Abstract
Background and Objectives
To evaluate costs and health-related quality of life (HRQoL) of neuromyelitis optica spectrum disorders (NMOSD) and myelin oligodendrocyte glycoprotein antibody–associated disease (MOGAD).
Methods
In this multicenter cross-sectional study, data on consumption of medical and nonmedical resources and work ability were assessed via patient questionnaires. Costs were analyzed in Euros for 2018 from the societal perspective. HRQoL was captured by the EuroQoL Group 5 Dimension 5 Level Scale (EQ-5D-5L) questionnaire. Clinical data were retrieved from the Neuromyelitis Optica Study Group (NEMOS) database.
Results
Two hundred twelve patients (80% women, median age 50 [19–83] years, median disease duration 7 [0–43] years, median Expanded Disability Status Scale [EDSS] score 3.5 [0–8.5], 66% aquaporin-4 immunoglobulin G [IgG] positive, 22% MOG IgG positive, 12% double seronegative) were analyzed. The mean total annual per capita cost of illness accounted for €59,574 (95% CI 51,225–68,293 or US dollars [USD] 70,297, 95% CI 60,445–80,586), and the mean index value of the EQ-5D-5L was 0.693 (95% CI 0.65–0.73). The most important cost drivers were informal care costs (28% of total costs), indirect costs (23%), and drugs (16%), especially immunotherapeutics. Costs showed a positive correlation with disease severity (ρ = 0.56, 95% CI 0.45–0.65); in the EDSS score 6.5 to 8.5 subgroup, the mean annual costs were €129,687 (95% CI 101,946–160,336 or USD 153,031, 95% CI 120,296–189,196). The HRQoL revealed a negative correlation to disease severity (ρ = −0.69, 95% CI −0.76 to −0.61); in the EDSS score 6.5 to 8.5 subgroup, the EQ-5D-5L mean index value was 0.195 (95% CI 0.13–0.28). Neither antibody status nor disease duration influenced the total annual costs or HRQoL.
Discussion
These German data from the era without approved preventive immunotherapies show enormous effects of the diseases on costs and quality of life. An early and cost-effective therapy should be provided to prevent long-term disability and to preserve quality of life.
Neuromyelitis optica spectrum disorders (NMOSD) are rare but well-characterized chronic autoimmune diseases of the CNS affecting mainly the optic nerves and spinal cord.1,2 Those affected can have severe physical disability even after the first attack.3,4 Recent data from smaller cohorts of 25 to 74 patients suggest a significant reduction in the quality of life of patients.5-8 However, exact data on the effects of NMOSD on patients' professional life, the need for long-term care, and the total cost of illness (COI) are still missing. Until summer 2019, the disease was globally treated off-label with standard immunotherapeutics, preferably rituximab, azathioprine, or mycophenolate mofetil.4,9 New treatments have been and are still being implemented,10 because 4 phase III trials indicate benefits for these new therapeutics of NMOSD.11-14 Approval has already been granted in several countries for eculizumab, satralizumab, and inebilizumab. Given the extraordinarily high costs of the new drugs, a standardized and up-to-date analysis of the “pre–new therapy era” costs of this disease is overdue as guidance for physicians, health policymakers, and health care providers. A recently published study reports patient experience and quality of life in NMOSD.15 This study did not include patients with home care needs and therefore missed a socioeconomically relevant part of patients with NMOSD. Myelin oligodendrocyte glycoprotein antibody–associated disease (MOGAD) is an autoimmune disorder affecting the CNS in a clinical pattern partly similar to that of classic NMOSD but now considered a disease entity pathophysiologically distinct from NMOSD with aquaporin-4 (AQP4) antibodies.16 There are no data on costs and health-related quality of life (HRQoL) for MOGAD to date. This underlines the need for independent research on disease costs and quality of life for these rare diseases. Accordingly, in September 2016, we initiated within the Neuromyelitis Optica Study Group (NEMOS) a Germany-wide study to assess the costs and HRQoL of patients with NMOSD (Costs and Health-related Quality of Life of Patients With NMO Spectrum Disorders [CHANCENMO Study]). The primary objective of this study was to assess the socioeconomic impact of these diseases from the societal perspective, together with the analysis of HRQoL and the main predictors thereof.
Methods
Study Design and Study Population
The study used a multicenter cross-sectional design and was conducted between April 2017 and April 2019 at 17 German NEMOS centers.17 Eligible patients were defined according to the following inclusion criteria: adult patients (≥18 years) with diagnoses of NMOSD according to International Panel for NMO Diagnosis (IPND) criteria 2015 and MOGAD who lived in Germany.1,18 Testing for serum antibodies for AQP4 and MOG immunoglobulin G (IgG) was performed with an established cell-based assay.19,20 The majority of patients (42 of 46) with MOG-IgG were tested with at least 2 different cell-based assays.18 Exclusion criteria were predominant treatment of a disease other than NMOSD/MOGAD and severe cognitive impairment (informed consent not possible).
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the ethics boards of the Hannover Medical School (No. 2016-7217) and other participating centers. All patients gave their written informed consent before enrollment.
Sample Selection
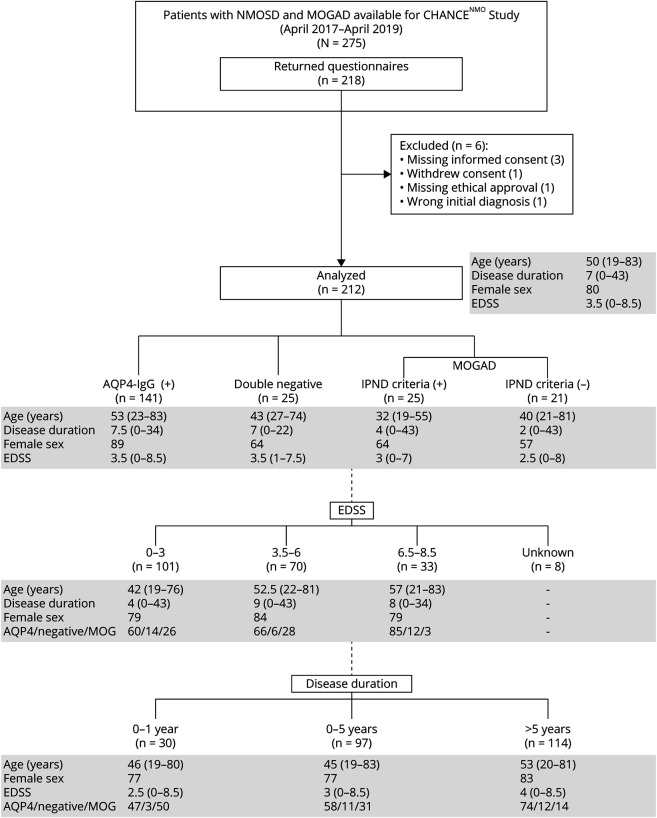
Patients were examined for eligibility by an experienced clinician in the field of neuroimmunology during routine primary or follow-up NEMOS cohort visits. Of 275 available patients, 218 patients returned a complete questionnaire, and 212 datasets were available for analysis (Figure 1).
Figure 1. CHANCENMO-Study Cohort Categorized by Serostatus, EDSS Score, and Disease Duration.
Age and disease duration are shown as median with range in years; female sex and serostatus (aquaporin-4 [AQP4]/neg/ myelin oligodendrocyte glycoprotein [MOG]) are shown as percentage. Patient subgroups were defined on the basis of serostatus, clinical disability (Expanded Disability Status Scale [EDSS] score [score is shown as median with range]), and disease duration. Category boundaries are shown above. EDSS scores for 8 patients were missing. In 1 patient, disease duration is unknown. In both cases, this had no effect on the representativeness of the group composition. IPND = International Panel for NMO Diagnosis; MOG(AD) = MOG immunoglobulin G (IgG) antibody (associated disease); neg = patients with AQP4 and MOG-antibody–negative NMOSD; NMOSD = neuromyelitis optica spectrum disorders.
Outcome Measures
Study participants were asked to answer a paper-based questionnaire (eQuestionnaire, links.lww.com/WNL/B773). Primary patient data on demographics, professional activity (and resulting impairment due to the disease), and retrospective consumption of medical and nonmedical resources were assessed.21 Clinical data on disease onset, severity, duration, serostatus, symptoms, immunotherapy, and management of attacks were retrieved from the NEMOS database in which all centers prospectively update the information of every individual patient. Expanded Disability Status Scale (EDSS) score was assessed by trained physicians during cohort visits. To investigate the patient self-reported HRQoL, we applied the validated EuroQoL Group 5 Dimension 5 Level Scale (EQ-5D-5L).22,23
Cost Estimation
Cost estimation was performed from the societal perspective and by use of a microcosting method following current recommendations for health economic evaluation.24-26 The use of medical and nonmedical resources was assessed retrospectively within different recall periods to reduce recall bias (eTable 8, links.lww.com/WNL/B773). Because we assumed a stable consumption of resources, we extrapolated costs to 1 year when appropriate. Costs were calculated in Euros for the year 2018 (main recruitment period; 2018 average: €1 = US dollar [USD] 1.18). The main cost categories (1) direct medical costs, (2) direct nonmedical costs, and (3) indirect costs yield the total COI. Direct costs consist of direct payments from social and health insurance agencies or the patients themselves. Thus, expenditures for drugs, medical consultations, or formal care, for example, are defined as direct medical costs, while travel expenses, investments in the home and car, and informal care result in direct nonmedical costs. In contrast to formal care, nontrained personnel such as relatives provide informal care. Last, indirect costs represent the loss of productivity of a patient and his or her caregivers due to absenteeism from work because of disability. A detailed explanation about cost valuation methods is provided in the Supplement (links.lww.com/WNL/B773).
Statistical Analysis
The paper-based data were recorded in an Excel 365 (Microsoft, Redmond, WA) database by an independent double data entry for descriptive statistics. Statistical analysis was performed in SPSS statistics version 26.0 (IBM, Armonk, NY) and Prism version 5.02 (GraphPad Software, La Jolla, CA). The D'Agostino-Pearson omnibus test was used to test for normal distribution of the data. Statistical significance of total COI, cost subcategories, and HRQoL parameters (index value, EuroQol Visual Analog Scale [EQ-VAS], and EQ-5D-5L) between disease duration subgroups (0–1 year vs >5 years and 0–5 years vs >5 years) was evaluated by nonparametric Mann-Whitney U test. In addition, the differences in resource use between mildly (EDSS score 0–3) and severely (EDSS score 6.5–8.5) affected patients were tested with this method. Multigroup comparisons were done with analysis of variance for nonparametric data (Kruskal-Wallis test with Dunn multiple-comparison test as a post hoc test). The different serogroups (AQP4 IgG–positive NMOSD, double seronegative NMOSD, MOGAD fulfilling and not fulfilling IPND criteria 2015) were analyzed for differences in total disease costs, cost subgroups, and HRQoL parameters (index value, EQ-VAS score, and EQ-5D-5L score). Likewise, subgroups of different disease severity (patients mildly affected [EDSS score 0–3], moderately affected [EDSS score 3.5–6], and severely affected [EDSS score 6.5–8.5]) were examined for differences in resource use. To evaluate influencing factors on costs and HRQoL as dependent variables, we studied a variety of independent candidate variables. Due to the skewed nature of these dependent variables, we used generalized linear models assuming that the dependent variables follow a gamma distribution rather a normal distribution. We picked those independents variables that showed statistically significant results in univariate regression analysis and information at what point in the joint distribution the nonnormality matters and analyzed them in 2 separated multiple linear regression models. In this context, the variables were assessed for collinearity and interaction. Data from multiple regressions were then entered into 2 generalized linear models with the appropriate link functions to generate the usability of the data regardless of their distribution. Due to this point and the right-skewness of our cost data, we calculated our 95% CIs of the means with SPSS bootstrap function.27,28 Because of the explorative character of the study, we did not correct for multiple testing. Correlations between 2 nonparametric variables were tested with the Spearman test (ρ). Data are expressed as mean and 95% CI. If not otherwise indicated, costs are presented in Euros (2018) per year. Values of P < 0.05 were considered statistically significant. Missing data resulted in different numbers of patients analyzed.
This study was reported following the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.29
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Characteristics of the Study Cohort
Two hundred twelve predominantly female (n = 170, 80%) mainly White (n = 199, 94%) patients were enrolled in the study (Figure 1). Regarding the current diagnostic criteria, two-thirds had AQP4 antibody–positive NMOSD (n = 141, 66%), and ≈1 in 10 patients was diagnosed with double seronegative NMOSD (n = 25, 12%).1 All other patients (n = 46, 22%) could be assigned to MOGAD, with 54% of these meeting the consensus criteria of the IPND 2015.1,18 Social, educational, and occupational information is given in more detail in eTable 1 (links.lww.com/WNL/B773). The number of study participants per federal state reflected the population distribution in German states (eTable 2). In addition, this cohort is representative of the current NEMOS overall study population along relevant dimensions (e.g., regarding EDSS score, age, and sex).
Direct Medical Costs
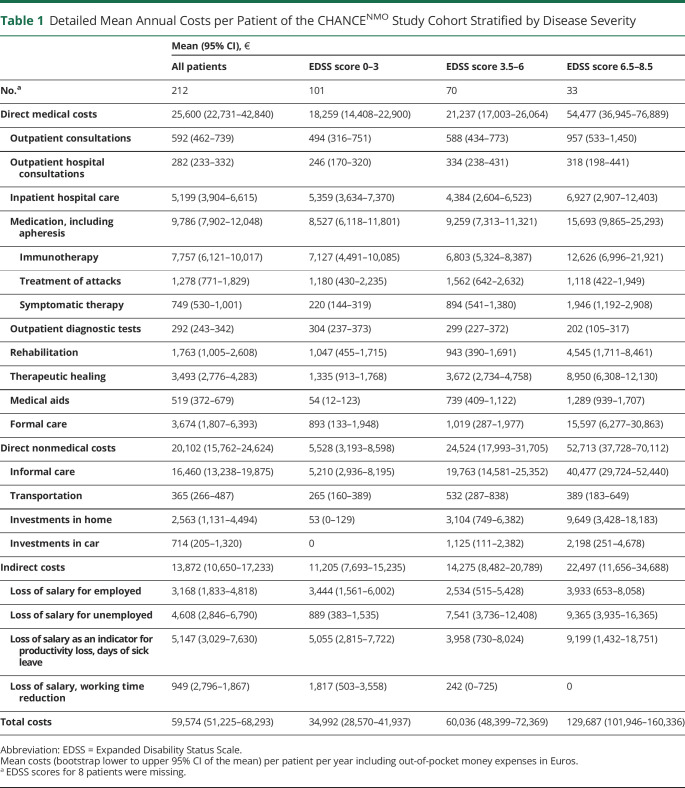
The mean direct medical costs per patient per year amounted to €25,600 (95% CI 22,731–42,840 or USD 30,208, 95% CI 26,823–50,551; Table 1). The most important cost driver was medication, including apheresis therapy (€9,786, 95% CI 7,902–12,048 or USD 11,547, 95% CI 9,324–14,216; 38% of direct medical costs). Immunotherapies were used by 91% of all patients. Rituximab was the treatment of choice in the majority of all patients treated (68%, n = 131). The distribution of immunotherapeutics within the patient cohort is shown in eTable 3 (links.lww.com/WNL/B773). The other 2 most important cost drivers were inpatient hospital care costs (€5,199, 95% CI 3,904–6,615 or USD 6,135, 95% CI 4,607–7,806; 20%) and costs for formal care (€3,674, 95% CI 1,807–6,393 or USD 4,335, 95% CI 2,132–7,544; 14%). Disease severity had an important impact on direct medical costs. This was particularly evident for formal care (Figure 2A and Table 1). eResults and eTable 4 provide detailed characterization of resource use.
Table 1.
Detailed Mean Annual Costs per Patient of the CHANCENMO Study Cohort Stratified by Disease Severity
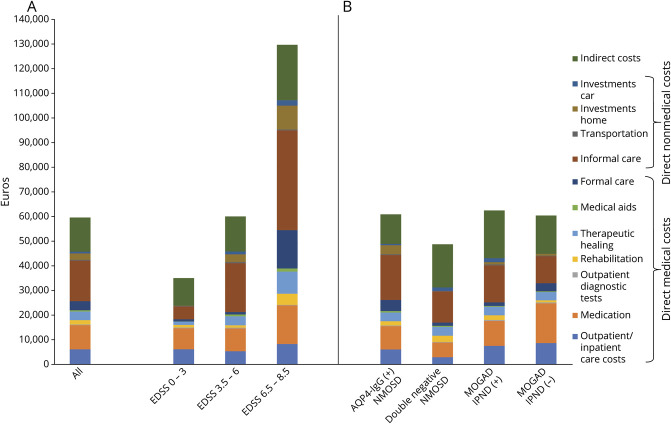
Figure 2. Mean Annual Costs per Patient Stratified by Disease Severity and Serostatus.
Mean total annual costs (2018 Euros) per patient per year of the entire study population and categorized according to disease severity groups (A) and serostatus (B). AQP4 = aquaporin-4; EDSS = Expanded Disability Status Scale; IPND = International Panel for NMO Diagnosis; MOGAD = myelin oligodendrocyte glycoprotein antibody-associated disease; NMOSD = neuromyelitis optica spectrum disorders.
Direct Nonmedical Costs
The mean direct nonmedical costs were calculated at €20,102 (95% CI 15,762–24,624, USD 23,720, 95% CI 18,599–29,056) per patient per year (Table 1). The main cost contributor was informal care (€16,460, 95% CI 13,238–19,875 or USD 19,423, 95% CI 15,621–3,453; 82% of direct nonmedical costs). Again, disease severity was a pivotal factor for the need of individual care. In total, 52% (n = 111) of patients required informal care. The mean hours per day for informal care added up to 1.8 (95% CI 1.4–2.2, eTable 4, links.lww.com/WNL/B773). Accordingly, the reduction of working time by caregivers showed a correlation with increasing EDSS score (ρ = 0.26, 95% CI 0.12–0.39): from 0.3 h/wk (95% CI 0.0–0.81) in the mildly affected group (EDSS score 0–3) to 4.4 h/wk (95% CI 1.5–7.8) in the severely affected group (EDSS score >6). Investments in home and car were made by 21% (n = 45): 5% (n = 5) of the mildly affected group and 52% (n = 17) of the severely affected group. Around 75% of these costs had to be financed by the patients themselves (eTable 5).
Indirect Costs
The mean indirect costs amounted to €13,872 (95% CI 10,650–17,233 or USD 16,369, 95% CI 12,567–20,335) per patient per year (Table 1). The 2 most important cost drivers were loss of salary as an indicator for productivity loss due to days of sick leave (€5,147, 95% CI 3,029–7,630 or USD 6,073, 95% CI 3,574–9,003; 37% of indirect costs) and unemployment (€4,608, 95% CI 2,846–6,790 or USD 5,437, 95% CI 3,358–8,012; 33%). Sick leave was reported by 56 patients (26%) with a mean duration of 32.5 days (95% CI 23.5–41.3) within the last 3 months (eTable 1, links.lww.com/WNL/B773). Sixty percent (n = 128) of the cohort was unemployed, the majority due to NMOSD/MOGAD (n = 41, 32%). There was a negative correlation between employment and EDSS score (ρ = −0.72, 95% CI −0.89 to −0.35). Furthermore, the disease-related reduction in working time was 6.9 h/wk (95% CI 4.2 to 9.2) among employed patients.
Overall Patient and Societal Economic Burden
The mean total annual COI per patient was estimated at €59,574 (95% CI 51,225–68,293 or USD 70,297, 95% CI 60,445–80,586, Table 1). Direct medical costs accounted for 43%, direct nonmedical costs for 34%, and indirect costs for 23% of the annual COI. On average, the out-of-pocket expenses of €3,548 (95% CI 2,116–5,474 or USD 4,187, 95% CI 2,497–6,459) per patient per year amounted to 6% of the annual COI (eTable 5, links.lww.com/WNL/B773). Annual costs rose with increasing EDSS score (ρ = 0.56, 95% CI 0.45–0.65), with the most notable increase in costs for informal care (Figure 2A).
On the basis of an estimated prevalence of NMOSD in Germany of 1.3 per 100,000, the annual burden from a societal perspective was calculated at €64.2 million (USD 75.8 million) for Germany.30
Cost of Illness Stratified by Serostatus and Disease Duration
Total annual COI showed no differences between serogroups (eTable 6, links.lww.com/WNL/B773). Furthermore, the individual cost categories revealed no difference between serogroups except for the cost of outpatient diagnostic tests, which differed significantly (p = 0.01) between AQP4 antibody–positive NMOSD (€248, 95% CI 200–304 or USD 293, 95% CI 236–727) and MOGAD not fulfilling the IPND criteria (€425, 95% CI 276–616 or USD 502, 95% CI 326–727) in the group comparison, likely due to the average disease duration (Figure 1). Considering the average costs, informal care has the largest impact on the costs of patients with AQP4–positive NMOSD (€18,220, 95% CI 14,239–22,686 or USD 21,500, 95% CI 16,802–26,769), and indirect costs were the largest cost driver in patients with MOGAD fulfilling the IPND criteria (€19,391, 95% CI 9,409–31,506 or USD 22,881, 95% CI 11,103–37,177), whereas the main cost driver in patients with MOGAD not fulfilling IPND criteria was medication costs (Figure 2B and eTable 6). There were no differences in total costs per patient for disease duration (eFigure 1).
Health-Related Quality of Life
In the EQ-5D-5L, more than two-thirds of all patients indicated slight to extreme problems regarding the following HRQoL dimensions: pain/discomfort 79% (n = 168), usual activities 69% (n = 146), mobility 67% (n = 142), and anxiety/depression 62% (n = 132). Approximately every third patient (35%, n = 75) stated an impairment in self-care. In all 5 dimensions, the problems revealed a positive correlation with EDSS score (mobility ρ = 0.72, 95% CI 0.65–0.79, self-care ρ = 0.68, 95% CI 0.60–0.75, usual activities ρ = 0.66, 95% CI 0.57–0.73, pain/discomfort ρ = 0.53, 95% CI 0.42–0.63, anxiety/depression ρ = 0.25, 95% CI 0.11–0.38; Figure 3). The mean EQ-5D-5L index value was 0.693 (95% CI 0.65–0.73) using the German value set, with a negative correlation with disease severity (EDSS score 0–3: 0.845, 95% CI 0.82–0.88; EDSS score 3.5–6: 0.705, 95% CI 0.66–0.75; EDSS score 6.5–8.5: 0.195, 95% CI 0.13–0.28; ρ = −0.69, 95% CI −0.76 to −0.61). Likewise, there was a decline in the global quality of life assessment with the EQ-VAS (all patients: 60.9, 95% CI 58.0–64.0; EDSS score 0–3: 70.5, 95% CI 66.5–74.5; EDSS score 3.5–6: 54.5, 95% CI 50.1–59.3; EDSS score 6.5–8.5: 45.6, 95% CI 38.6–52.4; ρ = −0.54, 95% CI −0.63 to −0.42).
Figure 3. Level of Problems Experienced by Patients Stratified by Disease Severity (A) and Serostatus (B).
Patients were able to provide levels on a scale from 0 to 5 (0 = no problems, 5 = unable/ extreme problems) for each of the 5 dimensions of the EuroQoL 5 Dimensions 5 Levels questionnaire. Pain/discomfort between the aquaporin-4 (AQP4) immunoglobulin G (IgG) antibody–positive neuromyelitis optica spectrum disorder (NMOSD) group and the double seronegative NMOSD group was the only dimension that differed significantly (p = 0.009). EDSS = Expanded Disability Status Scale; IPND = International Panel for NMO Diagnosis; MOGAD = myelin oligodendrocyte glycoprotein IgG antibody–positive disease.
For serostatus and disease duration groups, no relevant differences were found within the 5 HRQoL dimensions (Figure 3B and eFigure 2, links.lww.com/WNL/B773). Moreover, neither index value nor EQ-VAS score differed according to serostatus and disease duration groups. The mean EQ-5D-5L index values for the different serogroups are as follows: AQP4 antibody–positive NMOSD 0.663 (95% CI 0.61–0.72), double seronegative NMOSD 0.761 (95% CI 0.67–0.85), MOGAD fulfilling the IPND criteria 0.745 (95% CI 0.63–0.85), and MOGAD not fulfilling the IPND criteria 0.757 (95% CI 0.65–0.86).
Predictors for COI and HRQoL
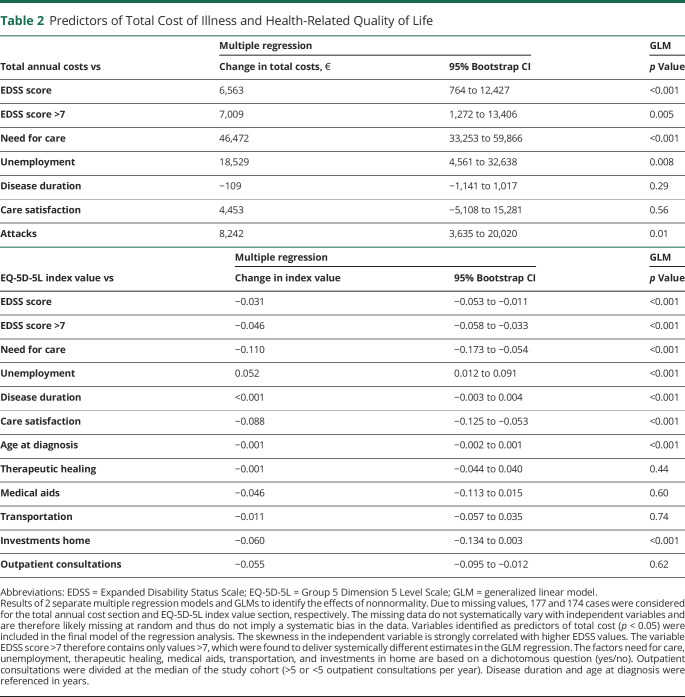
To identify factors that influence the total annual COI and HRQoL, cost categories and disease or patient characteristics were reviewed for any impact in a univariate analysis. In a subsequent generalized linear model analysis, the following independent predictors for COI were detected (Table 2): EDSS score (regression coefficients [RCs] 6,563, 95% CI 7,068–18,286 up to an EDSS score of 6.5 and 7,009, 95% CI 1,272–13,406 from an EDSS score of 7, per increase of 1 EDSS point), need for care (RC 46,472, 95% CI 33,253–59,866), unemployment (RC 18,529, 95% CI 4,561–32,638), and attacks (RC 8,242, 95% CI 3,635–20,020). Accordingly, factors with a significant impact on HRQoL were (Table 2) EDSS score (RC −0.031, 95% CI −0.053 to −0.011 up to an EDSS score of 6.5 and −0.046, 95% CI −0.058 to −0.033 from an EDSS score of 7), need for care (RC −0.110, 95% CI −0.173 to −0.054), unemployment (RC 0.052, 95% CI 0.012–0.091), disease duration (RC <0.001, 95% CI −0.003 to 0.004), care satisfaction (RC −0.088, 95% CI −0.125 to −0.053), age at diagnosis (RC −0.001, 95% CI −0.002 to 0.001), and investments at home (RC −0.060, 95% CI −0.134 to −0.003).
Table 2.
Predictors of Total Cost of Illness and Health-Related Quality of Life
Health Care Satisfaction
Nearly half of the patients stated they were very satisfied with health care, 40% stated they were mostly satisfied, and 10% said they were moderately dissatisfied (eTable 7, links.lww.com/WNL/B773). One hundred twelve patients made additional suggestions for improvement: 25% wanted more information about the disease, its therapy, or research results. About a quarter (24%) asked for more psychological support, and 23% of patients would like to be treated more consistently and preferably by 1 physician.
Discussion
The evaluation of disease-related costs and quality of life not only is important for patients, their families, their physicians, and the society but also descriptively depicts the total burden of an illness to create a basis for decision-making of policymakers, especially in light of new and costly therapies.31,32 There are numerous studies in multiple sclerosis (MS) that have undoubtedly contributed to the optimization of diagnosis and quality of care in patients with MS.33-36 To date, no sufficient data for NMOSD and MOGAD exist in this field. Thus, our aim was to address these issues with this study to improve the quality of care for patients with this rare but serious disease.
It is remarkable that we observed no differences of costs and quality of life regarding serostatus. Our study revealed an annual COI for NMOSD/MOGAD of €59,574 (95% CI 51,225–68,293 or USD 70,297, 95% CI 60,445–80,586). Comparing this study with the largest corresponding study in a German MS cohort indicates that the COI for NMOSD/MOGAD shows an inflation-adjusted higher mean cost (€41,207 or USD 48,624 for MS) despite higher disease severity and patient age in the MS cohort.36 Similarly, patients with NMOSD/MOGAD displayed a poorer quality of life than patients with MS when the mean index value for quality of life assessments of these 2 study collectives was compared (EQ-5D index value 0.693 CHANCENMO vs 0.756 for MS; best possible health condition 1.0). In both diseases, there was a distinct inverse relationship between quality of life and EDSS score.36 Despite a higher EDSS score compared to our study population (5.0 vs 3.5 in our study), a recent study of NMOSD in the United States did not show such a pronounced reduction in quality of life with an EQ-5D mean index value of 0.738; however, patient numbers in that study were small (n = 21 vs 212 in the present study).5 These differences might be due to sociocultural particularities or to their use of a mapping approach to calculate a country-specific value set. In contrast to the general population, the quality of life of patients with NMOSD/MOGAD is substantially impaired; the EQ-5D mean index value was 0.88 in a recently published representative German general population sample (n = 4,998) and 0.938 in a German reference sample (n = 3,552).37,38
In the analysis of total COI and quality of life, no relevant impact was found regarding the disease duration. This reflects the disease course because disability is mainly stable between relapses. Moreover, early treatment might prevent disability and might also have a positive side effect on COI. An annual cost increase for a 1-point rise in EDSS score of €6,563 (95% CI 764–12,427) ending with an EDSS score of 6.5 or €13,261 starting with an EDSS score of 7 emphasizes this. In light of these data, a consequent, albeit expensive, attack treatment (€8,242, 95% CI 3,635–20,020) with early application of apheresis techniques4,39 to avoid an accumulation of disability seems justified also from an economic point of view.
Informal care was the major cost driver at 28% of total COI. It reflects the immense burden on relatives and friends associated with the care of a loved one. These costs rise drastically with increasing disability. This information should be shared with patients and their caregivers, particularly as part of the decision process for an early start to therapy. Moreover, it should be an incentive for clinicians to prevent long-term disability by providing the most appropriate therapy possible and for scientists to gain more profound knowledge about this rare disease to be able to treat it as optimally as possible in the future.
Because this study was conducted in the era without approved standard medications, the costs for immunotherapeutics were moderate with 13% of the total COI. The new NMOSD therapeutics recently approved are considerably more expensive than the off-label therapies used so far and rank among the world's most expensive drugs.2 For example, the annual medication costs for eculizumab in Germany are >10-fold higher than the average total annual COI determined in this study. Recognizing the potentially dramatic disease course of this severe rare disease and its impact on HRQoL, our vision should be to make the best treatment options available to all our patients. Therefore, given the experience regarding the price increase of cancer drugs,40 controlled trials and registered-based studies to evaluate the cost-effectiveness of new NMOSD/MOGAD immunotherapeutics, especially compared with established treatments, are highly warranted.
The strength of this study, considering the rarity of the disease, is the large patient sample size. Furthermore, all clinical data were obtained from a cohort database for which, for example, EDSS score data were assessed by trained physicians and were not self-assessments by patients. The main limitation of our study is that the information on costs was based on patient reports from a questionnaire that inquired retrospective resource consumption with the risk of recall bias. In addition, there is the possibility of a selection bias that more severely affected or visually impaired patients refused to participate in the extensive and time-consuming survey. In contrast, the high recruitment rate of 79% and broad EDSS score distribution reinforce that the results of our study can be generalized. There were no obvious systematic reasons why 57 patients (6 patients were excluded from the original number of patients, as mentioned in Figure 1) did not participate in the survey. We verified that there were no relevant differences between participants and nonparticipants, especially regarding the interesting predictor EDSS score, but also, for example, disease duration, sex, and age. However, it cannot be excluded that, due to the nature of the study, different results would have been obtained if all identified patients had participated. Because our patient cohort had an earlier stage of disease compared to typical MS cohorts, there may be a bias in favor of more diagnostic procedures. In addition, an earlier disease stage may disfavor the need for assistive devices for people with disabilities, although neither of these were relevant cost factors.
In conclusion, NMOSD and MOGAD are extremely costly for the individual, for their families, and for society. The socioeconomic impact depends on the severity of the disease, which has a strong implication on the quality of life. These findings support an early, individually tailored, and cost-effective therapy to prevent long-term disability and to preserve quality of life.
Acknowledgment
The authors thank all patients for participating in the study, their family members, and all contributors of the NEMOS for their support. Special thanks also go to Sikinika Hache for excellent practical support and Tatjana Hümmert, Dr. Rasmus Schülke, Judith Kiehn, and Matthias Kehrig, PhD (Department of Economics, Duke University, Durham, NC) for statistical support. They thank Nirvana Morgan for her diligent proofreading of this manuscript, Jörg Ruge (deputy managing director, PVS Schleswig-Holstein) for his advice on private medical fee accounting in Germany, and Prof. Dr. rer. pol. Christian Krauth (Institute for Epidemiology, Social Medicine and Health Systems Research, Hannover Medical School, Germany) for his input to data evaluation.
Glossary
- AQP4
aquaporin-4
- CHANCENMO Study
Costs and Health-related Quality of Life of Patients With NMO Spectrum Disorders
- COI
cost of illness
- EDSS
Expanded Disability Status Scale
- EQ-5D-5L
EuroQoL Group 5 Dimensions 5 Levels
- EQ-VAS
EuroQol Visual Analog Scale
- HRQoL
health-related quality of life
- IgG
immunoglobulin G
- IPND
International Panel for NMO Diagnosis
- MOGAD
myelin oligodendrocyte glycoprotein antibody–associated disease
- MS
multiple sclerosis
- NEMOS
Neuromyelitis Optica Study Group
- NMOSD
neuromyelitis optica spectrum disorders
- RC
regression coefficient
- USD
US dollar
Appendix 1. Authors
Appendix 2. Coinvestigators

Footnotes
CME Course: NPub.org/cmelist
Study Funding
The authors report no targeted funding for this study. The NEMOS cohort/NationNMO is supported by the German Ministry for Education and Research (BMBF) as part of the German Competence Network Multiple Sclerosis (KKNMS). The contribution of the Berlin team (FP, NS, JBS, AD) was in part supported by DFG Exc 257.
Disclosure
M.W. Hümmert and L.M. Schöppe report no disclosures relevant to the manuscript. J. Bellmann-Strobl has received travel grants and speaking honoraria from Bayer Healthcare, Biogen Idec, Merck Serono, Sanofi Genzyme, Teva Pharmaceuticals, Roche, and Novartis, all unrelated to this work. N. Siebert reports no disclosures relevant to the manuscript. F. Paul receives honoraria for lecturing and travel expenses for attending meetings from Guthy Jackson Foundation, Sanofi Genzyme, Novartis, Alexion, Viela Bio, Roche, UCB, Mitsubishi Tanabe ,and Celgene. His research is funded by the German Ministry for Education and Research, Deutsche Forschungsgemeinschaft, Einstein Foundation, Guthy Jackson Charitable Foundation, EU FP7 Framework Program, Biogen, Genzyme, Merck Serono, Novartis, Bayer, Teva, Alexion, Roche, Parexel, Viela Bio, and Almirall. F.P. serves on advisory boards and steering committees for Novartis and Viela Bio and is associate editor of Neurology, Neuroimmunology & Neuroinflammation and academic editor for PLoS One. A. Duchow reports no disclosures relevant to the manuscript. H. Pellkofer received honoraria for lectures from Bayer Health Care, Biogen Idec, and Teva Pharma and travel reimbursement from Novartis. T. Kümpfel has received speaker honoraria including advisory boards from Bayer Healthcare, Teva Pharma, Merck, Novartis Pharma, Sanofi-Aventis/Genzyme, Roche Pharma, and Biogen, as well as grant support from Novartis and Chugai Pharma in the past. J. Havla reports grants for OCT research from Friedrich-Baur-Stiftung and Merck; personal fees and nonfinancial support from Celgene, Merck, Alexion, Novartis, Roche, Santhera, Biogen, Heidelberg Engineering, and Sanofi Genzyme; and nonfinancial support from the Guthy-Jackson Charitable Foundation, all outside the submitted work. S. Jarius reports no disclosures relevant to the manuscript. B. Wildemann received grants from the German Ministry of Education and Research, German Research Foundation, Dietmar Hopp Foundation, Klaus Tschira Foundation, and Merck Serono; grants and personal fees from Merck, Novartis, and Sanofi Genzyme; and personal fees from Bayer, Biogen, Roche, and TEVA, none related to this work. A. Berthele received speaker and consulting honoraria from Alexion, Biogen, Bayer Healthcare, Celgene, Merck, Novartis Pharma, and Roche, all outside the submitted work. F.T. Bergh received speaker honoraria from Actelion, Alexion, Bayer, Biogen, Genzyme, Merck-Serono, Novartis, Roche, and Teva; travel reimbursement to attend scientific meetings from Bayer, Biogen, Genzyme, Merck-Serono, Novartis, Roche, and Teva; and research support for investigator-initiated studies, through his institution, from the German Research Foundation, the German Federal Ministry of Education and Research, Actelion, Bayer, Merck-Serono, Novartis, and Teva; none of these funds were related to this study. M. Pawlitzki received travel/accommodation/meeting expenses from Novartis. L. Klotz received compensation for serving on Scientific Advisory Boards for Alexion, Genzyme, Janssen, Merck Serono, Novartis, and Roche. She received speaker honoraria and travel support from Bayer, Biogen, Genzyme, Grifols, Merck Serono, Novartis, Roche, Santhera, and Teva. She receives research support from the German Research Foundation, the IZKF Münster, IMF Münster, Biogen, Novartis, and Merck Serono. I. Kleiter has received speaker honoraria including advisory board speaker honoraria from Alexion, Biogen, Celgene, Chugai, IQVIA, Novartis, Merck, Mylan, Sanofi Genzyme, and Roche, as well as travel funding from the Guthy-Jackson Charitable Foundation. M. Stangel has received honoraria for scientific lectures or consultancy from Alexion, Bayer Healthcare, Biogen, Celgene, CSL Behring, Grifols, Janssen, MedDay, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Takeda, and Teva. His institution received research support from Sanofi-Aventis and Merck-Serono. S. Gingele has received speaker honoraria from Alnylam, not related to this manuscript. M.S. Weber receives research support from the Deutsche Forschungsgemeinschaft (WE 3547/5-1), Novartis, Teva, Biogen-Idec, Roche, Merck, and the ProFutura Programm of the Universitätsmedizin Göttingen. M.S.W. is serving as an editor for PLoS One. He received travel funding and/or speaker honoraria from Biogen-Idec, Merck Serono, Novartis, Roche, Teva, Bayer, and Genzyme. J.H. Faiss declares no conflicts of interest. R. Pul received honoraria for lectures from Alexion, Bayer Healthcare, Biogen, Celgene, Novartis, Merck, Roche, Sanofi-Aventis, and Teva. He received research grants from HERZ Burgdorf, Novartis, and Merck. A. Walter received speaker honoraria and meeting expenses from Novartis, Bayer, Biogen, Sanofi Genzyme, Teva, Roche, and Merck. U. Zettl received research support and support for other research activities as well as speaking fees and travel funds from Almirall, Bayer HealthCare, Biogen, Merck Serono, Novartis, Sanofi Genzyme, and Teva. None of this is related to the current study. M. Senel has received consulting and/or speaker honoraria from Alexion, Bayer, Biogen, Merck, Roche, and Sanofi Genzyme. She has received travel support from Celgene and Teva. She has received research funding from the Hertha-Nathorff-Program. None of this is related to the current study. J.P. Stellmann receives grants from Biogen and Genzyme outside the submitted work. He further received personal fees from Biogen and Alexion. V. Häußler reports no disclosures relevant to the manuscript. K. Hellwig received consultant and speaker honoraria from Bayer, Biogen, Merck, Novartis, Sanofi Genzyme, Roche, and Teva. I. Ayzenberg has received travel grants from Biogen Idec and Guthy-Jackson Charitable Foundation, served on scientific advisory boards for Roche and Alexion, and received research support from Diamed, not related to this manuscript. O. Aktas has received personal fees from Alexion, Bayer Healthcare, Biogen, Celgene, Merck Serono, MedImmune, Novartis, Roche, Teva, and Zambon, outside of the submitted work. M. Ringelstein received speaker honoraria from Novartis, Bayer Vital GmbH, Roche, Alexion, and Ipsen and travel reimbursement from Bayer Schering, Biogen Idec, Merz, Genzyme, Teva, and Merck, none related to this study. O. Schreiber-Katz has received honoraria as a speaker/consultant and/or funding for travel expenses from the German Neuromuscular Society “Deutsche Gesellschaft fuer Muskelkranke, Novartis, Biogen GmbH, Biermann Verlag GmbH, and the Jain Foundation. She received research support from the German Neuromuscular Society, 2019 to 2021, as well as academic research support from the Hannover Medical School Young Faculty Program, 2018 to 2020. None of this was related to the current report. C. Trebst has received honoraria for consultation and expert testimony from Alexion Pharma Germany GmbH, Biogen Idec/GmbH, Chugai Pharma Germany GmbH, Merck, Novartis Pharma GmbH, and Roche Pharma GmbH. None of this was related to the current report. Go to Neurology.org/N for full disclosures.
References
- 1.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, Wildemann B. Neuromyelitis optica. Nat Rev Dis Primers. 2020;6(1):85. [DOI] [PubMed] [Google Scholar]
- 3.Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. 2012;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleiter I, Gahlen A, Borisow N, et al. Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol. 2016;79(2):206-216. [DOI] [PubMed] [Google Scholar]
- 5.Mealy MA, Boscoe A, Caro J, Levy M. Assessment of patients with neuromyelitis optica spectrum disorder using the EQ-5D. Int J MS Care. 2019;21(3):129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanson JB, Zephir H, Collongues N, et al. Evaluation of health-related quality of life, fatigue and depression in neuromyelitis optica. Eur J Neurol. 2011;18(6):836-841. [DOI] [PubMed] [Google Scholar]
- 7.Eaneff S, Wang V, Hanger M, et al. Patient perspectives on neuromyelitis optica spectrum disorders: data from the PatientsLikeMe online community. Mult Scler Relat Disord. 2017;17:116-122. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt F, Zimmermann H, Mikolajczak J, et al. Severe structural and functional visual system damage leads to profound loss of vision-related quality of life in patients with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2017;11:45-50. [DOI] [PubMed] [Google Scholar]
- 9.Trebst C, Jarius S, Berthele A, et al. Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS). J Neurol. 2014;261(1):1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duchow A, Paul F, Bellmann-Strobl J. Current and emerging biologics for the treatment of neuromyelitis optica spectrum disorders. Expert Opin Biol Ther. 2020;20(9):1061-1072. [DOI] [PubMed] [Google Scholar]
- 11.Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(7):614-625. [DOI] [PubMed] [Google Scholar]
- 12.Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet. 2019;394(10206):1352-1363. [DOI] [PubMed] [Google Scholar]
- 13.Yamamura T, Kleiter I, Fujihara K, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(22):2114-2124. [DOI] [PubMed] [Google Scholar]
- 14.Traboulsee A, Greenberg BM, Bennett JL, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol. 2020;19(5):402-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beekman J, Keisler A, Pedraza O, et al. Neuromyelitis optica spectrum disorder: patient experience and quality of life. Neurol Neuroimmunol Neuroinflamm. 2019;6(4):e580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients, part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13(1):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuromyelitis Optica Study Group (NEMOS). Accessed February 2, 2022. https://nemos-net.de.
- 18.Jarius S, Paul F, Aktas O, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 2018;15(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waters P, Reindl M, Saiz A, et al. Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J Neurol Neurosurg Psychiatry. 2016;87(9):1005-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reindl M, Schanda K, Woodhall M, et al. International multicenter examination of MOG antibody assays. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreiber-Katz O, Klug C, Thiele S, et al. Comparative cost of illness analysis and assessment of health care burden of Duchenne and Becker muscular dystrophies in Germany. Orphanet J Rare Dis. 2014;9:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludwig K, Graf von der Schulenburg JM, Greiner W. German value set for the EQ-5D-5L. Pharmacoeconomics. 2018;36(6):663-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bock JO, Brettschneider C, Seidl H, et al. Calculation of standardised unit costs from a societal perspective for health economic evaluation [in German]. Gesundheitswesen. 2015;77(1):53-61. [DOI] [PubMed] [Google Scholar]
- 25.Bock JO, Brettschneider C, Seidl H, et al. Recommendations for Cost Valuation Methods From a Societal Perspective in Health Economic Studies [in German]. Nomos Verlagsgesellschaft; 2015. [Google Scholar]
- 26.Icks A, Chernyak N, Bestehorn K, et al. Methods of health economic evaluation for health services research [in German]. Gesundheitswesen. 2010;72(12):917-933. [DOI] [PubMed] [Google Scholar]
- 27.Franklin M, Lomas J, Walker S, Young T. An educational review about using cost data for the purpose of cost-effectiveness analysis. Pharmacoeconomics. 2019;37(5):631-643. [DOI] [PubMed] [Google Scholar]
- 28.Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy. 2004;9(4):197-204. [DOI] [PubMed] [Google Scholar]
- 29.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. [DOI] [PubMed] [Google Scholar]
- 30.Atlas of MS Database for 2013 of the Multiple Sclerosis International Federation. Accessed September 5, 2021. msif.org/about-us/who-we-are-and-what-we-do/advocacy/atlas/. [Google Scholar]
- 31.Jefferson T, Demicheli V, Mugford M. Cost-of-Illness Studies, Elementary Economic Evaluation in Health Care. 2nd ed. BMJ Publishing Group; 2000:17-29. [Google Scholar]
- 32.Jo C. Cost-of-illness studies: concepts, scopes, and methods. Clin Mol Hepatol. 2014;20(4):327-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy N, Confavreux C, Haas J, et al. Economic evaluation of multiple sclerosis in the UK, Germany and France. Pharmacoeconomics. 1998;13(5 pt 2):607-622. [DOI] [PubMed] [Google Scholar]
- 34.Parkin D, Jacoby A, McNamee P, Miller P, Thomas S, Bates D. Treatment of multiple sclerosis with interferon beta: an appraisal of cost-effectiveness and quality of life. J Neurol Neurosurg Psychiatry. 2000;68(2):144-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobelt G, Berg J, Lindgren P, et al. Costs and quality of life of multiple sclerosis in Germany. Eur J Health Econ. 2006;7(suppl 2):S34-S44. [DOI] [PubMed] [Google Scholar]
- 36.Flachenecker P, Kobelt G, Berg J, Capsa D, Gannedahl M; European Multiple Sclerosis Platform. New insights into the burden and costs of multiple sclerosis in Europe: results for Germany. Mult Scler. 2017;23(2_suppl):78-90. [DOI] [PubMed] [Google Scholar]
- 37.Grochtdreis T, Dams J, Konig HH, Konnopka A. Health-related quality of life measured with the EQ-5D-5L: estimation of normative index values based on a representative German population sample and value set. Eur J Health Econ. 2019;20(6):933-944. [DOI] [PubMed] [Google Scholar]
- 38.Janssen B, Szende A. Population norms for the EQ-5D. In: Szende A, Janssen B, Cabases J, eds. Self-Reported Population Health: An International Perspective Based on EQ-5D. Dordrecht (NL): Springer; 2014:19-30. [PubMed] [Google Scholar]
- 39.Kleiter I, Gahlen A, Borisow N, et al. Apheresis therapies for NMOSD attacks: a retrospective study of 207 therapeutic interventions. Neurol Neuroimmunol Neuroinflamm. 2018;5(6):e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saltz LB. Perspectives on cost and value in cancer care. JAMA Oncol. 2016;2(1):19-21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.