practice such as the importance of motor- and language-related structures. Indeed, both the bilateral precentral gyri of the frontal lobe, subserving primary motor function, and the bilateral postcentral gyri of the parietal lobe, subserving primary somatosensory function,31 were relevant for 90-day outcomes. Furthermore, the more pronounced importance of left-sided regions within the cortex, deep nuclei, and WM tracts may reflect that language is left-sided in the majority of patients.32
The importance of bilateral insular cortex was somewhat surprising but may be attributed to altered consciousness/emotions, autonomic dysregulation, and possibly aspiration.31,33 Opercular cortex, involving inferior precentral, inferior postcentral, and Heschl gyri,31 also stood out for its relevance to functional outcomes. Recently, right central opercular lesions were associated with depressive symptoms after stroke.34 The importance of lesions to the right frontal pole may be related to altered cognition or anxiety after stroke.31,35 In addition, the bilateral middle frontal gyri of the frontal lobes were implicated. The right middle frontal gyrus has been shown to play a role in attention.36 Other frontal lobe gyri with particular relevance included the bilateral inferior frontal gyri. This region is part of the prefrontal cortex and contains the Broca area31; right-sided infarcts may be related to poststroke psychosis.37
Subcortical regions, including both gray nuclei and WM tracts, were important for 90-day outcomes. The left-sided basal ganglia were more relevant than the right. The left putamen, while historically involved in motor control, has recently been shown to be involved in language.38 Similarly, the left caudate is often associated with cognitive or language problems when infarcted in isolation but can result in movement abnormalities and neuropsychiatric symptoms when infarcts extend to more of the basal ganglia.39 Several WM tracts were particularly relevant for long-term outcomes when infarcted. It is not surprising that the corticospinal tract, responsible for voluntary motor control,31 stood out among them. The corticospinal tract is the most-studied WM tract in stroke, and infarcts involving this tract have been related to long-term outcomes.40,41 Our results suggest that left-sided injury may be more deleterious. While speculative, this may relate to worsened disability in the setting of an affected dominant hand.32
Other WM tracts were identified in our study as having relevance for long-term outcomes after stroke. The left anterior thalamic radiation connects the prefrontal cortex and the thalamus through the anterior limb of the internal capsule.31 Lesions to this tract have been related to psychosis and apathy.42 Lesions to the bilateral forceps major were also important for long-term outcomes. This tract curves posterior from the splenium of the corpus callosum into the occipital lobe31; infarcts have been associated with visual hallucinations and topographic disorientation.43 Other tracts identified in our study were the bilateral inferior fronto-occipital fasciculi, which pass lateral to the caudate and medial to the corona radiata.31 They have bilateral involvement in language semantics.44 The bilateral superior longitudinal fasciculi, which pass lateral to the centrum semiovale and connect the frontal, parietal, occipital, and temporal lobes,31 were also relevant for outcomes in our study. Another small study of diffusion tensor imaging showed that infarction of this region correlated with worse outcomes after stroke.45 We also identified the left uncinate fasciculus as important for outcomes. It connects the anterior temporal lobe (parahippocampus and amygdala) with the inferior frontal lobe (orbitofrontal cortex).31 Lesions of this structure have been associated with psychiatric conditions, learning difficulty, and language impairment.46,47
While lesions affecting subcortical nuclei had the highest relevance in explaining acute stroke severity, lesions affecting WM tracts had the highest relevance in explaining 90-day mRS scores. In the case of acute stroke severity, the joint effect of all subcortical GM lesions was the only one substantially different from zero. Others have shown that basal ganglia infarction is associated with worse pre-EVT acute stroke severity and discharge stroke severity.48 This may relate to embolic strokes involving the M1 segment causing perforator occlusion. These patients often have higher NIHSS scores due to the lack of developed collaterals. Another group corroborated our results, showing that basal ganglia infarct after EVT was not associated with 90-day long-term outcomes.49 In the case of 90-day functional outcomes, WM tract lesions had the only posterior distribution not overlapping with zero and thus the highest relevance. We previously described the importance of WM infarct volume on post-EVT MRI for 90-day outcomes.13 Others have also supported the significance of WM infarction for 90-day outcomes using CT.50
Lesion network mapping is a powerful, novel technique to estimate the impact of local structural brain lesions on common whole-brain functional networks.51 It has facilitated insights into the origin of a multitude of neuropsychiatric symptoms.14 We used lesion network mapping to derive the overall burden of indirect lesion effects with respect to functional disconnection in our population of patients receiving EVT. Maximally functionally disconnected brain regions were in insular, opercular, and precentral and postcentral cortex. This finding suggests that even those stroke lesions not directly affecting cortical motor areas may interfere with their physiologic function through disconnection and thus may further promote deteriorated function. Patients without successful reperfusion, compared to those with successful reperfusion, were characterized by significantly more pronounced lesion connectivity to multiple brain regions, particularly motor cortex. These lesion connectivity differences could not be explained by differences in lesion volume alone. This suggests that specific lesion topographies, potentially linked to varying cerebral reorganization processes after stroke, modify lesion connectivity. While beyond the scope of our current work, these associations represent a promising starting point for further study.
Understanding the relevance of region-specific infarct topography for long-term outcomes after LVO stroke has both direct and indirect implications for patient care. Improving patient prognostication in the acute setting is instrumental for planning rehabilitative efforts and goals of care discussions with patients and their families.4,5 Patient selection for acute reperfusion therapies may also be influenced; while providers are unlikely to withhold reperfusion therapies on the basis of infarct topography alone, there are likely cases that could be considered for treatment despite large infarct volumes if highly relevant regions are spared. In addition, a better understanding of highly relevant regions allows the development of targeted agents for neuroprotection or neural repair. For instance, this work supports the importance of WM neuroprotection, which has been understudied given limitations of preclinical models.6
There are several limitations of this study. First, consecutive patients were identified retrospectively. Decisions regarding clinical management and imaging were made by treating clinicians, introducing the risk of selection bias. Nevertheless, this study is likely generalizable because baseline characteristics and outcomes, including TICI reperfusion and 90-day mRS disability, were similar to those in randomized trials.3 Second, errors can occur during registration of clinical-grade MRI scans to Montreal Neurological Institute 152 standard space. Compared to research-grade scans, those obtained clinically are often lower quality but lead to more translatable results. Furthermore, all scans were visually inspected and excluded if there was poor registration. The relatively large lesion volumes and high successful registration rate in this study make errors unlikely to affect the interpretation of results. Third, the coarse-grained nature of our 10 lesion patterns that each combined several individual brain regions may be considered a limitation. However, the anatomic configurations of stroke lesions are naturally constrained by human vasculature; our data-driven dimensionality reduction approach addresses this circumstance overtly by combining brain regions that tend to be lesioned simultaneously. Fourth, it is possible that the final chronic lesions differed slightly from those included in this study, but this is unlikely because the median recanalization-to-MRI time was 23 hours. Subsequent imaging after the acute hospitalization was unavailable because it is often not clinically indicated. Furthermore, other 90-day clinical outcomes were not available for the patients in our database. Future studies that build on our work are warranted to investigate differential effects of cortical, subcortical, and WM lesions on other outcomes after stroke. Last, this study included patients over a time frame spanning several trials that changed LVO stroke management. Despite this, we included TICI score as a covariate, and the study variable is post-EVT infarct topography, so interpretation of the results is unlikely to be affected.
These data show that MRI-defined region-specific infarct topography after EVT is associated with both acute stroke severity and long-term functional outcomes. They describe the significance for outcomes of specific brain regions involved in ischemic lesions. Future work in additional datasets is needed to confirm these granular findings.
Acknowledgment
Joyce A. McIntyre maintained the prospective Massachusetts General Hospital stroke database.
Glossary
- ASPECTS
Alberta Stroke Program Early Computed Tomography Score
- AUC
area under the curve
- DWI
diffusion-weighted imaging
- ECASS
European Cooperative Acute Stroke Study
- EVT
endovascular thrombectomy
- GM
gray matter
- HPDI
highest probability density interval
- IQR
interquartile range
- LVO
large vessel occlusion
- mRS
modified Rankin Scale
- NASCET
North American Symptomatic Carotid Endarterectomy Trial
- NIHSS
NIH Stroke Scale
- TICI
Thrombolysis in Cerebral Infarction
- WM
white matter
Appendix. Authors

Footnotes
Editorial, page 429
Study Funding
The NIH, National Institute of Neurologic Disorders and Stroke supported this work (R.W.R. by R25 NS065743; N.S.R. by R01 NS082285, R01 NS086905, U19 NS115388).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Regenhardt RW, Das AS, Stapleton CJ, et al. Blood pressure and penumbral sustenance in stroke from large vessel occlusion. Front Neurol. 2017;8(July):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Regenhardt RW, Biseko MR, Shayo AF, et al. Opportunities for intervention: stroke treatments, disability and mortality in urban Tanzania. Int J Qual Health Care. 2019;31(5):385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. [DOI] [PubMed] [Google Scholar]
- 4.Regenhardt RW, Takase H, Lo EH, Lin DJ. Translating concepts of neural repair after stroke: structural and functional targets for recovery. Restor Neurol Neurosci. 2020;38(1):67-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takase H, Regenhardt R. Motor tract reorganization after acute central nervous system injury: a translational perspective. Neural Regen Res. 2021;16(6):1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regenhardt RW, Das AS, Ohtomo R, Lo EH, Ayata C, Gurol ME. Pathophysiology of lacunar stroke: history's mysteries and modern interpretations. J Stroke Cerebrovasc Dis. 2019;28(8):2079-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng B, Forkert ND, Zavaglia M, et al. Influence of stroke infarct location on functional outcome measured by the modified Rankin Scale. Stroke. 2014;45(6):1695-1702. [DOI] [PubMed] [Google Scholar]
- 8.Wu O, Cloonan L, Mocking SJT, et al. Role of acute lesion topography in initial ischemic stroke severity and long-term functional outcomes. Stroke. 2015;46(9):2438-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonkhoff AK, Schirmer MD, Bretzner M, et al. Outcome after acute ischemic stroke is linked to sex-specific lesion patterns. Nat Commun. 2021;12(1):3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheth SA, Malhotra K, Liebeskind DS, et al. Regional contributions to poststroke disability in endovascular therapy. Interv Neurol. 2018;7(6):533-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholson P, Hilditch CA, Neuhaus A, et al. Per-region interobserver agreement of Alberta Stroke Program Early CT Scores (ASPECTS). J Neurointerv Surg .2020;12(11):1069-1071. [DOI] [PubMed] [Google Scholar]
- 12.Regenhardt RW, Etherton MR, Das AS, et al. Infarct growth despite endovascular thrombectomy recanalization in large vessel occlusive stroke. J Neuroimaging. 2020;31(1):155-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regenhardt RW, Etherton MR, Das AS, et al. White matter acute infarct volume After thrombectomy for anterior circulation large vessel occlusion stroke is associated with long term outcomes. J Stroke Cerebrovasc Dis. 2021;30(3):105567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox MD. Mapping symptoms to brain networks with the human connectome. N Engl J Med. 2018;379(23):2237-2245. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson MA, Lim C, Cooke D, et al. A human memory circuit derived from brain lesions causing amnesia. Nat Commun. 2019;10(1):3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regenhardt RW, Turner AC, Hirsch JA, et al. Sex-specific differences in presentations and determinants of outcomes after endovascular thrombectomy for large vessel occlusion stroke. J Neurol. 2022;269(1):307-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):E344–E418. [DOI] [PubMed] [Google Scholar]
- 18.Yu AT, Regenhardt RW, Whitney C, et al. CTA protocols in a telestroke network improve efficiency for both spoke and hub hospitals. AJNR Am J Neuroradiol. 2021;42(3):435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nolan NM, Regenhardt RW, Koch MJ, et al. Treatment approaches and outcomes for acute anterior circulation stroke patients with tandem lesions. J Stroke Cerebrovasc Dis. 2021;30(2):105478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274(13):1017-1025. [PubMed] [Google Scholar]
- 21.Young MJ, Regenhardt RW, Leslie-Mazwi TM, Stein MA. Disabling stroke in persons already with a disability: ethical dimensions and directives. Neurology. 2020;94(7):306-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regenhardt RW, Young MJ, Etherton MR, et al. Toward a more inclusive paradigm: thrombectomy for stroke patients with pre-existing disabilities. J Neurointerv Surg. 2021;13(10):865-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee DD, Seung HS. Learning the parts of objects by non-negative matrix factorization. Nature. 1999;401(6755):788-791. [DOI] [PubMed] [Google Scholar]
- 24.Bonkhoff AK, Lim JS, Bae HJ, Weaver NA, Kuijf HJ, Biesbroek JM, Rost NS, Bzdok D. Generative lesion pattern decomposition of cognitive impairment after stroke. Brain Commun. 2021;3(2): p.fcab110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James G, Witten D, Hastie T, Tibshirani R. An Introduction to Statistical Learning. Springer; 2013. [Google Scholar]
- 26.Yeo BTT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes AJ, Hollinshead MO, O'Keefe TM, et al. Brain Genomics Superstruct Project initial data release with structural, functional, and behavioral measures. Sci Data. 2015;2:150031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102(27):9673-9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101(6):3270-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefer A, Kong R, Gordon EM, et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 2018;28(9):3095-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haines DE. Neuroanatomy Atlas in Clinical Context. Wolters Kluwer; 2018. [Google Scholar]
- 32.Loring DW, Meador KJ, Lee GP, et al. Cerebral language lateralization: evidence from intracarotid amobarbital testing. Neuropsychologia. 1990;28(8):831-838. [DOI] [PubMed] [Google Scholar]
- 33.Raghu ALB, Parker T, Van Wyk A, Green AL. Insula stroke: the weird and the worrisome. Postgrad Med J. 2019;95(1127):497-504. [DOI] [PubMed] [Google Scholar]
- 34.Sutoko S, Atsumori H, Obata A, et al. Lesions in the right Rolandic operculum are associated with self-rating affective and apathetic depressive symptoms for post-stroke patients. Sci Rep. 2020;10(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang WK, Chen Y, Lu J, et al. Frontal infarcts and anxiety in stroke. Stroke. 2012;43(5):1426-1428. [DOI] [PubMed] [Google Scholar]
- 36.Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG. A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci. 2015;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devine MJ, Bentley P, Jones B, et al. The role of the right inferior frontal gyrus in the pathogenesis of post-stroke psychosis. J Neurol. 2014;261(3):600-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viñas-Guasch N, Wu YJ. The role of the putamen in language: a meta-analytic connectivity modeling study. Brain Struct Funct. 2017;222(9):3991-4004. [DOI] [PubMed] [Google Scholar]
- 39.Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108. [DOI] [PubMed] [Google Scholar]
- 40.Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41(5):910-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng W, Wang J, Chhatbar PY, et al. Corticospinal tract lesion load: an imaging biomarker for stroke motor outcomes. Ann Neurol. 2015;78(6):860-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torso M, Serra L, Giulietti G, et al. Strategic lesions in the anterior thalamic radiation and apathy in early Alzheimer's disease. PLoS One. 2015;10(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghosh GN, Wycoco V, Ghosh S. Transient visual hallucinations due to posterior callosal stroke. J Stroke Cerebrovasc Dis. 2015;24(6):e147–e148. [DOI] [PubMed] [Google Scholar]
- 44.Herbet G, Moritz-Gasser S, Duffau H. Direct evidence for the contributive role of the right inferior fronto-occipital fasciculus in non-verbal semantic cognition. Brain Struct Funct. 2017;222(4):1597-1610. [DOI] [PubMed] [Google Scholar]
- 45.Koyama T, Domen K. Diffusion tensor fractional anisotropy in the superior longitudinal fasciculus correlates with functional independence measure cognition scores in patients with cerebral infarction. J Stroke Cerebrovasc Dis. 2017;26(8):1704-1711. [DOI] [PubMed] [Google Scholar]
- 46.Papagno C, Miracapillo C, Casarotti A, et al. What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain. 2011;134(2):405-414. [DOI] [PubMed] [Google Scholar]
- 47.Zhang B, Chang J, Park J, et al. Uncinate fasciculus and its cortical terminals in aphasia after subcortical stroke: a multi-modal MRI study. Neuroimage Clin. 2021;30:102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loh Y, Towfighi A, Liebeskind DS, et al. Basal ganglionic infarction before mechanical thrombectomy predicts poor outcome. Stroke. 2009;40(10):3315-3320. [DOI] [PubMed] [Google Scholar]
- 49.Horie N, Morofuji Y, Iki Y, et al. Impact of basal ganglia damage after successful endovascular recanalization for acute ischemic stroke involving lenticulostriate arteries. J Neurosurg. 2020;132(6):1880-1888. [DOI] [PubMed] [Google Scholar]
- 50.Ospel JM, Menon BK, Qiu W, et al. A detailed analysis of infarct patterns and volumes at 24-hour noncontrast CT and diffusion-weighted MRI in acute ischemic stroke due to large vessel occlusion: results from the ESCAPE-NA1 trial. Radiology. 2021;300(1):152-159. [DOI] [PubMed] [Google Scholar]
- 51.Boes AD, Prasad S, Liu H, et al. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138(10):3061-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We will make available the data that support the findings of this study on reasonable request pending local institutional review board approval.