ABSTRACT
Introduction
Coronavirus Disease 19 (COVID-19) diagnosis has been a major problem in most Emergency Departments (EDs) and other senior care facilities. Various clinical manifestations, and the several radiologic and laboratory data combined with the misleading test results to identify the virus, are responsible for certain misdiagnoses, especially in suspected cases needing urgent management and treatment. Although emergency and other front-line physicians struggle to manage COVID-19 patients, still existent cases with ambiguous diagnosis trammel the ED safety and responsibility.
Areas Covered
This review article summarizes on a large scale the common information for the medical history, clinical examinations, radiology and laboratory data for SARS-CoV-2. We summarize the available literature using the PubMed, Science Direct and EMBASE databases published until December 2021 on the general information for COVID-19 diagnosis, and, finally, we propose algorithms for a precise and on-the-spot diagnosis the disease.
Expert Opinion
COVID-19 diagnosis has appeared to be such ambiguous, and physicians need to correlate medical history, medical examination, potential extrapulmonary manifestations, along with laboratory and radiologic data, for a prompt COVID-19 diagnosis.
KEYWORDS: COVID-19, diagnosis, Emergency Department
1. Introduction
A novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was identified from a cluster of pneumonia cases with unknown purpose in the province of Wuhan, China, in December 2019 [1]. On 30 January 2020, the World Health Organization (WHO) announced the Coronavirus Disease 19 (COVID-19) as a Public Health Emergency of International Concern, and a month and a half later, COVID-19 epidemic was portrayed as a pandemic [2].
Heretofore, the scientific community has exerted considerable attempts to surveil SARS-CoV-2 dynamics by its epidemiological blueprints [3]. Literature has already revealed the multifarious clinical manifestations in cases infected from SARS-CoV-2, from a mild to a severe COVID19, in a heterogeneous pathognomonic scenario [4]. Moreover, laboratory SARS-CoV-2 diagnosis has manifested false-positive and false-negative test results in all types of biochemical assays and physicians waffle back and forth in the various tests’ sensitivity spectrum [5]. Also, the ongoing vaccination era may affect the overall accurate diagnosis, with the heterogeneous clinical manifestations, especially in SARS-CoV-2 vaccinated individuals.
For such reasons, and while Emergency Departments (EDs) and other senior care facilities struggle to keep their front-line physicians and caretakers unscathed, since they are being overwhelmed by pandemic patient’s volume, malpractices cannot be kept at bay. Misdiagnoses or delayed diagnoses due to laboratory assays lead to a delayed medical care that can accelerate exacerbations placing infected people at a high risk of a severe COVID-19. Moreover, SARS-CoV-2 transmission in the emergency rooms is increased, placing other patients with comorbidities at a high risk of SARS-CoV-2 complications, and, generally, there occurs a cascade of several misfortunes. Also, front-line healthcare workers are facing several threats, such as the risk of infection, isolation from their families, and the higher number of daily deaths [6].
This review, by summarizing the medical history, clinical manifestations, radiologic findings and laboratory evidence of COVID-19, will provide recommended approaches to a more accurate management and a prompt diagnosis of COVID-19, particularly in cases where the suspicion is high [7]. We also illustrate general algorithms for extra emergency preparedness plans, and for a further COVID-19 diagnostic and management success. Literature review was performed by using the PubMed, Science Direct, and EMBASE databases using mainly the terms ‘COVID-19 diagnosis’, ‘SARS-CoV-2 infection’, ‘COVID-19 manifestations’, ‘COVID-19 symptoms’, ‘COVID-19 imaging’, ‘COVID-19 biochemical data’ or ‘COVID-19 tests’. The search was restricted to English language publications, and studies published until December 2021 were included.
2. The principles of diagnosing COVID-19
2.1. Medical history
A lot has been said about the heterogeneous pathognomonics of COVID-19. Fever, the most preserved symptom of infections, has shown a miscellaneous appearance, such that can even be expressed after hospitalization and not in the initial stage [8]. A persistent cough is thought to be a classic symptom of COVID-19 [9]. The acute respiratory illness by SARS-CoV-2 is also accompanied by dyspnea [10]. Pharyngalgia, shivering, chest pain and tightness have already been reported [11]. However, symptoms may appear in different days after SARS-CoV-2 exposure, with a median time of 4–5 days from exposure to symptom onset [12].
COVID-19 is believed to be a complex disease with several different symptoms. A bilateral, long-lasting headache has been reported as a symptom of COVID-19 [13]. Some patients report a present sore throat and a runny nose [14]. Some first-mentioned olfactory and gustatory dysfunctions include the decreased smell function, but not always anosmia, that could indicate a possible SARS-CoV-2 infection, and another reported chemosensory dysfunction, during COVID-19, is thought to be the loss or change of taste, especially in early stages or paucisymptomatic cases [15].
A common gastrointestinal manifestation of COVID-19 is considered to be diarrhea [16]. Even as atypical or occasional manifestations, abdominal and testicular pain have also been reported in cases tested positive for SARS-CoV-2 [17]. Nausea and vomiting have been reported as signs expressed in the early stages in some cases [18]. Other oral signs and widespread lesions have been reported, especially in older ages in parallel with a higher severity of COVID-19 [19]. Moreover, some studies describe audio-vestibular symptoms connected with COVID-19, such as sensorineural hearing loss, tinnitus, or rotatory vertigo in adults [20]. Ocular implications, while not with severity, rarely have been revealed, mainly in isolated cases with chemosis or conjunctivitis, as presenting signs of SARS-CoV-2 infection [21].
A lack of energy or slowed reactions could indicate a general unusual tiredness, as in several medical conditions, that it could even be similar to that of the post-severe acute respiratory syndrome fatigue, present in COVID-19 [22]. Furthermore, muscular complications in patients affected by COVID-19 have been reported, such as general muscle weakness, myalgia, myositis, rhabdomyolysis, or critical-illness myopathy [23]. Neurological presentations have increasingly been described in cases with SARS-CoV-2 infection, including nerve damage and peripheral nerve injury. Several other neurological disorders have been reported, not only related to the peripheral but also to the central nervous system involvement, such as an ischemic stroke or a cerebral hemorrhage [24]. Cutaneous signs of COVID-19 have also been described in the literature, regarding patients presenting acro-ischemia, chilblain-like edematous and erythematous eruptions, skin rash with petechiae, and other skin manifestations [25].
Finally, some patients infected from SARS-CoV-2 have shown a prevalence of psychiatric and mental health disorders, including depression, anxiety, and sleep disturbances [26]. Also, a sudden confusion or a delirium manifestation has been reported to affect mainly adults and older people, admitted to the intensive care unit [27].
Nevertheless, each patient is unique, so COVID-19 affects people in different ways, and each case may exhibit different combination of the known symptoms, or present a new, heretofore unknown symptom – for instance, as a result of future new viral mutants. Different age ranges may present different symptoms’ combinations; for example, respiratory symptoms are common especially in young people [28]. Even if acute COVID-19 and illness exacerbations have passed, a persistence of symptoms has thoroughly been reported [29].
2.2. Extrapulmonary manifestations
Although the main route of entry of SARS-CoV-2 to the body is the upper and lower respiratory tract, it can affect many other organs, or exacerbate preexisting medical conditions. Pulmonary manifestations include pneumonia, acute lung injury, endotheliitis, and thromboembolism [30]. Moreover, commonly encountered long-term clinical conditions are post-COVID interstitial lung disease, pulmonary embolism, and chronic cough, whereas cavitary lesions, small airway disease and development of pulmonary hypertension are rare [31]. Hypertension and cardiovascular diseases have generally been correlated with COVID-19, whereas they have been reported as the most common comorbidities along with diabetes mellitus [32]. Severe acute kidney injury and liver manifestations, even if they are not currently clearly elucidated, have long been reported [33,34].
Neurological complications have also been described in association with COVID-19, such as encephalitis and Guillain-Barré syndrome [35]. Similar to other critical illnesses, the complications of acute COVID-19, such as ischemic or hemorrhagic stroke, hypoxic–anoxic damage, posterior reversible encephalopathy syndrome and acute disseminated myelitis may lead to permanent neurological deficits [36]. Moreover, dermatological manifestations of COVID-19 have also been reported in the literature, including maculopapular rashes, urticaria, vesicles, petechiae, purpura, chilblains, livedo racemosa, and distal limb ischemia. Even if most of these dermatologic findings are self-resolving, they can help increase one’s suspicion for a SARS-CoV-2 infection [37]. COVID-19 also presents risk factors for bone demineralization related to systemic inflammation, immobilization, exposure to corticosteroids, vitamin D insufficiency and interruption of antiresorptive or anabolic agents for osteoporosis [38].
Multisystem inflammatory syndrome (MIS) which was stated to affect children in the first articles published in March 2020 was named MIS-C (child). A few months later a syndrome similar to MIS-C came to be noticed in adults as well (MIS-A). The syndrome manifests itself approximately 4 weeks after COVID-19 infection, with symptoms mimicking Kawasaki Disease and Kawasaki Disease Shock Syndrome. Patient presentation includes persistent fever, rash, gastrointestinal symptoms and cardiac complications including myocarditis. Blood tests reveal increased inflammatory biomarkers including C reactive protein, ferritin and interleukin-6. The syndrome can lead to multiorgan failure and death [39].
Even fully vaccinated individuals can become infected with the SARS-CoV-2. It has been demonstrated that older age, comorbidities, and immunosuppression may predispose to severe COVID-19 disease [40]. The current ranking of COVID-19 symptoms after two vaccinations is as follows: headache, runny nose, sneezing, sore throat and loss of smell as reported by infected people via an application in UK [41].
2.3. Imaging data
Several diagnostic methods providing radiology data have been considered for COVID-19, beginning with the chest radiography, that seems to be a reasonable preliminary test with a moderate complexity. A chest X-ray is vital to assess for COVID-19 mimics that includes pneumonia or pulmonary edema or other lung inflammations. Some COVID-19 findings involve bilateral or peripheral hazy opacities, consolidation, or lower zone predominance, but chest X-ray may not be such specific – such as in children-, or it may vary from the other radiological diagnostic methods [42,43].
Computed Tomography (CT) findings regarding the SARS-CoV-2 infection include multifocal bilateral ground glass opacity, peripheral predominant lesions without airway abnormalities, mediastinal features such as lymphadenopathy, and occasional pleural effusion [44]. Studies have also revealed several other chest CT manifestations, in patients infected from SARS-CoV-2 pneumonia, such as consolidation, crazy-paving pattern, interlobular septal thickening, reticulation, traction bronchiectasis, and a frequent unilateral pneumonia -especially in severe COVID-19 cases [45]. However, the disseminated COVID-19 may be absent, revealing a tropism of such illness. Also, lung abnormalities in chest CT findings may appear and develop in different days from the symptoms’ onset or window days, or a positive SARS-CoV-2 biochemical test [46]. Yet, the chest CT shows typical imaging findings, which can represent countless acute lung injuries, either along with an infection or another noninfectious etiology. Moreover, COVID-19 has led to several other extrapulmonary CT organ-related disorders, with gastrointestinal features, vascular enlargement or other hematological manifestations, or neurological CT findings [47].
COVID-19 can lead to lung pneumonia, especially in critically ill patients, and Lung Ultrasonography (LU) being a surface imaging technique, could be highly sensitive for an early diagnosis, presenting diffuse B pattern with spared areas, or other features [48]. Regarding COVID-19, the predominant pattern is in a spectrum of interstitial syndrome and alveolar consolidation, correlated with the severity of the illness [49]. The use of LU in patients with COVID-19 should be encouraged because of its intrinsic characteristics; a low cost, radiation free, practical method, with easy to sanitize equipment, which facilitates structural evaluation of lung damage caused by SARS-CoV-2 [50]. However, even if an LU provides an immediate point-of-care utility, it cannot detect inflammation manifestations occurring deep within the lung parenchyma.
Magnetic Resonance Imaging (MRI) has been reported in the literature for COVID-19 radiological diagnosis, concerning some neuroimaging findings characteristic in cases with a severe illness such as encephalopathy, or the cardiac findings in children with MIS-C [51]. Low-field MRIs could be possible for SARS-CoV-2 infection’s radiological manifestations, presenting ground glass opacity, but with differences compared to CT data, due to the breath mobility [52].
Nevertheless, not only are imaging findings unspecific, but also they are analogous to the stage of the disease, the severity of lung injury, and several other comorbidities – mainly the lung underlying medical conditions. As a result, each case is different and even if imaging methods show various specificity and sensitivity ranges, they should be assessed according to the afforementioned parameters, and in combination with the other criteria of the principles of diagnosis, in a spherical diagnostic frame.
2.4. Laboratory data
2.4.1. SARS-CoV-2 biochemical biomarkers
Several studies have reported the blood biochemical features of patients infected from SARS-CoV-2. High serum levels in alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBil), creatine kinase (CK) and lactate dehydrogenase (LDH) have been described in parallel with a decreased albumin (ALB), in the literature [53]. Also, abnormalities including both an increase or decrease have been reported, mainly for blood urea nitrogen (BUN) and creatinine (CRE). Furthermore, there is evidence that the elevated serum C-reactive protein (CRP), high procalcitonin (PCT) and D-dimer, and low ferritin levels are associated with poor outcomes in critically ill COVID-19 patients [54]. Also, elevated levels in serum N-terminal pro B-type natriuretic peptide (NT-proBNP) and cardiac Troponin-I (cTnI) have been correlated with acute cardiac injury in patients with COVID-19 [55]. Venous thromboembolism and arterial thrombosis seem to be common, in COVID-19 patients with arterial coagulopathy, revealing abnormalities in the prothrombin time (PT), levels of D-dimer and fibrin/fibrinogen degradation products (FDP) [56]. Also, may the elevated levels of glycated hemoglobin (HbA1c) and interleukin-6 (IL-6), measured on admission to the intensive care unit, can predict the outcome, mostly in critically ill COVID-19 patients with invasive mechanical ventilation [57,58]. Regarding blood cells, studies reveal that cases with a severe or fatal disease had significantly increased white blood cell (WBC) count, and decreased lymphocyte and platelet counts [59].
Yet, in hospitalized patients with acute respiratory distress, the WBC and platelet count, lymphopenia, serum ferritin and IL-6 might reveal a potential progression to critical illness. Also, strict observing of procalcitonin levels, WBC and neutrophils count, and CRP levels could be used for management in critically ill patients with a molecularly identified COVID-19. Nevertheless, differences in biochemical indices may exist among countries, races and ages. Furthermore, they may indicate a multiple-organ dysfunction, a possible co-infection or another covid-like illness—instead of a SARS-CoV-2 infection, or its mutants. Finally, front-line physicians should bear in mind that biochemical differences may occur simply due to the potential underlying medical conditions of a unique case.
2.5. SARS-CoV-2 identification tests
Antigen Rapid Diagnostic Tests (Ag-RDTs) have shown a various sensitivity, regarding the viral load [60]. Ag-RDTs have been utilized even as self-tests [61]. Ag-RDTs, sometimes lead to false results, due to errors in testing operation, poorly specific Ag-RDTs that detect other pathogens, detection of inactive or residual SARS-CoV-2, potential cross-reactions with antibodies, antigen degradation, cross-contaminations and cross-reactions with other substances in clinical samples, may lead to false-positive test results [5].
Serologic diagnosis for SARS-CoV-2 includes both rapid and classical laboratory serologic diagnosis, and Elisa-linked Immunosorbent Assays (ELISAs) and Immunoglobulin Rapid Diagnostic Tests (Ig-RDTs), detecting seroconverted IgA, IgM and IgG antibodies in blood or serum [5,62]. Yet, several reason could lead to a false-positive test result, including laboratory errors and technical reasons in each type, testing in window period, insufficient samples, antibody inhibitors, antibody degradation and factors that may impair antibody production. However, with serologic testing, we do not detect the virus itself but the case’s immune response.
As defined by WHO, a confirmed case is detected from nucleic acid amplification tests (NAATs) for SARS-CoV-2, such as real-time reverse transcription polymerase chain reaction (rRT-PCR), that is worldwide preferred. Nevertheless, as in all types of identification tests – rapid and laboratory ones, one or more negative tests do not rule out the possibility of SARS-CoV-2 infection. Retrospectively, test positivity does not always show an infection existing in reality. Approaching the so called ‘gold standard’, some false-positive results can be managed through standard curve or interim controls [5]. However, false-positive test results can occur due to an inadequate laboratory rRT-PCR experience, SARS-CoV-2 cross-contaminations, detection of other pathogens, SARS-CoV-2 inactive/residual detections, or technical reasons [5]. Also some common false-negative types occur in laboratory errors and technical issues, sample deficiency or degradation, SARS-CoV-2 mutations and RT-PCR inhibitors [5]. Yet, there are several reasons that trammel an accurate COVID-19 diagnosis.
3. Diagnostic algorithms
Typically, front-line clinicians evaluating patients presenting fever and acute respiratory illness should obtain information concerning exposure to infected individuals, except from the case that epidemiological rates of COVID-19 are tremendously high. However, places with a low SARS-CoV-2 prevalence render COVID-19 identified cases less reliable, due to a potential false-positive test result [5]. As a result, an individual arriving at an ED with a present coronavirus-like disease, having interacted with another identified individual in a low-prevalence COVID-19 city, should be tested for any potential disease and not only for a direct and sure SARS-CoV-2 infection. Reversely, individuals found positive in a SARS-CoV-2 identification test are not always presenting an existing infection, even a COVID-19. Figure 1 demonstrates an algorithm with possible COVID-19 diagnostic results, combining all parameters of diagnosing an infectious disease, and in places with high epidemiological rates of SARS-CoV-2:
Figure 1.
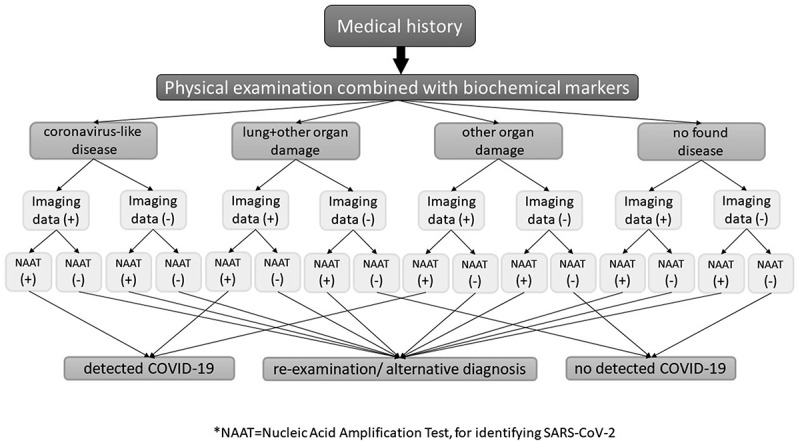
Diagnosing COVID-19 in the Emergency Department, in places with high SARS-CoV-2 prevalence.
In places with high SARS-CoV-2 prevalence, COVID-19 diagnosis seems to be easy, when imaging data in parallel with laboratory data, go along with the medical history and physical examination. COVID-19 identified cases are more likely to be real in places with high epidemiological rates; yet, precautions should always be taken, and emergency physicians should recognize that a case may present another coronavirus-like infection. Another straightforward diagnosis seems to be the not detected COVID-19, at the time of examination, that may occur due to a random false-positive SARS-CoV-2 identification test, or imaging data maybe due to other comorbidities. In cases with uncertain data, it is required that a reexamination or an alternative diagnosis be done, to identify a possible SARS-CoV-2 infection. Cases with symptoms, a positive SARS-CoV-2 identification test but no imaging data, may have passed the disease and show SARS-CoV-2 residues, or may they have recently caught the virus inside the emergency rooms.
Cases who report a medical history, and their examination mainly shows both lung and other organ damage, or at least another organ damage, with either imaging data or laboratory data, should have an alternative diagnosis or a reexamination, since may the virus has affected different organs. Since SARS-CoV-2 seems to attack vessels, we should admit, that, patients arriving in the ED may be in post-COVID stages, can arrive with other organ or generally other clinical manifestations, revealed in literature. Each case is unique, and each patient may present various clinical manifestations. Yet, another infection or a re-infection or a coinfection in parallel with SARS-CoV-2, should not be underrated. However, COVID-19 diagnosis seems tricky especially when influenza virus or other pathogens are in high circulation in some places, either with high SARS-CoV-2 circulation or not [5].
Alternative diagnosis could include alternative imaging method, and regarding SARS-CoV-2 identification, an alternative target of detection (for example, laboratory or rapid antigen combined with same antibody detection when there are suspected PCR inhibitors). Furthermore, an immediate repeated identification test could be done, or even an alternative sampling (bronchoalveolar lavage compared to nasopharyngeal sample), if case requires an urgent, possible or not, detection of COVID-19. Moreover, some laboratories are equipped to run more than one, different PCR kits from different manufacturers with various sensitivity. That means a case with misleading test results could have a more sensitive testing assay – an alternative PCR test result. Some studies have revealed attempts to monitor alternatively SARS-CoV-2 at an epidemiological level, via combining antigen and nucleic acid identification [63]. Retrospectively, Figure 2 shows an algorithm with possible COVID-19 diagnostic results, combining all parameters of diagnosing an infectious disease, in places with low SARS-CoV-2 prevalence:
Figure 2.
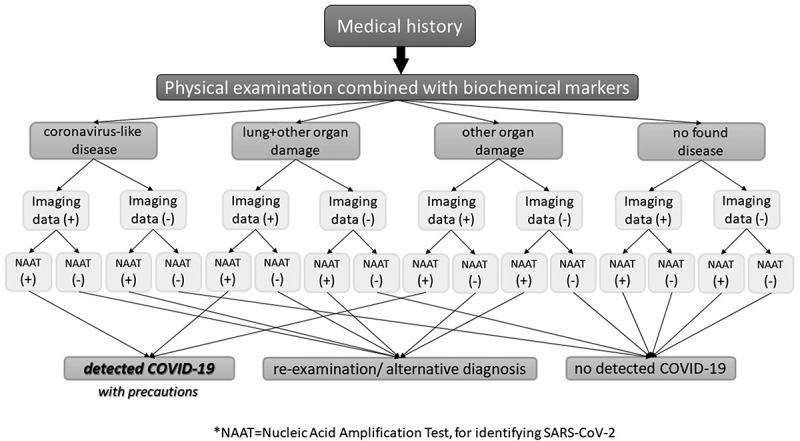
Diagnosing COVID-19 in the Emergency Department, in places with low SARS-CoV-2 prevalence.
In the previous Figure 1 and this one, we consider that mainly symptomatic cases with a medical history arrive in EDs. However, cases should have a different diagnostic management in places with a very low SARS-CoV-2 prevalence, and COVID-19 should be disclosed and identified with precautions. In such places, other pathogens as well as SARS-CoV-2 can cause an infection -or other antigens can lead to a respiratory tract allergy. It seems important for physicians to know if a suspected case has interacted with a confirmed case; nevertheless, a false test result could have had occurred, so as for the case to have another coronavirus-like pathogen infection, resulting even to flu-like symptoms and acute respiratory illness. In places with low SARS-CoV-2 circulation, misdiagnosis due to false results can be extremely risky and false-positive tests can be a reality. It is required that highly sensitive PCR kits be performed in such places, without identifying other pathogens, -that means emergency physicians should be assured that their facility’s laboratory personnel perform a highly sensitive PCR kit, detecting only SARS-CoV-2 genome. Rationally though more cases can lead to misdiagnosed COVID-19, whereas again some cases should count for an urgent reexamination or alternative diagnosis, for a more accurate and precise further urgent management and treatment. Doubtlessly, the various SARS-CoV-2 mutants can lead to different clinical phenotypes, and again, in this scenario, front-line providers should be able to recognize COVID-19 via the principles of diagnosis of infectious diseases, and identify a new symptom or finding. It is, therefore, evident that accurate diagnosis of COVID-19 can have an impact on healthcare resources.
Front-line healthcare providers should be aware of and recognize potential reasons that trigger a false test result. Alternative sampling, immediate re-testing and testing combinations -such as PCR combined with serologic testing, are a demand, for precarious patients requiring urgent management and treatment. Apart from the inadequate laboratory rRT-PCR experience, testing procedure errors or cross-contaminations, perhaps some possible falsely tested cases can be predicted. For instance, most PCR test kits cannot perform accurately in viscous or bloody samples, or saturated with nasal sprays [5]. Respiratory conditions may affect an upper or lower respiratory tract sample, and at the same way gastrointestinal conditions perhaps affect a stool/urine sample [5]. Another example regarding serologic testing, is that samples being saturated with other extra antibodies could lead to a false-positive or false-negative test result, regardless of the standard positivity or negativity [5]. In such manners, alternative or combined SARS-CoV-2 testing assays may enhance diagnostic accuracy, in some tricky cases. Figures 3, 4 and 5 summarize some already known causes that may affect a test result, concerning RT-PCR, antigen and antibody test results for diagnosing COVID-19 [5].
Figure 3.
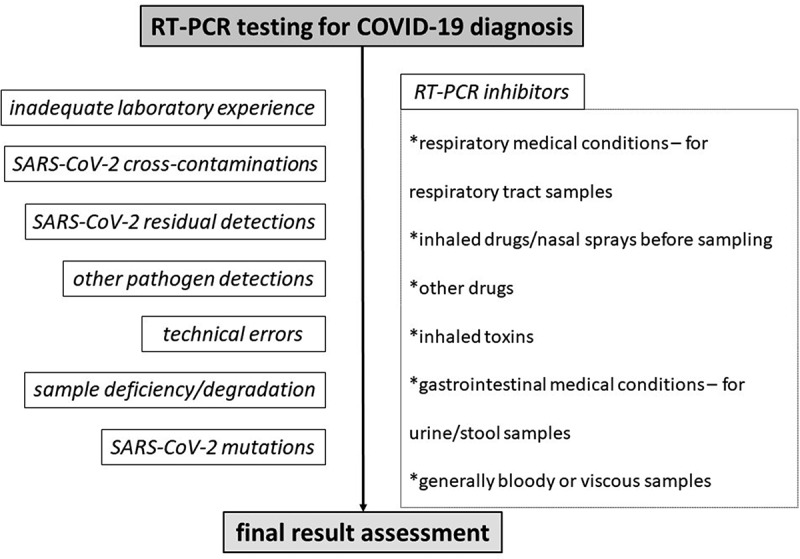
Algorithm for predicting and preventing potential misleading nucleic acid SARS-CoV-2 test results in the Emergency Department.
Figure 4.
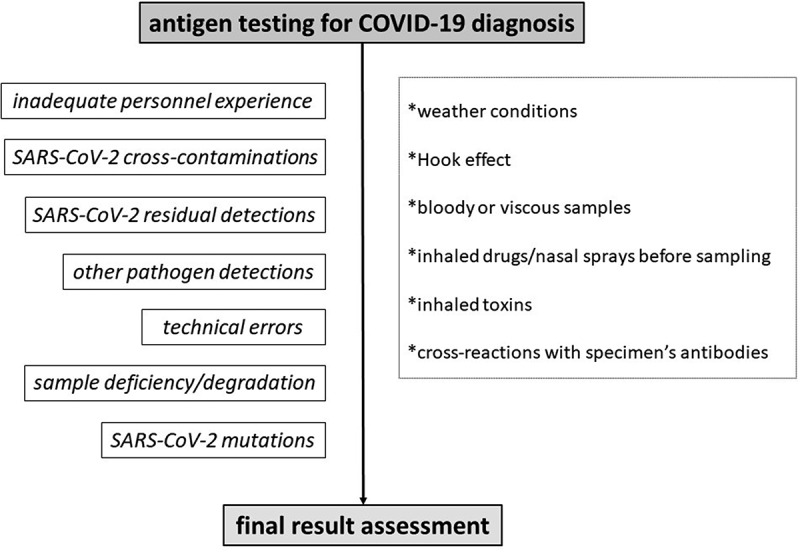
Algorithm for predicting and preventing potential misleading antigen SARS-CoV-2 test results in the Emergency Department.
Figure 5.
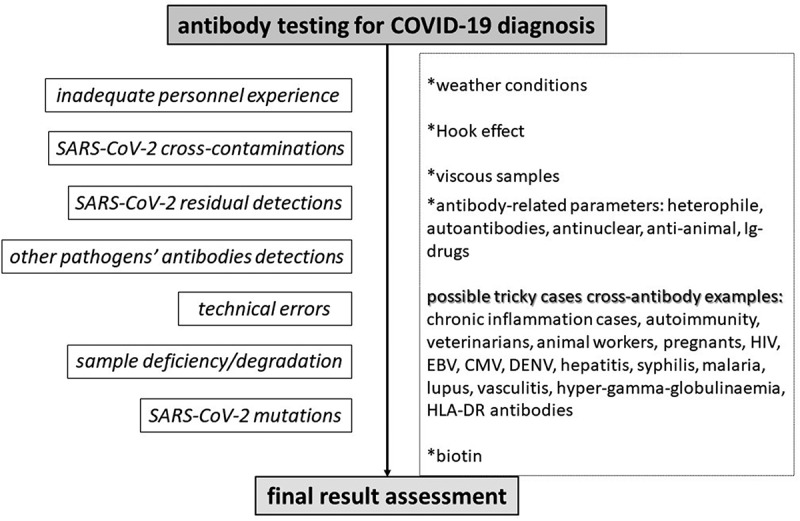
Algorithm for predicting and preventing potential misleading anti-SARS-CoV-2 antibodies test results in the Emergency Department.
It is required that front-line providers identify all possible reasons for a false PCR result, apart from laboratory errors. Respiratory and gastrointestinal medical conditions, that abound in modern society, and that can lead to a false result should be monitored from emergency physicians [5,64]. Also, cases with potential inhaled toxins or drug use should be highlighted [5].
Again, apart from laboratory errors, other issues regarding antigens, should be bore in mind, such as weather conditions, bloody or viscous samples, nasal sprays, toxins or potential antibodies in a sample [5]. Also, circulating SARS-CoV-2 mRNA vaccine antigen detected in the plasma of a few cases was reported; thus, antigen protein detection should be different from the vaccine-induced protein, so as the result to be accurate [65].
Serologic assays seem to be such tricky. Viscous samples and several underlying conditions may lead to a false result. Individuals with chronic inflammation and autoimmune comorbidities, veterinarians or animal workers or people with chronic indoor pets, pregnants, cases with specific viruses, or co-illnesses such as malaria and syphilis and hyper-gamma-globulinaemia, should be highlighted from front-line physicians, in case a test is not that sensitive and may lead to ambiguous test results [5]. Cases presenting the previous antibodies should be diagnosed accurately. It is required that emergency physicians act far away from the spectrum of each test’s sensitivity, and identify sole suspected cases that may lead to a false result.
Even if most PCR assays for identifying SARS-CoV-2 genome detect some other genes except the spike protein, laboratory clinicians should check the test kit interim guidances and assure front-line providers that vaccine’s protein cannot be detected – meaning in the few hours of vaccination before mRNA/inactivated microorganism degradation. Undeniably, vaccination rates are increasing [66]. Retrospectively, anti-SARS-CoV-2 antibodies should be diagnosed apart from the vaccine anti-spike protein antibodies, for an accurate and safe serologic diagnosis. Since, nowadays, we meet the viral mutants, front-line providers should bear in mind the various results that can arise from tests or vaccination in parallel with SARS-CoV-2 mutations. Last but not least, alternative nucleic acid amplification tests could be used in places with low SARS-CoV-2 circulation, for a better diagnosis of COVID-19.
Heretofore, for the first time is a pandemic monitored and managed through such testing strategies. After almost a year and a half of COVID-19 pandemic, the several case reports of falsely tested cases have revealed that physicians are far away from the real tests’ capacity and general diagnostic performance. Considering that these are the decades of multiplexed and rapid testing assays, emergency physicians should be familiar with such methods and their diagnostic amplitude, and prevent some potential misdiagnosis, for a better and on-the-spot response to COVID-19 cases. May the following epidemics, pandemic or the so-called ‘Disease X’, in the near future, be diagnosed with these new testing strategies; emergency physicians should be aware of the causes of false diagnosis, as long as the reasons for a false test result are the same in all pathogens, as in SARS-CoV-2.
4. Conclusion
In such pandemic eras, accurate diagnosis seems to be a demand for EDs. It is required that medical history and clinical manifestations combined with radiology and laboratory data be paralleled for a case’s precise diagnosis, mostly when further management and treatment are urgent. Emergency physicians should be well equipped with diagnostic algorithms and extra emergency preparedness plans above the sensitivity spectrum of each testing assay, for potential tricky cases, not only for SARS-CoV-2 diagnosis, but also for future pathogens recognition, prompt diagnosis and management.
5. Expert opinion
Emerging infectious diseases, as the cause of pathogenic agents, such as H1N1, Ebola virus and SARS-CoV-2, require immediate and precise strategies, and especially to molecularly identify the pathogens, so as to provide to critical cases acute care. Even if these decades are considered to revolutionize the diagnostic tools, and approach diagnosis outside the laboratory with rapid diagnostic testing, the several misuses of such devices can lead to false results and a potential misdiagnosis, that could delay the acute care to patients.
Principally, in order not to have delays and be waiting for laboratory responses to a severe or critical case, EDs could be well equipped with highly sensitive rapid diagnostic tools, so as front-line physicians to perform a rapid diagnosis by themselves. Thus, the first ideal would definitely be such an approach of scientific communities, to design suitable, flexible and highly specific and sensitive rapid diagnostic devices, for front-line urgent use – especially for severe or critical cases during a pandemic. Also, since misdiagnoses have become a common event – because of false-positivity and largely for false-negativity, emergency physicians should be aware of the interim guidances of each test kit performed in their hospital or other healthcare facilities, so as to figure out if a case could lead to a false test result, according to test capacity. These cases should be highlighted from emergency physicians so as to require a more sensitive or alternative laboratory method, or another test kit, and it is a need for future pandemics, for laboratories to be well equipped with more than one test kit, so as to have a highly sensitive testing assays for critical cases with potential false-negativity.
It is predicted that, in the near future, COVID-19 pandemic will have become a past event, may societies have acculturated SARS-CoV-2 and its mutants, and also the preexistent immunity due to previous infection of the virus or because of vaccinations may increase the odds for this scenario. However, as it is impossible for all humanity to be simultaneously vaccinated and also the antibody titers vary in each individual, all critical cases should be diagnosed urgently, accurately and in parallel with the principles of diagnosis of infectious diseases, regardless of the vaccination status.
Accurate, precise and highly sensitive algorithms for future emerging pathogenic agents are inevitably required, especially for front-line healthcare providers. Even if radiologic data can be partially precise, a correct and accurate nucleic acid, antigen or antibody test result can direct radiologic findings to a more logical trajectory, so as to avoid the heterogeneity of radiologic findings and lessen the radiologic spectrum of a pathogen’s resulted inflammation. Thus, in this scenario, too, it is undeniable that accurate test results are needed, so as to determine precisely the radiologic findings of future epidemics or pandemics. Also, the various clinical manifestations of the COVID-19, ranging from mild symptoms to definite death, have led to misinformation and misdirections in scientific communities, and these events could be a nightmare for future emerging infectious diseases. Nevertheless, we can have a prompter diagnosis, based on an accurate test result and a more direct and precise radiologic information.
In the near future, laboratory conventional assays may have been revolutionized, and a more sensitive rapid diagnosis may be a reality. Highly sensitive or alternative rapid methods could appear, and, thus, diagnostical trajectories of infectious diseases may be such different from the current ones. It should not be forgotten that rapid devices have appeared these decades, and COVID-19 pandemic was the first target of massive rapid tests – thus, there will be several lessons from SARS-CoV-2, for a better and on the spot future pathogen’s prompt diagnosis. Finally, a PCR with primers targeting various pathogens, or an LFIA with different monoclonal antibodies – that can identify different pathogenic agents, seems to have some favorable laboratory and rapid diagnostic trajectories for future infectious diseases.
Article highlights
COVID-19 emerged as a diagnostic burden for Emergency Departments
Diagnosis requires medical history, examination, radiology and laboratory data
Misleading radiologic and laboratory information can lead to misdiagnoses
Algorithms for a prompt and accurate COVID-19 diagnosis are the golden ideal
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020 . (cited 2021 Jul 31) https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020
- 3.Dawood FS, Ricks P, Njie GJ, et al. Observations of the global epidemiology of COVID-19 from the prepandemic period using web-based surveillance: a cross-sectional analysis. Lancet Infect Dis. 2020;20:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mouliou DS, Gourgoulianis KI.. False-positive and false-negative COVID-19 cases: respiratory prevention and management strategies, vaccination, and further perspectives. Expert Rev Respir Med. 2021;1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• this review summarizes the reasons for potential false results in all testing assays.
- 6.Rossettini G, Conti C, Suardelli M, et al. COVID-19 and health care leaders: how could emotional intelligence be a helpful resource during a pandemic? Phys Ther. 2021;101(pzab143). DOI: 10.1093/ptj/pzab143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Washington JA. Principles of Diagnosis. In: Baron S, editor. Medical Microbiology. Galveston: University of Texas Medical Branch at Galveston; 1996. (cited 2021 Jul 31). http://www.ncbi.nlm.nih.gov/books/NBK8014/ [PubMed] [Google Scholar]
- 8.Gul MH, Htun ZM, Inayat A. Role of fever and ambient temperature in COVID-19. Expert Rev Respir Med. 2021;15:171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song W-J, Hui CKM, Hull JH, et al. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir Med. 2021;9:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allali G, Marti C, Grosgurin O, et al. Dyspnea: the vanished warning symptom of COVID-19 pneumonia. J Med Virol. 2020;92:2272–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC. Coronavirus Disease 2019. (COVID-19) – Symptoms. Centers for Disease Control and Prevention. (cited 2021 Jul 31).https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html
- 13.Uygun Ö, Ertaş M, Ekizoğlu E, et al. Headache characteristics in COVID-19 pandemic-a survey study. J Headache Pain. 2020;21:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovato A, Rossettini G, Filippis C. Filippis C de. Sore throat in COVID-19: comment on “Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92:714–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xydakis MS, Dehgani-Mobaraki P, Holbrook EH, et al. Smell and taste dysfunction in patients with COVID-19. Lancet Infect Dis. 2020;20:1015–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang F, Zheng S, Zheng C, et al. Attaching clinical significance to COVID-19-associated diarrhea. Life Sci. 2020;260:118312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J, Thomsen T, Sell N, et al. Abdominal and testicular pain: an atypical presentation of COVID-19. Am J Emerg Med. 2020;38:1542.e1–1542.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews PLR, Cai W, Rudd JA, et al. COVID-19, nausea, and vomiting. J Gastroenterol Hepatol. 2021;36:646–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iranmanesh B, Khalili M, Amiri R, et al. Oral manifestations of COVID-19 disease: a review article. Dermatol Ther. 2021;34:e14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almufarrij I, Munro KJ. One year on: an updated systematic review of SARS-CoV-2, COVID-19 and audio-vestibular symptoms. Int J Audiol. 2021;1–11. DOI: 10.1080/14992027.2021.1962552 [DOI] [PubMed] [Google Scholar]
- 21.Willcox MD, Walsh K, Nichols JJ, et al. The ocular surface, coronaviruses and COVID‐19. Clin Exp Optometry. 2020;103:418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudroff T, Fietsam AC, Deters JR, et al. Post-COVID-19 Fatigue: potential Contributing Factors. Brain Sci. 2020;10:1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paliwal VK, Garg RK, Gupta A, et al. Neuromuscular presentations in patients with COVID-19. Neurol Sci. 2020;41:3039–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharifian-Dorche M, Huot P, Osherov M, et al. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J Neurol Sci. 2020;417. DOI: 10.1016/j.jns.2020.117085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wollina U, Karadağ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:e13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng J, Zhou F, Hou W, et al. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann N Y Acad Sci. 2021;1486:90–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawkins M, Sockalingam S, Bonato S, et al. A rapid review of the pathoetiology, presentation, and management of delirium in adults with COVID-19. J Psychosom Res. 2021;141:110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krajewska J, Krajewski W, Zub K, et al. COVID-19 in otolaryngologist practice: a review of current knowledge. Eur Arch Otorhinolaryngol. 2020;277:1885–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clinical Spectrum . COVID-19 treatment guidelines. (cited 2021 Sep 5). https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- 30.Aronson KI, Podolanczuk AJ. Lungs after COVID-19: evolving knowledge of post–COVID-19 interstitial lung disease. Ann ATS. 2021;18:773–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bompard F, Monnier H, Saab I, et al. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J. 2020;56. DOI: 10.1183/13993003.01365-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiffrin EL, Flack JM, Ito S, et al. Hypertension and COVID-19. Am J Hypertens. 2020;33:373–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strohbehn IA, Zhao S, Seethapathy H, et al. Acute kidney injury incidence, recovery, and long-term kidney outcomes among hospitalized patients with COVID-19 and influenza. Kidney Int Rep. 2021;2565–2574. DOI: 10.1016/j.ekir.2021.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu J, Song S, Cao H-C, et al. Liver diseases in COVID-19: etiology, treatment and prognosis. World J Gastroenterol. 2020;26:2286–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansson A, Mohamed MS, Moulin TC, et al. Neurological manifestations of COVID-19: a comprehensive literature review and discussion of mechanisms. J Neuroimmunol. 2021;358. DOI: 10.1016/j.jneuroim.2021.577658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharifian-Dorche M, Huot P, Osherov, M., Wen, D., Saveriano, A., Giacomini, P.S., Antel, J.P. and Mowla, A. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J Neurol Sci. 2020;417. DOI: 10.1016/j.jns.2020.117085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottlieb M, Long B. Dermatologic manifestations and complications of COVID-19. Am J Emerg Med. 2020;38:1715–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.COVID-19 and endocrine and metabolic disorders: critical points and suggestions for a correct therapeutic management from a tertiary endocrine center in Italy - minerva endocrinology 2021. Jul 26. (cited 2021 Jul 31). https://www.minervamedica.it/en/journals/minerva-endocrinology/article.php?cod=R07Y9999N00A21072601 [DOI] [PubMed]
- 39.Gorelik M. Learning about Kawasaki disease from COVID-19 and the multisystem inflammatory syndrome in children. Curr Opin Pediatr. 2021. July 23;33:603–609. Published online [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021;27(11):1652–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21:939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]; • this is an important article for SARS-CoV-2 infection in vaccinated individuals.
- 42.Serrano CO, Alonso E, Andrés M, et al. Pediatric chest x-ray in covid-19 infection. Eur J Radiol. 2020;131. DOI: 10.1016/j.ejrad.2020.109236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng M-Y, Lee EYP, Yang J, et al. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiology. 2020;2:e200034. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• this is an important literature review for radiologi findings in SARS-CoV-2 infection.
- 44.Himoto Y, Sakata A, Kirita M, et al. Diagnostic performance of chest CT to differentiate COVID-19 pneumonia in non-high-epidemic area in Japan. Jpn J Radiol. 2020;38:400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng Y, Wang L, Ben S. Meta-analysis of chest CT features of patients with COVID-19 pneumonia. J Med Virol. 2021;93:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology. 2020;295:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Behzad S, Aghaghazvini L, Radmard AR, et al. Extrapulmonary manifestations of COVID-19: radiologic and clinical overview. Clin Imaging. 2020;66:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poggiali E, Dacrema A, Bastoni D, et al. Can lung US help critical care clinicians in the early diagnosis of Novel Coronavirus (COVID-19) pneumonia? Radiology. 2020;295:E6–E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ravikanth R. Review of lung ultrasound findings in coronavirus disease 2019 (COVID-19): effectiveness, applications and approach to lung ultrasound during times of a pandemic. Saudi J Anaesth. 2021;15:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peixoto AO, Costa RM, Uzun R, et al. Applicability of lung ultrasound in COVID-19 diagnosis and evaluation of the disease progression: a systematic review. Pulmonology. 2021;27:529–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kremer S, Lersy F, de Sèze J, et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297:E242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heiss R, Grodzki DM, Horger W, et al. High-performance low field MRI enables visualization of persistent pulmonary damage after COVID-19. Magn Reson Imaging. 2021;76:49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng X, Liu B, Li J, et al. Blood biochemical characteristics of patients with coronavirus disease 2019 (COVID-19): a systemic review and meta-analysis. Clin Chem Lab Med. 2020;58:1172–1181. [DOI] [PubMed] [Google Scholar]
- 54.Huang I, Pranata R, Lim MA, et al. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He X, Wang L, Wang H, et al. Factors associated with acute cardiac injury and their effects on mortality in patients with COVID-19. Sci Rep. 2020;10:20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iba T, Levy JH, Connors JM, et al. The unique characteristics of COVID-19 coagulopathy. Crit Care. 2020;24:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Díaz JSS, Moguel KGP, Escudero EAG, et al. Glycosylated hemoglobin as a predictor of mortality in severe pneumonia by COVID-19. Expert Rev Respir Med. 2021;15:1–6. [DOI] [PubMed] [Google Scholar]
- 58.Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30:e2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henry BM, Oliveira MHSD, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021–1028. [DOI] [PubMed] [Google Scholar]
- 60.Yamayoshi S, Sakai-Tagawa Y, Koga M, et al. Comparison of rapid antigen tests for COVID-19. Viruses. 2020;12:1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mouliou DS, Pantazopoulos I, Gourgoulianis KI. Societal Criticism towards COVID-19: assessing the theory of self-diagnosis contrasted to medical diagnosis. Diagnostics. 2021;11:1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ejazi SA, Ghosh S, Ali N. Antibody detection assays for COVID-19 diagnosis: an early overview. Immunol Cell Biol. 2021;99:21–33. [DOI] [PubMed] [Google Scholar]
- 63.Kotsiou OS, Pantazopoulos I, Papagiannis D, et al. Repeated antigen-based rapid diagnostic testing for estimating the coronavirus disease 2019 prevalence from the perspective of the workers’ vulnerability before and during the lockdown. Int J Environ Res Public Health. 2021;18:1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mouliou DS, Kotsiou OS, Gourgoulianis KI. Estimates of COVID-19 risk factors among social strata and predictors for a vulnerability to the infection. Int J Environ Res Public Health. 2021;18:8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogata AF, Cheng C-A, Desjardins M, et al. Circulating SARS-CoV-2 vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients. Clin Infect Dis. 2021;ciab465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mouliou DS, Pantazopoulos I, Gourgoulianis KI. Social response to the vaccine against COVID-19: the underrated power of influence. JPM. 2021;12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]


