Abstract
With the advent of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic, several vaccines have been developed to mitigate its spread and prevent adverse consequences of the Coronavirus Disease 2019 (COVID-19). The mRNA technology is an unprecedented vaccine, usually given in two doses to prevent SARS-CoV-2 infections. Despite effectiveness and safety, inter-individual immune response heterogeneity has been observed in recipients of mRNA-based vaccines. As a novel disease, the specific immune response mechanism responsible for warding off COVID-19 remains unclear at this point. However, significant evidence suggests that humoral response plays a crucial role in affording immunoprotection and preventing debilitating sequelae from COVID-19. As such, this paper focused on the possible effects of age, sex, serostatus, and comorbidities on humoral response (i.e. total antibodies, IgG, and/or IgA) of different populations post-mRNA-based Pfizer-BioNTech vaccination. A systematic search of literature was performed through PubMed, Cochrane CENTRAL, Google Scholar, Science Direct, medRxiv, and Research Square. Studies were included if they reported humoral response to COVID-19 mRNA vaccines. A total of 32 studies were identified and reviewed, and the percent differences of means of reported antibody levels were calculated for comparison. Findings revealed that older individuals, male sex, seronegativity, and those with more comorbidities mounted less humoral immune response. Given these findings, several recommendations were proposed regarding the current vaccination practices. These include giving additional doses of vaccination for immunocompromised and elderly populations. Another recommendation is conducting clinical trials in giving a combined scheme of mRNA vaccines, protein vaccines, and vector-based vaccines.
Keywords: COVID-19, mRNA BNT162b2, antibodies, immunoglobulins, vaccines
Introduction
Over 200 million infected cases and over 4.6 million Coronavirus Disease 2019 (COVID-19) deaths have been reported globally [1]. The rapid transmissibility of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), including variants being monitored (VBM) and variants of concern (VoC), has sparked fear worldwide and forced many countries to deal with repeated surges in confirmed cases and deaths [2,3]. To control the pandemic, innovative therapeutic strategies have been formulated, with the specific aim to avert clinical outcomes and limit morbidity, disability, and death associated with COVID-19 [4]. A SARS-CoV-2 vaccination program that is cost-effective, safe, and efficacious has also been implemented globally. Among the different types of COVID-19 vaccines, the utilization of a new generation of mRNA-based vaccines is unprecedented and has shown high efficacy to trigger immunoprotective humoral response [5]. Despite their effectiveness in reducing the risk of infection and clinical deterioration, the considerable inter-individual heterogeneity in post-vaccine immune response has been increasingly observed in specific populations, particularly among the elderly and immunocompromised individuals [6,7]. To account for low vaccine responders or individuals with less effective production of anti-SARS-CoV-2 antibodies, this systematic review is focused on determining the possible effects of age, sex, serostatus, and underlying comorbidities on humoral response of individuals post-Pfizer-BioNTech mRNA vaccination.
Methods
Search strategy and eligibility criteria
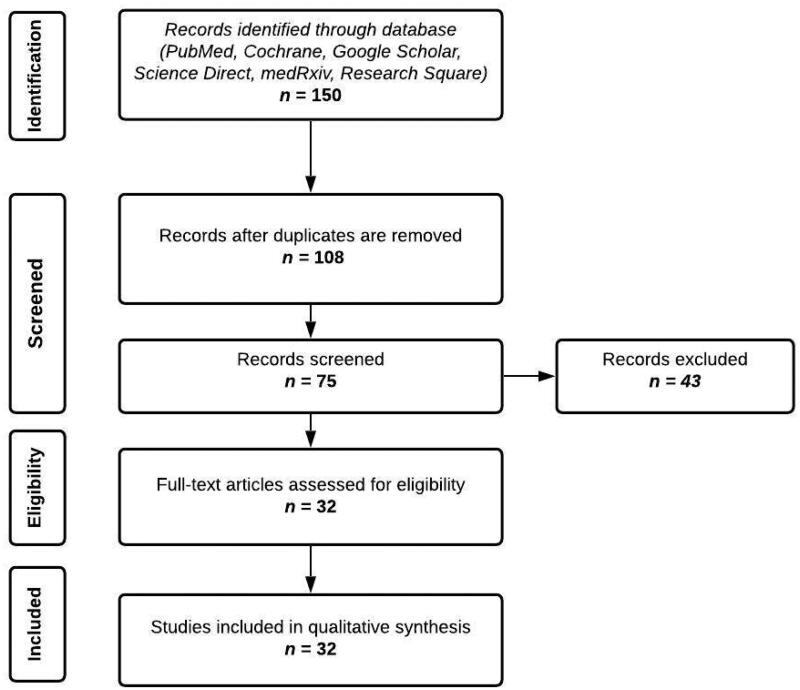
A systematic literature search was conducted to identify studies reporting the factors affecting humoral response of individuals who received the mRNA vaccines. As shown in Figure 1, a comprehensive search was carried out in PubMed, Cochrane CENTRAL, Google Scholar, Science Direct, medRxiv, and Research Square for articles published from January to end of July 2021. The search keywords include “SARS-CoV-2”, “COVID-19”, “age”, “sex”, “seropositivity”, “comorbidities”, “hemodialysis”, “malignancy”, “transplant”, “obesity”, “immunocompromised”, “humoral response”, “Pfizer-BioNTech”, and “BNT162b2”, which resulted in 150 journal articles. Key term combinations that were used are presented in full detail in Table S1 of the supplementary file.
Figure 1.
Screening and appraisal of journal articles for inclusion in this systematic review.
Articles that met the following inclusion criteria were considered: (1) sample size of 10 patients and above, (2) all participants received two complete doses of Pfizer-BioNTech (mRNA BNT162b2) only, (3) report of individual IgG or IgA, total IgG or IgA, or neutralizing antibody titers, (4) report of quantitative or semi-quantitative antibody tests, (5) report of humoral response, (6) available in the English language, as well as (7) randomized controlled or cohort studies and preprint or published papers as long as they provide extractable data, given the limited papers available for this novel disease and the mRNA vaccine.
On the other hand, exclusion criteria were: (1) inclusion of participants who received one dose of mRNA vaccine only, (2) inclusion of participants who received other vaccines such as Moderna (mRNA 1273), Sinovac, AstraZeneca, Johnson & Johnson, Novavax, and Sanofi-GSK, and (3) report of IgM response and cell-mediated immunity. IgM response was excluded because it is now increasingly clear that IgM plays a minor role against COVID-19, as it has lower sensitivity (64%), specificity (99%), and accuracy (94%) compared to IgG (93%, 100%, and 98%, respectively) [8]. IgM has also been found to decline early at day 20 and has lower neutralizing potential [9]. Cell-mediated immunity was excluded due to limited data and lack of standardization of available assays, hence imposing difficulty in data analysis, and thus we only focused on humoral immunity.
The search results were tabulated and duplicates were removed. Full text of each article was retrieved and assessed for final eligibility independently by any two authors. Any discrepancies between the authors with respect to eligibility were resolved by consensus among all the authors.
Data extraction
Data were extracted independently from each article by two authors into a spreadsheet. A third author checked the extracted data for completeness and accuracy. Any disagreements were resolved by consensus among the authors.
Descriptive and outcome data were extracted from the included studies. The extracted data include the sample size, country of origin, age range or median age of the population, type of immunoglobulin measured, antibody titer per time point, and percent mean difference between the control and factors affecting humoral immune response observed in various studies, as reported in Tables 1–4 and Tables S2–S5 in the supplementary file. Additional data were requested from the original study authors when necessary.
Table 1.
Effect of age in humoral response following Pfizer-BioNTech (mRNA BNT162b2) vaccine administration.
| Author | Country of origin | Sample size | Median age (range) | Measured immunoglobulin | % difference of means |
|---|---|---|---|---|---|
| Jabal et al. [10] | Israel | 514 | 57 y/o (30–60+) | Anti-S1/S2 IgG |
D21 IgG titer is 61% higher in <30 y/o vs. 30–39 y/o group IgG titer is 32% higher in <30 y/o vs. 40–49 y/o group IgG titer is 39% higher in <30 y/o vs. 50–59 y/o group IgG titer is 50% higher in <30 y/o vs. 60+ y/o group IgG titer is 19% higher in <30 y/o vs. 40–49 y/o group IgG titer is 23% higher in 30–39 y/o vs. 50–59 y/o group IgG titer is 41% higher in 30–39 y/o vs. 60+ y/o group IgG titer is 10% higher in 40–49 y/o vs. 50–59 y/o group IgG titer is 23% higher in 40–49 y/o vs. 60+ y/o group IgG titer is 19% higher in 50–59 y/o vs. 60+ y/o group |
| Naaber et al. [11] | Estonia | 118 | 34 y/o (21–68) | Anti-S-RBD IgG |
D21 IgG titer is 53% higher in <40 y/o vs. >40 y/o group D28 IgG titer is 26% higher in <40 y/o vs. >40 y/o group D63 IgG titer is 27% higher in <40 y/o vs. >40 y/o group |
| Salvagno et al. [12] | Italy | 925 | Mean age (range) Seropositive: 43 y/o (30–56) Seronegative: 44 y/o (31–57) |
Anti-S-RBD IgG |
D21 IgG titer is 84% higher in <60 y/o vs. ≥60 y/o group D50 IgG titer is 33% higher in <60 y/o vs. ≥60 y/o group |
| Ríos et al. [6] | Spain | 134 |
Total: 82.9 y/o (65–99) ≥80 y/o (n = 86) (ND) 65–79 y/o (n = 48) (ND) |
Anti-S IgG |
D43 IgG is 28% higher in ≥80 y/o vs. 65–79 y/o group |
| Pellini et al. [13] | Italy | 248 |
Total: 47 y/o (18–75) ≤37 y/o (n = 62) (ND) 37–47 y/o (n = 63) (ND) 47–56 y/o (n = 64) (ND) >56 y/o (n = 59) (ND) |
Anti-S1/S2 IgG |
D28 IgG titer is 27% higher in <37 y/o vs. 37–47 y/o group IgG titer is 47% higher in <37 y/o vs. 47–56 y/o group IgG titer is 60% higher in <37 y/o vs. >56 y/o group IgG titer is 28% higher in 37–47 y/o vs. 47–56 y/o group IgG titer is 45% higher in 37–47 y/o vs. >56 y/o group IgG titer is 24% higher in 47–56 y/o vs. >56 y/o group |
| Müller et al. [14] | Germany | 176 | Mean age (range) Younger group (<60 y/o, n=91): 42.2 y/o (19.5–59.5) Elderly group (>80 y/o, n=85): 87.9 y/o (80.1–100.5) |
Anti-S1 IgG |
D17–19 IgG is 87% higher in the younger vs. elderly group D38 IgG is 64% higher in the younger vs. elderly group |
| Frenck et al. [15] | 12–15 y/o group from United States 16–25 y/o group from other countries (Argentina, Brazil, Germany, South Africa, Turkey) |
3358 |
Received Pfizer Vaccine (n=1668) 12–15 y/o (n = 1131): 14 (12–15) 16–25 y/o (n = 537): 18 (16–25) Placebo (=1690) 12–15 y/o (n = 1129): 14 (12–15) 16–25 y/o (n = 561): 19 (16–25) |
Anti-SARS-CoV-2 serum neutralization assay |
D49 In those without evidence of infection, neutralizing titer is 43% higher in the 12–15 y/o vs. 16–25 y/o group In all participants who received Pfizer vaccine, regardless of serologic evidence of previous infection, neutralizing titer is 43% higher in the 12–15 y/o vs. 16–25 y/o group In all participants who received placebo, regardless of serologic evidence of previous infection, neutralizing titer is 29% higher in the 12–15 y/o vs. 16–25 y/o group |
Table 2.
Effect of sex in humoral response following Pfizer-BioNTech (mRNA BNT162b2) vaccine administration.
| Author | Country of origin | Sample size | Median age (range) | Measured immunoglobulin | % difference of means |
|---|---|---|---|---|---|
| Salvagno et al. [12] | Italy | 925 |
Seropositive: ND y/o (30–56) Seronegative: ND y/o (31–57) |
Anti-S-RBD IgG |
D30 Among all participants, IgG titer is 13% higher in females vs. males Among baseline seronegative participants, IgG titer is 16% higher in females vs. males |
| Pellini et al. [13] | Italy | 248 | 47 y/o (23–69) | Anti-S1/S2 IgG |
D28 IgG titer is 37% higher in females vs. males |
| Jabal et al. [10] | Israel | 514 | 57 y/o (30–60+) | Anti-N IgG |
D21 IgG titer is 15% higher in females vs. males |
Table 3.
Effect of serostatus in humoral response following Pfizer-BioNTech (mRNA BNT162b2) vaccine administration.
| Author | Country of origin | Sample size | Median age (range) | Measured immunoglobulin | % difference of means |
|---|---|---|---|---|---|
| Salvagno et al. [12] | Italy | 925 |
Seropositive: 43 y/o (ND) Seronegative: 44 y/o (ND) |
Anti-S-RBD IgG |
D21 IgG titer is 99% higher in seropositive vs. seronegative groups D50 IgG titer is 91% higher in seropositive vs. seronegative groups |
| Kelsen et al. [16] | USA | 61 |
Seropositive: 47 y/o (ND) Seronegative: 45 y/o (ND) |
Anti-S-RBD IgG |
D1 IgG titer is 100% higher in seropositive vs. seronegative groups D14 IgG titer is 95% higher in seropositive vs. seronegative groups D28 No significant difference D42 No significant difference D56 No significant difference |
| Callegaro et al. [17] | Italy | 184 |
Seropositive: 49 y/o (43–55) Seronegative: 51 y/o (39.7–56) |
Anti-S-RBD IgG |
D20 IgG titer is 36% higher in seropositive vs. seronegative groups |
| Efrati et al. [18] | Israel | 255 | >18 y/o (ND) | Anti-S1/S2 IgG |
D1 IgG titer is 89% higher in seropositive vs. seronegative groups D21 IgG titer is 83% higher in seropositive vs. seronegative groups |
| Ebinger et al. [19] | USA | 1090 | Mean (SD) Total: 41.89 y/o (12.18) Pre-vaccine: 41.60 y/o (12.05) Post-vaccine dose 1: 43.66 y/o (12.79) Post-vaccine dose 2: 44.12 y/o (12.65) |
Anti-SARS-CoV-2 IgG |
D1–3 Ab titer is 90% higher in participants with previous infection vs. those without D7–21 Ab titer is 30% higher in participants with previous infection vs. those without D28–42 Ab titer is 7% higher in participants with previous infection vs. those without |
| Prendecki et al. [20] | England | 72 | ND | Anti-S IgG |
D21–25 IgG titer is 96% higher in seropositive vs. seronegative groups |
| Ríos et al. [6] | Spain | 134 | 82.9 y/o (65–99) | Anti-S IgG |
D0 IgG titer is 69% higher in seropositive vs. seronegative groups D28–64 ND |
| Jabal et al. [10] | Israel | 514 | 57 y/o (19–77) | Anti-N IgG |
D21 Geometric mean IgG titer is 89% higher in participants with a (+) PCR test vs. participants who are IgG (–) at baseline and no prior positive PCR test Geometric mean IgG titer is 92% higher in participants who are IgG (+) at baseline vs. participants with IgG (–) at baseline and no prior positive PCR test Geometric mean IgG titer is 88% higher in participants who are IgG (–) with prior (+) PCR test vs. participants who are IgG (–) at baseline and no prior positive PCR test |
| Padoan et al. [21] | Italy | 163 | Mean (SD) 42.4 y/o (11.7) |
Anti-S-RBD IgG | The estimated mean cannot be computed since no sample size was given per group and per time point |
| Favresse et al. [22] | Belgium | 231 |
Female: 42.6 y/o (23–66) Male: 42.8 y/o (23–64) |
Anti-NCP IgG, Anti-S1 IgG |
D7 Ab titer is 99% higher in seropositive vs. seronegative groups D10 Ab titer is 99% higher in seropositive vs. seronegative groups D14 Ab titer is 99% higher in seropositive vs. seronegative groups D21 Ab titer is 99% higher in seropositive vs. seronegative groups D28 Ab titer is 89% higher in seropositive vs. seronegative groups |
| Sasso et al. [23] | Italy | 2607 |
Vaccinated: 57 y/o (41–65) Recovered: 51 y/o (36–56) COVID-19 recovered: 56 y/o (47–63) |
Anti-S-RBD IgG |
D31–41 Ab titer is 91% higher in vaccinated participants without previous SARS-CoV-2 infection vs. non-vaccinated participants who recovered from COVID-19 Ab titer is 89% higher in vaccinated participants who recovered from COVID-19 vs. non-vaccinated participants who recovered from COVID-19 Ab titer is 14% higher in vaccinated participants without previous SARS-CoV-2 infection vs. vaccinated participants who recovered from COVID-19 |
| Modenese et al. [24] | Italy | 74 | Mean (SD) 48.4 y/o (13.4) |
Anti-S-RBD IgG |
D56 Ab titer is 45% higher in seropositive vs. seronegative groups |
ND: no data.
Table 4.
Effect of comorbidities in humoral response following Pfizer-BioNTech (mRNA BNT162b2) vaccine administration.
| Author | Country of origin | Sample size | Median age (range) | Measured immunoglobulin | % difference of means |
|---|---|---|---|---|---|
| Hemodialysis/end-stage renal disease | |||||
| Jahn et al. [25] | Germany |
Controls (n=16) HDPs (n = 72) |
Controls: 45.5 y/o (39–65) HDPs: 68 y/o (37–90) 37–59 y/o: 54 y/o (ND) 60–69 y/o: 64.5 y/o (ND) 70–79 y/o: 76 y/o (ND) 80–90 y/o: 82 y/o (ND) |
Anti-SARS-CoV-2 IgG |
D38 Ab is 44% higher in HCW vs. all HDP Ab is 21% higher in HCW vs. 37–59 y/o HDP Ab is 40% higher in HCW vs. 60–69 y/o HDP Ab is 62% higher in HCW vs. 70–79 y/o HDP Ab is 66% higher in HCW vs. 80–90 y/o HDP Ab is 24% higher in 37–59 y/o HDP vs. 60–69 y/o HDP Ab is 52% higher in 37–59 y/o HDP vs. 70–79 y/o HDP Ab is 57% higher in 37–59 y/o HDP vs. 80–90 y/o HDP Ab is 37% higher in 60–69 y/o HDP vs. 70–79 y/o HDP Ab is 43% higher in 60–69 y/o HDP vs. 80–90 y/o HDP Ab is 9% higher in 70–79 y/o HDP vs. 80–90 y/o HDP |
| Goupil et al. [26] | Canada |
Controls (n = 40) w/o previous SARS-CoV-2 infection (n = 20) w/ previous SARS-CoV-2 infection (n = 20) HDPs (n = 150) w/o previous SARS-CoV-2 infection (n = 131) w/ previous SARS-CoV-2 infection (n = 19) |
Controls w/o previous SARS-CoV-2 infection: 52 y/o (21–59) w/ previous SARS-CoV-2 infection: 46 y/o (23–65) HDPs w/o previous SARS-CoV-2 infection: 73 y/o (33–92) w/ previous SARS-CoV-2 infection: 76 y/o (51–90) |
Anti-N |
D21 Controls IgG is 81% higher in those with previous infection vs. without previous infection D26 HDP IgG is 95% higher in those with previous infection vs. without previous infection D56 HDP IgG is 96% higher in those with previous infection vs. without previous infection D21 and D26 IgG is 89% higher in those controls without previous infection vs. HDP without previous infection IgG is 60% higher in those controls with previous infection vs. HDP with previous infection |
| Frantzen et al. [27] | France | HDPs (n = 244) | 71 y/o (63–80) | Anti-S | No control |
| Zitt et al. [28] | Austria |
Individuals who received the first dose (n = 50) Individuals who received the second dose (n = 48) |
67.6 y/o (ND) | Anti-RBD, anti-NTD IgG | No control |
| Torreggiani et al. [29] |
France |
101 |
68.89 (ND) |
Anti-S IgG |
No control
|
|
Cancer and autoimmune diseases
| |||||
| Herishanu et al. [30] | Israel |
Total (n = 219) CLL/SLL (n = 167) Only CLL w/ antibody response (n = 52) were compared to controls Control (n = 52) |
Control: 68 y/o (64–74) CLL/SLL: 71.0 y/o (63.0–76.0) |
D35–42 IgG titer of control is 80.65% higher vs. CLL patients |
|
| Pimpinelli et al. [31] | Italy |
Controls (n = 36) MM (n = 42) MPM (n = 50) |
>80 y/o (ND) | Anti-S1/S2 IgG |
D21 IgG titer of control is 56% higher vs. MM patients IgG titer of control is 5.26% higher vs. MPM patients D35 IgG titer of control is 69.8% higher vs. MM patients IgG titer of control is 51.06% higher vs. MPM patients |
| Massarweh et al. [32] | Israel |
Controls (n = 78) Solid tumor patients undergoing active IV anticancer treatment (n = 102) |
Control: 62 y/o (49–70) Solid tumor patients undergoing active IV anticancer treatment: 66 y/o (56–72) |
Anti-S-RBD IgG |
D59–61 IgG titer of control is 68.48% higher vs. cancer patients |
| Goshen-Lago et al. [33] | Israel |
Controls (n = 261) Men (n = 118) Women (n = 143) Solid tumor patients receiving active intravenous treatment (n = 232) Men (n = 132) Women (n = 100) |
Controls: 64 y/o (25–81) Solid tumor patients receiving active intravenous treatment: 68 y/o (25–88) |
Anti-S1/S2 IgG | Mean difference could not be computed |
| Furer et al. [34] | Israel |
Controls (n = 121) AIIRD (n = 686) RA (n = 263) PsA (n = 165) AxSpA (n = 68) SLE (n = 101) IIM (n = 19) LVV (n = 21) ANCA-AAV (n = 26) Other vasculitis (n = 23) |
Controls: 50 y/o (18–90) AIIRD: 59 y/o (19–88) RA: 64 y/o (20–88) PsA: 55 y/o (20–86) AxSpA: 49.5 y/o (21–83) SLE: 46 y/o (22–80) IIM: 64 y/o (34–76) LVV: 70 y/o (26–85) AAV: 60.5 y/o (26–85) Other vasculitis: 56 y/o (19–77) |
Anti-S1/S2 IgG |
D45–D63 Ab titer is 39% higher in controls vs. participants with autoimmune inflammatory rheumatic diseases Ab titer is 50% higher in controls vs. participants with rheumatoid arthritis Ab titer is 26% higher in controls vs. participants with psoriatic arthritis Ab titer is 21% higher in controls vs. participants with axial spondyloarthritis Ab titer is 26% higher in controls than participants with systemic lupus erythematosus Ab titer is 80% higher in controls vs. participants with idiopathic inflammatory myositis Ab titer is 34% higher in controls vs. participants with large vessel vasculitis Ab titer is 82% higher in controls vs. participants with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis Ab titer is 44% higher in controls vs. participants with other types of vasculitis |
| Gallo et al. [35] | Italy |
Controls (HCWs) (n = 121) pwMS on OCR (n = 4) |
Controls (HCWs): 41.2 y/o (31.9–55.9) pwMS Patient 1: 33 y/o Patient 2: 35 y/o Patient 3: 42 y/o Patient 4: 51 y/o |
Anti-S IgG |
D14 Geometric mean IgG titer is 92% higher in HCW controls vs. patients with multiple sclerosis D21 Geometric mean IgG titer is 90% higher in HCW controls vs. patients with multiple sclerosis D28 Geometric mean IgG titer is 97% higher in HCW controls vs. patients with multiple sclerosis |
| | |||||
|
Transplant patients
| |||||
| Korth et al. [36] | Germany |
Controls (HCWs) (n = 23) Renal transplant patients (n = 23) |
Controls (HCWs): 44.4 y/o (ND) Renal transplant patients: 57.7 y/o (ND) |
Anti-SARS-CoV-2 IgG |
D36 The IgG titer of control is 92.9% higher than renal transplant patients |
| Marinaki et al. [37] | Greece |
Controls (HCWs) (n = 116) SOT patients (n = 34) |
Controls (HCWs): ND SOT patients: ≤60 y/o: (ND) >60 y/o: (ND) |
Anti-SARS-CoV-2-RBD IgG |
IgG titer post-second dose of BNT162b2 vaccine Antibody response rate of Control is 41.2% higher vs. solid organ transplants |
| Shostak et al. [38] | Israel | 168 | 60.5 y/o (49.25–67.75) | Anti-SARS-CoV-2 IgG |
D22–28 IgG titers of previous seropositive individuals is 81.97% higher vs. seronegative individuals D35–42 IgG titers of previous seropositive individuals is 99.07% higher vs. seronegative individuals |
| Grupper et al. [39] |
Israel |
Controls (HCWs) (n = 25) Kidney transplant patients (n = 136) |
Controls (HCWs): 52.7 (ND) Kidney transplant patients: 58.6 y/o (ND) |
Anti-SARS-CoV-2 S1/S2 IgG |
D31–41 IgG titer of control group is 92.49% higher vs. kidney transplant group |
|
Metabolic derangements and smoking
| |||||
| Ríos et al. [6] | Spain | 134 | 82.9 y/o (65–99) | Anti-S-RBD IgG |
D43 IgG titer of participants with Charlson index <3 is 30% less vs. with Charlson index ≥3 |
| Pellini et al. [13] | Italy | 248 | 47 y/o (18–75) | Anti-S1/S2 IgG |
D28 Ab GMC is 28% higher in underweight vs. normal weight participants Ab GMC is 51% higher in underweight vs. pre-obese individuals Ab GMC is 63% higher in underweight vs. obese individuals Ab GMC is 32% higher in normal weight vs. pre-obese individuals Ab GMC is 49% higher in normal weight vs. obese individuals Ab GMC is 25% higher in pre-obese vs. obese individuals Ab GMC is 44% higher in normotensive vs. hypertensive individuals |
| Watanabe et al. [40] | Italy | 86 | 29 y/o (ND) | Anti-S total Ab |
D28–56 Ab titer is 43% higher in nonsmokers vs. smokers Ab titer is 66% higher in normotensive vs. hypertensive participants Ab titer is 71% higher in non-dyslipidemic vs. dyslipidemic participants |
ND: no data; HDP: hemodialysis patients; HCW: healthcare workers; CLL: chronic lymphocytic leukemia; SLL: small lymphocytic leukemia; MM: multiple myeloma; MPM: myeloproliferative malignancy; AIIRD: autoimmune inflammatory rheumatic disease; RA: rheumatoid arthritis; PsA: psoriatic arthritis; AxSpA: axial spondyloarthritis; SLE: systemic lupus erythematosus; IIM: idiopathic inflammatory myositis; LVV: large vessel vasculitis; ANCA-AAV: antineutrophil cytoplasmic antibody-associated vasculitis; pwMS: people with multiple sclerosis; OCR: ocrelizumab; SOT: solid organ transplant; GMC: geometric mean concentration.
Data analysis
All data reported in this study is in reference to the first dose after vaccine administration. However, due to significant heterogeneity in the assays used to probe antibody titers, the antibody measurements reported in the articles were standardized using the percent difference of means. This shows the absolute value of the ratio of the difference between two groups (groups A and B, which pertain to antibody titer measurements) and their average, expressed as a percentage to enable standard comparison of these data regardless of their units of measurements and the diagnostic tools used for their quantification. It is computed using the formula below:
In addition, when necessary, the authors also utilized the method established by Hozo et al. to convert median titers to mean titers, to enable calculation of percentage of means [41].
Scope and limitations
The demographic parameters used in this study were limited to age, sex, serostatus, and comorbidities such as hemodialysis or end-stage renal disease (ESRD), transplant recipients, cancer and autoimmune diseases, as well as metabolic derangements, including obesity, hypertension, and smoking. These factors were analyzed independently of each other, with the exception of concurrent effects of age with comorbidities.
In addition, only the Pfizer-BioNTech (mRNA BNT162b2) vaccine was discussed as it was the leading vaccine utilized worldwide and due to the wide range of available resources regarding this vaccine at the time of writing. Moreover, only studies published starting January to July 2021 were included while those published from August 2021 onwards [42–44] were not included in this analysis.
Lastly, a quantitative meta-analysis was not completed because of the heterogeneity determined between studies, as well as between different immunoassays used in each study, their units, sensitivities/specificities, and antigenic targets. Thus, percent (%) differences in titers between groups were calculated as an attempt to standardize the antibody data and enable comparisons between different studies.
Results
A total of 32 articles were included in our analysis. These were divided into four categories: humoral response influenced by (1) age, (2) sex, (3) baseline serostatus (i.e. seropositive or seronegative), and (4) presence of comorbidities. Seven articles were included under the age category, three under the sex category, 12 under the serostatus category, and 18 under the comorbidities category. The comorbidity category was further subdivided into four classes: hemodialysis or ESRD (five articles), cancer and autoimmune diseases (six articles), transplant patients (four articles), and metabolic derangements (three articles). The articles reviewed under each category were non-exclusive, as most studies analyzed their samples with at least two of the mentioned factors.
Tables 1–4 provide a summary of the following trends observed in this study. In general, mRNA vaccines were able to mount efficient antibody responses; however, the level of titers produced varied according to the factors of age, sex, serostatus, and comorbidities. The rate at which antibodies produced decline over time is also influenced by the aforementioned factors. Older individuals, males, seronegative individuals, and those with more underlying comorbidities mounted less humoral immune response. Aging, in particular, is a significant aggravating factor in the decline of humoral response among recipients with underlying comorbidities, especially when compounded with immunosuppressive medications.
Discussion
Factors affecting humoral response
Age
A critical factor that makes the elderly more susceptible to infectious diseases is immunosenescence or the decline of immune system functionality as people age [45]. Immunosenescence has been linked with diminished response to vaccination and could therefore influence the success of vaccination [46]. There is clear evidence that the decline in adaptive immunity results in dramatically reduced vaccine responses and vaccine longevity in older adults [45]. This is well-documented with influenza A/H1N1 vaccination, where age negatively correlates with humoral immunity [47,48]. Like any other vaccine, there is accumulating evidence that immunosenescence could also impact the effectiveness of COVID-19 mRNA vaccines, and may hence be less protective to the elderly [49].
Several journal articles reviewed in Table 1, regardless of age stratification, reported that younger individuals developed a greater antibody response after vaccination compared to older individuals [10–15]. Meanwhile, Ríos et al. reported no association between age and antibody response [6]; however, this article may be limited by the characteristics of its sample. The study used residents of long-term care facilities, with variable disability and frailty profiles, who had a mean age of 82.9 years, with a range of 65–99 years.
Nevertheless, the findings of most of the studies were consistent with the current knowledge that there is diminished humoral response among the elderly (>65 years), owing to qualitative differences in memory B cells and plasma cells as well as expansion of a pro-inflammatory subset of B cells [13]. Elderly individuals were noted to have decreased vaccine-specific antibody titers, and thus were more likely to be non- or low-responders [11]. In addition, it was found that the rate of change of the antibody titer of younger individuals (<50 years) was significantly lower in comparison to older participants (≥50 years) [15]. This difference in antibody response was most prominent following the first dose of COVID-19 mRNA but subsequently decreased over time, especially after the second dose [14,15,49].
Interestingly, the relationship between age and IgG or IgA antibody response was not limited to the elderly. Individuals across all age groups demonstrated this trend. Young individuals (12–64 years) consistently produced increased antibody titers compared to their older counterparts. This difference was even more pronounced between groups with large age gaps, further emphasizing the effect of age on antibody response [10–15].
These differences in antibody response have practical implications in COVID-19 vaccination programs. First, they highlight the importance of a second (or even a third) dose in order to boost the protective response in older individuals [11]. They also highlight the need to individualize vaccination programs and create strategies to account for possible age-related limitations of COVID-19 vaccination [14].
Sex
Females develop a greater antibody response due to hormonal differences compared to males, which regulates both adaptive and innate immune responses, with estradiol and testosterone having enhancing and suppressive effects, respectively [50]. However, levels of sex hormones change with age. Thus, after menopause, the drop in estradiol levels enhances immunosenescence [50]. Studies in childhood vaccination enable research that focuses on sex-dependent responses aside from those related to sex hormones that increase after puberty. Such is the case in studies on measles, mumps, and rubella (MMR) and diphtheria, pertussis, and tetanus (DPT) vaccines [51]. These suggest that genetic factors may play a role. The X chromosome expresses more genes, many of which influence immunity. This includes microRNAs (miRNAs) that are known to modulate immunity [51]. Several studies suggest that a similar trend is also observed among COVID-19 mRNA vaccines, noting a higher humoral response and adverse events among women.
Among the articles reviewed in Table 2, two studies showed a direct relationship between sex and humoral response. Both studies by Jabal et al. and Pellini et al. reported that the female sex is generally superior to male in terms of production of IgG on day 21 and day 28 post-vaccination with Pfizer-BioNTech, respectively [10,13]. These data further strengthen the previous studies of Ciarambino et al., which demonstrated that female sex is associated with generally decreased susceptibility to viral infections due to protection given by the X chromosome and/or sex hormones [52]. Specifically, the X chromosome provides females with greater inflammatory, antiviral, and humoral immune responses compared to males. In addition, estrogen, a key hormone in females, plays a significant part in immune regulation.
Furthermore, studies by Ma et al. showed that estrogen can directly inhibit SARS-CoV-2 replication by regulating cell metabolism and maintaining cell integrity through genetic modification and improving metabolic function, reducing incidence of SARS-CoV-2 infection [53]. In contrast, testosterone suppresses immune functions by acting on androgen receptors and immune cell activity, thereby decreasing inflammation and promoting anti-inflammatory responses. Thus, females have a baseline physiologic advantage in mounting immune response compared to males.
Serostatus
Vaccines act by triggering the body’s immune response, leading to development of humoral and cellular immune responses [12]. Several studies have provided evidence that individuals previously infected with SARS-CoV-2 develop early antibody responses right after the primary infection, resulting in inter-individual heterogeneity in post-vaccine immune response [54,55]. The preservation of B-cell-mediated memory immunity from patients’ previous SARS-CoV-2 infection has been theorized to be the primary cause of the boost-like immune response after COVID-19 vaccination in seropositive individuals [10,22].
Publications in Table 3 show a robust and accelerated humoral immune response after the first vaccine dose. However, results of antibody titers after the second dose displayed different trends. In three separate studies, baseline antibody titers of seropositive individuals were multiple folds higher than of seronegative subjects after the second vaccine dose [12, 17, 24]. On the other hand, some publications stated that there was no significant difference between these two groups after the second dose [16,19,21]. As a result, different recommendations can be gathered from different sources. Kelsen et al. recommended that subjects with prior COVID-19 may require only a single dose of vaccine [16]; although, they may eventually need a second dose when antibody levels decline significantly (i.e. typically, after 12 months) or when new VBM and VoC, characterized by the so-called “escape mutations”, become endemic. This is in contrast with the recommendation of Demonbreun et al., which state that one vaccine is not enough to produce strong protection against SARS-CoV-2 infection among most people previously infected [56]. Demonbreun et al. reported that the humoral response of the seropositive group was significantly lower than the response of the PCR (+) group. The study defined the seropositive group based on the presence of anti-RBD IgG antibodies, while participants who tested positive for SARS-CoV-2 on a clinical molecular diagnostic test for acute infection any time prior to vaccination were categorized as recovered COVID-19 under the PCR (+) group [24]. Yan et al. elaborated that difference among anti-SARS-CoV-2 titers may be attributed to higher initial amounts of viral antigens in cases of severe COVID-19. Higher titers may also be the result of an excessive immune response, selective B‐cell plasmablast amplification, and enhanced and prolonged B‐cell receptor stimulation in patients with severe COVID-19 [56].
Therefore, proper clinical investigation is necessary so that the heightened immune response among seropositive individuals may be used to incite strategic change in vaccine distribution, promoting faster and improved vaccine allocation, especially to high-risk populations [57].
Comorbidities
The review focuses on the comparison between a particular comorbidity to a control group to emphasize specifically which patients may be at highest risk of being low-responders who should be prioritized for boosters. The presence of comorbidities, such as chronic kidney diseases (CKD), diabetes mellitus (DM), and cardiovascular disease (CVD), is a clinical risk factor that is significantly associated with poorer prognosis in patients with COVID-19 [20]. This is greatly attributed to the disease mechanisms resulting in metabolic disorders that impair lymphocyte and macrophage functions, negatively affecting immune response after COVID-19 vaccination [58,59].
Additionally, Ríos et al. reported that immune response in the elderly with greater or more severe comorbidities is blunted by 30% as compared to those with lesser comorbidities [6]. This further highlights that increasing age (>60 years) as seen in Table 4, along with other factors such as sex in Table 2, and stage-specific severity for each comorbidity [6], collectively aggravate humoral response post-vaccination [60]. The following sections outline different comorbidities that may affect humoral response following COVID-19 mRNA vaccination, as highlighted in Table 4.
Hemodialysis or end-stage renal disease
Hemodialysis patients (HDPs) have been identified to be at high-risk of acquiring severe COVID-19 associated symptoms [61]. Most of these patients develop uremia, resulting in ineffective leukocyte function, decreased antigen processing and presentation, and subsequently disrupted innate and adaptive immune responses [61]. In the event of infection, these patients cannot fully observe quarantine protocols because of their dependencies on dialysis treatments [62]. Studies have shown that vaccination is the most cost- and resource-effective preventive measure available [25]. Herein, we tackle the immunological response of HDPs to mRNA vaccines.
Among five publications that discussed HDPs, only two studies compared HDPs to controls and both presented consistent findings that the control group had higher immune responses compared to HDPs [25,26]. Nonetheless, all five studies reported a response in the majority of their subjects after two doses of the vaccination. Frantzen et al. noted that the results go far beyond the hyporesponsive population [27].
Furthermore, Jahn et al. reported that HDPs under the age of 60 years responded equally to the control group [25]. However, a limitation of the study was the lack of control subjects over the age of 60 years. In a study by Torreggiani et al., it was found that younger HDPs (62.31 ± 16.20 years) with lower comorbidity burden were more likely to mount an antibody response and have higher response compared to older patients (73.72 ± 11.18 years) with more comorbidity burden [29]. This is consistent with the findings of Jahn et al. comparing the humoral response of HDPs across different age ranges as presented in Table 4 [25]. Torreggiani et al. raised concerns that after the first vaccination only a third of HDPs mounted an immune response [29]. Goupil et al. also stated that a single dose failed to elicit humoral response among HDPs with previous SARS-CoV-2 infection and it was delayed even in those previously infected [26]. Nevertheless, Goupil et al. noted that previously infected patients had higher response compared to the other HDPs.
Overall, there are concerns regarding lower vaccine efficacy and a shorter period of immunoprotection for HDPs. Suggestions on adjusting the vaccine dosage for this group, especially in the elderly population, were recommended in all studies.
Transplant recipients
Transplant recipients are at high-risk of infection resulting from induced immunosuppression, which is necessary to prevent organ rejection, and this is compounded by the immunosuppressive effects of organ failure and chronic disease [63]. Although vaccination has been used as a strategy to prevent infection among transplant recipients, there have been concerns that vaccination may trigger development of donor-specific anti-human leukocyte antigen (HLA) antibodies (DSA) and/or allograft rejection [64,65].
It is thought that vaccination could trigger T- and B-cell responses to vaccine antigens that directly cross-react with alloantigens, as in the case of viral infections [66,67]. Furthermore, vaccination can induce cytokine release that may stimulate previously quiescent alloreactive memory responses [37, 68]. Adjuvants in vaccines can also lead to nonspecific immunostimulating effects that could increase rates of rejection and DSA formation [65,66].
In line with the current pandemic, transplant recipients are a vulnerable group at higher risk for SARS-CoV-2 infection with poorer associated outcomes [68]. The US Centers for Disease Control and Prevention (CDC) affirmed the safety of the Pfizer-BioNTech and Moderna vaccines because neither vaccine contains live virus that could be dangerous to immunocompromised patients [68]. In this section, we describe humoral responses of transplant recipients who have received the mRNA vaccine.
Almost all of the studies reviewed had a mean difference of 90% between transplant recipients and control groups [36,38,39]. This is because of blunted immune response in transplant patients, which is most likely due to taking immunosuppressive medications. While antibody titers may develop, these usually develop late, are below the protective threshold, and unfortunately wane faster [67,69]. The observed failure to mount appreciable antibody immune response in transplant recipients is consistent with previous findings for other common vaccines [39]. Identified contributors to mount a response includes advanced age, need for high dose of corticosteroids during the past 12 months, maintenance with three immunosuppressive medications, and a regimen that includes mycophenolate, antimetabolites, or mTOR inhibitors [39].
Korth et al. proposed that novel vaccination strategies may be needed to address this failure [36]. This could either indicate the need for more than two booster doses or a combined scheme with mRNA vaccines, protein/subunit vaccines, and vector-based vaccines. Longitudinal evaluation may also be needed after vaccination to learn more about long-term immune response in this population.
Cancer and autoimmune disease
Cancer, as a systemic disease, induces functional and compositional changes to the immune system [70]. Some of these changes allow cancer cells to avoid destruction by the immune attack and even support its growth. Studies indicate that B cells responsible for mediating humoral immune response promote and support tumor growth [71]. Depending on the type of cancer, the immunosuppressive tumor microenvironment may vary [72]. In addition, immunosuppression observed among cancer patients may be attributed to their treatment. Similar to the type of cancer, different types of treatment cause different levels of immunosuppression [72]. In a recent study, a significant portion of patients with cancer developed proper humoral immune responses after vaccination [73]. However, recent chemotherapy treatment may be associated with low serologic response [73]. Similar findings were noted on influenza vaccinated patients undergoing chemotherapy compared to healthy controls [74]. Cancer patients who were vaccinated during the early chemotherapy cycle also had better response compared to those vaccinated later in the cycle of the treatment [74]. The possible immunosuppression in cancer patients receiving the Pfizer-BioNTech mRNA vaccine was documented by Herishanu et al., who demonstrated that IgG titers in patients diagnosed with chronic lymphocytic leukemia (CLL) were blunted by 80.65% compared to controls [30]. Moreover, the same pattern of immunosuppression was reported by Massarweh et al., wherein the IgG titers of the control group were 68.48% higher than in cancer patients [32]. Furthermore, similar observations were noted by Pimpinelli et al. in myeloproliferative malignancies (MPMs) and multiple myeloma (MM) [31]. Response in MPM patients was robust, while MM patients had significantly less response. MM patients undergoing regimens without daratumumab were associated with higher adaptive immune responses. This may be due to daratumumab’s mechanism of action that targets CD38 on the population of normal and tumor plasma cells, thus reducing vaccine immunogenicity by direct depletion of antibody producer cells. Nonetheless, Goshen-Lago et al. noted that while there was a pronounced lag in antibody production in cancer cases, seroconversion occurred in most patients after the second dose [33].
An autoimmune disease is a condition arising from an abnormal immune system response that mistakenly attacks healthy cells, tissues, and organs. This immune malfunction can affect any part of the body, weakening bodily function that can be potentially fatal [75]. The cornerstone to management of autoimmune disorders is the use of immunosuppressive therapies. However, various immunosuppressive treatments can impact vaccine-induced immunogenicity [76]. For instance, Gallo et al. reported that the geometric mean IgG titer of patients with multiple sclerosis (pwMS) treated with ocrelizumab is 97% lower than healthy participants [35]. However, this study is limited by the small number of tested patients and the inability to assess their cell-mediated and innate immune responses. Larger studies exploring the response to SARS-CoV-2 vaccines in pwMS treated with anti-CD20 drugs (e.g. rituximab) and other high efficacy disease-modifying therapies (DMTs) are necessary to confirm and expand these preliminary data.
Similarly, Furer et al. reported anti-SARS-CoV-2 S1/S2 IgG titers were 39% lower among participants diagnosed with autoimmune inflammatory rheumatic diseases (AIIRD) as compared to healthy controls [34]. Of note, age may have been a confounding factor that affected the reported results in this study since the majority of the AIIRD participants were elderly with a mean age of 59 years compared to the general population control group with a mean age of 50 years. Among the AIIRD participants, rheumatoid arthritis (RA), antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), and idiopathic inflammatory myositis (IIM) were associated with a low humoral response to the vaccine, which may be partially attributed to their underlying treatment. Furthermore, data presented in this study also have important implications for the management of COVID-19 vaccination in patients with a wide spectrum of AIIRD. Most immunosuppressive treatments, including conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), anticytokine, biologics, and janus kinase inhibitors (JAKi), can be safely continued without significantly attenuating vaccine-induced immunogenicity. In contrast to the recommendation of the American College of Rheumatology, this study does not support withholding methotrexate (MTX) and JAKi prior to administering COVID-19 vaccination [34]. Meanwhile, treatment with glucocorticoids, rituximab, or abatacept in combination with MTX and mycophenolate mofetil was associated with significantly decreased vaccine-induced immunogenicity. Therefore, timing of vaccination has a critical role in these cases. If clinically feasible, postponing administration of rituximab and abatacept, especially when combined with MTX, could improve vaccine-induced immunogenicity.
Metabolic derangements and smoking
Metabolic derangement is an important and prevalent comorbidity that needs to be considered regarding how it affects immune response. Most metabolic derangements are associated with high body mass index (BMI) and thus obesity. A recent review highlighted the effect of metabolic syndromes on immunity, pathogen defense, and coordination of innate and adaptive responses [77]. Changes in these systems are associated with decreased immunity from infection, higher risk for complications, and higher rates of vaccine failure [77]. In a study by Sheridan et al., BMI values correlated positively with higher initial fold increase in IgG antibodies detected after trivalent influenza vaccine [78]. However, 12 months after vaccination, subjects with higher BMI demonstrated a greater decline in antibody titers. The findings on vaccine failure in relation to BMI and obesity are also noted with mRNA-based vaccines [77]. For instance, Pellini et al. reported that there is higher anti-spike S1/S2 IgG production in individuals with lower BMI (BMI <18.5) as compared to pre-obese (BMI = 25.0–29.9) or obese (BMI >30.0) participants receiving the Pfizer-BioNTech COVID-19 mRNA vaccine [13]. Furthermore, anti-spike immunoglobulin production was 71% higher in non-dyslipidemic than dyslipidemic participants vaccinated with the Pfizer-BioNTech mRNA vaccine [40]. These findings may be associated with adipokines, cytokine-like hormones released by adipose tissues that bridge cellular metabolism to immune responses. In particular, leptin plays an important role in controlling the interplay between cellular energy metabolism and regulation of metabolic-immune responses. Indeed, leptin plays a role in modulating cell proliferation, responsiveness, and polarization of T cells. Conversely, leptin promotes B cell homeostasis through inhibition of apoptosis and induction of cell entry. Central leptin resistance is the main risk factor for obesity-related acute and chronic diseases. It also plays a role in dysglycemia, particularly in type 2 DM, as leptin is a therapeutic target for its impact on food intake, body weight, and potential to improve insulin action [79].
Hypertension is related to impaired metabolic homeostasis and thus is also regarded as a metabolic disorder [80]. It is important to note that the immune and autonomic systems play an important role in the cause of hypertension and other cardiovascular pathologies [81]. The blunted serologic response noted among hypertensive patients may actually be rooted in a dysfunctional immune system [40]. Both Pellini et al. and Watanabe et al. reported that IgG production against SARS-CoV-2 in normotensive individuals receiving the mRNA vaccine is significantly higher than in hypertensive participants [13,40].
In addition, ample evidence indicates that cigarette smoking could also affect both innate and adaptive immunity [79]. Cigarette smoking is known to attenuate the normal defensive function of the immune system by affecting nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling, as well as histone modification epigenetics [79]. As reported by Watanabe et al., the humoral response of smokers who received the mRNA vaccine was blunted by 43% as compared to nonsmokers [40]. Based on these studies, it is increasingly clear that suboptimal metabolic health and unhealthy lifestyle practices are associated with poor vaccine-induced immunogenicity.
Conclusions
Humoral immune response differs in every individual and is affected by many factors such as age, sex, serostatus, and underlying comorbidities. This systematic review showed that old individuals (>65 years) produce lower anti-SARS-CoV-2 antibody levels and are more likely to be low- or non-responders to Pfizer-BioNTech mRNA vaccination, especially when combined with other comorbidities. Interestingly, female sex appears to be associated with greater antibody production due to the immunomodulating properties of estrogen and the X chromosome. Moreover, due to early antibody response present among seropositive individuals, higher levels of antibodies were measured post-vaccination compared to seronegative individuals. Presence of comorbidities has also shown to correlate with a significant decline in antibody production, especially if present in the elderly population. Hemodialysis, transplantation, cancer and autoimmune diseases, as well as metabolic derangements and smoking, all demonstrate a blunted humoral immune response that could be rooted in a dysfunctional immune system and several factors such as aging, which serve as a significant aggravating factor, as well as the use of immunosuppressive and antimetabolite medications.
It is worth mentioning that additional mRNA-based vaccine boosters have already been attempted in some of these populations of low-responders. While presenting a relatively safe reactogenic response comparable to that seen after the first or second mRNA vaccine dose [82], the third vaccine dose was found to be effective in further boosting antibody levels in elderly people [80], in solid organ transplant recipients [28], and in patients receiving maintenance hemodialysis or peritoneal dialysis [83]. The third dose was also effective in reinforcing immunity against VoC [84]. Irrespective of the demographic or clinical conditions that would blunt the anti-SARS-CoV-2 antibody response, the importance of monitoring humoral immunity seems almost unquestionable for prioritizing vaccine boosters, including new vaccines able to efficiently protect against current and new SARS-CoV-2 VoC [85]. Their prioritized administration to especially vulnerable populations could provide the best compromise between limited vaccine availability and the highest clinical efficacy in averting or limiting SARS-CoV-2 infections and/or severe COVID-19 [86].
Recommendations
For future research, further investigation on the correlation of age, sex, serostatus, and comorbidities, as well as their interdependence, with humoral response is recommended. In addition, comparison between the level of humoral response elicited by other SARS-CoV-2 mRNA vaccines, such as Moderna (mRNA 1273), with Pfizer-BioNTech (mRNA BNT162b2) could also be explored using these demographic parameters.
Supplementary Material
Glossary
Abbreviations
- ANCA-AAV
antineutrophil cytoplasmic antibody-associated vasculitis
- AIIRD
autoimmune inflammatory rheumatic disease
- AxSpA
axial spondyloarthritis
- BMI
body mass index
- CVD
cardiovascular disease
- CDC
Centers for Disease Control and Prevention
- CKD
chronic kidney diseases
- CLL
chronic lymphocytic leukemia
- csDMARD
conventional synthetic disease-modifying antirheumatic drug
- COVID-19
Coronavirus Disease 2019
- DM
diabetes mellitus
- DPT
diphtheria, pertussis, and tetanus
- DMT
disease-modifying therapy
- DSA
donor-specific antibodies
- ESRD
end-stage renal disease
- GMC
geometric mean concentration
- HCW
healthcare workers
- HDP
hemodialysis patient
- HLA
human leukocyte antigen
- IIM
idiopathic inflammatory myositis
- JAKi
janus kinase inhibitors
- LVV
large vessel vasculitis
- MTX
methotrexate
- miRNAs
microRNAs
- MAPK
mitogen-activated protein kinase
- MM
multiple myeloma
- MMR
measles, mumps, and rubella
- MPM
myeloproliferative malignancy
- ND
no data
- NF-κB
nuclear factor-kappa B
- pwMS
patients with multiple sclerosis
- PsA
psoriatic arthritis
- RA
rheumatoid arthritis
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- SLL
small lymphocytic leukemia
- SOT
solid organ transplant
- SLE
systemic lupus erythematosus
- VBM
variants being monitored
- VoC
variants of concern
Disclosure statement
The authors report no conflict of interest.
References
- 1.Coronavirus Resource Center . COVID-19 dashboard; 2021. [cited 2021 Sep 19]. Available from: https://coronavirus.jhu.edu/map.html?fbclid=IwAR3YAUh1zt-Y0G1gUqLxBypXPQuI1PzhQItISdwOYNgRR0om-BjGOv6Ya-0
- 2.Koyama T, Weeraratne D, Snowdon JL, et al. Emergence of drift variants that may affect COVID-19 vaccine development. Pathogens. 2020;2020:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albano PM, Notarte KI, Macaranas I, et al. Cross-contamination in molecular diagnostic laboratories in low- and middle-income countries: a challenge to COVID-19 testing. PJP. 2020;5(2):7–11. [Google Scholar]
- 4.Lippi G, Sanchis-Gomar F, Henry BM.. COVID-19: unravelling the clinical progression of nature's virtually perfect biological weapon. Ann Transl Med. 2020;8(11):693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolgin E. How COVID unlocked the power of mRNA. Nature. 2021;589(7841):189–191. [DOI] [PubMed] [Google Scholar]
- 6.Ríos S, Mas Romero M, Cortés Zamora EB, et al. Immunogenicity of the BNT162b2 vaccine in frail or disabled nursing home residents: COVID-A study. J Am Geriatr Soc. 2021;69(6):1441–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80(10):1306–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tove H, Nissen K, Krambrich J, et al. Evaluation of a COVID-19 IgM and IgG rapid test; an efficient tool for assessment of past exposure to SARS-CoV-2. Infect Ecol Epidemiol. 2020;10(1):1754538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Wang J, Xu X, et al. Patterns of IgG and IgM antibody response in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabal KA, Ben-Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Eurosurveillance. 2021;26(6):2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naaber P, Jürjenson V, Ainika Adamson S, et al. Antibody response after COVID-19 mRNA vaccination in relation to age, sex, and side effects. medRxiv. 2021. [Google Scholar]
- 12.Salvagno GL, Henry BM, Di Piazza G, et al. Anti-SARS-Cov-2 receptor-binding domain total antibodies response in seropositive and seronegative healthcare workers undergoing COVID-19 mRNA BNT162b2 vaccination. Diagnostics. 2021;11(5):832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellini R, Venuti A, Pimpinelli F, et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine. 2021;36:100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller L, Andrée M, Moskorz W, et al. Age-dependent immune response to the BioNTech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis. 2021;73(11):2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frenck RW, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelsen SG, Braverman AS, Aksoy MO, et al. A longitudinal study of BNT162b2 vaccine-induced humoral response and reactogenicity in health care workers with prior COVID-19 disease. medRxiv. 2021;21253845. [Google Scholar]
- 17.Callegaro A, Borleri D, Farina C, et al. Antibody response to SARS-CoV-2 vaccination is extremely vivacious in subjects with previous SARS-CoV-2 infection. J Med Virol. 2021;93(7):4612–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Efrati S, Catalogna M, Abu Hamad R, et al. Safety and humoral responses to BNT162b2 mRNA vaccination of SARS-CoV-2 previously infected and naive populations. Sci Rep. 2021;11(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebinger JE, Fert-Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27(6):981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prendecki M, Clarke C, Brown J, et al. Effect of previous SARS-CoV-2 infection on humoral and T-Cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397(10280):1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padoan A, Dall'Olmo L, Rocca FD, et al. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin Chim Acta. 2021;519:60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Favresse J, Bayart J-L, Mullier F, et al. Early antibody response in health-care professionals after two doses of SARS-CoV-2 mRNA vaccine (BNT162b2). Clin Microbiol Infect. 2021;27(9):1351.e5–1351.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasso BL, Giglio RV, Vidali M, et al. Evaluation of anti-SARS-Cov-2 S-RBD IgG antibodies after COVID-19 mRNA BNT162b2 vaccine. Diagnostics. 2021;11(7):1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modenese A, Paduano S, Bargellini A, et al. Neutralizing anti-SARS-CoV-2 antibody titer and reported adverse effects, in a sample of Italian nursing home personnel after two doses of the BNT162b2 vaccine administered four weeks Apart. Vaccines. 2021;9(6):652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jahn M, Korth J, Dorsch O, et al. Humoral response to SARS-CoV-2-vaccination with BNT162b2 (Pfizer-BioNTech) in patients on hemodialysis. Vaccines. 2021;9(4):360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goupil R, Benlarbi M, Beaubien-Souligny W, et al. Short-term antibody response after 1 dose of BNT162b2 vaccine in patients receiving hemodialysis. CMAJ. 2021;193(22):E793–E800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frantzen L, Cavaillé G, Thibeaut S, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in a haemodialysis cohort. Nephrol Dial Transplant. 2021;36(9):1756–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zitt E, Davidovic T, Schimpf J, et al. The safety and immunogenicity of the mRNA-BNT162b2 SARS-CoV-2 vaccine in hemodialysis patients. Front Immunol. 2021;12(1):704773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torreggiani M, Blanchi S, Fois A, et al. Neutralizing SARS-CoV-2 antibody response in dialysis patients after the first dose of the BNT162b2 mRNA COVID-19 vaccine: the war is far from being won. Kidney Int. 2021;99(6):1494–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pimpinelli F, Marchesi F, Piaggio G, et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol. 2021;14(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massarweh A, Eliakim-Raz N, Stemmer A, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(8):1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goshen-Lago T, Waldhorn I, Holland R, et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(10):1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80(10):1330–1339. [DOI] [PubMed] [Google Scholar]
- 35.Gallo A, Capuano R, Donnarumma G, et al. Preliminary evidence of blunted humoral response to SARS-CoV-2 mRNA vaccine in multiple sclerosis patients treated with ocrelizumab. Neurol Sci. 2021;42(9):3523–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korth J, Jahn M, Dorsch O, et al. Impaired humoral response in renal transplant recipients to Sars-Cov-2 vaccination with Bnt162b2 (Pfizer-BioNTech). Viruses. 2021;13(5):756–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marinaki S, Adamopoulos S, Degiannis D, et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21(8):2913–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shostak Y, Shafran N, Heching M, et al. Early humoral response among lung transplant recipients vaccinated with BNT162b2 vaccine. Lancet Respir Med. 2021;9(6):e52–e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8):2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe M, Balena A, Tuccinardi D, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab Res Rev. 2021;38(1):e3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hozo SP, Djulbegovic B, Hozo I.. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vassilaki N, Gargalionis AN, Bletsa A, et al. Impact of age and sex on antibody response following the second dose of COVID-19 BNT162b2 mRNA vaccine in Greek healthcare workers. Microorganisms. 2021;9(8):1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butt A, Omer SB, Yan P, et al. SARS-CoV-2 vaccine effectiveness in a high-risk national population in a real-world setting. Ann Intern Med. 2021;174(10):1404–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danthu C, Hantz S, Dahlem A, et al. Humoral response after SARS-CoV-2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol. 2021;32(9):2153–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lord JM. The effect of ageing of the immune system on vaccination responses. Hum Vaccin Immunother. 2013;9(6):1364–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allen JC, Toapanta FR, Chen W, et al. Understanding immunosenescence and its impact on vaccination of older adults. Vaccine. 2020;38(52):8264–8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olafsdottir TA, Alexandersson KF, Sveinbjornsson G, et al. Age and influenza-specific pre-vaccination antibodies strongly affect influenza vaccine responses in the Icelandic population whereas disease and medication have small effects. Front Immunol. 2017;8:1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haralambieva IH, Painter SD, Kennedy RB, et al. The impact of immunosenescence on humoral immune response variation after influenza a/H1N1 vaccination in older subjects. PLOS One. 2015;10(3):e0122282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartleson JM, Radenkovic D, Covarrubias AJ, et al. SARS-CoV-2, COVID-19 and the ageing immune system. Nat Aging. 2021;1(9):769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giefing-Kröll C, Berger P, Lepperdinger G, et al. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14(3):309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischinger S, Boudreau CM, Butler AL, et al. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41(2):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciarambino T, Para O, Giordano M, et al. Immune system and COVID-19 by sex differences and age. Womens Health. 2021;17:17455065211022262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma Q, Hao ZW, Wang YF.. The effect of estrogen in coronavirus disease 2019. Am J Physiol Lung Cell Mol Physiol. 2021;321(1):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manica M, Pancheri S, Poletti P, et al. Risk of symptomatic infection during a second coronavirus disease 2019 wave in severe acute respiratory syndrome coronavirus 2-seropositive individuals. Clin Infect Dis. 2021;2019–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fraley E, LeMaster C, Geanes E, et al. Humoral immune responses during SARS-CoV-2 mRNA vaccine administration in seropositive and seronegative individuals. BMC Med. 2021;19(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demonbreun AR, Sancilio A, Velez ME, et al. Comparison of IgG and neutralizing antibody responses after one or two doses of COVID-19 mRNA vaccine in previously infected and uninfected persons. medRxiv. 2020;6(165):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krammer F, Srivastava K, Team P, et al. Robust spike antibody responses and increased reactogenicity in seropositive individuals after a single dose of SARS-CoV-2 mRNA vaccine. medRxiv. 2021;21250653. [Google Scholar]
- 58.Dooley KE, Chaisson RE.. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9(12):737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwetkat A, Heppner HJ.. Comorbidities in the elderly and their possible influence on vaccine response. Interdiscip Top Gerontol Geriatr. 2020;43:73–85. [DOI] [PubMed] [Google Scholar]
- 60.Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049–2055. [DOI] [PubMed] [Google Scholar]
- 61.Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3(5):1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Windpessl M, Bruchfeld A, Anders HJ, et al. COVID-19 vaccines and kidney disease. Nat Rev Nephrol. 2021;17(5):291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mulley WR, Dendle C, Ling JEH, et al. Does vaccination in solid-organ transplant recipients result in adverse immunologic sequelae? A systematic review and meta-analysis. J Heart Lung Transplant. 2018;37(7):844–852. [DOI] [PubMed] [Google Scholar]
- 64.Katerinis I, Hadaya K, Duquesnoy R, et al. De novo anti-HLA antibody after pandemic H1N1 and seasonal influenza immunization in kidney transplant recipients. Am J Transplant. 2011;11(8):1727–1733. [DOI] [PubMed] [Google Scholar]
- 65.Schaffer SA, Husain S, Delgado DH, et al. Impact of adjuvanted H1N1 vaccine on cell-mediated rejection in heart transplant recipients. Am J Transplant. 2011;11(12):2751–2754. [DOI] [PubMed] [Google Scholar]
- 66.Danziger-Isakov L, Cherkassky L, Siegel H, et al. Effects of influenza immunization on humoral and cellular alloreactivity in humans. Transplantation. 2013;89(7):838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar D, Blumberg EA, Danziger-Isakov L, et al. Influenza vaccination in the organ transplant recipient: review and summary recommendations. Am J Transplant. 2011;11(10):2020–2030. [DOI] [PubMed] [Google Scholar]
- 68.Aslam S, Goldstein DR, Vos R, et al. COVID-19 vaccination in our transplant recipients: the time is now. J Heart Lung Transplant. 2021;40(3):169–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hiam-Galvez KJ, Allen BM, Spitzer MH.. Systemic immunity in cancer. Nat Rev Cancer. 2021;21(6):345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gonzalez H, Hagerling C, Werb Z.. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19–20):1267–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Looi CK, Chung FFL, Leong CO, et al. Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. J Exp Clin Cancer Res. 2019;38(1):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Esperança-Martins M, Gonçalves L, Soares-Pinho I, et al. Humoral immune response of SARS-CoV-2-infected patients with cancer: influencing factors and mechanisms. Oncologist. 2021;26(9):e1619–e1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meerveld-Eggink A, de Weerdt O, van der Velden AMT, et al. Response to influenza virus vaccination during chemotherapy in patients with breast cancer. Ann Oncol. 2011;22(9):2031–2035. [DOI] [PubMed] [Google Scholar]
- 75.Wang L, Wang FS, Gershwin ME.. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278(4):369–395. [DOI] [PubMed] [Google Scholar]
- 76.Lerner A, Jeremias P, Matthias T.. The world incidence and prevalence of autoimmune diseases is increasing. Int J Celiac Dis. 2016;3(4):151–155. [Google Scholar]
- 77.Andersen CJ, Murphy KE, Fernandez ML.. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sheridan PA, Paich HA, Handy J, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes. 2012;36(8):1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Francisco V, Pino J, Campos-Cabaleiro V, et al. Obesity, fat mass and immune system: role for leptin. Front Physiol. 2018;9:640–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanaka M, Itoh H.. Hypertension as a metabolic disorder and the novel role of the gut. Curr Hypertens Rep. 2019;21(8):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh MV, Chapleau MW, Harwani SC, et al. The immune system and hypertension. Immunol Res. 2014;59(1–3):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flaxman A, Marchevsky NG, Jenkin D, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet. 2021;398(10304):981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bensouna I, Caudwell V, Kubab S, et al. SARS-CoV-2 antibody response after a third dose of the BNT162b2 vaccine in patients receiving maintenance hemodialysis or peritoneal dialysis. Am J Kidney Dis. 2021;79(2):185–192.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choi A, Koch M, Wu K, et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med. 2021;27(11):2025–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Notarte KI, Guerrero-Arguero I, Velasco JV, et al. Characterization of the significant decline in humoral immune response six months post-SARS-CoV-2 mRNA vaccination: a rapid systematic review. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lippi G, Henry BM, Plebani M.. Optimizing effectiveness of COVID-19 vaccination: will laboratory stewardship play a role? Clin Chem Lab Med. 2021;59(12):1885–1888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.