ABSTRACT
Introduction
The immunological response to COVID-19 is only partly understood. It is increasingly clear that the virus triggers an inappropriate host inflammatory reaction in patients experiencing severe disease.
Areas covered
The role of antibodies in COVID-19 remains to be fully defined. There is evidence for both protection and harm in different clinical syndromes triggered by SARS-CoV-2. Many patients dying from COVID-19 had both high titers of antibodies to SARS-CoV-2 and elevated viral loads. The uncertain protective role of humoral immunity is mirrored by the lack of benefit of therapeutic convalescent plasma infusions in COVID-19. In contrast, there is increasing evidence that a vigorous T-cell response is protective. Delayed or low avidity T cell reactions were seen in patients suffering severe COVID-19.
Expert opinion
These observations suggest T cell responses to SARS-CoV-2 are the dominant long-term protective mechanism following either infection or vaccination. The magnitude and quality of the antibody response is likely to reflect underlying T cell immunity to SARS-CoV-2. Much of what has been learned about COVID-19 will need to be revised following the recent rapid emergence and dominance of the omicron variant of SARS-CoV-2.
KEYWORDS: COVID-19, SARS-CoV-2, antibodies, monoclonal antibodies, vaccination, T cells
1. Introduction
Following its origin from Wuhan city in China, SARS-CoV-2 has spread to almost all continents on earth with calamitous health, economic and societal consequences. The origin of the virus is the subject of intense scrutiny [1–3]. Apart from a death toll exceeding 6 million, hundreds of millions of people have been infected with SARS-CoV-2, resulting in ongoing medical and psychiatric sequelae. A much larger number have been plunged into poverty, triggered by economic misery faced by many developing nations.
The infection appears to evolve in three overlapping clinical stages (Figure 1). In the first asymptomatic nasal phase, the virus targets cells bearing membrane ACE2 in the upper respiratory tract. Host proteases including TMPRSS-2 cleave the spike (S) glycoprotein [4]. The S2 subunit is then able to fuse with the cell, releasing the viral genome, which hijacks cellular machinery to produce daughter virus.
Figure 1.
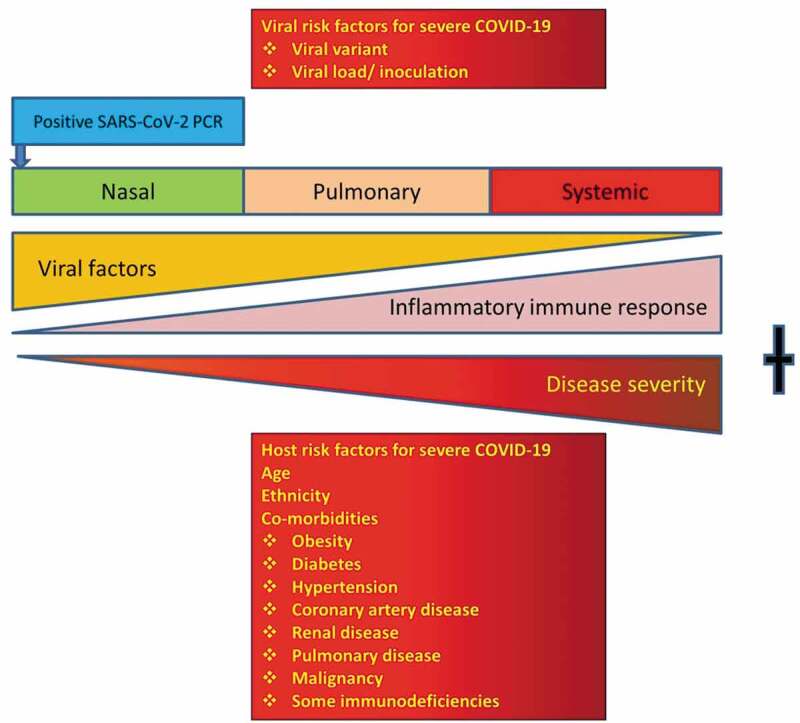
Factors determining the outcome of COVID-19. Both viral and host risk factors are important in progression from the nasal to the pulmonary and systemic phases. The omicron variant appears to produce milder pulmonary disease and may have a shorter nasal phase. New antiviral drugs such as molnupiravir (Merck) or paxlovid (Pfizer) are only likely to be effective early in disease. Later in disease, immunomodulatory drugs are more effective. Vaccines reduce the risk of aberrant immune responses, making the pulmonary and systemic phases less likely.
Following the nasal phase, some patients progress to the second pulmonary stage, probably by microaspiration from the nose and stomach [5]. Patients in this phase experience increasing breathlessness, lethargy and myalgia. High-resolution CT scans of the thorax may show ground glass appearance.
Patients progressing to the third systemic stage suffer multiple organ dysfunction including acute respiratory distress syndrome (ARDS) and activation of the coagulation cascade [6]. A cytokine storm is triggered by inappropriate activation of macrophages and neutrophils. In spite of invasive ventilation and extracorporeal membrane oxygenation, mortality is very high.
There is a steep age-related mortality rate, approaching 30% in those over 80 years [7]. In addition, comorbidities such as obesity, diabetes, malignancy, coronary artery disease, hypertension, renal and pulmonary disease are added risk factors for severe outcomes (Figure 1) [6–8]. Black, Hispanic and South Asian patients are at increased risk [9]. The immunological basis for these adverse outcomes is poorly understood. Part of this ethnic susceptibility may be due to sociodemographic factors, increased burden of comorbidities and inequitable access to healthcare [10].
In spite of intense study, the protective immunological correlates of COVID-19 remain uncertain [11,12]. The role of antibodies, in particular, during COVID-19 infection and following vaccination is incompletely understood. Since the beginning of the pandemic, several apparently contradictory observations have been made on the protective vs harmful roles of antibodies in COVID-19 syndromes/clinical scenarios. Some of these apparent paradoxes are reviewed and emerging explanations are discussed in this essay.
2. SARS-CoV-2 antibodies in the diagnosis of COVID-19
2.1. Variable antibody responses in COVID-19
Patients typically develop antibodies to the virus in the second week of infection. The magnitude of the antibody response may reflect the severity of the infection in some studies [13,14], but not in others [15]. Patients with more severe disease also appear to have longer-lasting antibody responses [13]. Antibodies are present for over 1 year in infected Health Care Workers (HCW) [16] and memory B cells are present for at least 6 or 8 months [17–19]. Memory B cells may be present long after antibodies wane. COVID-19 survivors generate long-lasting mucosal antibodies [20]. Their role in preventing reinfection remains to be defined.
Unexpectedly, many persons with documented infection have poor antibody responses to the virus and yet had only mild symptoms [21–23]. Mildly symptomatic HCWs had short-lived antibody responses [24] and asymptomatic infected persons frequently had low antibody responses or did not seroconvert [25–28]. Children also had lower antibody levels compared to adults and yet are less severely affected [29,30].
Lower viral loads as judged by the RT-qPCR cycle threshold (Ct) were associated with failure to seroconvert [31]. Self-prescribed ivermectin was associated with lower antibody titers but there was no difference in disease severity [32]. Ivermectin does not appear to be effective in the treatment of COVID-19 [33]. Overall, approximately 10% of infected patients with confirmed COVID-19 do not seroconvert [34–37]. It is uncertain if these individuals are susceptible to reinfection.
2.2. Variable sensitivity of diagnostic serology tests for COVID-19
Currently, there are a large number of commercial and in-house assays for SARS-CoV-2 antibodies. Potential targets include the nucleocapsid (N), envelope (E), membrane (M) proteins or the S glycoprotein. The reader should seek up-to-date information as many vendors offer tests to several target antigens.
The S glycoprotein is post-translationally modified by carbohydrates, which may reduce its immunogenicity and antibodies may not be durable [27]. Yet it appears antibodies to the S glycoprotein are longer lasting than those to other proteins. Anti N antibodies decline more rapidly than anti S antibodies [38–42]. IgA and IgM antibodies to SARS-CoV-2 also rapidly wane. Antibodies to the M, E and N proteins have an important role in distinguishing COVID-19 survivors from those who have been vaccinated. First-generation COVID-19 vaccines comprise the S glycoprotein presented in different formats.
Recent studies have shown considerable variability in sensitivity in head-to-head comparisons of COVID-19 antibody assays [43,44]. While most of the antibody tests are specific for SARS-CoV-2, there is variable sensitivity. The sensitivity of the assay is determined by the antigen used and the relevant detection system. Currently, there are many laboratories offering in-house SARS-CoV-2 assays and it will be important for these assays to be verified and validated to ISO 9001 and 17,025 standards. External quality assurance (EQA) programs will improve the performance of these assays.
2.3. Inconsistent neutralising antibody titers in the protection against COVID-19
Measuring neutralizing antibodies by preventing in vitro infection of Vero cells requires access to Physical Containment Class 3 (PC3) laboratories. PC3 facilities are typically found in regional government laboratories or in academic institutions [45]. A surrogate virus neutralization assay has been created by a competitive ELISA, blocking antibody binding to soluble ACE2 [46,47]. A similar assay could be configured with pseudovirus [48].
Neutralizing antibodies can persist up to 9 months in mildly affected young adults [49] and were present 13 months after disease in another study [50]. The mechanism of action of neutralizing antibodies has not been completely defined. Very recent data suggest that both linear and conformation epitopes are important in neutralizing activity [51]. This indicates immunization strategies with linear peptides alone, may not be successful in conferring protective immunity.
There have been paradoxical observations on the role of neutralizing antibodies following infection. Some data indicate neutralizing antibodies predict protection [35] and patients who developed delayed neutralizing antibody responses had more severe disease [52]. Specific neutralizing antibody titers however do not reliably predict protection from breakthrough SARS-CoV-2 infection [53,54]. As discussed below, there is no consistent, predetermined protective antibody level following infection or vaccination. As outlined below, many of these apparently contradictory observations may be explained by the underappreciated critical protective role of T cell responses to SARS-CoV-2 [55,56].
3. The role of antibodies in the pathogenesis of various COVID-19 syndromes
3.1. The risk of Antibody Dependent Enhancement (ADE)
Early in the pandemic it was realized antibody responses did not necessarily confer protection. Many patients dying from COVID-19 had both high viral loads as well as high antibody titers [57,58]. The antibody response was not protective in these individuals [59] and there was concern this was evidence of antibody-dependent enhancement (ADE).
Recent studies have explored possible mechanisms for ADE in vitro [57]. One study identified four regions within the viral receptor-binding domain (RBD) of SARS-CoV-2 [57]. Antibodies to specific epitopes resulted in either neutralization or enhancement by allowing the virus to infect Raji cells. What may be neutralizing antibodies in vitro may be enhancing in vivo. Another recent study implicated inappropriate antibody-dependent phagocytosis of SARS-CoV-2 into macrophages, triggering inflammasome activation leading to pyroptosis and a cytokine storm [60]. As discussed below, these inconsistent observations underscore the importance of T cell responses to SARS-CoV-2.
3.2. Multisystem Inflammatory Syndrome in Children (MISC)
ADE may be occurring in the multisystem inflammatory syndrome in children (MISC) and adults. Here, a severe inflammatory response occurs 4–6 weeks after infection with SARS-CoV-2. These patients have high titers of antibodies, particularly IgA to the virus. In vitro studies have shown these antibodies are proinflammatory and trigger neutrophil and macrophage responses. The latter cells may be the source of multiple cytokines, which could be the basis of this rare but severe syndrome. Whether MISC is an example of ADE is the subject of ongoing study [61].
3.3. Passive transfer of SARS-CoV-2 antibodies through the placenta and breast milk
Antibodies can be transferred through the placenta or via breast milk to protect the infant. Antibodies to SARS-CoV-2 have also been noted in breast milk of mothers after vaccination [62] or infection [63,64]. The role of these passively acquired antibodies in protecting infants is the subject of ongoing investigation. To date, there is no evidence these antibodies cause ADE/MISC in infants [65].
3.4. Antibody response in long COVID
Long COVID is a recently recognized syndrome following COVID-19 where patients experience a range of disabling symptoms including severe fatigue, chest pain, breathlessness and neurological symptoms including autonomic instability, loss of memory and clarity of thought (brain fog) [66,67]. There is ongoing debate about the case-definition of Long-COVID. It seems likely this is a heterogeneous disorder with disparate symptoms of variable duration [68]. Current data indicate vaccines reduce the risk of long-COVID, suggesting the disorder may be a consequence of either a suboptimal immune response to the infection or ongoing immune dysregulation. Some data indicate endothelial dysfunction and microthrombi may be contributing to ongoing organ dysfunction [69,70]. It remains to be determined if the new omicron variant will cause a similar syndrome in COVID-19 survivors.
Many of these patients were infected early in the pandemic, did not have access to reliable RT-qPCR tests and their antibodies may have waned [71]. Some patients are in a diagnostic vacuum, without an explanation for their disorder [72]. There is an urgent need for diagnostic SARS-CoV-2 T cell assays for these patients [73,74].
3.5. The antibody response in chronic COVID-19
Chronic COVID-19 is a dangerous stalemate between SARS-CoV-2 and a suboptimal cellular immune response [75]. Patients may shed the virus for months before either succumbing or recovering. Chronic COVID-19 can lead to intra-host viral evolution with the emergence of dangerous mutants evading vaccines and monoclonal antibodies [76].
Some patients with Chronic COVID-19 have received passive immunotherapy with convalescent plasma. Intra-host viral evolution caused the emergence of new resistant variants, indicating passive immunotherapy was not protective [77]. There is a risk of variants of high consequence evolving. Chronic COVID-19, although rare, is a public health emergency within a global crisis and must be prevented at any cost.
3.6. Antibody responses in immunodeficient patients
Many patients with primary and secondary immunodeficiencies are unable to generate antibodies. Some of these patients respond poorly to vaccines and are susceptible to breakthrough infections [78,79]. Patients with Common Variable Immunodeficiency Disorders (CVID) often have poor responses to vaccines, although this can be inconsistent [80–82]. Recent studies indicate such patients will derive at least partial protection from COVID-19 vaccines [83,84].
The severity of COVID-19 may depend on the nature of the immune defect [74]. Patients with innate immune defects and those with T cell defects are at increased risk [85–87]. Paradoxically, patients with X-linked agammaglobulinemia (XLA) without co-morbidities, may be at lower risk of severe disease [88–91]. This indicates antibodies can be harmful in some circumstances and it infers T cell responses, which are intact in XLA, are protective. Patients with secondary immunodeficiency may also have suboptimal T cell responses to vaccines and infection.
Immunodeficient patients are frequently treated with subcutaneous or intravenous immunoglobulin (SCIG/IVIG). With increasing rates of COVID-19 infection and immunization, most plasma donors are likely to have SARS-CoV-2 antibodies [92]. It will be impossible to judge humoral responses to vaccines or infections in these patients. It will also not be possible to use SARS-CoV-2 antibody responses as a neoantigen for patients on SCIG/IVIG. At this time, it is uncertain if passively acquired SARS-CoV-2 antibodies will influence the severity of SARS-CoV-2 infections or if they will modulate responses to COVID-19 vaccines. Again, a T cell assay for SARS-CoV-2 may be very helpful in identifying infection in immunodeficient patients including those on SCIG/IVIG.
4. Limited therapeutic and protective role of SARS-CoV-2 antibodies following vaccination or infection
4.1. Limited role of passive immunotherapy
Early in the pandemic, there was enthusiasm for passive immunotherapy with convalescent plasma [93]. It was hoped that high titers of neutralizing antibodies would provide effective antiviral activity in the absence of proven therapeutics for COVID-19. Some studies have shown that convalescent plasma from patients infected with the original Wuhan virus are able to neutralize the alpha (B.1.1.7) variant in vitro [94]. In other studies, convalescent plasma from the Wuhan variant was less effective against newer variants including delta (B.1.617.2) [95,96], but may still be protective [97].
ADE may be a risk with passive immunotherapy [98]. ADE could amplify the inflammatory response to the virus and precipitate multiple-organ failure [58]. Anti-interferon antibodies in convalescent plasma could aggravate COVID-19. Ongoing studies aim to identify the risk of ADE with various therapeutic antibody preparations.
Meta-analysis of the results of randomized studies was disappointing, and the therapeutic role of such infusions is uncertain [99]. Any benefit seen in some trials may have been obscured by the variability in the quality and quantity of neutralizing antibodies. Recently, other components of plasma have been stated to have either beneficial or detrimental effects, increasing the complexity of assessing this form of treatment [100].
There has been interest in using post-vaccination serum for passive immunotherapy. As noted above, there was concern, however, that some of the new variants may be less susceptible to viral neutralization in vivo [101].
Newer biological products such as nanobodies may prove to be useful, but like many therapeutics, their role will need to investigated in patients suffering from the omicron variant. Detailed discussion of nanobodies [102] is beyond the scope of this article, which aims to provide an overview of the current status of human antibodies in COVID-19.
4.2. The role of monoclonal antibodies remains to be determined
Monoclonal antibodies (mabs) have revolutionized the treatment of several disorders including malignancy, autoimmunity and more recently infectious diseases. These antibodies are derived from immunized mice or convalescent humans. In the case of mice, murine complementarity determining regions can be grafted to human immunoglobulins to minimize anti-mab responses.
The FDA has recently granted emergency use authorization to several monoclonal antibodies directed to SARS-CoV-2. Therapeutic monoclonal antibodies include imdevimab, casirivimab, etesivimab sotrovimab, regdanivimab and bamlanivimab. These can be used individually (bamlanivimab) or as combinations (casirivimab and imdevimab or bamlanivimab and etesivimab) to prevent viral resistance [103].
Their main role appears to be in high-risk patients early in disease or as prophylaxis. Most current mabs are directed to the RBD. These antibodies block the attachment of the S glycoprotein to ACE2. There are multiple mechanisms of action including complement activation and opsonophagocytosis. Some can prematurely trigger or lock the S glycoprotein conformation, leading to viral inactivation. The role of the Fc fragment in mabs is uncertain. Some therapeutic mabs have inactivated Fc components. Fc function may be needed for therapy but not prophylaxis. In mice, neutralizing antibodies appear to require Fc function [104].
Mutations at the E484 and N501 position of the RBD confer resistance to several mabs [105–107]. The E484K mutation is present in the highly infectious delta variant [95]. Emergence of E484K mutants were identified in patients treated with the bamlanivimab [108]. A recent meta-analysis of published studies showed there was insufficient evidence for the efficacy of mabs in terms of hospital admissions or mortality [109]. The results of future trials will determine their efficacy [109]. A newly described mab, VIR-7831 (sotrovimab) appears to have broad neutralizing activity across the subgenus of beta coronaviridae [103]. Such antibodies may be resistant to viral evolution [103]. Other long-acting mabs such as AZD442 are undergoing phase 3 trials for prophylaxis. These trials may have to be repeated as the new omicron SARS-CoV-2 variant appears to be resistant to many of these monoclonal antibodies [110–112]. Monoclonal antibodies are not representative of a physiological polyclonal antibody response to SARS-CoV-2 and cannot be construed as evidence for the protective role of antibodies in COVID-19.
4.3. Vaccines and inconsistent protective antibody titers in breakthrough infections
Vaccines against SARS-CoV-2 have proved effective. Vaccines can boost immunological responses in COVID-19 survivors [113]. However, there are increasing reports of breakthrough infections in fully immunized persons. Antibodies wane after 3-4 months and boosters may be needed [114] Some countries are now advocating a third booster injection after 4 months or less [115]. A booster dose may improve cross-protection to other variants of concern. It appears heterologous vaccination with Astra Zeneca and mRNA vaccines lead to a robust antibody response [116]. There may be a move to include the N protein in future vaccines, which may confer acute protection for the brain and lung in infected persons [117].
In spite of declining antibody titers [118], vaccines do however provide partial protection beyond 6 months and shift the severity of the disease to the milder end of the spectrum. Hospitalizations and deaths are much less common in vaccinated persons than those who are unvaccinated.
Several factors appear to predispose to these breakthrough infections; age, comorbidities, waning immunity and the emergence and dominance of the delta and omicron variants [119–121]. Breakthrough infections in vaccinated individuals were more common with SARS-CoV-2 strains bearing mutations at the E484 and N501 positions, including the delta (E484K/N501Y) and omicron (E484A/N501Y) variants [122]. As noted, vaccines using the founder (Wuhan) strain may not induce optimal cross-protective antibodies (and therefore T cell responses) to other variants [123].
Although some articles suggest levels of neutralizing antibodies are relevant to breakthrough infections [54], there is no predefined protective level, as noted above. Deaths are age-related and not dependent on the titer of neutralizing antibodies [53]. As discussed below, it seems likely age, ethnicity and the well-known comorbidities are more important than antibody titers in breakthrough infections leading to death.
4.4. The NZACE2-Pātari project (Pātari – Māori verb for decoy, which will lead to interception)
Given the propensity for rapid viral evolution, one option has been to intercept and block SARS-CoV-2 in the nasal phase of the infection [124,125]. The NZACE2-Pātari project proposes using modified ACE2 molecules (N90D/R273A) to bind the virus early in the nasal phase to mitigate the pulmonary and systemic phases. Because the project uses ACE2 molecules, it is resistant to viral evolution, unlike most monoclonal antibodies or polyclonal antibodies. SARS-CoV-2 cannot evade therapeutics based on ACE2. The molecules would be administered as soon as the patient receives a positive RT-qPCR or rapid antigen test. The lower burden of SARS-CoV-2 in the nose would in turn mitigate the severity of the pulmonary and systemic phases. This treatment is likely to be well suited for the newer viral variants including omicron and delta. Clinical trials will confirm the efficacy of this type of treatment in the future.
5. Emerging explanations for the (apparent) antibody paradox in COVID-19
5.1. The role of age, ethnicity and comorbidities in COVID-19
The role of age, ethnicity and comorbidities in the outcome of COVID-19 is poorly understood. Recently, a study suggested that a higher prevalence of anti-interferon autoantibodies in patients with severe outcomes, particularly in older persons [126]. This may aggravate the inflammatory response.
It has been noted in multiple studies that patients prescribed proton pump inhibitors (PPIs) are at increased risk of severe disease [127]. The stomach may serve as a reservoir for intact SARS-CoV-2 and be aspirated to the lungs [125]. Data from China showed exposure to a higher viral inoculum was associated with a risk of death even in younger HCWs, before the use of personal protective equipment.
Similarly, inappropriate macrophage activation has been noted in patients with type 2 diabetes [128]. This could contribute to the cytokine storm and multiple-organ dysfunction seen in the systemic phase of the infection. Such observations might also be relevant to worse outcomes in persons of Black, Hispanic and South Asian ethnicity, who have increased rates of comorbidities including obesity, gastroesophageal reflux and diabetes.
Apart from biological factors, sociodemographic determinants including inequitable access to healthcare are likely to be major factors responsible for adverse outcomes in some disadvantaged ethnic groups [9].
5.2. The critical role of T cells in COVID-19
The role of T cells is being defined in the acute phase of COVID-19. Current data indicate uncoordinated over or under activation of acute T cell responses lead to severe disease [129,130]. Patients with severe disease were noted to have low avidity T cell responses [131]. Unbalanced T cell subsets in acute disease may be responsible for an ineffective, non-protective antibody response [132]. In contrast, patients who were either asymptomatic or mildly symptomatic had robust T-cell responses to the virus [28,133,134]. Data suggest patients exposed to the virus, who do not develop symptoms may be protected [135]. Such patients with borderline RT qPCR and negative antibodies may have a robust T cell response [27]. Pre-existing high avidity T cells aborted COVID-19 in exposed healthcare workers (HCWs) before these patients developed positive RT-qPCR tests to the virus. These individuals remained seronegative [136].
In individuals who succumbed to COVID-19, a recent study showed poor T cell responses in spite of a high antibody titer and viral loads [134]. This may explain early paradoxical observations from China, where individuals died from COVID-19 in spite of having high titers of antibodies to the virus. It is possible the difference between neutralizing function in vitro and ADE in vivo is an early and vigorous protective T cell response to vaccination or infection.
A robust memory T cell response following infection, confers long-term protection [19,27,133]. There are excellent T cell responses to the virus 6 months later [135,137] and T cell responses persist in spite of waning antibody responses [138–140]. A recent community-based survey from Sweden showed 17% of patients with T cell responses to SARS-CoV-2 were seronegative [141]. This highlights the importance of diagnostic T cell assays for SARS-CoV-2.
Vaccination provides protection against an unbalanced, uncoordinated cellular immune response causing severe disease and death [135]. Vaccination does not prevent infection but does alter the prognostic trajectory of COVID-19 in most patients. Robust T cell responses are likely to be the critical outcome of vaccination, which prevents severe disease [135]. Similarly, an effective T cell response to vaccines may protect against long-term sequelae of COVID-19 including Long COVID, Chronic COVID-19 and MISC [142]. It seems likely the T cell response to vaccines also protects against ADE.
Passive immunotherapy with convalescent plasma and monoclonal antibodies does not confer T cell immunity. This might explain why convalescent plasma has not been as successful as hoped. Given the critical role of cellular immunity, current data suggests antibodies to SARS-CoV-2 are arguably at best, epiphenomena and at worst, bad actors.
6. Expert opinion
Science will ultimately prevail against SARS-CoV-2. In spite of the havoc caused by the virus, there have been major advances including the development, testing and deployment of effective vaccines and therapeutics. Currently, there is a race between global vaccination and emergence of escape mutants. The ultimate death toll will be determined by the outcome of this race. The uncoordinated global response to the pandemic has allowed the virus to continue evolving, resulting in the selection and emergence of vaccine and antibody resistant SARS-CoV-2 variants.
This was seen with the emergence and dominance of the new SARS-CoV-2 omicron variant. This variant has multiple nucleotide substitutions in the S glycoprotein and N protein. The new omicron variant has far-reaching implications for the diagnosis, treatment and prevention of COVID-19 [143]. This essay has explored the current role of antibodies in the diagnosis and treatment of previous SARS-CoV-2 variants. Much of what has been written here may need to be revised with the emergence of the omicron variant. From preliminary data, omicron may need to be considered an entirely new viral infection and many aspects of what has been learned about previous SARS-CoV-2 variants will need to be amended.
Antibody-based assays will need to be reviewed for their sensitivity for this variant. New antibody assays may need to be developed with the specific mutations in the omicron RBD as the antigenic target. Assays will need to be compared in EQA programs and it is possible different antibody tests will be needed to determine the specific variant causing infection. This is particularly important if RT-qPCR tests were not undertaken at the time of viral shedding.
Evidence has been presented here that antibodies do not play a critical role in protecting against COVID-19. This again will have to be reviewed. It is possible antibodies will have a greater role in protecting against omicron than previous variants. Serum from omicron convalescent donors may be more effective than against previous SARS-CoV-2 variants. New trials of convalescent and post-vaccine plasma will be needed in the future.
It is becoming apparent omicron will evade many monoclonal antibodies currently in use. Current monoclonal antibodies in development will need to be tested against the RBD of omicron. It is hoped that at least some will be effective. New monoclonal antibodies generated from patients recovering from omicron infection will need to be developed.
In spite of the many mutations in the RBD, NZACE2-Pātari project is likely to prevail against omicron. Omicron requires binding to ACE2 for cellular entry and NZACE2-Pātari is likely to remain effective. Viral evolution of the RBD will not be tolerated without loss of infectivity. It appears the omicron variant has a shorter nasal phase than other SARS-CoV-2 variants, so treatment with NZACE2-Pātari will need to commence very early to alter the prognostic trajectory of patients.
The response of immunodeficient patients to omicron remains to be determined. Given omicron appears to be less prone to cause ARDS, the case fatality rate in immunodeficient patients may be less. Partial responses to three or four primary doses of current COVID-19 vaccines may suffice in protecting immunodeficient patients from severe outcomes. If omicron is less virulent, it may be less prone to causing Chronic COVID-19 in immunocompromised persons. Unlike previous variants, therapeutic plasma infusions may be more effective in immunocompromised persons infected with omicron.
The role of omicron in the outcomes in patients with comorbidities and the elderly will need to be revisited. Patients with pre-existing pulmonary disease may have milder disease given omicron is less likely to cause ARDS. All of this remains to be confirmed by future studies.
Drugs, such as remdesivir or favipiravir, which were previously shown to be ineffective against other SARS-CoV-2 variants, will need to be reassessed in new randomized trials of omicron infected patients. Similarly, the role of drugs such as dexamethasone will also need to be re-evaluated, as ARDS is less of a problem with omicron. Dexamethasone may increase the risk of secondary bacterial and fungal infections such as mucormycosis, which may alter the therapeutic index. It is possible machine learning and artificial intelligence may provide helpful information determining the optimal treatment strategies, based on the viral variant and detailed immunological parameters.
The role of T cells in protection against omicron will have to be revisited. Evidence has been presented here that cellular immunity is critical for recovery from COVID-19 caused by previous SARS-CoV-2 variants. It is possible existing vaccines confer effective cellular immunity to omicron and this may be enhanced by heterologous primary or booster vaccinations. Entirely new vaccines may need to be produced, but there is always the risk yet newer variants will emerge in the interim.
The final chapter on COVID-19 has not been written. If omicron has a low case fatality rate, it may serve as an efficient ‘live attenuated viral vaccine’. Omicron infection may confer protection against more virulent variants of concern. Inadvertent infection of immunized persons by omicron could confer a ‘booster’ effect, with robust long-term immune protection. Omicron may signal the beginning of end rather than the end of the beginning of the COVID-19 pandemic. This optimistic perspective must be tempered against hospitals being overwhelmed by COVID-19 infections in predominantly unvaccinated individuals.
Funding Statement
This paper was not funded.
Article highlights
SARS-CoV-2 the agent responsible for COVID-19 has caused calamitous social, economic and health consequences globally
The virus is able to evade the innate immune system and is also able to subvert the adaptive immune system in those who are severely affected
The role of antibodies in protection against the infection is incompletely understood. In some circumstances, the humoral immune response appears to be protective, while in other situations it may be aggravating the inflammatory response
At this time, it is not possible to predict specific protective antibody levels that prevent breakthrough infections in patients who have been vaccinated
The antibody response may be best viewed as a biomarker of either infection or vaccination.
It is becoming increasingly clear that cellular immunity is the most critical part of the response to either the virus or immunization, which allows long-term protection.
In the future, it is possible measurement of T cell responses will predict protective responses to vaccines in vulnerable individuals
This article seeks to provide an overview of the current status of knowledge on the role of antibodies in COVID-19
What is currently understood about COVID-19 may need to be revised with the rapid emergence and dominance of the omicron variant of SARS-CoV-2.
Omicron appears to be fundamentally different to previous SARS-CoV-2 variants and may need to be considered a new infection
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Further information
Neither the diagnostic T cell assay for SARS-CoV-2 nor the NZACE2-Pātari project has received support at this time.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Tiwari R, Dhama K, Sharun K, et al. COVID-19: animals, veterinary and zoonotic links. Vet Q. 2020;40(1):169–182. DOI: 10.1080/01652176.2020.1766725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segreto R, Deigin Y.. The genetic structure of SARS-CoV-2 does not rule out a laboratory origin: SARS-COV-2 chimeric structure and furin cleavage site might be the result of genetic manipulation. Bioessays. 2021;43(3):e2000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes EC, Goldstein SA, and Rasmussen AL, et al. The origins of SARS-CoV-2: a critical review. Cell. 2021;184:2848–4856. DOI: 10.1016/j.cell.2021.08.017. 1097-4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;4(20):271–280e278. DOI: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baek WK, Sohn SY, Mahgoub A, et al. A comprehensive review of severe acute respiratory syndrome Coronavirus 2. Cureus. 2020;12(5):e7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395(10229):1014–1015. DOI: 10.1016/S0140-6736(20)30633-4. London, England [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. DOI: 10.1016/S0140-6736(20)30566-3. London, England [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Y, Chen Y, Liu M, et al. Impacts of immunosuppression and immunodeficiency on COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):e93–e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirby T. Evidence mounts on the disproportionate effect of COVID-19 on ethnic minorities. Lancet Respir Med. 2020;8(6):547–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abedi V, Olulana O, Avula V, et al., Racial, economic, and health inequality and COVID-19 infection in the United States. J Racial Ethn Health Disparities. 8(3): 732–742. 2021. DOI: 10.1007/s40615-020-00833-4. . [DOI] [PMC free article] [PubMed] [Google Scholar]; •• disparties in outcomes in disadvantaged minorites
- 11.Wiersinga WJ, Rhodes A, and Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. [DOI] [PubMed] [Google Scholar]
- 12.Mathew D, Giles JR, Baxter AE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369(6508):eabc8511. DOI: 10.1126/science.abc8511. New York, N.Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan X, Chen G, Jin Z, et al. Anti-SARS-CoV-2 IgG levels in relation to disease severity of COVID-19. J Med Virol. 2022;94(1):380–383. DOI: 10.1002/jmv.27274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Legros V, Denolly S, and Vogrig M, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18:318–327. DOI: 10.1038/s41423-020-00588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandberg JK, Varnaitė R, Christ W, et al. SARS-CoV-2-specific humoral and cellular immunity persists through 9 months irrespective of COVID-19 severity at hospitalisation. Clin Transl Immunology. 2021;10(7):e1306. DOI: 10.1002/cti2.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobano C, Ramirez-Morros A, Alonso S, et al. Persistence and baseline determinants of seropositivity and reinfection rates in health care workers up to 12.5 months after COVID-19. BMC Med. 2021;19(1):155. DOI: 10.1186/s12916-021-02032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaebler C, Wang Z, and Lorenzi J, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:eabf1555. DOI: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartley G, Edwards ESJ, and Aui P, et al. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Sci Immunol. 2020;5. DOI: 10.1126/sciimmunol.abf8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. DOI: 10.1126/science.abf4063. New York, N.Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fröberg J, Gillard J, and Philipsen R, et al. SARS-CoV-2 mucosal antibody development and persistence and their relation to viral load and COVID-19 symptoms. Nat Commun. 2021;12:5621. DOI: 10.1038/s41467-021-25949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kowitdamrong E, Puthanakit T, Jantarabenjakul W, et al. Antibody responses to SARS-CoV-2 in patients with differing severities of coronavirus disease 2019. PloS one. 2020;15(10):e0240502. DOI: 10.1371/journal.pone.0240502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. DOI: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 23.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of Anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383(11):1085–1087. DOI: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marot S, Malet I, and Leducq V, et al. Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers. Nat Commun. 2021;12:844. DOI: 10.1038/s41467-021-21111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flieder T, Vollmer T, Muller B, et al. Retrospective analysis of 426 donors of a convalescent collective after mild COVID-19. PloS one. 2021;16(2):e0247665. DOI: 10.1371/journal.pone.0247665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Wang X, Du RH, et al. Serological investigation of asymptomatic cases of SARS-CoV-2 infection reveals weak and declining antibody responses. Emerg Microbes Infect. 2021;10(1):905–912. DOI: 10.1080/22221751.2021.1919032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al., Robust T Cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 183(1): 158–168 e114. 2020. DOI: 10.1016/j.cell.2020.08.017. . [DOI] [PMC free article] [PubMed] [Google Scholar]; •• the role of T cells in COVID-19
- 28.Rodda LB, Netland J, Shehata L, et al. Functional SARS-CoV-2-Specific immune memory persists after mild COVID-19. Cell. 2020;184(1):169–183e117. DOI: 10.1016/j.cell.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloise S, Marcellino A, Testa A, et al. Serum IgG levels in children 6 months after SARS-CoV-2 infection and comparison with adults. Eur J Pediatr. 2021;180(11):3335–3342. DOI: 10.1007/s00431-021-04124-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierce CA, Preston-Hurlburt P, Dai Y, et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med. 2020;12(564):eabd5487. DOI: 10.1126/scitranslmed.abd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, R R, B-r F, et al. Predictors of nonseroconversion after SARS-CoV-2 Infection. Emerg Infect Dis. 2021;27(9):2454–2458. DOI: 10.3201/eid2709.211042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedroso C, Vaz S, Netto EM, et al. Self-prescribed Ivermectin use is associated with a lower rate of seroconversion in health care workers diagnosed with COVID, in a dose-dependent response. Braz J Infect Dis. 2021;25(4):101603. DOI: 10.1016/j.bjid.2021.101603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill A, Garratt A, and Levi J, et al. Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection. Open Forum Infect Dis. 2021;8:ofab358. DOI: 10.1093/ofid/ofab358. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Ali AM, Ali KM, and Fatah MH, et al. SARS-CoV-2 reinfection in patients negative for immunoglobulin G following recovery from COVID-19. New Microbes New Infect. 2021;43:100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marklund E, Leach S, Axelsson H, et al. Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders. PloS one. 2020;15(10):e0241104. DOI: 10.1371/journal.pone.0241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cota G, Freire ML, de Souza CS, et al. Diagnostic performance of commercially available COVID-19 serology tests in Brazil. Int J Infect Dis. 2020;101:382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383(18):1724–1734. DOI: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lumley SF, Wei J, O’Donnell D, et al. The duration, dynamics, and determinants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody responses in individual healthcare workers. Clin Infect Dis. 2021;73(3):e699–e709. DOI: 10.1093/cid/ciab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo X, Zeng L, Huang Z, et al. Longer duration of SARS-CoV-2 infection in a case of mild COVID-19 with weak production of the specific IgM and IgG antibodies. Front Immunol. 2020;11:1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5(12):1598–1607. DOI: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roltgen K, Powell AE, Wirz OF, et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5(54):eabe0240. DOI: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAndrews KM, Dowlatshahi DP, Dai J, et al. Heterogeneous antibodies against SARS-CoV-2 spike receptor binding domain and nucleocapsid with implications for COVID-19 immunity. JCI Insight. 2020;5(18):e142386. DOI: 10.1172/jci.insight.142386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naaber P, Hunt K, Pesukova J, et al. Evaluation of SARS-CoV-2 IgG antibody response in PCR positive patients: comparison of nine tests in relation to clinical data. PloS one. 2020;15(10):e0237548. DOI: 10.1371/journal.pone.0237548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chua KYL, Vogrin S, Bittar I, et al. Clinical evaluation of four commercial immunoassays for the detection of antibodies against established SARS-CoV-2 infection. Pathology. 2020;52(7):778–782. DOI: 10.1016/j.pathol.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braun J, Loyal L, Frentsch M, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587(7833):270–274. DOI: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 46.Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38(9):1073–1078. DOI: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 47.Perera RAPM, Ko R, Tsang OTY, et al. Evaluation of a SARS-CoV-2 surrogate virus neutralization test for detection of antibody in human, canine, cat and hamster sera. J Clin Microbiol. 2021. Jan 21;59(2):e02504–20. doi: 10.1128/JCM.02504-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Septisetyani EP, Prasetyaningrum PW, Anam K, et al. SARS-CoV-2 antibody neutralization assay platforms based on epitopes sources: live virus, pseudovirus, and recombinant s glycoprotein RBD. Immune Netw. 2021. Dec;21(6):e39. doi: 10.4110/in.2021.21.e39-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bylicki O, Delarbre D, and Mayet A, et al. Neutralizing antibodies response to SARS-CoV-2 are persistent 9 months post symptom onset in mild or asymptomatic patients. Int J Infect Dis. 2021;112:8–12. DOI: 10.1016/j.ijid.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dehgani-Mobaraki P, Kamber Zaidi A, Porreca A, et al. Neutralizing antibody responses against SARS-CoV-2 spike receptor-binding domain 13 months after the recovery from the disease, Ann Ig. 2021. Aug 3. doi: 10.7416/ai.2021.2466. Epub ahead of print. PMID: 34328495. [DOI] [PubMed] [Google Scholar]
- 51.Gattinger P, Niespodziana K, Stiasny K, et al. Neutralization of SARS-CoV-2 requires antibodies against conformational receptor-binding domain epitopes. Allergy. 2022;77. 1398-9995 DOI 10.1111/all.15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawasuji H, Morinaga Y, Tani H, et al. Delayed neutralizing antibody response in the acute phase correlates with severe progression of COVID-19. Sci Rep. 2021;11(1):16535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faggiano F, Rossi M, and Cena T, et al. An outbreak of COVID-19 among mRNA-Vaccinated nursing home residents. Vaccines (Basel). 2021;9:859. DOI: 10.3390/vaccines9080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–1484. DOI: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tarke A, Coelho CH, Zhang Z, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022. DOI: 10.1016/j.cell.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186–193. Epub 42022 Feb 41591. DOI: 10.1038/s41590-41021-01122-w [DOI] [PubMed] [Google Scholar]
- 57.Zhou Y, Liu Z, Li S, et al. Enhancement versus neutralization by SARS-CoV-2 antibodies from a convalescent donor associates with distinct epitopes on the RBD. Cell Rep. 2021;34(5):108699. DOI: 10.1016/j.celrep.2021.108699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Negro F. Is antibody-dependent enhancement playing a role in COVID-19 pathogenesis?. Swiss Med Wkly. 2020;150:w20249. DOI: 10.4414/smw.2020.20249. eCollection 22020 Apr. [DOI] [PubMed] [Google Scholar]
- 59.Lambert PH, Ambrosino DM, Andersen SR, et al. Consensus summary report for CEPI/BC March 12-13, 2020 meeting: assessment of risk of disease enhancement with COVID-19 vaccines. Vaccine. 2020;38(31):4783–4791. DOI: 10.1016/j.vaccine.2020.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Junqueira C, Crespo Ã, Ranjbar S, et al. SARS-CoV-2 infects blood monocytes to activate NLRP3 and AIM2 inflammasomes, pyroptosis and cytokine release. Res Sq. 2021;Preprint (2921). DOI: 10.21203/rs.3.rs-153628/v1. [DOI] [Google Scholar]
- 61.Rothan HA, Byrareddy SN. The potential threat of multisystem inflammatory syndrome in children during the COVID-19 pandemic. Pediatr Allergy Immunol. 2021;32(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guida M, Terracciano D, Cennamo M, et al. COVID-19 vaccine mRNABNT162b2 elicits human antibody response in milk of breastfeeding women. Vaccines (Basel). 2021;9:785. DOI: 10.3390/vaccines9070785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duncombe CJ, McCulloch DJ, and Shuey KD, et al. Dynamics of breast milk antibody titer in the six months following SARS-CoV-2 infection. J Clin Virol. 2021;142:104916. DOI: 10.1016/j.jcv.2021.104916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Juncker HG, Romijn M, Loth VN, et al. Human milk antibodies against SARS-CoV-2: a longitudinal follow-up study. J Hum Lact. 2021;37(3):485–491. DOI: 10.1177/08903344211030171. [DOI] [PubMed] [Google Scholar]
- 65.Douxfils J, Gillot C, and De Gottal É, et al. Efficient maternal to neonate transfer of neutralizing antibodies after SARS-CoV-2 vaccination with BNT162b2: a case-report and discussion of the literature. Vaccines (Basel). 2021;9:907. DOI: 10.3390/vaccines9080907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Editorial. Facing up to long COVID. Lancet. 2020;396(10266):1861. DOI: 10.1016/S0140-6736(20)32662-3. Editorial [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–264. [DOI] [PubMed] [Google Scholar]
- 68.Jennings G, Monaghan A, Xue F, et al. A systematic review of persistent symptoms and residual abnormal functioning following acute COVID-19: ongoing symptomatic phase vs. Post-COVID-19 syndrome. J Clin Med. 2021;10(24):5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Charfeddine S, Ibn Hadj Amor H, and Jdidi J, et al. Long COVID 19 syndrome: is it related to microcirculation and endothelial dysfunction? Insights from TUN-EndCOV study. Front Cardiovasc Med. 2021;8:745758. DOI: 10.3389/fcvm.2021.745758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pretorius E, Vlok M, Venter C, et al. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 2021;20(1):172. DOI: 10.1186/s12933-021-01359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.García-Abellán J, Padilla S, and Fernández-González M, et al. Antibody response to SARS-CoV-2 is associated with long-term clinical outcome in patients with COVID-19: a longitudinal study. J Clin Immunol. 2021;41:1490–1501. DOI: 10.1007/s10875-021-01083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Augustin M, Schommers P, and Stecher M, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021;6:100122. DOI: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ameratunga R, Woon ST, Jordan A, et al. Perspective: diagnostic laboratories should urgently develop T cell assays for SARS-CoV-2 infection. Expert Rev Clin Immunol. 2021;17(5):421–430. DOI: 10.1080/1744666X.2021.1905525. [DOI] [PubMed] [Google Scholar]
- 74.Ameratunga R, Longhurst H, Steele R, et al. Common variable immunodeficiency disorders, T-Cell responses to SARS-CoV-2 vaccines, and the risk of chronic COVID-19. J Allergy Clin Immunol Pract. 2021;9(10):3575–3583. DOI: 10.1016/j.jaip.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hensley MK, Bain WG, Jacobs J, et al., Intractable coronavirus disease 2019 (COVID-19) and prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication in a chimeric antigen receptor-modified T-Cell therapy recipient: a case study. Clin Infect Dis. 73(3): e815–e821. 2021. DOI: 10.1093/cid/ciab072. . [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Illustrating Chronic COVID-19
- 76.Choi B, Choudhary MC, Regan J, et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med. 2020;383(23):2291–2293. DOI: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen L, Zody MC, Di Germanio C, et al. Emergence of multiple SARS-CoV-2 antibody escape variants in an immunocompromised host undergoing convalescent plasma treatment. mSphere. 2021;6(4):e0048021. DOI: 10.1128/mSphere.00480-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ferri C, Ursini F, and Gragnani L, et al. Impaired immunogenicity to COVID-19 vaccines in autoimmune systemic diseases. High prevalence of non-response in different patients’ subgroups. J Autoimmun. 2021;125:102744. DOI: 10.1016/j.jaut.2021.102744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brosh-Nissimov T, Orenbuch-Harroch E, and Chowers M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully-vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021;27:1652–1657. DOI: 10.1016/j.cmi.2021.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ameratunga R, Ahn Y, Steele R, et al. The natural history of untreated primary hypogammaglobulinemia in adults: implications for the diagnosis and treatment of Common Variable Immunodeficiency Disorders (CVID). Front Immunol. 2019;10:1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ameratunga R, Allan C, Woon ST. Defining common variable immunodeficiency disorders in 2020. Immunol Allergy Clin North Am. 2020;40(3):403–420. [DOI] [PubMed] [Google Scholar]
- 82.Ameratunga R, Jordan A, Cavadino A, et al. Bronchiectasis is associated with delayed diagnosis and adverse outcomes in the New Zealand Common Variable Immunodeficiency Disorders cohort study. Clin Exp Immunol. 2021;204(3):352–360. DOI: 10.1111/cei.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amodio D, Ruggiero A, Sgrulletti M, et al. Humoral and cellular response following vaccination with the BNT162b2 mRNA COVID-19 vaccine in patients affected by primary immunodeficiencies. Front Immunol. 2021;12:727850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salinas AF, Mortari EP, Terreri S, et al. SARS-CoV-2 vaccine induced atypical immune responses in antibody defects: everybody does their best. J Clin Immunol. 2021;41(8):1709–1722. DOI: 10.1007/s10875-021-01133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Esenboga S, Ocak M, Akarsu A, et al. COVID-19 in patients with primary immunodeficiency. J Clin Immunol. 2021;41(7):1515–1522. DOI: 10.1007/s10875-021-01065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bastard P, Rosen LB, Zhang Q, et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020. Oct 23;370(6515):eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020. Oct 23;370(6515):eabd4570. doi: 10.1126/science.abd4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Quinti I, Lougaris V, Milito C, et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146(1):211–213 e214. DOI: 10.1016/j.jaci.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jin H, Reed JC, Liu STH, et al. Three patients with X-linked agammaglobulinemia hospitalized for COVID-19 improved with convalescent plasma. J Allergy Clin Immunol Pract. 2020;8(10):3594–3596 e3593. DOI: 10.1016/j.jaip.2020.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mira E, Yarce OA, Ortega C, et al. Rapid recovery of a SARS-CoV-2-infected X-linked agammaglobulinemia patient after infusion of COVID-19 convalescent plasma. J Allergy Clin Immunol Pract. 2020;8(8):2793–2795. DOI: 10.1016/j.jaip.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Damme KFA, Tavernier S, and Van Roy N, et al. Case report: convalescent plasma, a targeted therapy for patients with CVID and Severe COVID-19. Front Immunol. 2020;11:596761. DOI: 10.3389/fimmu.2020.596761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jones JM, Stone M, and Sulaeman H, et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations. JAMA. 1538-3598 2020-July 2021;326:1400–1409. DOI: 10.1001/jama.2021.15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abraham J. Passive antibody therapy in COVID-19. Nat Rev Immunol. 2020;20(7):401–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shen X, Tang H, McDanal C, et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021;29(4):529–539.e523. DOI: 10.1016/j.chom.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mlcochova P, Kemp S, Dhar MS, et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599(7883):114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tea F, Ospina Stella A, Aggarwal A, et al. SARS-CoV-2 neutralizing antibodies: longevity, breadth, and evasion by emerging viral variants. PLoS Med. 2021;18(7):e1003656. DOI: 10.1371/journal.pmed.1003656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Choi A, Koch M, Wu K, et al. Serum neutralizing activity of mRNA-1273 against SARS-CoV-2 variants. J Virol. 2021;95(23):e0131321. DOI: 10.1128/JVI.01313-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bégin P, Callum J, Jamula E, et al. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat Med. 2021 Nov;27(11):2012-2024. doi: 10.1038/s41591-021-01488-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Valk SJ, Piechotta V, Chai KL, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;5(5):CD013600. DOI: 10.1002/14651858.CD013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Focosi D, Franchini M, and Pirofski LA, et al. COVID-19 convalescent plasma is more than neutralizing antibodies: a narrative review of potential beneficial and detrimental co-factors. Viruses. 2021;13:1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bates TA, Leier HC, Lyski ZL, et al. Neutralization of SARS-CoV-2 variants by convalescent and BNT162b2 vaccinated serum. Nat Commun. 2021;12(1):5135. DOI: 10.1038/s41467-021-25479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aria H, Mahmoodi F, and Ghaheh HS, et al. Outlook of therapeutic and diagnostic competency of nanobodies against SARS-CoV-2: a systematic review. Anal Biochem. 2022;4. 1096-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Corti D, Purcell LA, Snell G, et al. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell. 2021;184:3086–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ullah I, Prévost J, Ladinsky MS, et al. Live imaging of SARS-CoV-2 infection in mice reveals that neutralizing antibodies require Fc function for optimal efficacy. Immunity. 2021;54:2143–2158.e15. DOI: 10.1016/j.immuni.2021.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Z, VanBlargan LA, Bloyet LM, et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe. 2021;27(21):447–488.e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan M, Huang D, Lee CD, et al. Structural and functional ramifications of antigenic drift in recent SARS-CoV-2 variants. Science. 2021;373(6556):818–823. DOI: 10.1126/science.abh1139. New York, N.Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen RE, Winkler ES, Case JB, et al. In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains. Nature. 2021;596(7870):103–108. DOI: 10.1038/s41586-021-03720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peiffer-Smadja N, Bridier-Nahmias A, Ferré VM, et al. Emergence of E484K mutation following bamlanivimab monotherapy among high-risk patients infected with the alpha variant of SARS-CoV-2. Viruses. 2021;13(8):1462. DOI: 10.3390/v13081642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kreuzberger N, Hirsch C, and Chai KL, et al. SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19. Cochrane Database Syst Rev. 2021;9:CD013825. DOI: 10.1002/14651858.CD013825.pub2. 1469-493X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xiang Y, Nambulli S, Xiao Z, et al. Versatile and multivalent nanobodies efficiently neutralize SARS-CoV-2. Science. 2020;370(6523):1479–1484. DOI: 10.1126/science.abe4747. New York, N.Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Planas D, Saunders NA, and Maes PA, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2021;10. 1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- 112.Liu L, Iketani S, and Guo YA-OX, et al. Striking antibody evasion manifested by the omicron variant of SARS-CoV-2. Naturev 2021;10:. . 10.1038/s41586-021-04388-0 [DOI] [PubMed] [Google Scholar]
- 113.Goel R, Apostolidis SA, and Painter M, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6. DOI: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pegu A, O’Connell S, Schmidt SD, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science. 2021;373(6561):1372–1377. New York, N.Y. 2921. DOI: 10.1126/science.abj4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bar-On YM, Goldberg Y, and Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385. 1533-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Importance of booster vaccines in protection
- 116.Benning L, Töllner M, and Hidmark A, et al. Heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccination induces strong humoral responses among health care workers. Vaccines (Basel). 2021;9:857. DOI: 10.3390/vaccines9080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dangi T, Class J, Palacio N, et al. Combining spike- and nucleocapsid-based vaccines improves distal control of SARS-CoV-2. Cell Rep. 2021;36(10):109664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Erice A, Varillas-Delgado D, and Caballero C. Decline of antibody titres three months after two doses of BNT162b2 in non-immunocompromised adults. Clin Microbiol Infect. 2022;28:139.e131–139.e134. DOI: 10.1016/j.cmi.2021.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fowlkes A, Gaglani M, Groover K, et al. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection among frontline workers before and during B.1.617.2 (delta) variant predominance - eight U.S. locations. 2020. December -August 2021. MMWR Morb Mortal Wkly Rep. 2021 Aug 27;70(34):1167-1169. doi: 10.15585/mmwr.mm7034e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Harder T, Külper-Schiek W, and Reda S, et al. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection with the Delta (B.1.617.2) variant: second interim results of a living systematic review and meta-analysis. Euro Surveill. 2021;1 January to 1 January ;26:2100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Spicer KB, Glick C, and Cavanaugh AM, et al. Protective immunity after natural infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) - Kentucky, USA, 2020. Int J Infect Dis. 2022;114(114):21–28. DOI: 10.1016/j.ijid.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Feder K, Patel A, and Vepachedu VR, et al. Association of E484K spike protein mutation with SARS-CoV-2 infection in vaccinated persons. Clin Infect Dis. 2021 Sep 2;ciab762. doi: 10.1093/cid/ciab762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen LL, Lu L, and Choi CY, et al. Impact of SARS-CoV-2 variant-associated RBD mutations on the susceptibility to serum antibodies elicited by COVID-19 infection or vaccination. Clin Infect Dis. 2021;26:656. DOI: 10.1093/cid/ciab656. [DOI] [PubMed] [Google Scholar]
- 124.Ameratunga R, Lehnert K, Leung E, et al. Inhaled modified angiotensin converting enzyme 2 (ACE2) as a decoy to mitigate SARS-CoV-2 infection. N Z Med J. 2020;133(1515):112–118. [PubMed] [Google Scholar]
- 125.Ameratunga R, Woon ST, Steele R, et al. Perspective: the nose and the stomach play a critical role in the NZACE2-Patari* (modified ACE2) drug treatment project of SARS-CoV-2 infection. Expert Rev Clin Immunol. 2021;17(6):553–560. DOI: 10.1080/1744666X.2021.1912596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goncalves D, Mezidi M, Bastard P, et al. Antibodies against type I interferon: detection and association with severe clinical outcome in COVID-19 patients. Clin Transl Immunology. 2021;10(8):e1327. DOI: 10.1002/cti2.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Almario CV, Chey WD, Spiegel BMR. Increased risk of COVID-19 among users of proton pump inhibitors. Am J Gastroenterol. 2020;115(10):1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Melvin W, Audu C, Davis F, et al. Coronavirus induces diabetic macrophage-mediated inflammation via SETDB2. Proc Natl Acad Sci U S A. 2021. Sep 21;118(38):e2101071118. doi: 10.1073/pnas.2101071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weiskopf D, Schmitz KS, Raadsen MP, et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5(48):eabd2071. DOI: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meckiff BJ, Ramirez-Suastegui C, Fajardo V, et al. Imbalance of regulatory and cytotoxic SARS-CoV-2-Reactive CD4(+) T Cells in COVID-19. Cell. 2020;183(5):1340–1353 e1316. DOI: 10.1016/j.cell.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bacher P, Rosati E, Esser D, et al. Low-Avidity CD4(+) T Cell responses to SARS-CoV-2 in unexposed individuals and humans with severe COVID-19. Immunity. 2020;53(6):1258–1271 e1255. DOI: 10.1016/j.immuni.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Golovkin A, Kalinina O, and Bezrukikh V, et al. Imbalanced immune response of T-Cell and B-Cell subsets in patients with moderate and severe COVID-19. Viruses. 2021;13:1966. DOI: 10.3390/v13101966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-Specific adaptive immunity to SARS-CoV-2 in Acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012 e1019. DOI: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tan AT, Linster M, Tan CW, et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34(6):108728. DOI: 10.1016/j.celrep.2021.108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bertoletti A, Le Bert N, Qui M, et al. SARS-CoV-2-specific T cells in infection and vaccination. Cell Mol Immunol. 2021;18(10):2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Swadling L, Diniz MO, and Schmidt N, et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature. 2022;601:110–117. DOI: 10.1038/s41586-021-04186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zuo J, Dowell AC, Pearce H, et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nature. 2021;22(5):620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cassaniti I, Percivalle E, Bergami F, et al. SARS-CoV-2 specific T-cell immunity in COVID-19 convalescent patients and unexposed controls measured by ex vivo ELISpot assay. Clin Microbiol Infect. 2021;27(7):1029–1034. DOI: 10.1016/j.cmi.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen J, Liu X, and Zhang X, et al. Decline in neutralising antibody responses, but sustained T-cell immunity, in COVID-19 patients at 7 months post-infection. Clin Transl Immunology. 2021;10:e1319. DOI: 10.1002/cti2.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bonifacius A, Tischer-Zimmermann S, Dragon AC, et al. COVID-19 immune signatures reveal stable antiviral T cell function despite declining humoral responses. Immunity. 2021;54(2):340–354 e346. DOI: 10.1016/j.immuni.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Björkander S, Du L, and Zuo F, et al. SARS-CoV-2 specific B- and T-cell immunity in a population-based study of young Swedish adults. J Allergy Clin Immunol. 2021;21:S0091–6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22(1):43–55. DOI: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hoffmann M, Krüger N, Schulz S, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022 Feb 3;185(3):447-456.e11. doi: 10.1016/j.cell.2021.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]


