ABSTRACT
Multiple vaccines have recently been developed, and almost all the countries are presently vaccinating their population to tackle the COVID-19 pandemic. Most of the COVID-19 vaccines in use are administered via intramuscular (IM) injection, eliciting protective humor and cellular immunity. COVID-19 intranasal (IN) vaccines are also being developed that have shown promising ability to induce a significant amount of antibody-mediated immune response and a robust cell-mediated immunity as well as hold the added ability to stimulate protective mucosal immunity along with the additional advantage of the ease of administration as compared to IM injected vaccines. By inducing secretory IgA antibody responses specifically in the nasal compartment, the intranasal SARS-CoV-2 vaccine can prevent virus infection, replication, shedding, and disease development, as well as possibly limits virus transmission. This article highlights the current progress, advantages, prospects, and challenges in developing intranasal COVID-19 vaccines for countering the ongoing pandemic.
KEYWORDS: COVID-19, SARS-CoV-2, intranasal vaccine, mucosal immunity
Introduction
The ongoing coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has till date (February 8, 2022) affected hundreds of millions of people while leading to >5.7 million deaths worldwide.1 A very rapid global spread of COVID-19 posed an international health emergency as a devastating pandemic of the 21st Century. Though several drugs, therapies, and immunomodulatory regimens such as remdesivir, ivermectin, dexamethasone, convalescent plasma therapy, antibody-based immunotherapies, and monoclonal antibodies (MAbs) have been identified and used in emergency purposes for reducing the disease severity in patients with COVID-19 as well as others are being investigated; however, the choice of effective curative drugs and medicines are yet to be identified.2–7
Various research advances paved the way for developing multiple COVID-19 vaccines in less than a year by exploring several vaccine platforms and advances. After very high efforts, researchers have developed COVID-19 vaccines such as mRNA vaccine, DNA vaccine, viral vector vaccine, virus-like particles (VLPs), recombinant vaccine, protein subunit-based vaccines, live attenuated and inactivated virus vaccines that are being used for vaccinating people across the globe under different vaccination programs that are in progress in several countries. A few vaccines have been approved for use, and vaccination programs are currently underway in various countries8–12 while others newer and versatile vaccine production platforms including recombinant vaccines, plant-based vaccine, immunoinformatics-based multi-epitope subunit vaccine, artificial intelligence, and CRISPR/Cas technology-based vaccine, nanotechnology-based vaccines (nano-vaccine) and others are also under high progress and currently in the pipeline for developing appropriate vaccine candidates to counter SARS-CoV-2.11–18
Different kinds of vaccines in use have shown a high degree of efficacy with variable protective levels of up to 95% (70–95% range) in vaccinated individuals against COVID-19.2,3,12,19–21 In the era of various advances in developing vaccines, few issues of concerns, debates, and challenges related to vaccines include induction of variable protective levels, defining booster doses, the feasibility of virus re-infection and outcome of disease course in vaccinated individuals particularly amidst emerging variants and mutants, need for further modifying the vaccines as per main mutants/variants, levels of herd immunity developed, separate clinical trials in elderly, pregnant women and children or need same/special vaccine for these, vaccine hesitancy, diplomacy, and equitable access to the worldwide population and completing vaccination process at the earliest of worldwide population, these need to be addressed adequately.22–33
Currently, all available COVID-19 vaccines are administered by intramuscular (IM) injection, which is an invasive method, while many researchers are focusing on developing an effective vaccine that can be administered through nasal or oral routes. For instance, in mucosally transmitted illnesses like influenza and SARS-CoV-2 viruses, administering immunizations through the nasal route is regarded as extremely appealing since it induces a dual systemic and a robust local mucosal immune response. Since there is no need for trained medical personnel to deliver IN dosage, the nasal vaccines would have greater ease to administer and render desired efficacy. This is advantageous, particularly in developing nations, and therefore nasal vaccination provides a more cost-effective and convenient approach to administering vaccinations during disease outbreaks.34,35
COVID-19 approved vaccines delivered intramuscularly elicit antibody mediated and cell-mediated immunity in order to avoid viral replication and to provide resistance against the development of COVID-19. However, existing IM vaccinations are meant to induce systemic immune response without generating mucosal protection. Therefore, protections offered by IM vaccines may not be sufficient to deal with virus replication and shedding in the upper respiratory and so may not stop nasal SARS-CoV-2 infection. The absence of a local secretory IgA (sIgA) antibody immune response could pose a risk of SARS-CoV-2 transmission from vaccinated people as they still can be infected and therefore could spread the infection36(Figure 1).
Figure 1.
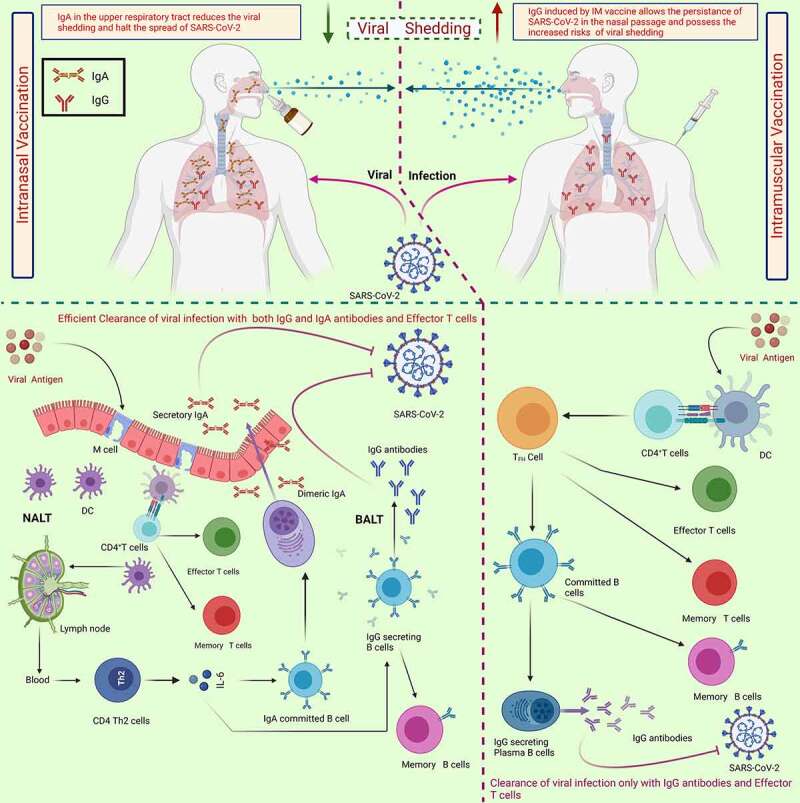
The schematic differentiation of immune response elicited by intranasal vaccine to intramuscular vaccine which is substantially efficient in reduction of the viral shedding as in case of intramuscular vaccination the shedding of viral particles is comparatively higher and possess the greater risks of transmission. the generation of secretory IgA in upper respiratory tract along with generation of IgG and effector T cells has been linked with the efficient and robust immune response against SARS-CoV-2. the robust mucosal immune response elicited by in vaccine lead to reduced spread of viral particles. Abbreviations: DC, dendritic cells; NALT, nasal-associated lymphoid tissues; BALT, Bronchus-associated lymphoid tissue; Ig., Immunoglobulin; Th, T helper cells. the figure was designed by Biorender.Com program (https://biorender.com/, accessed on 15 August 2021).
Recently, good progress has been made to develop vaccines and drugs which can be given via intranasal route that have benefits of ease of administration as being noninvasive route, and especially generating mucosal immunity apart from humoral and cellular immunity to render protection against COVID-19.34,37–41 Despite significant progress in developing a safe and reliable vaccine; there is still a need to discover better vaccine candidates that are safe and efficient for the great majority of the population. In this context, few of the novel intranasal COVID-19 vaccines are being developed, with encouraging preclinical findings in non-human primates and other animal models.35,39,42–46 An IM dosage followed by an internasal vaccination might lead to a robust immune response, which might be a reliable approach to attain herd immunity in the population.34 Furthermore, IN vaccines can elicit a substantial amount of B cells mediated and T cell-mediated immune response along with desired mucosal immunity. This article presents an overview of the efforts and progress being made in designing and developing vaccines that could be administered through nasal route and as nasal spray to counter SARS-CoV-2/COVID-19.
Intranasal COVID-19 vaccines
The primary entry portal for coronaviruses (CoVs) in the human body is constituted by oral and nasal mucosal surfaces, and the nasal compartment is the first-line barrier to SARS-CoV-2 entry that needs to be breached by the virus, after which the virus spread and disseminate to the lungs, therefore mucosal (IN) vaccination can render a safe and effective way for the generation of long-lasting systemic and humoral immune responses as well as mucosal immunity (sIgA) in both upper and lower respiratory tracts to bestow defense against SARS-CoV-2 infection.34,37,38,41 Intranasal (IN) administration of SARS-CoV-2 vaccines can prevent from virus infection, replication, shedding, and disease development as well as possibly limits virus transmission.34 Priming with IM vaccine and a booster with IN vaccination may likely lead to superior immune responses while preventing or sturdy dropping of viral replication in respiratory tracts.34 A variety of intranasal COVID-19 vaccines are currently under development, and these have shown attractive and promising avenues in countering COVID-19, which are discussed briefly in this section (Table 1).
Table 1.
Various nasal vaccines under various phases of clinical trials for efficient containment of COVID-19
| Intranasal Vaccine | Type of Vaccine | Developer/Developers | Antigen | Development Phase | ClinicalTrials.gov, Identifier | References |
|---|---|---|---|---|---|---|
| ChAdOx1 nCoV-19 | Adenovirus vector-based vaccine | University of Oxford in collaboration with AstraZeneca | SARS-CoV-2 spike protein | In a Phase I trial clinical trial using an intranasal spray | NCT04816019 | 47 |
| ChAd-SARS-CoV-2-S | Washington University School of Medicine in St Louis, USA | SARS-CoV-2 spike protein | Phase I clinical trials in India under the name BBV154 | NCT04751682 | 48 | |
| Ad5-nCoV vaccine | CanSino Biologics Inc. with Beijing Institute of Biotechnology and Jiangsu Province Centers for Disease Control and Prevention, China | Receptor Binding Domain (RBD) of S protein | In Phase I/II clinical trials | NCT04840992 | 49, 50 | |
| AdCOVID | Altimmune, Inc. USA | SARS-CoV-2 S-protein | Phase 1 | NCT04679909 | 43 | |
| NasoVAX | Altimmune, Inc. USA | SARS-CoV-2 S-protein | Phase 2 | NCT04442230 | 51 | |
| DelNS1-nCoV-RBD LAIV | Live attenuated vaccine | Beijing Wantai Biological Pharmacy Enterprise with researchers from Xiamen University and Hong Kong University | SARS-CoV-2 S-protein | Phase 1 | NCT04809389 | 52 |
| Mv-014-212 | Meissa Vaccines, Inc. | SARS-CoV-2 S-protein | Phase 1 | NCT04798001 | 53 | |
| COVI-VAC | CODAGENIX Inc., 3 Bioscience Park Drive, Farmingdale, NY, USA | Phase 1 | NCT04619628 | 54 | ||
| CROWNase | Subunit vaccines | Illinois Institute of Technology, Chicago | An enzyme removes the coating from the spike glycoprotein | Preclinical studies | N.A.* | 55 |
| CovOMV | Intravacc, Antonie van Leeuwenhoek laan 9, 3721 MA Bilthoven, Netherlands | Bacterial outer membrane vesicles (OMVs) mixed with recombinant S-protein (rSp) i.e., (CovOMV) | Preclinical studies | N.A.* | 56, 57 | |
| CIBG-669 | Center for Genetic Engineering and Biotechnology, Cuba | Protein subunit AgnHB (RBD) | Phase I/II | N.A.* | 58 | |
| STINGa-liposomes | AuraVax Therapeutics, Ina Mae Rude Entrepreneur Center 4200 James Ray Drive, Grand Forks, North Dakota, United States |
Trimeric S proteins with the STINGa-liposomes | Preclinical studies | N.A.* | 59 |
*Not Available.
NCT number National Clinical Trial number.
An intranasal vaccine, namely AdCOVID, has been developed to induce a robust and concentrated immune response to SARS-CoV-2 receptor-binding domain (RBD) by inducing mucosal IgA, serum neutralizing antibodies, and T cells (CD4+ and CD8+) along with the expressions of cytokines belonging to Th1 cells. By potentially enhancing both the systemic and local mucosal immunity, AdCOVID is considered a reliable and efficient IN vaccine candidate against COVID-19.43
Recently, a comparative study of IN and IM administration of a chimpanzee adenovirus-vectored vaccine expressing a pre-fusion stabilized S protein (ChAd-SARS-CoV-2-S) was conducted. IM vaccination produced a strong antibody-mediated immune response capable of neutralizing the SARS-CoV-2 infection, whereas immune response was greater in hamsters receiving IN vaccine. Further, the immunized hamsters were protected from SARS-CoV-2 exposure, and viral infection was unable to cause weight loss in the hamsters. In addition, lower viral load in both intranasal and pulmonary swabs was observed along with decreased transcripts levels of inflammatory genes and improved disease conditions. The vaccine offered greater protection from SARS-CoV-2 infection and inflammation by IN vaccination and reduced the dissemination of viral particles.42 Moreover, receptor-binding domain (RBD)-specific immune responses in mice inoculated with recombinant SARS-CoV-2-RBD-based subunit vaccine administered through IN inoculation and IM injection were compared in another recent study. The IN immunization resulted in a strong antibody-mediated immune response with the increased levels of IgG antibodies and also a considerable amount of mucosal immune response. As a result of these findings, noninvasive IN vaccines should be considered for SARS-CoV-2 vaccine development in the coming time.44
Immunization triggered by transduction of respiratory tract cells of mice with a Lentiviral vector (LV) though elicited neutralizing antibodies with high levels of serum neutralizing activity against S (spike) glycoprotein of SARS-CoV-2 but provided only partial protection. Intranasal stimulation of the immune system in the respiratory system, on the other hand, results in a reduction in lung infection rates and local inflammation. In addition, in golden hamsters, which are normally restrictive to SARS-CoV-2 replication and closely mimic COVID-19 physiopathology in humans, both integrative and non-integrative LV systems revealed strong clinical effectiveness and impeded detrimental damage of lungs. The findings have shown that SARS-CoV-2 LV-based vaccination approach has a significant prophylactic effect, and IN vaccination against COVID-19 is an effective strategy.45 Furthermore, to stimulate mucosal immunity as well as systemic immunity, the trimeric or monomeric spike protein was combined with a liposomal STING agonist as an adjuvant in an IN subunit vaccination. A strong antibody-mediated immune response was observed along with a higher concentration of IgA in the lung and nasal compartments. Despite strong B cell-mediated response, a substantial amount of T cell-mediated immune response was observed in the lungs of the immunized mice with this vaccine. Simultaneous activation of both antibody-mediated and cell-mediated immune responses in a germinal center-like manner was observed within the nasal-associated lymphoid tissues (NALT), supporting the significance of this approach for the achievement of long-lasting immunity.60
Another study generated a cold-adapted live-attenuated vaccine by changing SARS-CoV-2 growth in Vero cells from 37°C to 22°C, which could be given as a nasal spray in humans. In intranasally inoculated K18-hACE2 mice that are highly susceptible to SARS-CoV-2 infections, a single dose of vaccine-elicited a significant B and T cell-mediated immune response as well as mucosal IgA antibodies. The vaccinated mice were fully protected against SARS-CoV-2 infection, with minor bodyweight loss, fewer deaths, and slight viral expression in numerous vital organs, such as the brain and kidneys.61 These findings imply that nasal vaccination could be a viable method for eliciting a robust immune response.9,61 Furthermore, the SARS-CoV-2 nucleocapsid (N) protein has been recommended as a viable vaccine target. Mice injected with N protein had a significant number of T cells as well as an antibody-mediated immunological response. The majority of vaccination methods have concentrated on inducing a considerable B cell driven immune response; however, it is critical to discover the T cell responses generated by SARS-CoV-2 N protein. In this regard, IN vaccination has been proposed to elicit a protective T cell-mediated immune response, which is generally minimal when immunized with SARS-CoV N protein intradermally. In BALB/c mice immunized intranasally with recombinant adenovirus type-5 expressing SARS-CoV-2 N protein, a significant amount of T cell mediated immune response was reported in the lungs. Moreover, a substantial amount of CD4 T cells mediated immune response was recorded in the spleen, which was linked with enhanced antibody-mediated immune response. These findings lend credence to the idea that the IN vaccines are efficient and reliable in inducing the immune response.39
In addition, whereas most vaccination techniques have concentrated on systemic immunization, the preventive effect of two adjuvanted subunit vaccines, including an IM-primed/boosted vaccine and an IM-primed/IN-boosted mucosal vaccine, was examined. The IM vaccination with alum caused a strong antibody-mediated immune response, while the IN vaccine with nanoparticles such as IL-15 and TLR agonists generated lower T cell and antibody responses, yet greater dimeric IgA and IFN-alpha production. Nonetheless, upon SARS-CoV-2 exposure, no subject had detectable sub-genomic RNA in the upper or lower respiratory system, proving adequate immunity against viral infection. In all instances, both vaccinations have been shown to protect against respiratory SARS-CoV-2 exposure.46
Designing a molecular vaccine based on nanotechnology advancements has been proposed by utilizing nanoconjugate containing inorganic nanoparticle layered double hydroxide intercalated with shRNA-plasmid possessing a sequence targeting viral genome or viral mRNA. This vaccine could be used as a nasal spray for delivering shRNA-plasmid to the target site, having the advantages of being biocompatible, facilitating stable knockdown to the target cells, and considered stable in the nasal mucosa.62
In collaboration with Codagenix (United States), the Serum Institute of India has initiated manufacturing COVI-VAC as a live-attenuated intranasal COVID-19 vaccine that is presently undergoing Phase 1 clinical trial (NCT04619628) with regard to safety and immunogenicity against SARS-CoV-2.63,64 Bharat Biotech has also initiated the phase 1 trial (NCT04751682) of single-dose IN vaccine in India (BBV154, replication-deficient adenovirus vectored vaccine) against COVID-19.65,66
In several recent studies, the IN route of vaccination has been an efficient way to decrease viral shedding while generating a significant immune response.67,68 Hassan et al. reported that IM injections of ChAd-SARS-CoV-2-S stimulate vigorous humoral and cell-mediated immunity and helps in providing protection against lung infection and inflammation but do not render sterilizing immunity, as indicated by viral RNA detection and activation of anti-nucleoprotein antibodies upon SARS-CoV-2 infection. On the other hand, in both upper and lower respiratory tracts, a single IN dosage of ChAd-SARS-CoV-2-S generated large amounts of neutralizing antibodies, enhanced systemic and mucosal immunoglobulin A (IgA) and T cell responses, and nearly provided complete prevention from SARS-CoV-2 infection.68 The same research group recently released a follow-up study in which Rhesus macaques were inoculated with ChAd-control or ChAd-SARS-CoV-2-S and then challenged with SARS-CoV-2 through a combination of intranasal and intrabronchial methods one month later. After the SARS-CoV-2 infection, a single IN dosage of ChAd-SARS-CoV-2-S generated neutralizing antibodies and T cell immunity and limited or prevented viral infection in the upper and lower respiratory tracts. Because ChAd-SARS-CoV-2-S protects nonhuman primates against SARS-CoV-2 infection and transmission, it is a good option for minimizing SARS-CoV-2 infections and transmission in people.69 In another study, employing a SARS-CoV-2 virus with D614 G mutation in its S protein, the intranasally delivered ChAdOx1 nCoV-19 was found to lower virus dissemination as observed by a reduction in viral shedding. In both a direct challenge and a transmission scenario, the viral load in nasal swabs of vaccinated hamsters was observed to be much lower than controls. Any viral RNA or infectious virus was not identified in lung tissues. Intranasal immunization of rhesus macaques lowered the viral load in bronchoalveolar lavage and lower respiratory tract tissues, and decreased viral shedding. The IN vaccination decreased viral shedding as observed in two separate SARS-CoV-2 animal models, indicating that it should be investigated further as a possible immunization route for COVID-19 vaccines.67
For COVID-19 vaccination, a recombinant type 5 adenovirus vector carrying gene for the SARS-CoV-2 S1 subunit antigen (Ad5.SARS-CoV-2-S1) was utilized to immunize mice through the IN route. A single Ad5.SARS-CoV-2-S1 vaccination delivered by IN route elicited potent antibody and cellular immune responses. Considerable levels of S1-specific immunoglobulins (IgGs), including IgG1 and IgG2a endpoint titers, were seen after two weeks of vaccination, and the antibodies produced were long-lasting. In comparison to unvaccinated control groups, Ad5.SARS-CoV-2-S1 injection resulted in S1-specific B cells as well as antigen-specific T cell responses. The IN administration has been shown to be a potential method for triggering cellular immune responses.70
The potential of nanoparticles (NPs) synthesized with inulin acetate (InAc) (InAc-NPs) as an IN-vaccine delivery strategy to produce both mucosal and systemic immunity has been reported. Compared to PLGA (Poly lactic-co-glycolic acid)-NPs as a delivery strategy, IN vaccination with antigen-loaded InAc-NPs resulted in 65-fold and 19-fold greater serum IgG1 and IgG2a titers, respectively. InAc-NPs also enhanced the production of sIgA at numerous mucosal locations, including nasal-associated lymphoid tissues (NALTs), the lungs, and the colon, resulting in a significant memory response suggestive of both humoral and cellular immune system activation. Studies with InAc-NPs provided the groundwork for a prospective IN delivery strategy for mucosal immunization by simultaneously activating both systemic and mucosal immunity.71
In a recent study, N, N, N-trimethyl chitosan nanoparticles (TMC NPs) were incorporated with RBD (receptor-binding domain) of SARS-CoV-2 S-protein and tested for their ability to induce an immune response when administered through the intranasal route. The presence of IgG and IgA responses in BALT (Bronchus-associated lymphoid tissue) and the lungs of vaccinated mice demonstrated that intranasal administration of RBD-TMC NPs to mice generated significant local mucosal immunity. In addition, rodents given this immunogen substrate intranasally produced a robust systemic antibody mediated immune response, comprising serum IgG, IgG1, IgG2a, IgA, and neutralizing antibodies. Furthermore, compared to animals given soluble RBD immunogen, these immunized mice showed considerably more significant numbers of activated splenic CD4+ and CD8+ cells. Such data, taken together, point to an alternate vaccination pathway that closely resembles the natural path of SARS-CoV-2 infection. Not only did this mode of delivery activates local mucosal responses, but it also activated the immune system’s systemic component.72
Immune responses generated by mucosal homologous plasmid and a heterologous vaccination method, combining a plasmid vaccine and a Modified Vaccinia Ankara (MVA) expressing SARS-CoV-2 S and N antigens, revealed that only the heterologous IN vaccination technique resulted in neutralizing antibodies against SARS-CoV-2 in mice’s serum. Neutralizing antibodies against SARS-CoV-2 were also reported in the bronchoalveolar lavage of mice which suggested significant levels of efficiency and reliability of this vaccination as compared to IM vaccination. In the lungs and spleens of immunized mice, the same prime/boost method resulted in the production of type 1 and type 17 T cell responses and polyfunctional T cells producing various type 1 cytokines (e.g., IFN-alpha, TNF, IL-2). The plasmid homologous vaccination method, on the other hand, resulted in the production of local mono and polyfunctional T cells that secrete IFN-gamma. The findings of this investigation suggest that QAC-nano vaccines and intranasal immunization can generate significant mucosal immune responses toward respiratory coronaviruses.73
Potential advantages of intranasal COVID-19 vaccines over the intramuscular vaccines
Several of the experiments described above have demonstrated the efficacy of IN immunization and attracted scientists’ curiosity in learning more about the potential of intranasal vaccinations against COVID-19. In this regard, various research groups have emphasized certain benefits of intranasal vaccinations over intramuscular immunization.34,67,68,74
A single dose of an effective SARS-CoV-2 vaccine candidate via IN route may induce the substantial amount of neutralizing antibodies, boosts mucosal IgA and T cell responses, and almost completely protects viral infection in both the upper and lower respiratory tracts (Figure 1).
Intranasal immunization can be an effective approach to minimize viral shedding and spread, which might be advantageous over IM vaccines.
The viral load in the upper and lower respiratory tract tissues can be reduced by IN immunization.
Nasal vaccinations are appealing as an alternative to injectable vaccinations since they may allow for a lower dosage than IM administration.
The IN vaccine can be administered at the appropriate region, such as nasal-associated lymphoid tissues (NALT), to induce a substantial amount of mucosal immunity.
Owing to ease of administration, nasal vaccinations may not always need to be given by a health-care professional.
It is indeed a better option for infants who do not like injections in nature. Additionally, nasal vaccinations may be administered using simple devices, which eliminates the requirement for sterilized settings during vaccination, which is particularly beneficial for immunization programs in developing nations.
Dry powder nasal vaccines have been created, which may allow the easy storage and transportation of the vaccines. In addition, IN vaccinations permit self-administration and may be manufactured to persist at room temperature, easing transportation and storage procedures. This approach can be highly advantageous in developing countries such as India. However, the preliminary studies are yet to be approved in the coming time.
The potential strengths associated with the use of an IN vaccine to boost a subject previously immunized systemically with IM vaccines include induction of a robust immune response in terms of significant protective humoral and cellular immunity, activation of mucosal immunity (IgA production) in both the upper and lower respiratory tracts, which could inhibit viral multiplication and decrease virus shedding via nasal mucosa that acts as the first-line barrier to the virus entrance before dissemination into the lungs, thus preventing the transmission and spread of SARS-CoV-2 as well as to attain herd immunity in the population. The early restriction of viral replication in the nasal mucosa and clearance of SARS-CoV-2 infection via robust protective immunity including mucosal immune responses reflects the potency of intranasal COVID-19 vaccines in the mitigation of the rising cases of virus reinfection during the pandemic.75–77
Challenges in developing COVID-19 intranasal vaccines
Determining immunization platforms, the number of doses required, route of delivery, and time to acquire maximal protection have been discussed in recent conversations on vaccination tactics against SARS-CoV-2. The VSV-SARS2-EBOV vaccine, fast-acting vesicular stomatitis virus-based vaccine produced from licensed Ebola virus (EBOV) vaccine that expresses SARS-CoV-2 S protein and the EBOV glycoprotein, when given in a single dose via IM route to rhesus macaques, showed protection over ten days with no symptoms of COVID-19 pneumonia. It’s IN immunization resulted in reduced immunogenicity and increased COVID-19 pneumonia as compared to control animals. While both the IM and IN vaccination resulted in the induction of neutralizing antibody titers, only the IM vaccine elicited a strong cellular immunological response. These findings were corroborated with RNA sequencing data, indicating strong activation of innate and adaptive immune transcriptional markers in the lungs of only IM-vaccinated animals. Such findings show that injecting VSV-SARS2-EBOV into the bloodstream provides fast protection.78 These findings, however, contradict with those of other researchers suggesting the potentialities of IN vaccines.
Moreover, there is scarcity of concrete evidences about the effectiveness of IN vaccines, however preliminary data has shown that the IN immunization can protect the host from illness. The effectiveness of an IN vaccination may be influenced by the dose or vaccine platform used. Nasal immunization, on the other hand, could be a successful route to acquire herd immunity in the vast majority of the population since it can offer sterilizing immunity, blocking interhuman transmission.79 While clinical trials of various intranasal vaccines are now underway, including AdCOVID (Altimmune, Gaitersburg, USA), further research is needed to establish the best effective vaccination method.80 Furthermore, elaborative studies should discover the most efficient routes of vaccine administration based on the different vaccine platforms being potentially explored, as well as the processes that underpin the efficacy of various delivery routes.81 In addition to the debatable efficacy of IN vaccines, there are a number of challenges and shortcomings that must be addressed. These are described below:
Recently, the inability of IN vaccine to induce effective and long-lasting immunity has been considered a serious concern; this might lead to the waning of protection rapidly. This has been associated with the sticky mucus in the respiratory system, which acts as a barrier for pathogens that may obstruct vaccine accessibility and immune activation, resulting in low immunogenicity and rapid loss of protective immunity.82
IN vaccines may be created using a variety of platforms, including viral vectors and protein subunit vaccinations. The safety of IN vaccines is a critical factor to consider. Whole pathogen-based vaccinations have raised some concerns due to the likelihood of reverting to a replicating form.82,83 This condition has also been found with the oral polio vaccination; however, it is quite unusual. The safety of live attenuated vaccines must be proven over a lengthy period of time. Berna Biotech, a Swiss firm, canceled an intranasal flu vaccination because it was related to a greater incidence of Bell’s palsy.82,84
Despite the fact that IN vaccines can induce both IgA and IgG antibodies in the upper and lower respiratory tract and provide a substantial amount of humoral immune response. However, it is to be considered that some IN vaccines are not effective in generating IgG antibodies in the lower respiratory tract,82 which can lead to the reduced protectiveness of IN vaccines.
Moreover, the likelihood of retrograde transport to the brain via olfactory nerves, which has been reported previously in live attenuated adenovirus, is a serious concern associated with IN vaccines.83,85,86
Adjuvants, on the other hand, are critical for significant immune responses, particularly with protein subunit vaccines. However, because of their immunomodulatory qualities, adjuvants might cause complications in terms of the safety of IN vaccines.83 In the following section, we will shed some light on the possible roles of adjuvants in the development of IN vaccine and any possible concerns associated with them.
Adjuvants in the development of intranasal vaccines against COVID-19
The inclusion of immunostimulatory adjuvants to vaccines may be required to enhance the immune reaction, particularly as an integral component in most of the inactivated and subunit vaccine formulations, vaccinations containing purified protein- or antigen-based vaccines, including component or recombinant vaccines. As vaccine components, adjuvants activate the innate immune system- and trigger-specific adaptive immune responses, and enhance the magnitude, breadth, and durability of the immune response.87–89 Alum, chitosan, and bacterial toxins like cholera toxin (CT), as well as inactivated viral envelopes like recombinant adenoviruses, liposomes, cytokines, CpG oligodeoxynucleotides, Toll-like receptor-4 (TLR) agonists, nanoparticles, and others have been utilized as potential adjuvants for the mucosal vaccines including IN vaccines.90–94 After the use of bacterial toxins in nasal vaccinations, certain adverse consequences have been recorded.95 Moreover, allergic reactions have been reported in the subjects administered with a registered IN vaccine, namely tetravalent cold-adapted live-attenuated influenza vaccine (LAIV). The allergic reactions have been correlated with the utilization of eggs in LAIV vaccine production process, which causes problems in asthma patients.96 Various scientific advances in gaining knowledge about innate immunity and systems vaccinology are paving ways to design and develop different kinds of novel adjuvants, including molecular adjuvants for use in vaccines against infectious diseases, challenging pathogens including coronaviruses, tackling COVID-19, and future pandemics.88,89,94,97–102 Incorporation of effective adjuvants in IN vaccines would enhance the protective immune responses of these vaccines and aid in fighting COVID-19 in a better way. SARS-CoV-2 virus has been reported to enter the brain by a transneural pathway into the olfactory epithelium (where ACE2 is expressed) and then spreads.103 It is yet not established that the recombinant vectors harboring the S-protein-producing gene are able to penetrate the olfactory region and cause significant side effects. Adenovirus has been used to transfer medications from the nose to the brain, although it has not been proven that the adenovirus directly penetrated the brain, just like medicine action mechanisms.104 Nanoparticles (NPs) entering the olfactory tissues through tight junctions must be less than 20 nm, but particles entering via the transcellular pathway into olfactory tissue cells or olfactory neuron cells need to ideally be less than 100 nm.105,106 Many NPs used for COVID-19 intranasal vaccines have a diameter of around 90 nm, and it is critical to remember that the endocytosis process is also influenced by particle features such as charge and surface attributes as well as particle size. Additionally, there is a difference between SARS-CoV-2 virus particles that enter the nasal cavity by normal inhalation and vaccines’ particles injected with greater power into the cavity. As a result, unless a sophisticated nasal delivery system is utilized, a nasal spray reaching the olfactory area at the top of the nasal cavity is exceedingly difficult. Hence, it’s hard to ascertain whether immunization carriers will penetrate the olfactory tissues or whether any harmful occurrences will appear as a result of this entry. Toxicological findings will be required in accordance with standard regulatory requirements prior to the nasal SARS-CoV-2 vaccines are approved for vaccination purposes.34
Conclusion and future prospects
Almost all available vaccines against COVID-19 are delivered by IM administrations. IM injected vaccines are primarily intended to generate both antibody mediated and T cell mediated immune responses. These IM vaccines have shown high levels of effectiveness by eliciting a significant amount of immune response. Still, it has been found that these vaccines are inefficient in stimulating IgA secretion in mucosal cells, which may not be efficient in controlling the shedding of viral particles in the upper respiratory tract. Preclinical results in numerous animal models have demonstrated the promising potentials of IN COVID-19 vaccination, which can induce a significant amount of protective humoral antibody immune response, cellular immunity (T cell mediated) as well as mucosal immune response (IgA production) in the respiratory tract, and can prevent or reduce viral multiplication, viral particle shedding, and transmission. A range of IN vaccines have been generated by clinical investigations in mouse models and rhesus macaques, which have demonstrated activation of the mucosal immunity (sIgA) in both the upper and lower respiratory tracts along with a significant degree of humoral and cell mediated immune responses that can inhibit viral reproduction and transmission/spread. The intranasal vaccine is an exciting method for preventing COVID-19 since the nasal mucosa provides the first-line barrier to SARS-CoV-2 entrance before dissemination into the lungs. Hence, developing effective and reliable intranasal vaccines is crucial at this time.
There is no doubt that IN vaccines have their own set of potential advantages over the IM vaccine. However, the shortcomings associated with them cannot be ignored. The overall immunostimulatory effectiveness of a vaccine is determined by the immunization type chosen, functional ingredients such as vaccine adjuvants, and vaccine carriers such as NPs (nanoparticles), surface-modified NPs, and virus-like particles. Large-scale production, in-process quality control, and satisfying regulatory standards are all challenges in manufacturing the IN vaccine at the commercial level. Hopefully, the availability of intranasal vaccines may be made feasible in the coming future after further evaluation of such vaccines being developed with large-scale clinical studies and trials to incorporate them in worldwide vaccination programs. According to current statistics, more than 10 pharmaceutical companies are developing intranasal vaccinations, with five of them having reached the early phases of clinical trials. Nonetheless, we believe that an intranasal COVID-19 immunization may be available soon. Moreover, in our opinion, the second generation IN vaccines could significantly increase the capacity of several developing countries to restrain the deleterious consequences of COVID-19, where immunization is still a significant concern for the government.
Acknowledgements
All the authors acknowledge and thank their respective Institutes and Universities.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Author contributions
All the authors contributed significantly in this manuscript. KD and MD conceptualized the manuscript. KD wrote the first draft with input from MD. Authors RT, TBE, SM, AAR, SA, ZAA, and AAM reviewed and updated the manuscript. All authors contributed to revisions and approved the final manuscript.
Disclosure statement
All authors declare that there exist no commercial or financial relationships that could, in any way, lead to a potential conflict of interest.
References
- 1.WHO . WHO COVID-19 dashboard - up to date data on pandemic. WHO Heal Emerg Dasboard 2021. https://covid19.who.int/region/searo/country/id [Accessed on February 8, 2022]
- 2.Dhama K, Khan S, Tiwari R, Sircar S, Bhat S, Malik YS, Singh KP, Chaicumpa W, Bonilla-Aldana DK, Rodriguez-Morales AJ.. Coronavirus disease 2019–COVID-19. Clin Microbiol Rev. 2020;33(4):1–11. doi: 10.1128/CMR.00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharun K, Tiwari R, Iqbal Yatoo M, Patel SK, Natesan S, Dhama J, Malik YS, Harapan H, Singh RK, Dhama K.. Antibody-Based immunotherapeutics and use of convalescent plasma to counter COVID-19: advances and prospects. Expert Opin Biol Ther. 2020;20(9):1033–46. doi: 10.1080/14712598.2020.1796963. [DOI] [PubMed] [Google Scholar]
- 4.Ghareeb DA, Saleh SR, Nofal MS, Kaddah MMY, Hassan SF, Seif IK, El-Zahaby SA, Khedr SM, Kenawy MY, Masoud AA. Potential therapeutic and pharmacological strategies for SARS-CoV2. J Pharm Investig. 2021;51(3):281–96. doi: 10.1007/s40005-021-00520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyder Pottoo F, Abu-Izneid T, Mohammad Ibrahim A, Noushad Javed M, AlHajri N, Hamrouni AM. Immune system response during viral infections: immunomodulators, cytokine storm (CS) and Immunotherapeutics in COVID-19. Saudi Pharm J. 2021;29(2):173–87. doi: 10.1016/j.jsps.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ita K. Coronavirus disease (COVID-19): current status and prospects for drug and vaccine development. Arch Med Res. 2021;52(1):15–24. doi: 10.1016/j.arcmed.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iqbal Yatoo M, Hamid Z, Rather I, Nazir QUA, Bhat RA, Ul Haq A, Magray SN, Haq Z, Sah R, Tiwari R, et al. Immunotherapies and immunomodulatory approaches in clinical trials - a mini review. Hum Vaccines Immunother. 2021;17(7):1897–909. doi: 10.1080/21645515.2020.1871295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iqbal Yatoo M, Hamid Z, Parray OR, Wani AH, Ul Haq A, Saxena A, Patel SK, Pathak M, Tiwari R, Malik YS, et al. COVID-19 - Recent advancements in identifying novel vaccine candidates and current status of upcoming SARS-CoV-2 vaccines. Hum Vaccines Immunother. 2020;16(12):2891–904. doi: 10.1080/21645515.2020.1788310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashraf MU, Kim Y, Kumar S, Seo D, Ashraf M, Bae YS. Covid-19 vaccines (Revisited) and oral-mucosal vector system as a potential vaccine platform. Vaccines. 2021;9(2):1–24. doi: 10.3390/vaccines9020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García-Montero C, Fraile-Martínez O, Bravo C, Torres-Carranza D, Sanchez-Trujillo L, Gómez-Lahoz AM, Guijarro LG, García-Honduvilla N, Asúnsoloúnsolo A, Bujan J, et al. An updated review of sars-cov-2 vaccines and the importance of effective vaccination programs in pandemic times. Vaccines. 2021;9(5):433. doi: 10.3390/vaccines9050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawat K, Kumari P, and Saha L. COVID-19 vaccine: A recent update in pipeline vaccines, their design and development strategies. Eur J Pharmacol. 2021;892:173751. doi: 10.1016/j.ejphar.2020.173751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO . WHO Covid-19. Draft landscape of COVID-19 candidate vaccines. Who; 2020. 3. Available from. [Google Scholar]
- 13.Dhama K, Natesan S, Iqbal Yatoo M, Patel SK, Tiwari R, Saxena SK, Harapan H. Plant-Based vaccines and antibodies to combat COVID-19: current status and prospects. Hum Vaccines Immunother. 2020;16(12):2913–20. doi: 10.1080/21645515.2020.1842034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malik YS, Sircar S, Bhat S, Ansari MI, Pande T, Kumar P, Mathapati B, Balasubramanian G, Kaushik R, Natesan S, et al. How artificial intelligence may help the Covid-19 pandemic: pitfalls and lessons for the future. Rev Med Virol. 2020;31(5):1–11. doi: 10.1002/rmv.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monrad JT, Sandbrink JB, Cherian NG. Promoting versatile vaccine development for emerging pandemics. NPJ Vaccines. 2021;6(1):6. doi: 10.1038/s41541-021-00290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nooraei S, Bahrulolum H, Hoseini ZS, Katalani C, Hajizade A, Easton AJ, Ahmadian G. Virus-Like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J Nanobiotechnology. 2021;19(1). doi: 10.1186/s12951-021-00806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ura T, Yamashita A, Mizuki N, Okuda K, Shimada M. New vaccine production platforms used in developing SARS-CoV-2 vaccine candidates. Vaccine. 2021;39:197–201. doi: 10.1016/j.vaccine.2020.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao VB, Zhu J, Ananthaswamy N, Jain S, Batra H, Tang W-C, Lewry DA, Richards ML, David SA, Kilgore PB, et al. A universal Bacteriophage T4 nanoparticle platform to design Multiplex SARS-CoV-2 vaccine candidates by CRISPR Engineering. bioRxiv. 2021;2021.01.19.427310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2021;384:1576–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knoll MD, Wonodi C. Oxford–astrazeneca COVID-19 vaccine efficacy. Lancet. 2021;397(10269):72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen AL. Vaccination is the only acceptable path to herd immunity. Med. 2020;1:21–23. doi: 10.1016/j.medj.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vora KS, Sundararajan A, Saiyed S, Dhama K, Natesan S. Impact of COVID-19 on women and children and the need for a gendered approach in vaccine development. Hum Vaccines Immunother. 2020;16(12):2932–37. doi: 10.1080/21645515.2020.1826249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharun K, Dhama K. COVID-19 vaccine diplomacy and equitable access to vaccines amid ongoing pandemic. Arch Med Res. 2021;52(7):761–63. doi: 10.1016/j.arcmed.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharun K, Tiwari R, Dhama K, Emran TB, Rabaan AA, Al Mutair MA. Emerging SARS-CoV-2 variants: impact on vaccine efficacy and neutralizing antibodies. Hum Vaccines Immunother. 2021;17(10):3491–94. doi: 10.1080/21645515.2021.1923350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aschwanden C. Five reasons why COVID herd immunity is probably impossible. Nature. 2021;591:520–22. doi: 10.1038/d41586-021-00728-2. [DOI] [PubMed] [Google Scholar]
- 27.Baldo V, Reno C, Cocchio S, Fantini MP. SARS-CoV-2/COVID-19 vaccines: the promises and the challenges ahead. Vaccines. 2021;9:1–4. doi: 10.3390/vaccines9010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhama K, Patel SK, Natesan S, Vora KS, Iqbal Yatoo M, Tiwari R, Saxena SK, Singh KP, Singh R, Malik YS. COVID-19 in the elderly people and advances in vaccination approaches. Hum Vaccines Immunother. 2020;16(12):2938–43. doi: 10.1080/21645515.2020.1842683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhama K, Sharun K, Tiwari R, Dhawan M, Emran TE, Rabaan AA, Alhumaid S. COVID-19 vaccine hesitancy – reasons and solutions to achieve a successful global vaccination campaign to tackle the ongoing pandemic. Hum Vaccines Immunother. 2021;17(10):3495–99. doi: 10.1080/21645515.2021.1926183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.dos Santos WG. Impact of virus genetic variability and host immunity for the success of COVID-19 vaccines. Biomed Pharmacother. 2021;136:111272. doi: 10.1016/j.biopha.2021.111272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piret J, Boivin G, Soleimanpour S, Yaghoubi A, Poland GA, Baviskar T, Raut D, Bhatt LK, Forni G, Mantovani A, et al. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;11:8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pennington TH. Herd immunity: Could it bring the COVID-19 pandemic to an end? Future Microbiol. 2021;16(6):371–74. doi: 10.2217/fmb-2020-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redwan EM. COVID-19 pandemic and vaccination build herd immunity. Eur Rev Med Pharmacol Sci. 2021;25(2):577–79. doi: 10.26355/eurrev_202101_24613. [DOI] [PubMed] [Google Scholar]
- 34.Tiboni M, Casettari L, Illum L. Nasal vaccination against SARS-CoV-2: synergistic or alternative to intramuscular vaccines? Int J Pharm. 2021;603:120686. doi: 10.1016/j.ijpharm.2021.120686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J-G, Oladunni FS, Rohaim MA, Whittingham-Dowd J, Tollitt J, Assas BM, Alhazmi W, Almilaibary A, Iqbal M, Chang P, et al. Immunogenicity and protective efficacy of an intranasal live-attenuated vaccine against SARS-CoV-2 in preclinical animal models. bioRxiv 2021; [DOI] [PMC free article] [PubMed]
- 36.Bleier BS, Ramanathan M, Lane AP. COVID-19 vaccines may not prevent nasal SARS-CoV-2 infection and asymptomatic transmission. Otolaryngol - Head Neck Surg (United States). 2021;164(2):305–07. doi: 10.1177/0194599820982633. [DOI] [PubMed] [Google Scholar]
- 37.Mudgal R, Nehul S, Tomar S. Prospects for mucosal vaccine: shutting the door on SARS-CoV-2. Hum Vaccines Immunother. 2020;16(12):2921–31. doi: 10.1080/21645515.2020.1805992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Travis CR. As plain as the nose on your face: the case for a nasal (mucosal) route of vaccine administration for Covid-19 disease prevention. Front Immunol. 2020;11:11. doi: 10.3389/fimmu.2020.591897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He J, Huang JR, Zhang YL, Zhang J. SARS-CoV-2 nucleocapsid protein intranasal inoculation induces local and systemic T cell responses in mice. J Med Virol. 2021;93(4):1923–25. doi: 10.1002/jmv.26769. [DOI] [PubMed] [Google Scholar]
- 40.Hoseini-Tavassol Z, Ejtahed H-S, Soroush A-R, Sajjadpour Z, Hasani-Ranjbar S, Larijani B. Natural derived nasal spray; a proposed approach for COVID-19 disease control. Infect Disord - Drug Targets. 2021;21(8):21. doi: 10.2174/1871526521666210218201113. [DOI] [PubMed] [Google Scholar]
- 41.Kumar A, Kumar A. Mucosal and transdermal vaccine delivery strategies against COVID-19. Drug Deliv Transl Res. 2021. doi: 10.1007/s13346-021-01001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bricker T, Darling T, Hassan AO, Harastani H, Soung A, Jiang X, Dai Y-N, Zhao H, Adams LJ, Holtzman MJ, et al. A single intranasal or intramuscular immunization with Chimpanzee adenovirus vectored SARS-CoV-2 vaccine protects against pneumonia in Hamsters. SSRN Electron J. 2021. doi: 10.2139/ssrn.3773804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King RG, Silva-Sanchez A, Peel JN, Botta D, Dickson AM, Pinto AK, Meza-Perez S, Allie SR, Schultz MD, Liu M et al. Single-Dose intranasal administration of AdCOVID Elicits systemic and mucosal immunity against SARS-CoV-2 and fully protects Mice from lethal challenge. Vaccines (Basel). ;9(8):881. doi: 10.3390/vaccines9080881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du Y, Xu Y, Feng J, Hu L, Zhang Y, Zhang B, Guo W, Mai R, Chen L, Fang J, et al. Intranasal administration of a recombinant RBD vaccine induced protective immunity against SARS-CoV-2 in mouse. Vaccine. 2021;39:2280–87. doi: 10.1016/j.vaccine.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ku MW, Bourgine M, Authié P, Lopez J, Nemirov K, Moncoq F, Noirat A, Vesin B, Nevo F, Blanc C, et al. Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models. Cell Host Microbe. 2021;29(2):236–49.e6. doi: 10.1016/j.chom.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sui Y, Li J, Zhang R, Prabhu SK, Andersen H, Venzon D, Cook A, Brown R, Teow E, Velasco J, et al. Protection against SARS-CoV-2 infection by a mucosal vaccine in rhesus macaques. JCI Insight. 2021;6(10):6. doi: 10.1172/jci.insight.148494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Doremalen N, Purushotham JN, Schulz JE, Holbrook MG, Bushmaker T, Carmody A, Port JR, Yinda CK, Okumura A, Saturday G, et al. Intranasal ChAdox1 nCov-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci Transl Med. 2021;13(607):eabh0755. doi: 10.1126/scitranslmed.abh0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hassan AO, Shrihari S, Gorman MJ, Ying B, Yaun D, Raju S, Chen RE, Dmitriev IP, Kashentseva E, Adams LJ, et al. An intranasal vaccine durably protects against SARS-CoV-2 variants in mice. Cell Rep. 2021;36(4):109452. doi: 10.1016/j.celrep.2021.109452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.CanSino Biologics Inc . A randomized, double-blind, placebo-controlled phase I/II clinical trial to evaluate the safety and immunogenicity of Ad5-nCov for inhalation in adults 18 years of age and older. 2021; clinicaltrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT04840992
- 50.Wu S, Huang J, Zhang Z, Wu J, Zhang J, Hu H, Zhu T, Zhang J, Luo L, Fan P, et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCov) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect Dis. 2021;21(12):1654–64. doi: 10.1016/S1473-3099(21)00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Altimmune, Inc . Phase 2, double-blind, randomized, placebo-controlled study of NasoVAX in the prevention of clinical worsening in patients with early coronavirus infectious disease 2019 (COVID-19). clinicaltrials.gov. USA: Altimmune, Inc. 2020. [Google Scholar]
- 52.Kong TU of H. A phase 1, randomized, double-blinded, placebo- controlled, dose-escalation and dose-expansion study to evaluate the safety and immunogenicity of DelNS1-NCoV-RBD LAIV for COVID-19 in Healthy Adults. The University of Hong Kong, Hong Kong. https://clinicaltrials.gov/ct2/show/NCT04809389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meissa Vaccines I Phase 1, open-label, dose-escalation study to evaluate tolerability, safety, and immunogenicity of an intranasal live attenuated respiratory syncytial virus vaccine expressing Spike protein of SARS-CoV-2 in healthy adults ages 18–69 Years.
- 54.First patient dosed with COVI-VAC, an intranasal COVID-19 vaccine candidate. https://www.europeanpharmaceuticalreview.com/news/139089/first-patient-dosed-with-covi-vac-an-intranasal-covid-19-vaccine-candidate/
- 55.Chavda VP, Vora LK, Pandya AK, Patravale VB. Intranasal vaccines for SARS-CoV-2: From challenges to potential in COVID-19 management. Drug Discov Today. 26(11): 2619–2636. doi: 10.1016/j.drudis.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klouwens MJ, Salverda ML, Trentelman JJ, Ersoz JI, Wagemakers A, Gerritzen MJ, van der Ley PA, Hovius JW. Vaccination with meningococcal outer membrane vesicles carrying Borrelia OspA protects against experimental Lyme borreliosis. Vaccines. 39(18): 2561–2567. doi: 10.1016/j.vaccine.2021.03.059. [DOI] [PubMed] [Google Scholar]
- 57.Gaspar EB, Prudencio CR, De Gaspari E. Experimental studies using OMV in a new platform of SARS-CoV-2 vaccines. Hum Vaccines Immunother. 2021;17(9):2965–68. doi: 10.1080/21645515.2021.1920272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.https://covid19.trackvaccines.org/vaccines/66/#trial-rpcec00000345 [Accessed on August 12, 2021].
- 59.AuraVax Therapeutics licences intranasal vaccine adjuvant technology from Massachusetts General Hospital. www.oindpnews.com/2021/01/auravax-therapeutics-licences-intranasal-vaccine-adjuvant-technology-from-massachusetts-general-Hosp
- 60.An X, Martinez MP, Rezvan A, Sefat SR, Fathi M, Singh S, Biswas S, Pourpak M, Yee C, Liu X, et al. Single-Dose intranasal vaccination Elicits systemic and mucosal immunity against SARS-CoV-2. SSRN Electron J. 2020. doi: 10.2139/ssrn.3751056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seo SH, Jang Y. Cold-Adapted live attenuated sars-cov-2 vaccine completely protects human ace2 transgenic mice from sars-cov-2 infection. Vaccines. 2020;8:1–17. doi: 10.3390/vaccines8040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Acharya R. Prospective vaccination of COVID-19 using shRNA-plasmid-LDH nanoconjugate. Med Hypotheses. 2020;143:143. doi: 10.1016/j.mehy.2020.110084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chakraborty C, Agoramoorthy G. India’s cost-effective COVID-19 vaccine development initiatives. Vaccine. 2020;38:7883–84. doi: 10.1016/j.vaccine.2020.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Codagenix Inc . Codagenix and Serum Institute of India initiate dosing in phase 1 trial of COVI-VAC, a single dose, intranasal, live attenuated vaccine for COVID-19. 2021;. https://www.prnewswire.com/news-releases/codagenix-and-serum-institute-of-india-initiate-dosing-in-phase-1-trial-of-covi-vac-a-single-dose-intranasal-live-attenuated-vaccine-for-covid-19-301203130.html
- 65.Sharun K, Dhama K. India’s role in COVID-19 vaccine diplomacy. J Travel Med. 2021;28(7). doi: 10.1093/jtm/taab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bharat Biotech’s nasal vaccine can be a gamechanger in fight against Covid: all you need to know.
- 67.van Doremalen N, Purushotham J, Schulz J, Holbrook M, Bushmaker T, Carmody A, Port J, Yinda KC, Okumura A, Saturday G, et al. Intranasal ChAdox1 nCov-19/AZD1222 vaccination reduces shedding of SARS-CoV-2 D614G in rhesus macaques. bioRxiv Prepr Serv Biol; 2021. https://pubmed.ncbi.nlm.nih.gov/33447831/ [DOI] [PMC free article] [PubMed]
- 68.Hassan AO, Kafai NM, Dmitriev IP, Fox JM, Smith BK, Harvey IB, Chen RE, Winkler ES, Wessel AW, Case JB, et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183(1):169–84.e13. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hassan AO, Feldmann F, Zhao H, Curiel DT, Okumura A, Tang-Huau TL, Case JB, Meade-White K, Callison J, Chen RE, et al. A single intranasal dose of chimpanzee adenovirus-vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. Cell Reports Med. 2021;2(4):100230. doi: 10.1016/j.xcrm.2021.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim E, Weisel FJ, Balmert SC, Khan MS, Huang S, Erdos G, Kenniston TW, Carey CD, Joachim SM, Conter LJ, et al. A single subcutaneous or intranasal immunization with adenovirus-based SARS-CoV-2 vaccine induces robust humoral and cellular immune responses in mice. Eur J Immunol. 2021;51(7):1774–84. doi: 10.1002/eji.202149167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bakkari MA, Valiveti CK, Kaushik RS, Tummala H. Toll-Like receptor-4 (TLR4) agonist-based intranasal nanovaccine delivery system for inducing systemic and mucosal immunity. Mol Pharm. 2021;18(6):2233–41. doi: 10.1021/acs.molpharmaceut.0c01256. [DOI] [PubMed] [Google Scholar]
- 72.Jearanaiwitayakul T, Seesen M, Chawengkirttikul R, Limthongkul J, Apichirapokey S, Sapsutthipas S, Phumiamorn S, Sunintaboon P, Ubol S. Intranasal administration of RBD nanoparticles confers induction of mucosal and systemic immunity against SARS-CoV-2. Vaccines. 2021;9:768. doi: 10.3390/vaccines9070768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chandrasekar SS, Phanse Y, Hildebrand RE, Hanafy M, Wu CW, Hansen CH, Osorio JE, Suresh M, Talaat AM. Localized and systemic immune responses against sars-cov-2 following mucosal immunization. Vaccines. 2021;9(2):1–17. doi: 10.3390/vaccines9020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chavda VP, Vora LK, Pandya AK, Patravale VB. Intranasal vaccines for SARS-CoV-2: from challenges to potential in COVID-19 management. Drug Discov Today. 2021;26(11):2619–36. doi: 10.1016/j.drudis.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nainu F, Abidin RS, Bahar MA, Frediansyah A, Emran TB, Rabaan AA, Dhama K, Harapan H. SARS-CoV-2 reinfection and implications for vaccine development. Hum Vaccines Immunother. 2020;16(12):3061–73. doi: 10.1080/21645515.2020.1830683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.A H. Antibody responses to natural SARS-CoV-2 Infection or after COVID-19 vaccination. Vaccines. 2021;9:910. doi: 10.3390/vaccines9080910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fröberg J, Diavatopoulos DA. Mucosal immunity to severe acute respiratory syndrome coronavirus 2 infection. Curr Opin Infect Dis. 2021;34(3):181–86. doi: 10.1097/QCO.0000000000000724. [DOI] [PubMed] [Google Scholar]
- 78.Furuyama W, Shifflett K, Pinski AN, Griffin AJ, Feldmann F, Okumura A, Gourdine T, Jankeel A, Lovaglio J, Hanley PW, et al. Rapid protection from COVID-19 in nonhuman primates vaccinated intramuscularly but not intranasally with a single dose of a recombinant vaccine. bioRxiv Prepr Serv Biol; 2021. http://www.ncbi.nlm.nih.gov/pubmed/33501447%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC7836117 [DOI] [PMC free article] [PubMed]
- 79.Gallo O, Locatello LG, Mazzoni A, Novelli L, Annunziato F. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol. 2021;14(2):305–16. doi: 10.1038/s41385-020-00359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6(1):6. doi: 10.1038/s41541-021-00292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park JH, Lee HK. Delivery routes for COVID-19 vaccines. Vaccines. 2021;9:524. doi: 10.3390/vaccines9050524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choudhary OP, Mohammed TA, Singh I, Singh I. Intranasal COVID-19 vaccines: is it a boon or bane? Int J Surg. 2021;94:106119. doi: 10.1016/j.ijsu.2021.106119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alu A, Chen L, Lei H, Wei Y, Tian X, Wei X. Intranasal COVID-19 vaccines: from bench to bed. eBiomedicine. 2022;76. doi: 10.1016/j.ebiom.2022.103841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R. Use of the inactivated intranasal Influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med. 2004;350(9):896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 85.Lemiale F, Kong W, Akyürek LM, Ling X, Huang Y, Chakrabarti BK, Eckhaus M, Nabel GJ. Enhanced mucosal immunoglobulin a response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J Virol. 2003;77(18):10078–87. doi: 10.1128/JVI.77.18.10078-10087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6(2):148–58. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 87.Bernasconi V, Norling K, Bally M, Höök F, Lycke NY. Mucosal vaccine development based on Liposome technology. J Immunol Res. 2016;2016:1–16. doi: 10.1155/2016/5482087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Del Giudice G, Rappuoli R, Didierlaurent AM. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin Immunol. 2018;39:14–21. doi: 10.1016/j.smim.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 89.Pulendran B, Arunachalam SP, O’-Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021;20(6):454–75. doi: 10.1038/s41573-021-00163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X, Meng D. Innate endogenous adjuvants prime to desirable immune responses via mucosal routes. Protein Cell. 2015;6(3):170–84. doi: 10.1007/s13238-014-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aoshi T. Modes of action for mucosal vaccine adjuvants. Viral Immunol. 2017;30(6):463–70. doi: 10.1089/vim.2017.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jin Z, Gao S, Cui X, Sun D, Zhao K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int J Pharm. 2019;572:572. doi: 10.1016/j.ijpharm.2019.118731. [DOI] [PubMed] [Google Scholar]
- 93.O’-Hagan DT, Lodaya RN, Lofano G. The continued advance of vaccine adjuvants - 'we can work it out'. Semin Immunol. 2020;50:50. doi: 10.1016/j.smim.2020.101426. [DOI] [PubMed] [Google Scholar]
- 94.Mallakpour S, Azadi E, Hussain M. Chitosan, alginate, hyaluronic acid, gums, and β-glucan as potent adjuvants and vaccine delivery systems for viral threats including SARS-CoV-2: A review. Int J Biol Macromol. 2021;182:1931–40. doi: 10.1016/j.ijbiomac.2021.05.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van Ginkel FW, Jackson RJ, Yuki Y, McGhee JR. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into Olfactory tissues. J Immunol. 2000;165(9):4778–82. doi: 10.4049/jimmunol.165.9.4778. [DOI] [PubMed] [Google Scholar]
- 96.Vasu N, Ghaffari G, Craig ET, Craig TJ. Adverse events associated with intranasal influenza vaccine in the United States. Ther Adv Respir Dis. 2008;2(4):193–98. doi: 10.1177/1753465808093933. [DOI] [PubMed] [Google Scholar]
- 97.Sarkar I, Garg R, van Drunen Littel-van den Hurk S. Selection of adjuvants for vaccines targeting specific pathogens. Expert Rev Vaccines. 2019;18(5):505–21. doi: 10.1080/14760584.2019.1604231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Islam F, Bibi S, Meem AF, Islam M, Rahaman M, Bepary S, Rahman M, Elzaki A, Kajoak S, Osman H, et al. Natural Bioactive Molecules: An Alternative Approach to the Treatment and Control of COVID-19. Int J Mol Sci. 22(23):2021;12638. doi: 10.3390/ijms222312638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharma R, Palanisamy A, Dhama K, Mal G, Singh B, Singh KP. Exploring the possible use of saponin adjuvants in COVID-19 vaccine. Hum Vaccines Immunother. 2020;16(12):2944–53. doi: 10.1080/21645515.2020.1833579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abbasi S, Uchida S. Multifunctional immunoadjuvants for use in minimalist nucleic acid vaccines. Pharmaceutics. 2021;13(5):13. doi: 10.3390/pharmaceutics13050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bouazzaoui A, Abdellatif AAH, Al-Allaf FA, Bogari NM, Al-Dehlawi S, Qari SH. Strategies for vaccination: conventional vaccine approaches versus new-generation strategies in combination with adjuvants. Pharmaceutics. 2021;13:1–20. doi: 10.3390/pharmaceutics13020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mao L, Chen Z, Wang Y, Chen C. Design and application of nanoparticles as vaccine adjuvants against human corona virus infection. J Inorg Biochem. 2021;219:219. doi: 10.1016/j.jinorgbio.2021.111454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Butowt R, Bilinska K. SARS-CoV-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem Neurosci. 2020;11(9):1200–03. doi: 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]
- 104.Ma XC, Liu P, Zhang XL, Jiang WH, Jia M, Wang CX, Dong YY, Dang YH, Gao CG. Intranasal delivery of recombinant AAV containing BDNF fused with HA2TAT: a potential promising therapy strategy for major depressive disorder. Sci Rep. 2016;6:6. doi: 10.1038/srep22404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Illum L. Nanoparticulate systems for nasal delivery of drugs: A real improvement over simple systems? J Pharm Sci. 2007;96(3):473–83. doi: 10.1002/jps.20718. [DOI] [PubMed] [Google Scholar]
- 106.Francisco ARL, Sherman W, Repasky M, Beuming T. Improved docking of polypeptides with glide. J Chem Inf Model. 2013;53(7):1689–99. doi: 10.1021/ci400128m. [DOI] [PubMed] [Google Scholar]


