ABSTRACT
Background
Acute viral infections, including coronavirus disease 2019 (COVID-19), are characterized by the dysregulation of iron metabolism, resulting in high serum ferritin and low iron levels.
Research design and methods
This study aimed to evaluate the prospective impact of iron metabolism dysregulation, as expressed by serum Ferritin-to-Iron Ratio (FIR), on the in-hospital prognosis of patients with COVID-19. Serum levels of ferritin and iron, as well as other iron metabolism markers and recognized prognostic indicators of COVID-19 severity, were measured in 362 patients consecutively hospitalized for COVID-19. The prospective relationship between FIR and the risk of the composite outcome of intensive care unit (ICU) admission/in-hospital death was analyzed.
Results
In the population examined (mean age 74 ± 15 years, males 55%), the rates of radiographic signs of pneumonia, respiratory distress, and the need for noninvasive ventilation were higher in patients with high FIR (≥29.2, the 75th percentile) than in those with low FIR (<29.2, the 75th percentile) (p < 0.05 for all comparisons). High FIR was associated with a 1.7-fold (HR 1.709, 95% CI 1.017–2.871, p = 0.043) higher risk of ICU admission/in-hospital death.
Conclusions
Increasing FIR values significantly and independently predicts worse in-hospital prognosis in hospitalized patients with COVID-19.
KEYWORDS: COVID-19, ferritin, ferritin to iron ratio, inflammation, iron, SARS-CoV-2
1. Introduction
Since the start of the SARS-CoV-2 pandemic, hundreds of millions of people have been affected by coronavirus disease 2019 (COVID-19) and over 5 million people have died from this viral disease [1]. SARS-CoV-2-mediated injury of the lower respiratory tract along with the hyperinflammatory state and coagulopathy has a crucial role in the progression toward the most severe and life-threatening complications of COVID-19 (acute respiratory distress syndrome and multi-organ dysfunction) [2–6]. Accordingly, the measurement of circulating levels of different markers of inflammation [7], the detection of laboratory and instrumental anomalies indicative of thrombotic phenomena [8,9], the search for clinical indicators of respiratory distress [10,11] as well as the evaluation of organ dysfunction by means of different scores [12,13], are routinely put into practice during the clinical management of hospitalized patients with COVID-19. However, despite enormous efforts having been made since the beginning of the pandemic in the search for possible prognostic indicators to refine the clinical management and treatment models of COVID-19 [14,15], the in-hospital outcome of this infectious disease is often unfavorable [16–18].
Acute infections, including SARS-CoV-2 infection, are accompanied by a typical acute-phase response that is aimed primarily at eliminating the causative pathogen [19]. Serum ferritin level, a conventional indicator of the adequacy of the body’s iron stores, increases during viral infections along with the levels of other acute-phase proteins [20,21]. Such a ferritin increase, beyond being a consequence of the activation of acute inflammation, may itself enhance the inflammatory response, thereby possibly exerting a pathogenic role in viral infections [22]. In addition, hyperferritinemia may be associated with tissue toxicity due to an excessive production of reactive oxygen species and oxidative stress [23–25]; this has been clearly shown in the liver but may also be of importance in the lungs and other organs because of COVID-19. In conjunction with hyperferritinemia, decreased serum iron levels are also observed during acute viral infections because of the hepcidin-mediated inhibition of ferroportin [21,23]. Although the resulting hypoferremia may be protective by subtracting iron from the needs of pathogenic microorganisms [20–24], low iron levels may be harmful; accordingly, hypoferremia may reflect a detrimental intracellular iron overload during inflammatory processes [24] and impair oxygen delivery to peripheral tissues by limiting erythropoiesis [26].
It is important to note that the concomitant presence of high serum ferritin levels and low iron levels can be observed during COVID-19 [26]. Furthermore, despite some inconsistency, many retrospective studies [26–36] and a smaller number of prospective studies [37–40] have shown that either the state of hyperferritinemia or hypoferremia are accompanied by a greater severity/worse prognosis of COVID-19 both in its acute and post-acute phase. In light of the unfavorable prognostic impact of either hyperferritinemia or hypoferremia in COVID-19, we may hypothesize that our ability to stratify the prognosis of hospitalized patients with COVID-19 might be further increased by integrating the value gathered from these individual variables (serum ferritin and iron levels) into a combined index, that is the Ferritin-to-Iron Ratio (FIR). Furthermore, given the biological plausibility of a pathogenic link between iron metabolism and COVID-19 severity, it is possible that the putative negative impact of elevated FIR on the clinical outcome of patients with COVID-19 might be independent of that of established indicators of poor prognosis, such as comorbidities [e.g. Charlson Comorbidity Index, CCI)], markers of inflammation [e.g. erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)] and organ damage [e.g. Sequential Organ Failure Assessment (SOFA)].
The objective of this prospective study was to evaluate the ability of elevated FIR, as the expression of a possible unfavorable interaction between hyperferritinemia and hypoferremia, to predict the in-hospital prognosis of COVID-19 patients.
2. Patients and methods
2.1. Study population
In this prospective study, hospitalized COVID-19 patients referring to the Internal Medicine and Infectious Diseases wards of the ‘Santa Maria della Misericordia Hospital’ of Perugia (Italy) from December 2020 to February 2021, mainly coming from the Umbria region of central Italy, were consecutively enrolled. The study protocol was developed in accordance with the principles of the Helsinki Declaration and was approved by the local ethics committee (Comitato Etico Regionale Umbria). Inclusion criteria were the following ones: 1) age ≥18 years; 2) a positive result on real-time reverse-transcriptase-PCR (RT-PCR) assays testing for SARS-CoV-2 on nasal or pharyngeal swab specimens at hospital admission; 3) informed written consent. The estimated study sample size was of 308 patients by assuming the type I error = 0.05, the type II error = 0.1, the ratio of the unexposed group (FIR ≤ the 75th percentile) to the exposed group (FIR > the 75th percentile) = 3, the probability of event (ICU admission/in-hospital death) in the unexposed group = 0.25, and the probability of event in the exposed group = 0.45. Noteworthy, the composite endpoint of ICU admission/in-hospital death was a priori selected to ensure the observation of a sufficient number of events providing an acceptable statistical power to the analyses. In addition, the probabilities of the event (ICU admission/in-hospital death) were arbitrarily established for this pilot study, as no previous literature data on the association between FIR and in-hospital outcomes in COVID-19 patients were available. Also, given the absence of literature data reporting a preferable/universally accepted cutoff for high FIR, the 75th percentile was arbitrarily chosen as the cutoff for high FIR, in that it was hypothesized to provide the best dichotomy for FIR values.
2.2. Data collection
For each patient data on demographic characteristics, coexisting medical conditions, current treatments, laboratory tests as well as physical and instrumental examinations performed within 48 hours since hospital admission were collected and registered in medical records. Tests for SARS-CoV-2 on nasal or pharyngeal swab specimens were performed through RT-PCR assays (Allplex 2019-nCoV Assay, Seegene, Seoul, South Korea or the Xpert Xpress SARS-CoV-2, Cepheid, Sunnyvale, USA). Arterial and venous blood samples were collected at hospital admission and processed according to standard laboratory techniques in order to determine the following laboratory variables: blood gas parameters (ABL90 FLEX blood gas analyzer, Radiometer, Brønshøj, Denmark), leukocytes, platelets, hemoglobin, and red blood cell count (Sysmex XT-2000i, Dasit, Milano, Italy), D-dimer (BCS XP Coagulation Analyzer, Siemens, Munich, Germany), high-sensitivity cardiac troponin (hs-cTn) (UniCel DxI 800 analyzer, Beckman Coulter, Brea, USA), C-reactive protein (CRP), blood urea nitrogen (BUN), creatinine, lactate dehydrogenase (LDH), bilirubin, ferritin, serum iron, total iron-binding capacity (TIBC), and fasting glucose (AU5800 Clinical Chemistry System, Beckman Coulter, Brea, USA). Transferrin was calculated according to the following formula: transferrin = (0.8 X TIBC) – 43. Transferrin saturation was calculated as serum iron/TIBC X 100. Estimated glomerular filtration rate (eGFR) was calculated through the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Radiological diagnosis of pneumonia was made based on the presence of at least one of the following radiographic signs in either chest X-ray or high-resolution computed tomography: mono or bilateral consolidations, ground glass opacities, and crazy paving pattern. Respiratory insufficiency was defined as the presence of peripheral oxygen saturation (SpO2) ≤90% and/or arterial partial pressure of oxygen (PaO2) ≤60 mmHg and/or need of oxygen support at admission. Calculated PaO2/fraction of inspiration oxygen ratio (PaO2/FiO2) ≤300 was used to define the presence of respiratory distress. The SOFA score was estimated for each patient by integrating six clinical/laboratory data at admission [i.e. 1- PaO2/FiO2; 2 – platelets; 3 – bilirubin; 4 – mean arterial pressure as the compositum of systolic and diastolic blood pressures assessed through sphygmomanometer; 5 – creatinine; 6 – Glasgow Coma Scale] [41–43]. The CCI was calculated for each patient by integrating information on coexisting medical conditions [44]. Data on clinical course [in-hospital medical treatments and need of noninvasive ventilation (NIV)] and in-hospital outcomes (ICU admission, in-hospital death or hospital discharge) were collected and registered in medical records.
2.3. Statistical analysis
The SPSS statistical package, release 24.0 (SPSS Inc, Chicago, IL), was used for all statistical analyses. The Shapiro test was used to verify the normality of the study variables. Categorical variables were expressed as percentages, while continuous variables were expressed as mean ± standard deviation (SD) or median and interquartile ranges. The independent samples t-test, the Mann-Whitney U-test, and the chi-squared test were used for two-group comparisons. Correlation analyses between the study variables were performed using the Pearson’s and Spearman’s coefficients of correlation. Time-to-event analyses were performed to assess the association between FIR and the composite endpoint of ICU admission/in-hospital death. For six patients, who did not meet the composite endpoint and were still hospitalized at the time of analysis, the event date was censored on 28 April 2021.
Multivariable linear regression analyses were performed to determine the independent predictors of FIR. The association between either serum iron, ferritin, or both serum iron and ferritin and the composite endpoint of ICU admission/in-hospital death was investigated through three different Cox proportional Hazard models including multiple potential confounders. The Chi squared test was performed to assess the association between high FIR (FIR ≥the 75th percentile) and the composite endpoint of ICU admission/in-hospital death, as well the sensitivity, specificity, positive predictive value, and negative predictive value of high FIR (FIR ≥the 75th percentile) toward the same endpoint. In order to assess the risk of the composite endpoint of ICU admission/in-hospital death according to high versus low FIR (FIR ≥the 75th percentile versus FIR 75th percentile], both in the entire study population and in the severe COVID-19 subgroup, a Kaplan–Meier plot was run and the Log-rank test was performed. The association between FIR, either as continuous or categorical variable (FIR quartiles and high FIR), and the composite endpoint of ICU admission/in-hospital death was further evaluated through three Cox proportional Hazard models including multiple potential confounders, both in the entire study population and in the severe COVID-19 subgroup. The following exploratory analyses were also performed: 1) multi-adjusted Cox regression analyses to assess the association between FIR, either as continuous or categorical variable, and either ICU admission or in-hospital death as single endpoints; 2) a multi-adjusted Cox regression analysis to assess the association between FIR ≥the median value and the composite endpoint of ICU admission/in-hospital death; 3) univariate Cox regression analyses to assess the association between Ferritin-to-Transferrin Ratio (FTR) and either ICU admission or in-hospital death as single endpoints, in analogy with a previous study [45]. Statistical significance was assumed if a null hypothesis could be rejected at p ≤0.05.
3. Results
3.1. Baseline characteristics of the study population
Three hundred and sixty-two COVID-19 patients were enrolled (mean age 74 ± 15 years, males 55%). The characteristics of the study population categorized according to FIR ≥29.2 (FIR ≥the 75th percentile) versus FIR < 29.2 (FIR <the 75th percentile) are reported in Table 1, while the characteristics of the study population dichotomized according to the occurrence of the composite outcome of ICU admission/in-hospital death are described in Table 2. Hyperferritinemia (ferritin >300 ng/mL, the upper limit of the normal range) was found in 64% of the enrolled patients, while hypoferremia (serum iron <80 μg/dL in males or serum iron <60 μg/dL in females) was detected in 86/80% of males/females, respectively. Noteworthy, only 3 out of 362 patients had extremely high ferritin levels (ferritin >4420 ng/mL), which are compatible with the macrophage activation syndrome [46]. Patients who were admitted to ICU/died had reduced serum iron levels, TIBC, transferrin, and transferrin saturation, and increased ferritin levels and FIR as compared to those who were not admitted to ICU/were discharged alive (Table 3).
Table 1.
Baseline characteristics of COVID-19 patients according to FIR ≥ 29.2 (i.e. ≥ the 75th percentile) versus FIR <29.2 (i.e. < the 75th percentile)
| FIR < 29.2 n = 272 |
FIR ≥ 29.2 n = 90 |
p | |
|---|---|---|---|
| Age, years | 72 (16) | 76 (13) | 0.170 |
| Male gender, % | 53 | 63 | 0.085 |
| BMI, kg/m2 | 26.3 (4.3) | 25.8 (4.5) | 0.227 |
| Current smoking, % | 18 | 20 | 0.705 |
| Hypertension, % | 63 | 68 | 0.400 |
| Type 2 diabetes, % | 20 | 24 | 0.441 |
| CKD, % | 13 | 11 | 0.662 |
| Previous CV event, % | 19 | 20 | 0.976 |
| Active cancer, % | 11 | 9 | 0.506 |
| Previous VTE, % | 29 | 78 | 0.046 |
| AF, % | 19 | 13 | 0.212 |
| COPD, % | 14 | 8 | 0.123 |
| Obesity, % | 33 | 23 | 0.079 |
| CCI | 4 (3–6) | 5 (4–6) | 0.125 |
| ACE inhibitors, % | 25 | 32 | 0.239 |
| ARBs, % | 14 | 18 | 0.431 |
| Statins, % | 15 | 15 | 1.000 |
| DOACs, % | 12 | 10 | 0.647 |
| VKAs, % | 2 | 4 | 0.370 |
| LMWH, % | 17 | 25 | 0.085 |
| Anti-platelets, % | 28 | 34 | 0.318 |
| BBs, % | 30 | 31 | 0.915 |
| CCBs, % | 23 | 21 | 0.740 |
| Diuretics, % | 39 | 32 | 0.277 |
| Insulin, % | 12 | 14 | 0.584 |
| Oral hypoglycemic agents, % | 10 | 12 | 0.609 |
| SBP, mmHg | 132 (20) | 127 (24) | 0.036 |
| DBP, mmHg | 77 (11) | 73 (12) | 0.014 |
| Leukocytes, X 103/μL | 7.6 (5.4–10.6) | 7.9 (5.4–11.6) | 0.396 |
| Platelets, X 103/μL | 211 (160–269) | 197(141–259) | 0.111 |
| Hb, g/dL | 13.2 (11.7–14.4) | 12.7 (10.7–14.4) | 0.254 |
| Red blood cells, X 106/μL | 4.4 (3.9–4.9) | 4.3 (3.6–4.8) | 0.071 |
| D-dimer, ng/mL | 844 (533–1538) | 1405 (600–2799) | 0.002 |
| Hs-cTn, ng/L | 13 (7–30) | 27 (14–44) | <0.001 |
| CRP, mg/dL | 5.8 (2.8–9.5) | 11.9 (5.7–17.1) | <0.001 |
| ESR, mm/h | 52 (34–79) | 68 (51–94) | <0.001 |
| Fasting glucose, mg/dL | 120 (102–145) | 127 (105–162) | 0.222 |
| eGFR, ml/min | 71 (27) | 61 (26) | 0.032 |
| Albumin, g/dL | 3.5 (3.2–3.7) | 3.2 (2.7–3.5) | <0.001 |
| LDH, UI/L | 288 (225–387) | 354 (257–466) | <0.001 |
| PaO2/FiO2 | 266 (195–310) | 197 (126–276) | <0.001 |
| SOFA score | 2 (2–4) | 4 (2–5) | <0.001 |
ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blockers; AF, atrial fibrillation; BBs, beta-blockers; BMI, body mass index; CCBs, calcium channel blockers; CCI, Charlson Comorbidity Index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CV, cardiovascular; DBP, diastolic blood pressure; DOACs, direct oral anticoagulants; eGFR, estimated glomerular filtration rate; ESR, erythrocyte sedimentation rate; FiO2, fraction of inspiration oxygen; FIR, ferritin to iron ratio; Hb, hemoglobin; hs-cTn, high sensitivity cardiac troponin; LDH lactate dehydrogenase; LMWH, low molecular weight heparin; NLR, neutrophil-to-lymphocyte ratio; PaO2, arterial partial pressure of oxygen; SBP, systolic blood pressure; SOFA, Sequential Organ Failure Assessment; VKAs, vitamin-K antagonists; VTE, venous thromboembolism.
Table 2.
Baseline characteristics of COVID-19 patients according to the composite endpoint of ICU admission/in-hospital death
| Non-ICU admitted/Discharged alive n = 261 |
ICU admitted/ Non-survivors n = 101 |
p | |
|---|---|---|---|
| Age, years | 72 (16) | 79 (12) | <0.001 |
| Male gender, % | 53 | 62 | 0.103 |
| BMI, kg/m2 | 26.6 (4.5) | 25.7 (4.1) | 0.128 |
| Current smoking, % | 19 | 18 | 0.762 |
| Hypertension, % | 62 | 68 | 0.297 |
| Type 2 diabetes, % | 20 | 26 | 0.227 |
| CKD, % | 10 | 18 | 0.053 |
| Previous CV event, % | 18 | 25 | 0.126 |
| Active cancer, % | 11 | 10 | 0.739 |
| Previous VTE, % | 5 | 3 | 0.486 |
| AF, % | 16 | 22 | 0.203 |
| COPD, % | 12 | 14 | 0.608 |
| Obesity, % | 32 | 26 | 0.280 |
| CCI | 4 (2–6) | 5 (4–7) | 0.001 |
| ACE inhibitors, % | 27 | 29 | 0.732 |
| ARBs, % | 14 | 17 | 0.589 |
| Statins, % | 15 | 13 | 0.614 |
| DOACs, % | 11 | 12 | 0.836 |
| VKAs, % | 2 | 5 | 0.187 |
| LMWH, % | 19 | 21 | 0.663 |
| Anti-platelets, % | 24 | 34 | 0.079 |
| BBs, % | 31 | 31 | 0.994 |
| CCBs, % | 19 | 30 | 0.037 |
| Diuretics, % | 35 | 42 | 0.173 |
| Insulin, % | 12 | 15 | 0.433 |
| Oral hypoglycemic agents, % | 10 | 12 | 0.072 |
| SBP, mmHg | 132 (21) | 126 (20) | 0.019 |
| DBP, mmHg | 77 (11) | 73 (11) | 0.007 |
| Leukocytes, X 103/μL | 7.7 (5.2–10.8) | 7.6 (5.6–11.1) | 0.643 |
| Platelets, X 103/μL | 217 (163–274) | 185 (144–235) | 0.003 |
| Hb, g/dL | 13.2 (11.7–14.4) | 12.7 (10.8–14.3) | 0.162 |
| Red blood cells, X 106/μL | 4.4 (3.9–4.9) | 4.3 (3.5–4.8) | 0.042 |
| D-dimer, ng/mL | 860 (565–1760) | 1150 (533–1803) | 0.283 |
| Hs-cTn, ng/L | 12.5 (6.8–27.3) | 28.6 (17.6–51.9) | <0.001 |
| CRP, mg/dL | 6.1 (2.8–10.8) | 9.2 (4.5–15.7) | <0.001 |
| ESR, mm/h | 54 (36–83) | 65 (47–88) | 0.106 |
| Fasting glucose, mg/dL | 120 (102–146) | 131 (105–160) | 0.282 |
| eGFR, ml/min | 73 (26) | 58 (27) | <0.001 |
| Albumin, g/dL | 3.4 (3.1–3.7) | 3.3 (3–3.6) | 0.010 |
| LDH, UI/L | 281 (224–385) | 366 (283–468) | <0.001 |
| PaO2/FiO2 | 271 (206–312) | 161 (114–261) | <0.001 |
| SOFA score | 2 (2–3) | 4 (2–5) | <0.001 |
ACE, angiotensin converting enzyme; ARBs, angiotensin II receptor blockers; AF, atrial fibrillation; BBs, beta-blockers; BMI, body mass index; CCBs, calcium channel blockers; CCI, Charlson Comorbidity Index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CV, cardiovascular; DBP, diastolic blood pressure; DOACs, direct oral anticoagulants; eGFR, estimated glomerular filtration rate; ESR, erythrocyte sedimentation rate; FiO2, fraction of inspiration oxygen; Hb, hemoglobin; hs-cTn, high sensitivity cardiac troponin; ICU, intensive care unit; LDH lactate dehydrogenase; LMWH, low molecular weight heparin; NLR, neutrophil-to-lymphocyte ratio; PaO2, arterial partial pressure of oxygen; SBP, systolic blood pressure; SOFA, Sequential Organ Failure Assessment; VKAs, vitamin-K antagonists; VTE, venous thromboembolism.
Table 3.
Baseline iron metabolism parameters according to the composite endpoint of ICU admission/in-hospital death
| Non-ICU admitted/Discharged alive n = 261 |
ICU admitted/ Non-survivors n = 101 |
p | |
|---|---|---|---|
| Serum iron, μg/dL | 35 (23–58) | 26 (17–37) | <0.001 |
| Ferritin, ng/mL | 402 (185–790) | 772 (313–1375) | <0.001 |
| FIR | 10 (5–24) | 27 (11–56) | <0.001 |
| TIBC, μg/dL | 213 (184–250) | 195 (156–219) | <0.001 |
| Transferrin, mg/dL | 130 (104–157) | 113 (82–133) | <0.001 |
| Transferrin saturation, % | 16 (10–29) | 13 (8–20) | 0.008 |
| FTR | 3 (1–6) | 8 (2–14) | <0.001 |
FIR, Ferritin to Iron Ratio; FTR, Ferritin-to-Transferrin Ratio; ICU, intensive care unit; TIBC, total iron binding capacity.
3.2. Clinical course and in-hospital outcomes
At hospital admission, 25 (7%), 59 (16%), and 278 (77%) patients had mild COVID-19 (signs and symptoms of COVID-19 without shortness of breath, dyspnea, or abnormal chest imaging), moderate COVID-19 (lower respiratory disease during clinical assessment or imaging and SpO2 ≥ 94% on room air at sea level) and severe COVID-19 (SpO2 < 94% on room air at sea level, PaO2/FiO2 < 300 mmHg, respiratory frequency >30 breaths/min, or lung infiltrates >50%), respectively, according to the National Institutes of Health (NIH) classification of COVID-19 severity [47]. Mild (200< PaO2/FiO2 ≤ 300), moderate (100< PaO2/FiO2 ≤ 200), and severe respiratory distress (PaO2/FiO2 ≤ 100) were documented in 141 (39%), 91 (25%) and 24 (7%) patients, respectively. During the hospital stay, the therapeutic management of patients followed the scientific recommendations formulated during the pandemic evolution. Thirty-five patients (10%) underwent ICU admission, 66 (18%) patients died, and 101 patients (28%) met the composite endpoint of ICU admission/in-hospital death. The median time from in-hospital admission to ICU admission was 2 (2–5) days, while the median time from in-hospital admission to death was 10 (6–16) days. Supplementary Table 1 shows the main medical therapies and clinical complications that were recorded in the study population during the hospital stay according to the composite outcome of ICU admission/in-hospital death.
3.3. Serum iron, ferritin, FIR, and their covariates
Serum iron levels were negatively associated with age (rho = −0.242, p < 0.001), CCI (rho = −0.225, p < 0.001), ESR (rho = −0.166, p = 0.009), SOFA score (rho = −0.198, p < 0.001), and hs-cTn (rho = −0.238, p < 0.001), whereas they were positively associated with LDH (rho = 0.125, p = 0.018), albumin (rho = 0.121, p = 0.022), eGFR (rho = 0.203, p < 0.001), TIBC (rho = 0.159, p = 0.003), ferritin (rho = 0.120, p = 0.022), transferrin saturation (rho = 0.923, p < 0.001), red blood cells (rho = 0.169, p = 0.001), and hemoglobin (rho = 0.199, p < 0.001). Ferritin was higher in males than females (p < 0.001). In addition, it correlated positively with ESR (rho = 0.239, p < 0.001), CRP (rho = 0.451, p < 0.001), SOFA score (rho = 0.191, p > 0.001), LDH (rho = 0.402, p < 0.001), hs-cTn (rho = 0.165, p = 0.005), serum iron (rho = 0.120, p = 0.022), transferrin saturation (rho = 0.282, p < 0.001), hemoglobin (rho = 0.144, p = 0.006), leukocytes (rho = 0.115, p = 0.029), and D-dimer (rho = 0.114, p = 0.047), whereas it was negatively associated with PaO2/FiO2 (rho = −0.275, p < 0.001), albumin (rho = −0.173, p = 0.001) and TIBC (rho = −0.468, p < 0.001). FIR was higher in men than in women (p < 0.001). In addition, it correlated positively with ESR (rho = 0.328, p < 0.001), CRP (rho = 0.409, p < 0.001), SOFA score (rho = 0.294, p < 0.001), LDH (rho = 0.274, p < 0.001), hs-cTn (rho = 0.292, p < 0.001), and D-dimer (rho = 0.159, p = 0.005), whereas it was negatively associated with albumin (rho = −0.222, p < 0.001), eGFR (rho = −0.194, p < 0.001), TIBC (rho = −0.526, p < 0.001), transferrin saturation (rho = −0.254, p < 0.001), and PaO2/FiO2 (rho = −0.293, p < 0.001).
In a multivariable linear regression analysis including logarithmic (LG)-FIR as the dependent variable and non-iron metabolism-related FIR covariates (sex, either LG-ESR or LG-CRP, LG-SOFA score, LG-LDH, LG-hs-cTn, LG-D-dimer, LG-albumin, and eGFR) as the independent variables, inflammatory markers (LG-ESR and LG-CRP, which were included one at time) were independent predictors of LG-FIR (β = 0.262 and p < 0.001 for LG-ESR, β = 0.279 and p < 0.001 for LG-CRP, in models including either LG-ESR or LG-CRP, respectively).
3.4. Association between iron metabolism parameters and the composite endpoint of ICU admission/in-hospital death
Six models of Cox regression analysis were plotted to explore the association between iron metabolism parameters and risk of ICU admission/in-hospital death. In a model in which serum iron was included as an independent variable, the latter one was associated with an increased risk of worse in-hospital prognosis independently of confounders (age, sex, CCI, red blood cell count, platelet count, hs-cTn, albumin, CRP, SOFA score, LDH, and D-dimer) (Table 4, Model 1). In another model, in which the independent variable serum iron was replaced by ferritin (Table 4, Model 2), the latter one was significantly associated with the in-hospital outcome. Upon the inclusion in the same model of both serum iron and ferritin (Table 4, Model 3), both these independent variables were able to predict in-hospital prognosis irrespective of confounders.
Table 4.
Association between iron metabolism parameters (i.e. serum iron, ferritin, and FIR) and the composite endpoint of ICU admission/in-hospital death in the entire study population
| Model 1 | Variables | HR | 95% CI | p |
|---|---|---|---|---|
| Serum iron | 0.988 | 0.978–0.998 | 0.017 | |
| Age | 1.004 | 0.981–1.027 | 0.745 | |
| Sex | 1.261 | 0.771–2.065 | 0.356 | |
| CCI | 1.032 | 0.919–1.160 | 0.592 | |
| Red blood cell count | 0.944 | 0.655–1.360 | 0.756 | |
| Platelet count | 0.999 | 0.996–1.001 | 0.285 | |
| Hs-cTn | 1.000 | 0.999–1.001 | 0.779 | |
| Albumin | 1.165 | 0.672–2.019 | 0.587 | |
| CRP | 1.026 | 0.997–1.057 | 0.080 | |
| SOFA score | 1.288 | 1.122–1.479 | <0.001 | |
| LDH | 1.002 | 1.000–1.003 | 0.017 | |
| D-dimer | 1.000 | 1.000–1.000 | 0.371 | |
| Model 2 | Variables | HR | 95% CI | p |
| Ferritin | 1.001 | 1.000–1.001 | <0.001 | |
| Age | 1.009 | 0.984–1.034 | 0.484 | |
| Sex | 1.113 | 0.674–1.841 | 0.675 | |
| CCI | 1.065 | 0.944–1.201 | 0.308 | |
| Red blood cell count | 1.063 | 0.741–1.525 | 0.741 | |
| Platelet count | 0.999 | 0.997–1.002 | 0.485 | |
| Hs-cTn | 1.000 | 0.999–1.001 | 0.592 | |
| Albumin | 1.127 | 0.631–2.012 | 0.686 | |
| CRP | 1.028 | 0.996–1.060 | 0.084 | |
| SOFA score | 1.286 | 1.126–1.469 | <0.001 | |
| LDH | 1.000 | 0.999–1.002 | 0.559 | |
| D-dimer | 1.000 | 1.000–1.000 | 0.382 | |
| Model 3 | Variables | HR | 95% CI | p |
| Serum iron | 0.989 | 0.980–0.999 | 0.028 | |
| Ferritin | 1.001 | 1.000–1.001 | 0.001 | |
| Age | 1.003 | 0.979–1.028 | 0.806 | |
| Sex | 1.124 | 0.682–1.853 | 0.645 | |
| CCI | 1.073 | 0.955–1.206 | 0.236 | |
| Red blood cell count | 1.074 | 0.750–1.536 | 0.698 | |
| Platelet count | 0.999 | 0.996–1.002 | 0.426 | |
| Hs-cTn | 1.000 | 0.999–1.001 | 0.680 | |
| Albumin | 1.115 | 0.644–1.931 | 0.696 | |
| CRP | 1.025 | 0.994–1.057 | 0.113 | |
| SOFA score | 1.266 | 1.108–1.447 | 0.001 | |
| LDH | 1.001 | 0.999–1.002 | 0.314 | |
| D-dimer | 1.000 | 1.000–1.000 | 0.265 | |
| Model 4 | Variables | HR | 95% CI | p |
| FIR | 1.009 | 1.004–1.014 | 0.001 | |
| Age | 1.007 | 0.983–1.031 | 0.593 | |
| Sex | 1.179 | 0.715–1.942 | 0.519 | |
| CCI | 1.061 | 0.942–1.196 | 0.326 | |
| Red blood cell count | 1.033 | 0.724–1.475 | 0.858 | |
| Platelet count | 0.999 | 0.997–1.002 | 0.591 | |
| Hs-cTn | 1.000 | 0.999–1.001 | 0.745 | |
| Albumin | 1.265 | 0.702–2.280 | 0.434 | |
| CRP | 1.027 | 0.996–1.060 | 0.088 | |
| SOFA score | 1.245 | 1.087–1.426 | 0.002 | |
| LDH | 1.001 | 1.000–1.002 | 0.092 | |
| D-dimer | 1.000 | 1.000–1.000 | 0.331 | |
| Model 5 | Variables | HR | 95% CI | p |
| FIR quartiles | 1.481 | 1.162–1.888 | 0.002 | |
| Age | 1.007 | 0.983–1.031 | 0.587 | |
| Sex | 1.084 | 0.657–1.789 | 0.751 | |
| CCI | 1.045 | 0.929–1.175 | 0.467 | |
| Red blood cell count | 0.942 | 0.663–1.340 | 0.741 | |
| Platelet count | 0.999 | 0.996–1.001 | 0.370 | |
| Hs-cTn | 1.000 | 0.999–1.001 | 0.643 | |
| Albumin | 1.214 | 0.693–2.128 | 0.498 | |
| CRP | 1.025 | 0.994–1.057 | 0.116 | |
| SOFA score | 1.301 | 1.133–1.494 | <0.001 | |
| LDH | 1.001 | 1.000–1.002 | 0.131 | |
| D-dimer | 1.000 | 1.000–1.000 | 0.241 | |
| Model 6 | Variables | HR | 95% CI | p |
| High FIR (FIR ≥29.2) | 1.709 | 1.017–2.871 | 0.043 | |
| Age | 1.007 | 0.984–1.031 | 0.558 | |
| Sex | 1.095 | 0.655–1.829 | 0.730 | |
| CCI | 1.031 | 0.916–1.160 | 0.611 | |
| Red blood cell count | 0.929 | 0.646–1.335 | 0.690 | |
| Platelet count | 0.999 | 0.996–1.001 | 0.351 | |
| Hs-cTn | 1.000 | 0.999–1.001 | 0.691 | |
| Albumin | 1.186 | 0.671–2.097 | 0.558 | |
| CRP | 1.027 | 0.997–1.059 | 0.082 | |
| SOFA score | 1.319 | 1.149–1.515 | <0.001 | |
| LDH | 1.001 | 1.000–1.002 | 0.071 | |
| D-dimer | 1.000 | 1.000–1.000 | 0.370 |
CCI, Charlson comorbidity index; CI, confidence interval; CRP, C-reactive protein; hs-cTn, high-sensitivity cardiac troponin; FIR, ferritin to iron ratio; LDH, lactate dehydrogenase; HR, hazard ratio; SOFA, Sequential Organ Failure Assessment.
A significant association emerged between high FIR (FIR ≥29.2, the 75th percentile) and the composite endpoint of ICU admission/in-hospital death (p < 0.001), with a 45.5% sensitivity, 83.1% specificity, 51.1% positive predictive value, and 79.8% negative predictive value of high FIR (FIR ≥29.2, the 75th percentile) in the prediction of the same endpoint. Figure 1 shows the Kaplan-Meier analysis stratified according to high versus low FIR (FIR ≥29.2 or <29.2, the 75th percentile) in the entire study population (Panel A) (Log-rank p < 0.001) and in the severe COVID-19 subgroup (Panel B) (Log-rank p < 0.001). In three Cox regression analyses (Table 4, Models 4–6) including FIR, either as continuous variable (FIR) or categorical variable [either FIR quartiles (1st FIR <5.9, 2nd 5.9≤ FIR >13.9, 3rd 13.9≤ FIR >29.2, 4th FIR ≥29.2) or high FIR], FIR, FIR quartiles, and high FIR were able to predict the in-hospital prognosis of COVID-19 patients regardless of confounders (age, sex, CCI, red blood cell count, platelet count, hs-cTn, albumin, CRP, SOFA score, LDH, and D-dimer). Similarly, FIR, FIR quartiles, and high FIR were able to predict the in-hospital prognosis of patients with severe COVID-19 regardless of confounders (age, sex, CCI, red blood cell count, platelet count, hs-cTn, albumin, CRP, SOFA score, LDH, and D-dimer) (Supplementary Table 2).
Figure 1.
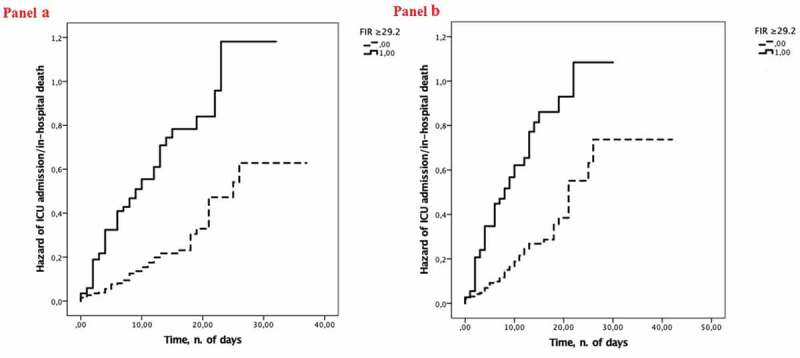
Cumulative hazard of ICU admission/in-hospital death according to low versus high FIR (i.e. FIR ≥29.2 versus FIR <29.2, the 75th percentile) at hospital admission in the entire study population (panel A) and in the severe COVID-19 subgroup (panel B).
3.5. Exploratory analyses
At multi-adjusted Cox regression analysis, a significant association emerged between either FIR quartiles or high FIR and ICU admission as single endpoint, while the association between FIR as continuous variable and ICU admission as single endpoint was not significant (Supplementary Table 3). In addition, a significant association emerged between either FIR, FIR quartiles or high FIR and in-hospital death as single endpoint (Supplementary Table 4). At the same multi-adjusted model, FIR ≥ 13.9 (the median value) was independently associated with the composite endpoint of ICU admission/in-hospital death (Supplementary Table 5). At univariate Cox regression analysis, FTR as continuous variable was significantly associated with the single endpoint of in-hospital death (HR 1.037, 95%CI 1.027–1.046, p < 0.001), but not with the single endpoint of ICU admission (HR 1.015, 95% CI 0.997–1.034, p = 0.102).
4. Discussion
Three main results of this study deserve discussion: 1) hyperferritinemia and hypoferremia were prevalent conditions in hospitalized COVID-19 patients; 2) both ferritin and serum iron levels were associated with the composite endpoint of ICU admission/in-hospital death in hospitalized COVID-19 patients; 3) high FIR, as an integrated marker of ferritin and iron status, was an independent predictor of in-hospital prognosis in COVID-19 patients.
The high prevalence of hyperferritinemia (ferritin >300 ng/mL) [48] and hypoferremia (serum iron <80 μg/dL in males or serum iron <60 μg/dL in females) [49] which emerged in this study strongly suggests that iron metabolism is dysregulated in COVID-19. Consistently, previous studies have shown significantly higher ferritin levels and reduced serum iron levels in hospitalized COVID-19 patients as compared to COVID-19 negative subjects [50–52]. From a biological perspective, different mechanisms might explain the derangement of iron metabolism occurring in COVID-19. First, inflammatory response may mediate both the increase of ferritin levels and the decrease of serum iron levels in COVID-19 patients. Indeed, ferritin is a well-known acute-phase protein, whose expression may be induced by pro-inflammatory cytokines during infections [53]. Also, hypoferremia occurs during infections due to the hepcidin-mediated inhibition of ferroportin and subsequent iron retention in the intracellular compartment [54]. In agreement with this, different markers of inflammation were independent predictors of FIR, as the compositum of ferritin and serum iron levels, in the present study. Second, it has been speculated that SARS-CoV-2 may directly affect iron metabolism. Indeed, SARS-CoV-2 has been shown to exhibit hepcidin-like properties, potentially contributing to reduce serum iron availability, independently from the inflammatory response, through the inhibition of ferroportin activity [55,56]. Also, SARS-CoV-2, by promoting the disruption of hemoglobin 1-beta chain and the dissociation of porphyrins from iron, has been speculated to increase ferritin expression [57].
In this study a significant prospective association emerged between both ferritin and serum iron and COVID-19 prognosis. This result supports previous literature data, mainly derived from retrospective analyses, showing a significant association between either hyperferritinemia or hypoferremia, and COVID-19 severity/prognosis [26–40].
As an unprecedented finding, the present study showed a significant and independent association between FIR, either as continuous or categorical variable, and in-hospital prognosis of patients with COVID-19. Indeed, to the best of our knowledge, the association of the high ferritin/low iron binomial with COVID-19 prognosis has never been explored so far. A plausible biological explanation of this result may rely on the possible pathogenic role, either individual or synergistical, of the two elements combined in FIR, in the context of COVID-19. In this regard, there is evidence suggesting that both hyperferritinemia and hypoferremia, beyond being induced by COVID-19, may themselves promote some pathophysiological mechanisms leading to the most severe clinical manifestations of COVID-19 (enhanced inflammatory response and multi-organ dysfunction) [23,58]. Supporting this notion, ferritin stimulates intracellular pro-inflammatory pathways culminating in the activation of NF-κB and in the increased expression of pro-inflammatory mediators [23,58]. In addition, iron excess bound to ferritin within the intracellular compartment may promote the generation of reactive oxygen species and oxidative damage, ultimately leading to ferroptosis (the programmed cell death induced by iron-dependent peroxidation mechanisms) [59]. Finally, hypoferremia may impair tissue oxygen supply, thereby affecting negatively COVID-19 outcome [26]. Overall, these processes are likely to be implicated in the onset of multi-organ damage in COVID-19.
From a clinical perspective, the existence of a significant and independent association between FIR and COVID-19 prognosis may have important implications: 1) the utility of FIR measurement in the prognostic stratification of COVID-19 patients; 2) the need of a better understanding of FIR as a possible therapeutic target in COVID-19. Regarding the first issue, it should be emphasized that FIR is an inexpensive and easily available laboratory parameter, which can be rapidly obtained from venous blood samples at hospitalization. Regarding the second issue, it should be considered that, although different therapeutic strategies targeting iron metabolism have been proposed in patients with COVID-19 (iron chelation, therapeutic plasma exchange, iron depletion) [60], there is no evidence from randomized controlled trials of the impact of this therapeutic approach on COVID-19 outcomes.
Some limitations of this study, mainly considering its observational character, should be acknowledged. First, this is a single-center prospective study enrolling patients from a quite restricted Italian region, in a short period of time, and with variable COVID-19 severity. A multi-center design with an extended enrollment period might have allowed for the evaluation of a greater number of participants from different geographical areas and in different seasons, potentially overcoming geographical and seasonal variations of iron parameters, which have been reported in previous studies [61,62]; in addition, it might have allowed for sub-group analyses. Second, due to the study design, comparison of ferritin, iron, and FIR values between COVID-19 cases and COVID-19 negative controls was not possible; a case–control analysis could have strengthened the study results. Third, the observation lasted until the occurrence of the composite endpoint of ICU admission/death or hospital discharge; this did allow for the investigation of the association between FIR and in-hospital prognosis but not the association between FIR and long-term prognosis. Fourth, the possible residual confounding effect due to unmeasured variables cannot be ruled out. To this regard, it should be considered that serum hepcidin levels were not measured, although they could have displayed a high informative value in support of the hypothetical pathophysiological mechanisms underlying iron metabolism derangement in COVID-19; therefore, future studies exploring this issue are warranted. Fifth, as in the nature of observational analysis, our study did suggest but not prove any causality between iron metabolism derangement and COVID-19 severity/prognosis. Finally, as a possible methodological limitation of the study, blood samples were not performed in the same daytime hours; therefore, possible diurnal variations of serum iron levels, which have been extensively recognized [63], may compromise, at least partially, the reliability of the observed results.
5. Conclusions
This study shows that FIR directly correlates with COVID-19 severity and predict worse in-hospital clinical outcomes in COVID-19 patients. Accordingly, FIR, as an integrated parameter of iron metabolism derangement, may be worthy of attention to refine the prognostic stratification of COVID-19 patients at hospital admission. Potential therapeutic strategies aimed at restoring iron homeostasis are worthy of being investigated to prevent the most severe complications of COVID-19.
Supplementary Material
Author contributions
Conception and design: V.B and M.P.; analysis and interpretation of the data: V.B., M.R.M., F.F., E.C., G.B., E.M., M.B., A.S., and M.P.; the drafting of the paper or revising it critically for intellectual content: V.B., M.R.M., M.B., A.S., and M.P.; final approval of the version to be published: V.B., M.R.M., F.F., E.C., G.B., E.M., M.B., A.S., and M.P. All authors agree to be accountable for all aspects of the work.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [M.R.M.], upon reasonable request.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants, or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary material
Supplemental data for this article can be accessed here.
References
- 1.Coronavirus disease (COVID-19) pandemic. World Health Organization. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed on November 30 2021.
- 2.Bohn MK, Hall A, Sepiashvili L, et al. Pathophysiology of COVID-19: mechanisms underlying disease severity and progression. Physiology (Bethesda). 2020;35(5):288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romanova ES, Vasilyev VV, Startseva G, et al. Cause of death based on systematic post-mortem studies in patients with positive SARS-CoV-2 tissue PCR during the COVID-19 pandemic. J Intern Med. 2021;290(3):655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao YM, Xu G, Wang B, et al. Cytokine storm syndrome in coronavirus disease 2019: a narrative review. J Intern Med. 2021;289(2):147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhaskar S, Sinha A, Banach M, et al. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol. 2020;11:1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrovic V, Radenkovic D, Radenkovic G, et al. Pathophysiology of cardiovascular complications in COVID-19. Front Physiol. 2020;11:575600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji P, Zhu J, Zhong Z, et al. Association of elevated inflammatory markers and severe COVID-19: a meta-analysis. Medicine (Baltimore). 2020;99(47):e23315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juneja GK, Castelo M, Yeh CH, et al. COVID-BEACONS investigators. Biomarkers of coagulation, endothelial function and fibrinolysis in critically-ill patients with COVID-19: a single-centre prospective longitudinal study. J Thromb Haemost. 2021;19(6):1546–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alfageme M, González Plaza J, Méndez S, et al. Venous doppler ultrasound in critically ill COVID-19 patients: game changer in anticoagulation therapy. Ultrasound J. 2020;12(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balfanz P, Hartmann B, Müller-Wieland D, et al. Early risk markers for severe clinical course and fatal outcome in German patients with COVID-19. PLoS One. 2021;16(1):e0246182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pennica A, Conforti G, Falangone F, et al. Clinical management of adult coronavirus infection disease 2019 (COVID-19) positive in the setting of low and medium intensity of care: a short practical review. SN Compr Clin Med. 2020;2(6):694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raschke RA, Agarwal S, Rangan P, et al. Discriminant accuracy of the SOFA score for determining the probable mortality of patients with COVID-19 pneumonia requiring mechanical ventilation. JAMA. 2021;325(14):1469–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan G, Tu C, Zhou F, et al. Comparison of severity scores for COVID-19 patients with pneumonia: a retrospective study. Eur Respir J. 2020;56(3):2002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianconi V, Mannarino MR, Figorilli F, et al. Prevalence of vitamin D deficiency and its prognostic impact on patients hospitalized with COVID-19. Nutrition. 2021;91-92:111408 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianconi V, Mannarino MR, Figorilli F, et al. Low brachial artery flow-mediated dilation predicts worse prognosis in hospitalized patients with COVID-19. J Clin Med. 2021;10(22):5456 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madahar P, Wunsch H, Jha P, et al. Trends in COVID-19-related in-hospital mortality: lessons learned from nationwide samples. Lancet Respir Med. 2021;9(4):322–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellan M, Patti G, Hayden E, et al. Fatality rate and predictors of mortality in an Italian cohort of hospitalized COVID-19 patients. Sci Rep. 2020;10(1):20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bianconi V, Violi F, Fallarino F, et al. Is acetylsalicylic acid a safe and potentially useful choice for adult patients with COVID-19 ? Drugs. 2020;80(14):1383–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Knovich MA, Coffman LG, et al. Serum ferritin: past, present and future. Biochim Biophys Acta. 2010;1800(8):760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drakesmith H, Prentice A.. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6(7):541–552 . [DOI] [PubMed] [Google Scholar]
- 22.Ruscitti P, Berardicurti O, Barile A, et al. Severe COVID-19 and related hyperferritinaemia: more than an innocent bystander? Ann Rheum Dis. 2020;79(11):1515–1516 . [DOI] [PubMed] [Google Scholar]
- 23.Gomes AC, Moreira AC, Mesquita G, et al. Modulation of iron metabolism in response to infection: twists for all tastes. Pharmaceuticals (Basel). 2018. Sep 1;11(3):84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galaris D, Barbouti A, Pantopoulos K. Iron homeostasis and oxidative stress: an intimate relationship. Biochim Biophys Acta Mol Cell Res. 2019;1866(12):118535. [DOI] [PubMed] [Google Scholar]
- 25.Ruddell RG, Hoang-Le D, Barwood JM, et al. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology. 2009;49(3):887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hippchen T, Altamura S, Muckenthaler MU, et al. Hypoferremia is associated with increased hospitalization and oxygen demand in COVID-19 patients. Hemasphere. 2020;4(6):e492. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this retrospective study, low serum iron levels predicted hospitalization due to COVD-19.
- 27.Zhao K, Huang J, Dai D, et al. Serum iron level as a potential predictor of coronavirus disease 2019 severity and mortality: a retrospective study. Open Forum Infect Dis. 2020;7(7):ofaa250 . [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this retrospective study, low serum iron levels predicted COVID-19 severity and mortality.
- 28.Lino K, Guimarães GMC, Alves LS, et al. Serum ferritin at admission in hospitalized COVID-19 patients as a predictor of mortality. Braz J Infect Dis. 2021;25(2):101569. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this retrospective study, high ferritin levels predicted COVID-19 mortality.
- 29.Taneri PE, Gómez-Ochoa SA, Llanaj E, et al. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol. 2020;35(8):763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• In this meta-analysis of 189 studies (57,563 COVID-19 patients), severe COVID-19 cases had higher ferritin levels as compared to moderate cases; in addition, serum ferritin levels were significantly lower in survivors as compared to non-survivors.
- 30.Ashktorab H, Pizuorno A, Aduli F, et al. Elevated liver enzymes, ferritin, C-reactive protein, D-dimer, and age are predictive markers of outcomes among African American and Hispanic patients with coronavirus disease 2019. Gastroenterology. 2021;161(1):345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng HL, Yang Q, Yuan P, et al. Associations of essential and toxic metals/metalloids in whole blood with both disease severity and mortality in patients with COVID-19. FASEB J. 2021;35(3):e21392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bia Biamonte F, Botta C, Mazzitelli M, et al. Combined lymphocyte/monocyte count, D-dimer and iron status predict COVID-19 course and outcome in a long-term care facility. J Transl Med. 2021;19(1):79 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah A, Frost JN, Aaron L, et al. Systemic hypoferremia and severity of hypoxemic respiratory failure in COVID-19. Crit Care. 2020;24(1):320. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this retrospective study, serum iron levels were significantly lower in patients with severe hypoxemia as compared to patients with non-severe hypoxemia.
- 34.Deng F, Zhang L, Lyu L, et al. Increased levels of ferritin on admission predicts intensive care unit mortality in patients with COVID-19. Med Clin (Engl Ed). 2021;156(7):324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this retrospective study, ferritin levels were positively associated with mortality in patients with COVID-19 admitted to Intensive Care Unit.
- 35.Nai A, Lorè NI, Pagani A, et al. Hepcidin levels predict COVID-19 severity and mortality in a cohort of hospitalized Italian patients. Am J Hematol. 2021;96(1):E32–E35. [DOI] [PubMed] [Google Scholar]
- 36.Bozkurt FT, Tercan M, Patmano G, et al. Can ferritin levels predict the severity of illness in patients with COVID-19? Cureus. 2021;13(1):e12832 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonnweber T, Boehm A, Sahanic S, et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: a prospective observational cohort study. Respir Res. 2020;21(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• In this prospective multicentre study, alterations of iron homeostasis were shown to persist for at least two months after the onset of COVID-19 and to be closely associated with non-resolving lung pathologies and impaired physical performance.
- 38.Arnold DT, Attwood M, Barratt S, et al. Predicting outcomes of COVID-19 from admission biomarkers: a prospective UK cohort study. Emerg Med J. 2021;21:emermed-2020-210380. [DOI] [PubMed] [Google Scholar]
- 39.Elhadi M, Alsoufi A, Abusalama A, et al. Epidemiology, outcomes, and utilization of intensive care unit resources for critically ill COVID-19 patients in libya: a prospective multi-center cohort study. PLoS One. 2021;16(4):e0251085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al Sulaiman KA, Aljuhani O, Eljaaly K, et al. Clinical features and outcomes of critically ill patients with coronavirus disease 2019 (COVID-19): a multicenter cohort study. Int J Infect Dis. 2021;105:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schillaci G, Mannarino MR, Pucci G, et al. Age-specific relationship of aortic pulse wave velocity with left ventricular geometry and function in hypertension. Hypertension. 2007;49(2):317–321. [DOI] [PubMed] [Google Scholar]
- 42.Schillaci G, Pirro M, Ronti T, et al. Prognostic impact of prolonged ventricular repolarization in hypertension. Arch Intern Med. 2006;166(8):909–913. [DOI] [PubMed] [Google Scholar]
- 43.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 45.Bellmann-Weiler R, Lanser L, Barket R, et al. Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID-19 infection. J Clin Med. 2020. Jul 29;9(8):2429 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.COVID-19 treatment guidelines. National Institute of Health. Available from: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Accessed on November 30 2021. [Google Scholar]
- 48.Lee J, Park HK, Kwon MJ, et al. Decreased lung function is associated with elevated ferritin but not iron or transferrin saturation in 42,927 healthy Korean men: a cross-sectional study. PLoS One. 2020;15(4):e0231057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pagana KD, Pagana TJ, Pagana TN. Mosby’s diagnostic and laboratory test reference. 14th ed. St Louis (MO): Elsevier; 2019. [Google Scholar]
- 50.Banchini F, Cattaneo GM, Capelli P. Serum ferritin levels in inflammation: a retrospective comparative analysis between COVID-19 and emergency surgical non-COVID-19 patients. World J Emerg Surg. 2021;16(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gharamti AA, Mei F, Jankousky KC, et al. Diagnostic utility of a ferritin-to-procalcitonin ratio to differentiate patients with COVID-19 from those with bacterial pneumonia: a multicenter study. medRxiv. 2020;2020.10.20.20216309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tapan OO, Gursoy C, Dogan E, et al. Evaluation of iron deficiency in COVID-19 pneumonia. Authorea. 2021. [Google Scholar]
- 53.Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29(9):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rivera S, Nemeth E, Gabayan V, et al. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. 2005;106(6):2196–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Habib HM, Ibrahim S, Zaim A, et al. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed Pharmacother. 2021;136:111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ehsani S. COVID-19 and iron dysregulation: distant sequence similarity between hepcidin and the novel coronavirus spike glycoprotein. Biol Direct. 2020;15(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu W, Li H. COVID-19: attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. ChemRxiv 2020.
- 58.Shoenfeld Y. Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev. 2020;19(6):102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edeas M, Saleh J, Peyssonnaux CI. Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int J Infect Dis. 2020;97:303–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Menshawey R, Menshawey E, Alserr AHK, et al. Low iron mitigates viral survival: insights from evolution, genetics, and pandemics—a review of current hypothesis. Egypt J Med Hum Genet. 2020;21(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodrigues PCO, Ignotti E, Hacon SS. Association between weather seasonality and blood parameters in riverine populations of the Brazilian amazon. J Pediatr (Rio J). 2017;93(5):482–489. [DOI] [PubMed] [Google Scholar]
- 62.Nicolau GY, Haus E, Lakatua DJ, et al. Chronobiology of serum iron concentration in subjects of different ages at different geographic locations. Endocrinologie. 1987;25(2):63–82. [PubMed] [Google Scholar]
- 63.Nguyen LT, Buse JD, Baskin L, et al. Influence of diurnal variation and fasting on serum iron concentrations in a community-based population. Clin Biochem. 2017;50(18):1237–1242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [M.R.M.], upon reasonable request.


