Abstract
Objective
Post-COVID syndrome (PCS) is a poorly known entity. An underlying chronic, low-grade inflammation (LGI) has been theorized as a pathophysiological mechanism. Available data on biomarkers in PCS show conflicting results. Our aim was to know whether subjects with PCS present higher levels of inflammatory markers, after a mild COVID-19.
Methods
Analytical cross-sectional study. Cases of mild COVID-19 in a community setting were included. We collected epidemiological data (age, sex, BMI, smoking, comorbidities), variables of the acute COVID-19 (duration, symptoms), and data at 3 months after the acute phase (symptoms and laboratory test). Serum C-reactive protein (CRP), neutrophil and lymphocyte counts, neutrophil/lymphocyte ratio (NLR), lactate dehydrogenase, ferritin, fibrinogen, and D-dimer levels were analysed. LGI was defined as CRP >0.3 and <1.0 mg/dL. A subject was classified as PCS + if presented signs and symptoms >12 weeks after an infection consistent with COVID-19. Five composite indices (C1–C5) were developed, combining the upper ranges of biomarkers distributions. Multivariate analyses were performed.
Results
We analysed 121 mild COVID-19 cases (mean age = 45.7 years, 56.2% women). Among the acute symptoms, women presented a higher frequency of fatigue (54.4% vs 30.2%; p = .008). PCS affected 35.8% of women and 20.8% of men (p = .07), and the most reported symptoms were fatigue (42.8%), anosmia (40%), ageusia (22.8%), dyspnea (17.1%) and myalgia (11.4%). Neutrophil count, NLR, CRP and fibrinogen showed the best correlations with PCS and were selected to develop the indices. In women PCS+, C1, C3 and C4 indices were more frequently met, while in men PCS+, C2, C5 and CRP were in the range of LGI. Anosmia, ageusia and fatigue were related to higher neutrophil counts, with sex differences. Fibrinogen levels were higher in persistent myalgia (510 ± 82 mg/dL vs 394 ± 87; p = .013). In multivariable analysis, a woman with a neutrophil count above the median, or with fibrinogen level or NLR in the highest tertile, had a 4–5-fold increased risk of prevalent PCS. A man with CRP in the range of LGI, or fibrinogen level or a neutrophil count in the highest tertile, had a 10–17-fold increased risk of prevalent PCS.
Conclusions
The data obtained in the present cross-sectional study seems to demonstrate a consistent association between PCS and upper ranges of the neutrophil count, NLR, fibrinogen, and CRP in the LGI range. Furthermore, composite indices appear useful in detecting relationships between slight elevations of biomarkers and PCS, and our study identifies relevant sex differences in symptoms and markers regarding the PCS.
Keywords: Post-COVID syndrome, low-grade inflammation, inflammatory markers, mild COVID-19
Introduction
In December 2019, a novel coronavirus was identified as the cause of a cluster of pneumonia cases in Wuhan, a city in the Hubei province in central China. It rapidly spread, resulting in an epidemic throughout China, followed by a global pandemic. In February 2020, the World Health Organization denominated the disease as COVID-19, and the causative agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1. To date, over 240 million confirmed cases and 5 million deaths have been reported. Since late 2019, prophylactic and therapeutic alternatives to SARS-CoV-2 have been evolving. Vaccines based on non-replicating viral vectors and RNA are showing very high efficacies2 and trial data currently suggest (November 2021) a mortality benefit with dexamethasone as well as with adjunctive tocilizumab (IL-6 pathway inhibitor) or baricitinib (Janus kinase inhibitor), and a possible clinical benefit with the nucleoside analog remdesivir3,4.
The number of cases, hospitalizations and deaths are not the only negative consequences of SARS-CoV-2 infection. Many patients who had the infection go on to develop lasting symptoms that fluctuate over time and can have disabling consequences5. Fatigue, myalgia, dyspnea, anosmia, ageusia, autonomic dysregulation (manifested as orthostatic hypotension, tachycardia, thermoregulatory or gastrointestinal disturbances), anxiety, depression and cognitive problems are frequently reported5,6. These prolonged symptoms significantly affect patients' quality of life7,8, cause economic and productivity losses and increase the burden of care9. The condition has been called Long Covid or Post-COVID syndrome (PCS)7, and there is currently no consensus on its definition or diagnostic criteria. However, it should be noted that as of 1 October 2021 there is a new ICD-10-CM code for this syndrome (U09.9, “Post-COVID conditions, unspecified”)10.
The PCS has led healthcare organizations to develop new care units with multidisciplinary teams, in which rehabilitation services play a key role11,12. A systematic review analysing 12 international models of PCS care has shown that most of them include primary care and/or specialized clinics, and they all include rehabilitation services tailored to the patient’s needs13. With regard to assessment, the COVID-19 Yorkshire Rehabilitation Scale (C19-YRS), the Newcastle post-COVID screening tool and the Post-COVID-19 Functional Status scale (PCFS), have been proposed to track functional outcomes in patients affected by this complex multi-organ disorder13–15.
It is unclear if the PCS is a final prolongation of the COVID-19 infection or a different entity. Risk factors for having severe acute COVID-19, such as age, male gender, or obesity, have not been fully related to the development of PCS16. On the other hand, there is compelling evidence that patients who suffered from mild or moderate forms can present symptoms unassociated with organ dysfunctions from the acute COVID-19 [17]. Specific underlying mechanisms in the PCS still need to be unravelled. Virus-driven tissue damage, microbiome alterations or a dysregulated immune/inflammatory reaction in response to the infection, might be involved16–21. It is well-known that the disruption of immune and inflammatory responses caused by an acute viral infection (such as Chikungunya, Ebstein-Barr, SARS-CoV or MERS-CoV viruses) can lead to long-term disorders22.
Another relevant issue regarding the PCS is the differences in gender: while men, perhaps due to a previous pro-inflammatory state, are at greater risk in acute COVID-19, women have a higher specific T-cell response to the SARS-CoV-223 and are more frequently affected by PCS18.
It has been suggested that after an acute COVID-19 and in predisposed women, a low-grade and continuous inflammatory reaction would be activated20,24. Low-grade inflammation (LGI) is a chronic, ineffective inflammatory state that leads to oxidative stress and causes tissue damage25,26. The demonstration of higher levels of inflammatory markers in subjects with PCS could provide evidence of an underlying LGI and would allow further investigation into the relationship between immunity and post-COVID symptoms23. However, available data on biomarkers in PCS are very scarce18, poorly systematised and with conflicting results. Elevation of C-reactive protein (CRP) serum levels has been reported8, while other studies found no association27.
Furthermore, most of the PCS reports have been carried out in post-hospitalization settings, on patients with high comorbidity and after a moderate or severe disease7,17. In this regard, it has been recommended28 to assess the PCS in outpatient settings after a mild COVID-19, to reasonably dismiss persistent symptoms due to comorbidities or sequelae due to organ damage in the acute phase.
Taking into account the above considerations, we aimed to know whether subjects with PCS after a mild COVID-19, present higher levels of inflammatory markers than subjects without PCS. Also, given the sex differences in the immune response to COVID-19, a secondary objective was to determine whether there are gender differences in the variables related to the development of PCS.
Methods
Design
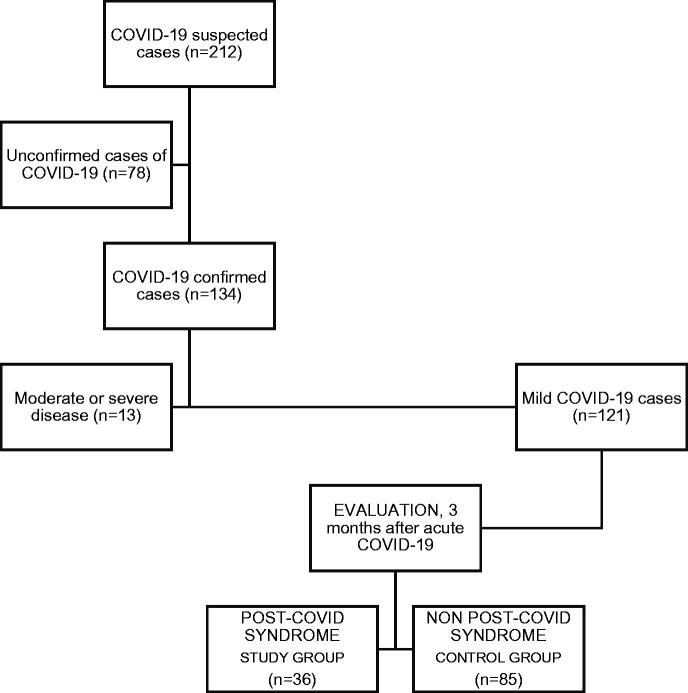
Analytical cross-sectional study with a control group. Figure 1 summarises the study design.
Figure 1.
Flow chart of the study design. 1/Suspected cases of COVID-19 from the medical records of family physicians between April and September 2020. 2/Seventy-eight cases could not be confirmed as COVID-19 (negative or unavailable RT-PCR and/or negative or unavailable IgG anti-SARS-CoV-2) and were discarded. 3/Confirmed cases of COVID-19. 4/Thirteen cases with moderate or severe disease were ruled out (all of them required hospital care, and 1 of them was admitted to the ICU). 5/One hundred and twenty-one cases of mild COVID-19 were included in the study. 6/Clinical interview and laboratory test (inflammatory biomarkers), 3 months after acute COVID-19. 7–8/Classification of participants according to the post-COVID syndrome. The control group consisted of patients affected by COVID-19 who did not develop PCS, as recommended (ref.17)
Participants
The study was carried out on the general population of a semi-urban Basic Health Zone covered by a Primary Health Care center (Camargo-Interior) in Santander, Northern Spain. The sample was obtained from the medical records of family physicians based on COVID-19 cases between April and September 2020. All cases were followed up exclusively at the Primary Care level and none of them had been vaccinated against SARS-CoV-2. We included confirmed COVID-19 cases by a positive result in the real-time reverse transcription-polymerase chain reaction test (RT-PCR) or by the presence of anti-SARS-CoV-2 IgG, three months after the acute COVID-19. A second inclusion criterion was a mild course of infection, according to the WHO definition29, and characterized by fever, malaise, cough, upper respiratory symptoms, and/or less common features of COVID-19, in the absence of dyspnea. No exclusion criteria were considered.
Study variables
Three groups of variables were collected: epidemiological data, variables related to the acute COVID-19, and data obtained 3 months after the acute episode. In the first group, sex, age, body mass index -BMI, measured in kg/m2-, smoking habit, past medical history and Charlson comorbidity index, were evaluated. Moreover, disorders related to a worse outcome of acute COVID-1930 such as cerebrovascular disease, ischemic heart disease, hypertension, diabetes mellitus, asthma, chronic kidney disease or immunosuppression, were registered. Variables related to acute COVID-19 were duration (in days), the number of symptoms and specific symptoms31. Finally, 3 months after the onset of the acute COVID-19 (median = 115 days), clinical symptoms and biomarkers levels were collected. The interviews were carried out by 3 of the co-authors, using a structured questionnaire (Supporting information) and after specific training. The diagnosis of PCS was established if the National Institute for Care and Excellence (NICE) criteria were met: signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks and are not explained by an alternative diagnosis32. Patients who were diagnosed with PCS formed the study group, whereas the control group consisted of patients who had experienced COVID-19 but did not developed PCS. There were 3 sources of data: The patient’s medical history (baseline variables, comorbidities, Charlson index), the clinical interview (symptoms) and the laboratory tests (inflammation markers).
Inflammation markers
CRP serum levels, ferritin, lactate dehydrogenase (LDH), fibrinogen, D-dimer levels, and neutrophil and lymphocyte counts have been evaluated. These markers are routinely used in acute COVID-19 and have demonstrated their usefulness in chronic inflammation25,33–36. CRP has been measured in mg/dL, and the detection limit was 0.4 mg/dL. LGI has been defined by a conventionally accepted CRP serum level >0.3 mg/dL and <1.0 mg/dL36. Normal ranges of LDH (U/L), ferritin (ng/mL), fibrinogen (mg/dL), D-dimer (ng/mL), neutrophil count and lymphocyte count were 120–246, 22–322, 180–500, 0–500, 1.4–7.5 (×103/µL) and 1.2–5 (×103/µL), respectively. The neutrophil/lymphocyte ratio (NL ratio), a demonstrated marker for chronic inflammation37, was also determined.
Blood samples were obtained from an antecubital vein by the standard vein puncture procedure in the morning and after 12-hour fasting. LDH and ferritin were analysed by spectrophotometric assay in one Atellica CH Analyzer (Siemens Healthcare Diagnostics Inc, Tarrytown, NY, USA). CRP was quantified by immunonephelometric assay in one Atellica CH Analyzer (Siemens Healthcare Diagnostics Inc, Tarrytown, NY, USA). Haematological cell counts were analysed in one DXH900 (Beckman Coulter), and fibrinogen and D-dimer in one ACL TOP 750 (Werfen).
Statistical analysis
The sample was analysed after a double stratification, by sex, and by PCS. After assessing for normality with the Shapiro-Wilk test, quantitative variables normally distributed were expressed as mean ± standard deviation (SD), and as median [interquartile range (IQR)] if data came from a non-normal distribution. Student's t and ANOVA tests were used as parametric tests and the median and Kruskal-Wallis tests as non-parametric tests. Categorical variables have been expressed as percentages, and Chi-Square tests have been used for their comparison. Correlation analyses have been performed using Pearson's r, and Phi coefficient for categorical variables. The strength of an association has been expressed as an odds ratio (OR) with its corresponding 95% confidence interval (CI).
Composite indices represent heightened inflammatory activity and provide strong predictors over individualized or single measures25. To this end, we divided the sample into two random halves and used one of them to build and select the indices. We first selected the biomarkers that showed the highest correlations with prevalent PCS. Combining the upper ranges of their distributions, several indices were developed. They were correlatively designated C1–C5 (Tables 1 and 3).
Table 1.
Composite indices of inflammation.
| Composite index | Definition |
|---|---|
| C1 | [Neutrophil count ≥3.10* (×10³/μL)] or [NL Ratio ≥1.86**] |
| C2 | [CRp >0.3 and <1.0 mg/dL] or [Fibrinogen ≥421** mg/dL] |
| C3 | [Neutrophil count ≥3.10* (×10³/μL)] or [Fibrinogen ≥421** mg/dL] |
| C4 | [NL Ratio ≥1.86**] or [Fibrinogen ≥421** mg/dL] |
| C5 | [CRp >0.3 and <1.0 mg/dL] or [Neutrophil count ≥3.40** (×0³/μL)] |
Composite indices development process: Division of the sample into two random halves and selection of one of them to construct the indices. Selection of the biomarkers that showed the highest correlations with prevalent PCS. Development of the indices by combining the upper ranges of their distributions. Selection of the indices that best-classified subjects according to PCS. Receiver-operating characteristic (ROC) analyses of the composite indices for validation.
Abbreviations. CRP, C-reactive protein; NL Ratio, Neutrophil/Lymphocyte Ratio.
*Values above the median; **Values in the third tertile.
Table 3.
Epidemiological variables, acute COVID-19 variables and inflammatory markers, stratifying by sex and post-COVID syndrome.
| Variables | Mild COVID-19 cases (n = 121) |
|||||
|---|---|---|---|---|---|---|
| Women (n = 68) |
Men (n = 53) |
|||||
| Post-COVID syndrome (n = 25) | Non post-COVID syndrome (n = 43) | p* | Post-COVID syndrome (n = 11) | Non post-COVID syndrome (n = 42) | p* | |
| Personal antecedents | ||||||
| Age (years) | 47.2 ± 13 | 47.7 ± 17 | 0.90 | 45.7 ± 17 | 42.3 ± 17 | 0.56 |
| BMI (kg/m²) | 24.3 ± 3 | 25.1 ± 4 | 0.43 | 25.6 ± 4 | 26 ± 3 | 0.75 |
| Tobacco; n (%) | 7 (29.2) | 15 (36.6) | 0.54 | 4 (36.4) | 17 (41.5) | 0.76 |
| Charlson comorbidity index | 0.17 ± 0.3 | 0.28 ± 0.5 | 0.29 | 0.27 ± 0.4 | 0.66 ± 1 | 0.27 |
| Acute COVID-19 | ||||||
| Number of symptoms | 7 [3] | 3 [5] | 0.0001 | 5 [4] | 4 [4] | 0.37 |
| Duration (days) | 14 [28] | 10 [12] | 0.37 | 10 [8] | 10 [10] | 0.78 |
| Cough; n (%) | 12 (50) | 20 (46.5) | 0.78 | 4 (36.4) | 20 (47.6) | 0.50 |
| Odynophagia; n (%) | 8 (33.3) | 15 (34.9) | 0.89 | 4 (36.4) | 12 (28.6) | 0.61 |
| Low-grade fever/fever; n (%) | 18 (75) | 23 (53.5) | 0.08 | 8 (72.7) | 24 (57.1) | 0.34 |
| Diarrhea; n (%) | 2 (8.3) | 9 (20.9) | 0.18 | 1 (9.1) | 6 (14.3) | 0.65 |
| Anosmia/ageusia; n (%) | 21 (87.5) | 18 (41.9) | 0.0001 | 7 (63.6) | 18 (42.9) | 0.21 |
| Fatigue; n (%) | 17 (70.8) | 20 (46.5) | 0.05 | 4 (36.4) | 12 (28.6) | 0.61 |
| Myalgia; n (%) | 16 (66.7) | 15 (34.9) | 0.012 | 7 (63.6) | 18 (42.9) | 0.21 |
| Headache; n (%) | 16 (66.7) | 12 (27.9) | 0.002 | 5 (45.5) | 18 (42.9) | 0.87 |
| Dyspnea; n (%) | 11 (45.8) | 3 (7) | 0.0001 | 1 (9.1) | 6 (14.3) | 0.65 |
| Chest pain; n (%) | 4 (16.7) | 1 (2.3) | 0.05 | – | 3 (7.1) | 0.36 |
| Rhinitis; n (%) | 9 (37.5) | 5 (11.6) | 0.013 | 2 (18.2) | 10 (23.8) | 0.69 |
| Inflammatory markers | ||||||
| CRp > 0.3 and < 1.0 mg/dL; n (%) | 4 (18.2) | 4 (11.4) | 0.47 | 3 (37.5) | 2 (5.7) | 0.037 |
| Neutrophil count (x10³/μL) | 2.85 ± 1 | 2.67 ± 0.9 | 0.50 | 4.07 ± 1.2 | 3.26 ± 1.1 | 0.057 |
| Lymphocyte count (x10³/μL) | 1.88 ± 0.8 | 1.65 ± 0.7 | 0.26 | 2.18 ± 0.5 | 1.92 ± 0.8 | 0.37 |
| Neutrophil/Lymphocyte Ratio | 1.65 [0.9] | 1.45 [0.6] | 0.16 | 1.28 [2] | 1.70 [1] | 0.58 |
| Ferritin (ng/mL) | 40 [86] | 47 [82] | 0.87 | 141 [134] | 117 [278] | 0.99 |
| Lactate dehydrogenase (UI/L) | 177 [42] | 179 [36] | 0.66 | 172 [26] | 178 [58] | 0.84 |
| Fibrinogen (mg/dL) | 417 [111] | 392 [83] | 0.59 | 345 [211] | 369 [93] | 0.69 |
| D-dimer (ng/mL) | 323 [314] | 310 [268] | 0.70 | 149 [602] | 218 [157] | 0.69 |
| C1 index (+); n (%) | 17 (70.8) | 15 (38.5) | 0.013 | 9 (81.8) | 26 (68.4) | 0.38 |
| C2 index (+); n (%) | 11 (50) | 12 (32.4) | 0.18 | 5 (62.5) | 7 (21.2) | 0.021 |
| C3 index (+); n (%) | 17 (73.9) | 16 (43.2) | 0.020 | 9 (90) | 24 (63.2) | 0.10 |
| C4 index (+); n (%) | 17 (73.9) | 18 (47.4) | 0.042 | 5 (55.6) | 16 (44.4) | 0.55 |
| C5 index (+); n (%) | 8 (36.4) | 11 (31.4) | 0.70 | 8 (80) | 14 (37.8) | 0.030 |
Quantitative variables expressed as mean ± standard deviation or median [interquartile range].
p*: Chi-Square for categorical variables. Student´s t test (contrast between means) or median test (contrast between medians) for continuous variables.
Abbreviation. BMI, Body Mass Index.
Logistic regressions and general linear models, stratified by sex, were performed to ascertain the relationships between biomarkers and PCS, after controlling for confounding variables. Logistic regression models were validated by calculating the areas under the receiver-operating characteristic (ROC) curves (AUC), and a value >0.70 has been considered acceptable38. The validation of the general linear models was carried out with the analysis of the standardised residuals. A two-sided p-value <.05 was considered significant in all the calculations.
Ethical aspects
Postulates of the Declaration of Helsinki have been carried out. All patients who met the inclusion criteria were informed of the purpose of the study with the delivery of an information sheet and were invited to participate. All of them expressed their verbal consent and there was no refusal to participate. The study was approved by the Cantabria Clinical Research Ethics Committee (Internal Code 2021.102)
Results
Descriptive analysis
One hundred and thirty-four patients with confirmed COVID-19 were recruited, and 13 were discarded for presenting a moderate or severe disease (Figure 1). The final sample consisted of 121 subjects, all of them with mild COVID-19, of which 68 (56.2%) were women. The mean age of the sample was 45.7 ± 16 years (range, 18–88 years). Table 2 shows the baseline characteristics of the population and acute COVID-19 variables. Among the acute COVID-19 symptoms, women had a higher frequency of fatigue compared to men (54.4% vs 30.2%; p = .008).
Table 2.
Clinical characteristics of the participants and variables related to acute COVID-19, according to sex.
| Variables | Mild COVID-19 cases |
p* | ||
|---|---|---|---|---|
| Total sample (n = 121) | Women (n = 68) | Men (n = 53) | ||
| Personal antecedents | ||||
| Age (years) | 45.4 ± 16 | 47.7 ± 15 | 43 ± 17 | 0.12 |
| BMI (kg/m²) | 24.9 ± 3.8 | 24.8 ± 4 | 25.9 ± 3.9 | 0.14 |
| Tobacco; n (%) | 43 (36.4) | 22 (33.3) | 21 (40.4) | 0.42 |
| Charlson comorbidity index | 0.4 ± 0.7 | 0.23 ± 0.4 | 0.58 ± 1 | 0.02 |
| Asthma; n (%) | 13 (10.7) | 6 (8.7) | 7 (11.3) | 0.62 |
| Immunosuppression; n (%) | 3 (2.5) | 2 (3) | 1 (1.9) | 0.70 |
| Chronic kidney disease; n (%) | 1 (0.8) | – | 1 (1.9) | 0.44 |
| Cerebrovascular disease; n (%) | 1 (0.8) | – | 1 (1.6) | 0.29 |
| Hypertension; n (%) | 21 (17.8) | 11 (16.7) | 10 (19.2) | 0.71 |
| Diabetes mellitus; n (%) | 6 (5.1) | 1 (1.5) | 5 (9.6) | 0.05 |
| Dyslipidemia; n (%) | 38 (32.2) | 23 (34.8) | 15 (28.8) | 0.48 |
| Ischemic heart disease; n (%) | 6 (5.1) | 3 (4.5) | 3 (5.8) | 0.54 |
| Acute COVID-19 | ||||
| Number of symptoms | 5 [4] | 5 [4] | 4 [4] | 0.50 |
| Duration (days) | 10 [9] | 11 [15] | 10 [9] | 0.40 |
| Cough; n (%) | 56 (46.3) | 32 (47.1) | 24 (45.3) | 0.84 |
| Odynophagia; n (%) | 39 (32.2) | 23 (33.8) | 16 (30.2) | 0.67 |
| Low-grade fever/fever; n (%) | 73 (60.3) | 41 (60.3) | 32 (60.4) | 0.99 |
| Diarrhea; n (%) | 18 (14.9) | 11 (16.2) | 7 (13.2) | 0.65 |
| Anosmia/ageusia; n (%) | 64 (52.9) | 39 (57.4) | 25 (47.2) | 0.26 |
| Fatigue; n (%) | 53 (43.8) | 37 (54.4) | 16 (30.2) | 0.008 |
| Myalgia; n (%) | 56 (46.3) | 31 (45.6) | 25 (47.2) | 0.86 |
| Headache; n (%) | 51 (42.1) | 28 (41.2) | 23 (43.4) | 0.80 |
| Dyspnea; n (%) | 21 (17.4) | 14 (20.6) | 7 (13.2) | 0.28 |
| Chest pain; n (%) | 8 (6.6) | 5 (7.4) | 3 (5.7) | 0.71 |
| Rhinitis; n (%) | 26 (21.5) | 14 (20.6) | 12 (22.6) | 0.78 |
Quantitative variables are expressed as mean ± standard deviation or median [interquartile range].
p*: Chi-Square for categorical variables. Student´s t test (contrast between means) or median test (contrast between medians) for continuous variables.
Abbreviation. BMI, Body mass index.
Regarding PCS, 36 subjects (29.7%, 25 women and 11 men) were classified as PCS+, and 85 as PCS- (Table 3). Therefore, 35.8% of women and 20.8% of men developed PCS (p = .07). The most reported symptoms were fatigue (42.8%), anosmia (40%), ageusia (22.8%), dyspnea (17.1%), myalgia (11.4%), and palpitations (11.4%).
Bivariable analysis
PCS + women experienced an acute episode of COVID-19 with a greater number of symptoms than PCS- women (7[3] vs 3[5]; p = .0001), and they also had a significantly higher frequency of anosmia/ageusia, myalgia, headache, dyspnea and rhinitis. In the male group, no differences were observed between PCS + and PCS- concerning the initial symptoms.
Neutrophil count, NLR, CRP and fibrinogen showed the best correlations with PCS and were used to develop the indices. C1, C3 and C4 indices were more frequently met in women PCS+, while C2, C5 and CRP in range of chronic inflammation were more frequently met in men PCS+ (Table 3). Correlation analyses between composite indices and PCS showed values of 0.314, 0.360, 0.300, 0.260 and 0.346 (all of them p < .05), for C1, C2, C3, C4 and C5, respectively. In men, CRP in the range of LGI was correlated with PCS prevalent with a coefficient value of 0.386 (p = .011).
Multivariable analysis
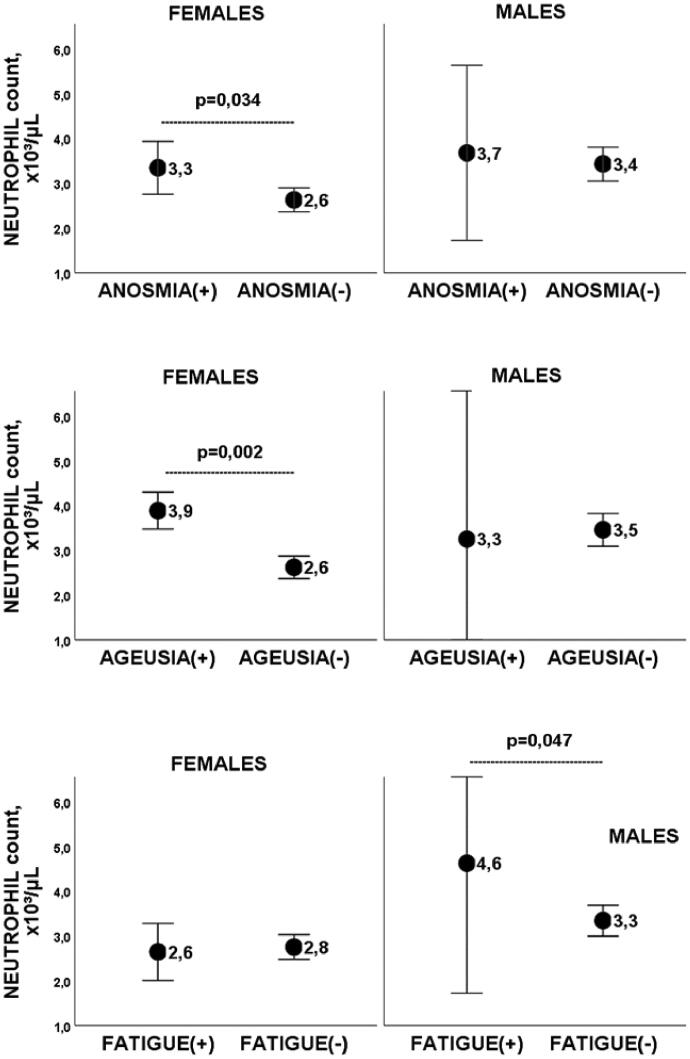
Anosmia, ageusia and fatigue have shown to be associated with higher neutrophil counts, after adjusting for age, BMI, Charlson index, and tobacco consumption (Figure 2 and Table 4). Fibrinogen levels were higher in subjects with persistent myalgia (510 ± 82 mg/dL vs 394.6 ± 87; p = .013), a difference that remained significant after controlling for age, sex, BMI, hypertension, diabetes and dyslipidemia.
Figure 2.
Neutrophil count in anosmia, ageusia, and fatigue, according to gender. Graphs showing mean and error bars (95% confidence interval) of neutrophil count in (PCS+) subjects with anosmia, ageusia or fatigue, compared to same-sex (PCS−) subjects without the symptom. Differences remained significant after adjusting for age, BMI, Charlson index and tobacco consumption.
Table 4.
Multivariable analysis, effect size, and validation of models.
| Composite index/inflammatory marker | Model | Effect size* |
Validation |
|||
|---|---|---|---|---|---|---|
| OR (CI 95%) | p | AUC (CI 95%) | p | Standardized residuals | ||
| Mean ± SD | ||||||
| Women | ||||||
| C1 index | Logistic regression (DV: PCS) | Adjusted OR = 5.14 (1.6–16.4) | 0.006 | 0.76 (0.63–0.89) | 0.001 | – |
| C3 index | Logistic regression (DV: PCS) | Adjusted OR = 4.20 (1.3–13.3) | 0.015 | 0.72 (0.57–0.86) | 0.005 | – |
| C4 index | Logistic regression (DV: PCS) | Adjusted OR = 4.12 (1.3–13.1) | 0.016 | 0.71 (0.57–0.85) | 0.006 | – |
| Neutrophil count (×103/µL) | General linear model (Fixed factor: Anosmia) | (Anosmia+) 3.43 ± 0.3 (Anosmia−) 2.58 ± 0.1 | 0.014 | – | – | 0 ± 0.95 |
| Neutrophil count (×103/µL) | General linear model (Fixed factor: Ageusia) | (Ageusia+) 3.89 ± 0.3 (Ageusia−) 2.59 ± 0.1 | 0.002 | – | – | 0 ± 0.95 |
| Men | ||||||
| CRp > 0.3 and < 1.0 mg/dL | Logistic regression (DV: PCS) | Adjusted OR = 12.9 (1.3–121) | 0.025 | 0.76 (0.58–0.94) | 0.025 | – |
| C2 index | Logistic regression (DV: PCS) | Adjusted OR = 10.1 (1.2–85) | 0.033 | 0.75 (0.55–0.95) | 0.029 | – |
| C5 index | Logistic regression (DV: PCS) | Adjusted OR = 17.5 (2–153) | 0.010 | 0.82 (0.65–0.98) | 0.003 | – |
| Neutrophil count (×103/µL) | General linear model (Fixed factor: Fatigue) | (Fatigue+) 4.68 ± 0.6 (Fatigue−) 3.37 ± 0.1 | 0.041 | – | 0 ± 0.93 | |
* After adjusting for age, BMI, Charlson index and tobacco consumption.
Abbreviations. DV, Dependent variable; PCS, Post-COVID syndrome; CRP, C-reactive protein; BMI, Body mass index; OR, odds ratio; CI, confidence interval; AUC, Area under the (ROC) curve; SD, Standard deviation.
Validation of the models: AUC values ranged between 0.71 and 0.82, and the standardised residuals showed a normal distribution, with a curve of mean = 0 and SD = 1. R2 values were 0.27, 0.54, 0.23, 0.23, 0.58 and 0.54, for composite indices C1, C2, C3, C4 and C5, respectively.
After adjusting for confounders, a woman with a neutrophil count above the median, or with fibrinogen levels or NL ratio in the highest tertile, had a 4–5-fold increased risk of prevalent PCS. A man with serum CRP >0.3 and <1.0 mg/dL, or fibrinogen levels or a neutrophil count in the highest tertile, had a 10–17-fold increased risk of prevalent PCS (Table 4).
Discussion
The present study, aimed at assessing the levels of inflammation markers in PCS, has shown results of interest. Data supporting a possible relationship between a chronic inflammatory state and PCS, and the difference by sex observed regarding symptoms and biomarkers, are mentioned afterwards.
Low-grade inflammation and post-COVID-19 syndrome
While acute inflammation is intense, short-lived, and results in tissue repair, LGI is persistent, ineffective, and leads to collateral damage25. LGI is typically triggered by damage-associated molecular patterns (DAMPs) 25, which are released by damaged cells or tissues as endogenous danger signals, alerting the immune system to non-programmed cell death, invasion by pathogens, and in response to stress39. Frequent symptoms of LGI are chronic fatigue, arthralgia, myalgia, anxiety, depression, constipation or diarrhea, and it is associated with slight elevations of acute-phase reactants and cytokines26.
In turn, COVID-19 infection can cause a release of DAMPs40,41 and therefore could trigger an LGI, symptoms of PCS correlate with those of LGI, and our study has shown slightly but significantly higher values of serum CRP, fibrinogen and neutrophil count in patients with PCS.
Low et al. point out the coincident symptoms between PCS and cytokine release syndrome, with fever, fatigue, headache, skin rash, arthralgia and myalgia, and propose a model in which SARS-CoV-2 infection is hypothesized to trigger a dysregulated peripheral immune system activation with subsequent cytokine release. Chronic low-grade inflammation could lead to dysregulated brain microglia with an exaggerated release of central cytokines, producing neuroinflammation, brain fog, intermittent fatigue, post-exertional malaise, arthralgias and paresthesias, among others42.
Doykov et al.43 analysed 96 proteins associated with the immune response in subjects with a positive test for SARS-CoV-2 and compared their mass spectrometry profiles with those of similar negative controls. They observed that those who had suffered from COVID-19 had a significant elevation of biomarkers involved in inflammation 40 days after infection, such as the mitochondrial protein PRDX3 or the cytosol protein NDRG1. Holmes et al.44 analysed plasma samples from patients with past COVID-19 infection, hospitalized patients with severe COVID-19 respiratory symptoms, and controls. Three months after the acute episode, elevated levels of plasma taurine and the persistence of a reduced glutamine/glutamate ratio were observed, possibly related to a metabolic and inflammatory disturbance.
Post-COVID syndrome and biomarkers
The most frequent symptom of PCS has been fatigue (45.8% of women and 36.4% of men). This high frequency is consistent with previous reports45,46. We observed a higher neutrophil count in men with post-COVID fatigue, a difference that remained significant after adjusting for confounders. A pathophysiological link between neutrophils and fatigue could be the production of reactive oxygen species (ROS) by activated neutrophils, causing oxidative tissue damage. Increased ROS release by neutrophils has been described in situations associated with chronic inflammation, such as age, hyperlipidemia, or hyperglycemia47. In contrast, Townsend et al.27 evaluated 128 people of both sexes, after an acute COVID-19, and found no relationship between the white blood cell count, neutrophils, lymphocytes, NL ratio, LDH or CRP, and post-COVID fatigue. The authors analysed sex as an adjustment variable in the regression model, but a gender analysis was not assessed in the paper.
The second most frequent symptom of PCS was anosmia (41.7% of women and 36.4% of men). As previously published, COVID-19 anosmia is more common in women48, in young patients7, and mild forms of the disease49. We found that women with anosmia had a significantly higher neutrophil count. One possible explanation is that young women, probably due to exposure to sex hormones50, have an active neutrophil profile characterised by strong type I IFN activity and an increased pro-inflammatory response, which may cause persistent inflammation51. The results seem to point to a higher local immunity in young patients, presenting with upper respiratory tract symptoms, compared to patients with severe forms, probably older, with lower local immunity and predominantly lower tract symptoms52.
Regarding markers, mild but significant elevations of neutrophil count, CRP and fibrinogen levels have been registered in subjects affected by PCS. Neutrophils are the main effectors of the immune system and play a complex role in chronic inflammation. It is known that they are a source of DAMPs, and in the tissue repair process, they can simultaneously release highly immunogenic products that could trigger and/or amplify an inflammatory response53. Furthermore, chronic inflammation may in turn stimulate extramedullary neutrophil production and increase their peripheral blood count47.
In addition to neutrophil count, CRP serum level in the range of chronic inflammation has shown a significant relationship with PCS. Interestingly, this association has been noted only in men. CRP is an acute-phase protein produced by hepatocytes, and cytokines such as IL-6 play a key role in stimulating its synthesis35. The biological response of pro-inflammatory cytokines during COVID-19 is higher in men than in women41 and this fact could be reflected in our results. Evans et al.8 evaluated 1170 patients, discharged from the hospital following treatment for COVID-19 and identified 4 clusters of patients suffering from PCS, according to mental and physical impairment. They analysed plasma CRP according to the clusters and found that patients included in clusters 1 and 2, with very severe and severe impairment, showed frequencies of CRP >1.0 mg/dL of 16.5% and 18%, respectively. The authors stated that the result was possibly due to post-COVID-19 systemic inflammation. Unfortunately, data on serum CRP levels in women and men separately were not available in the published work.
Fibrinogen levels were significantly higher in subjects with post-COVID myalgia, and the difference remained significant after adjusting for confounders. Likewise, values in the highest tertile were included in 3 of the 5 final composite indices, all of them presenting good discrimination between subjects with and without PCS. Fibrinogen, as well as CRP, serum amyloid A (SAA) and haptoglobin, are closely linked to IL-6 activation, hence higher levels of fibrinogen may be a reflection of the events driven by the production of IL-6 during the COVID-19 infection. Moreover, fibrinogen seems to exert pleiotropic effects in tissue injury34, pain54, and chronic LGI34, since it may act as a DAMP55. This relationship between fibrinogen levels and post-COVID myalgia is in line with a study by Wåhlén et al.54, focused on fibromyalgia and based on plasma proteome profile. The authors identified the presence of α and β-fibrinogen chains in a cluster of proteins involved in pain intensity, a lower pain threshold and psychological distress.
Symptoms and sex differences
We have observed several differences between women and men. In addition to the mentioned the higher frequency of fatigue among women with acute COVID-19, PCS has affected women more frequently, with a trend toward significance and in line with published papers8,18,20,45. The number of PCS symptoms was another difference: men reported 1 or 2 symptoms, while up to 20% of women with PCS reported 3 or more symptoms.
Stratified analysis by sex showed other clinical differences. One example is the previously reported association between an acute polysymptomatic phase and PCS45. In our study, such an association was observed only in women. Specifically, a woman with ≥6 symptoms in the acute phase had an increased risk of prevalent PCS, with an adjusted OR (aOR) of 7.1 (95% CI: 1.8–28), after controlling for age, BMI, Charlson index, and smoking. In men, this association could not be demonstrated.
Additionally, in women, certain acute COVID-19 symptoms that were significantly more frequent in PCS + than in PCS- acted as independent predictors of PCS: Anosmia/ageusia, aOR = 12.8 (2.9–56); myalgia, aOR = 5.1 (1.7–15); headache, aOR = 6.9 (2.1–23); dyspnea, aOR = 14.8 (2.8–78) and rhinitis, aOR = 14.2 (2.3–85). In contrast, in men, no initial symptoms showed a relationship with PCS. These results point to a relationship between acute COVID-19 symptoms and PCS in women and could probably be explained by immunological bases56.
Limitations
The study has several limitations that should be considered. Firstly, its cross-sectional design, allows us to establish associations but not to infer causality. It was carried out in patients from a semi-urban and Caucasian population in northern Spain, so the results may not be extrapolated to other populations or geographical areas. We have performed a double stratification (by sex and by PCS), that has provided additional data but may result in a loss of statistical power and a type 2 error. Composite indices have proven to be a useful methodology, improving performance in the detection of associations between PCS and inflammation markers. However, large samples studies are necessary to define the cut-off points for biomarkers levels at which the diagnosis of PCS could be established.
Conclusion
After mild COVID-19, consistent associations have been observed between PCS and the upper ranges of neutrophil count and fibrinogen, and between PCS and CRP in the range of chronic inflammation in men. Such relationships may provide evidence of an underlying LGI in patients affected by PCS.
Composite indices have shown to be useful in detecting the association between PCS and mild elevations of some biomarkers, but their clinical relevance should be confirmed in future research. The study has demonstrated substantial gender differences in symptoms and inflammatory biomarkers, probably related to the different immune responses of women and men to COVID-19. A gender-differentiated analysis is mandatory in the approach to this new and heterogeneous entity.
Supplementary Material
Transparency
Declaration of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Conception and design of the work: MM, AA, EP; Acquisition of data: YR, SG, MT; Analysis of data: SDS, MT; Interpretation of data: EP, PF, SDS; Drafting the manuscript: CR, EP; Revising the manuscript critically: JMO, JLH; Approval of the final version: MM, AA, EP, PF, YR, SG, MT, SDS, CR, JMO, JLH
Data availability statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics statement
The study was approved by the Cantabria Clinical Research Ethics Committee (Internal Code 2021.102). All patients who met the inclusion criteria were informed of the purpose of the study with the delivery of an information sheet and were invited to participate. All of them expressed their verbal consent and there was no refusal to participate.
References
- 1.World Health Organization (WHO). Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. [cited 2020 February 12]. Available from: http://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-.
- 2.Costanzo M, De Giglio MAR, Roviello GN.. Anti-coronavirus vaccines: past investigations on SARS-CoV-1 and MERS-CoV, the approved vaccines from BioNTech/Pfizer, Moderna, Oxford/AstraZeneca and others under development against SARS-CoV-2 infection. Curr Med Chem. 2022;29(1):4–18. [DOI] [PubMed] [Google Scholar]
- 3.Kim AY, Gandhi RT.. Coronavirus disease 2019 (COVID-19): management in hospitalized adults. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc; 2022. [Google Scholar]
- 4.Borbone N, Piccialli G, Roviello GN, et al. . Nucleoside analogs and nucleoside precursors as drugs in the fight against SARS-CoV-2 and other coronaviruses. Molecules. 2021;26(4):986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meeting the challenge of long COVID (editorial). Nat Med. 2020;26(12):1803. [DOI] [PubMed] [Google Scholar]
- 6.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27(1):28–33. [DOI] [PubMed] [Google Scholar]
- 7.Akbarialiabad H, Taghrir MH, Abdollahi A, et al. . Long COVID, a comprehensive systematic scoping review. Infection. 2021;49(6):1163–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans RA, McAuley H, Harrison EM, et al. . Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9(11):1275–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Understanding long COVID: a modern medical challenge (editorial). Lancet. 2021;398(10302):725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikkelsen ME, Abramoff B.. COVID-19: evaluation and management of adults following acute viral illness. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. (Accessed on October 30, 2021. [Google Scholar]
- 11.de Sire A, Andrenelli E, Negrini F, et al. . International multiprofessional steering committee of Cochrane rehabilitation REH-COVER action. Rehabilitation and COVID-19: update of the rapid living systematic review by Cochrane rehabilitation field as of August 31st, 2021. Eur J Phys Rehabil Med. 2021;57(6):1045–1048. [DOI] [PubMed] [Google Scholar]
- 12.Ferraro F, Calafiore D, Dambruoso F, et al. . COVID-19 related fatigue: which role for rehabilitation in post-COVID-19 patients? A case series. J Med Virol. 2021;93(4):1896–1899. [DOI] [PubMed] [Google Scholar]
- 13.Decary S, Dugas M, Stefan T, et al. Care Models for Long COVID - A Rapid Systematic Review. SPOR Evidence Alliance, COVID-END Network. 2021. [Google Scholar]
- 14.O'Connor RJ, Preston N, Parkin A, et al. . The COVID-19 Yorkshire Rehabilitation Scale (C19-YRS): application and psychometric analysis in a post-COVID-19 syndrome cohort. J Med Virol. 2022;94(3):1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klok FA, Boon GJ, Barco S, et al. . The Post-COVID-19 functional status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020;56(1):2001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altmann DM, Boyton RJ.. Confronting the pathophysiology of long COVID. London: BMJ Opinion; 2020. [Google Scholar]
- 17.Castanares-Zapatero D, Chalon P, Van den Heede K.. Pathophysiology of long COVID: a preliminary report. COVID-19 KCE contributions. Belgian Health Care Knowledge Centre. 2021. [Google Scholar]
- 18.Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis. 2021;53(10):737–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salmon-Ceron D, Slama D, De Broucker T, et al. . Clinical, virological and imaging profile in patients with prolonged forms of COVID-19: a cross-sectional study. J Infect. 2021;82(2):e1–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torjesen I. Covid-19: middle aged women face greater risk of debilitating long term symptoms. BMJ. 2021;372:n829. [DOI] [PubMed] [Google Scholar]
- 21.Yeoh YK, Zuo T, Lui GC, et al. . Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70(4):698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amenta EM, Spallone A, Rodriguez-Barradas MC, et al. . Postacute COVID-19: an overview and approach to classification. Open Forum Infect Dis. 2020;7(12):ofaa509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.British Society for Immunology. Report: Long-term immunological health consequences of COVID-19; 2020. [Google Scholar]
- 24.Saini G, Aneja R.. Cancer as a prospective sequela of long COVID-19. Bioessays. 2021;43(6):e2000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furman D, Campisi J, Verdin E, et al. . Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pahwa R, Goyal A, Bansal P, et al. . Chronic inflammation. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. [Google Scholar]
- 27.Townsend L, Dyer AH, Jones K, et al. . Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLOS One. 2020;15(11):e0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halpin S, O'Connor R, Sivan M. Long COVID and chronic COVID syndromes. J Med Virol. 2021;93(3):1242–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization (WHO) Publications: Clinical management of COVID-19: living guidance (2nd version); 2021. [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) Science Brief: Coronavirus Disease 2019 (COVID-19): Evidence used to update the list of underlying medical conditions that increase a person's risk of severe illness from COVID-19. 2021. [PubMed] [Google Scholar]
- 31.Jin X, Lian JS, Hu JH, et al. . Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institute for Care and Excellence (NICE) guideline [NG188]: managing the long–term effects of COVID-19; 2020. [Google Scholar]
- 33.Brenner DR, Scherer D, Muir K, et al. . A review of the application of inflammatory biomarkers in epidemiologic cancer research. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1729–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luyendyk JP, Schoenecker JG, Flick MJ.. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133(6):511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wannamethee SG, Whincup PH, Lennon L, et al. . Associations between fibrin D-dimer, markers of inflammation, incident self-reported mobility limitation, and all-cause mortality in older men. J Am Geriatr Soc. 2014;62(12):2357–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinh KM, Kaspersen KA, Mikkelsen S, et al. . Low-grade inflammation is negatively associated with physical Health-Related quality of life in healthy individuals: results from the Danish Blood Donor Study (DBDS). PLOS One. 2019;14(3):e0214468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imtiaz F, Shafique K, Mirza SS, et al. . Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med. 2012;5(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–1316. [DOI] [PubMed] [Google Scholar]
- 39.Tang D, Kang R, Coyne CB, et al. . PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249(1):158–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shenoy S. Coronavirus (covid-19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflamm Res. 2020;69(11):1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cicco S, Cicco G, Racanelli V, et al. . Neutrophil Extracellular Traps (NETs) and Damage-Associated Molecular Patterns (DAMPs): two potential targets for COVID-19 treatment. Mediators Inflamm. 2020;2020:7527953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Low RN, Low RJ, Akrami A. A Cytokine-based Model for the Pathophysiology of Long COVID Symptoms. OSF preprints. 2020. [Google Scholar]
- 43.Doykov I, Hällqvist J, Gilmour KC, et al. . The long tail of covid-19' – the detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Res. 2020;9:1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmes E, Wist J, Masuda R, et al. . Incomplete systemic recovery and metabolic phenoreversion in post-acute-phase nonhospitalized COVID-19 patients: implications for assessment of post-acute COVID-19 syndrome. J Proteome Res. 2021;20(6):3315–3329. [DOI] [PubMed] [Google Scholar]
- 45.Sudre CH, Murray B, Varsavsky T, et al. . Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. . More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soehnlein O, Steffens S, Hidalgo A, et al. . Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol. 2017;17(4):248–261. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen NN, Hoang VT, Lagier JC, et al. . Long-term persistence of olfactory and gustatory disorders in COVID-19 patients. Clin Microbiol Infect. 2021;27(6):931–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han AY, Mukdad L, Long JL, et al. . Anosmia in COVID-19: mechanisms and significance. Chem Senses. 2020;17:bjaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein SL, Marriott I, Fish EN.. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015;109(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta S, Nakabo S, Blanco LP, et al. . Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc Natl Acad Sci USA. 2020;117(28):16481–16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saussez S, Lechien JR, Hopkins C.. Anosmia: an evolution of our understanding of its importance in COVID-19 and what questions remain to be answered. Eur Arch Otorhinolaryngol. 2021;278(7):2187–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caielli S, Banchereau J, Pascual V.. Neutrophils come of age in chronic inflammation. Curr Opin Immunol. 2012;24(6):671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wåhlén K, Ernberg M, Kosek E, et al. . Significant correlation between plasma proteome profile and pain intensity, sensitivity, and psychological distress in women with fibromyalgia. Sci Rep. 2020;10(1):12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosin DL, Okusa MD.. Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol. 2011;22(3):416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi T, Wong P, Ellingson MK, et al. . Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.