ABSTRACT
Introduction
The coronavirus 19 (COVID-19) pandemic triggered a simultaneous global demand for preventative vaccines, which quickly became a high priority among governments as well as academia and the pharmaceutical industry. Within less than a year after COVID-19 was declared a pandemic, vaccines had received emergency approvals and vaccination campaigns were initiated.
Areas covered
We discuss the several factors that led to the unprecedented, accelerated development and approval of COVID-19 vaccines, which includes optimization of processes by regulatory authorities, redesign of sequential development processes, learnings from previous pandemics, and prior development of novel vaccine platforms.
Expert Opinion
Despite unanticipated and complex challenges presented by real-time vaccine development in the context of the evolving COVID-19 pandemic and subsequent ever-changing landscape of public health measures and recommendations, important milestones were reached within extraordinarily short periods and, following roll-out to billions worldwide, the approved vaccines have proven to be well tolerated and effective. Whilst this is an exceptional feat and an example of what can be achieved with collaboration and innovation, there are lessons that can still be learned, including the need for further harmonization between regulatory authorities, modes to react to the pandemic’s ever-evolving challenges, and ensuring equitable vaccine access among low-income countries.
KEYWORDS: COVID-19, pandemic, public health, regulatory processes, vaccine development
1. Introduction
On 21 February 2020, the World Health Organization (WHO) raised its threat assessment to its highest level [1] and, subsequently, on 11 March 2020 announced that coronavirus disease 19 (COVID-19) was a pandemic caused by a novel coronavirus, Severe Acute Respiratory Syndrome coronavirus-2 (SARS-CoV-2) [2]. By this point, nearly 120,000 cases had been reported and transmission was observed in more than 114 countries [2]. The emergency triggered an intense and rapid large-scale response; by 8 April 2020 just weeks after the pandemic was declared, 115 vaccine candidates had entered pre-clinical development and five were being investigated in Phase I clinical trials [3]. These immense efforts were not in vain; extensive immunization programs have now been rolled out globally following expedited authorizations of vaccines by major regulatory authorities including the United States (US) Food and Drug Administration (FDA) [4–6], and the European Medicines Agency (EMA) [7–10]. Earliest approvals occurred shortly after the first safety and efficacy data from Phase III became available, less than 10 months after the start of respective Phase I trials [7,9]. As of 31 January 2022, 9.9 billion vaccine doses have been administered worldwide [11].
The reasons behind this unprecedented development and approval time were multifactorial and included overlapping and condensed pre-clinical and clinical development phases, collaboration with academia, optimized use of existing regulatory pathways, utilization of novel vaccine platform technologies with shorter design-to-production turnaround times, increased funding, and up-scaled manufacturing capacities.
This review explores the ways in which vaccine development and approval processes have been utilized to enable rapid licensure of COVID-19 vaccines. We will primarily focus on the FDA and EMA regulatory frameworks and the respective COVID-19 vaccines approved by these bodies. Although the decision-making pathways and guidance from the WHO as a key leader in driving the worldwide pandemic response particularly in low- and middle-income countries, and of national and international public health decision makers will not be included in the scope of this review, one of the learnings is the need for a highly integrated real-time alignment of regulatory pathways and developed pandemic response policies.
2. Vaccine development
2.1. Standard vaccine development: the need for innovation
Prior to COVID-19, the time between vaccine development start and product licensure/marketing typically spanned a period of 10 to 15 years or more (Figure 1) [12]. These lengthy timelines are partly due to the multiple sequential steps involved in previous regulatory processes outlined by the FDA and EMA that are broadly segmented into exploratory and pre-clinical stages, clinical development (i.e. Phase I, II, and III trials), regulatory review and approval, manufacturing, quality control and long-term post-authorization studies. The shortest time in which a vaccine had been approved previously was for mumps, which took 4 years (approved in 1967) [13,14], although, unlike COVID-19, the disease had been prevalent in the decades before, and live-attenuated mumps vaccines were already in use [14].
Figure 1.
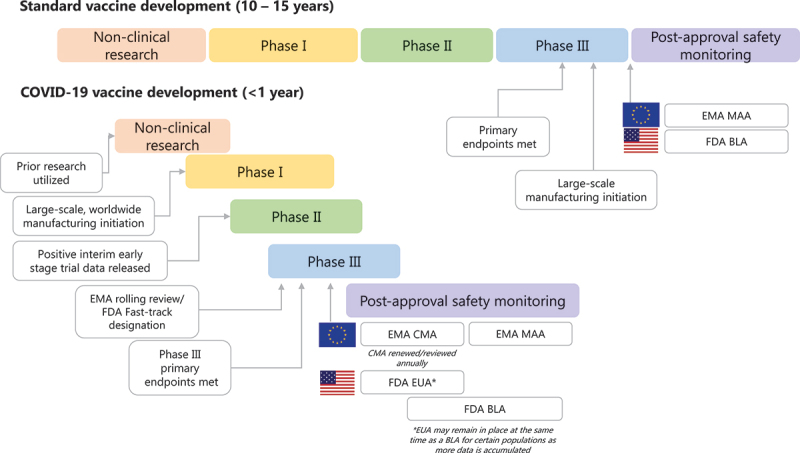
Standard vs. COVID-19 vaccine development process.
EMA, European Medicines Agency; FDA, US Food and Drug Administration; ICMRA, WHO, World Health Organization. References: [4-6], [12].
Although the conventional way of developing vaccines has led to numerous successes throughout the years, in the stark light of global infectious emergencies where rapid access to vaccines and treatments is paramount, it was clear that such lengthy timelines would not be fit for purpose.
2.2. Pandemic vaccine development: lessons learned pre-COVID-19
Prior to COVID-19, the world had already grappled with a number of pandemics, most recent and notable of which are human immunodeficiency virus (1981 – ongoing), SARS-CoV (2002–2003), Swine flu (A/H1N1; 2009–2010), and Middle East Respiratory Syndrome coronavirus (MERS-CoV; 2015 – ongoing) [15]. As well as having drastic implications on public health and causing significant global disruption, these events exposed weaknesses and deficiencies in existing vaccine development systems, including the lack of harmonization among regulatory authorities and the need for real-time guidance and optimization of regulatory processes.
Emergency or expedited approval systems were already in place at the time of these pandemics (i.e. Emergency Use Authorization [EUA] by the FDA, Conditional Marketing Authorization [CMA] by the EMA) as were methods to streamline the regulatory review process (fast-track designation [FDA] [16] and rolling data submissions [EMA] [17]). Both the EUA and CMA expedited approvals can be applied for a medicine that addresses an unmet medical need (such as pandemics) and require a less comprehensive dataset than standard marketing authorization but should demonstrate that the benefits of immediate availability outweigh the risks of the medicine, including vaccines [18,19]. Following an EUA or CMA, robust safeguards and controls are in place, and manufacturers must continue to collect and submit placebo-controlled data in ongoing trials according to negotiated schedules [18,19].
These temporary authorizations are not planned to remain conditional: a CMA is reviewed annually and, as soon as the relevant data needed in order to confirm the positive benefit/risk relationship are provided within a full dossier in the specified timeframe, it will become a standard marketing authorization (MAA) [18,19]. Indeed, one of the key requirements for a CMA to be issued is that it will be possible to generate comprehensive data post-authorization [19]. The FDA expects that the manufacturer will subsequently submit a Biologics License Application (BLA) as soon as longer-term data are available [20].
Although previously in place and designed to allow marketing authorizations as soon as possible, the EUA and CMA had not been implemented in a public health crisis to the scale of COVID-19, which was an unprecedented and rapidly evolving situation, and scaffolds, e.g. specific guidance to expedite approval, were needed.
The EMA initiated pandemic preparedness strategies in 2003 following the SARS-CoV pandemic [21]. This included creation of a crisis management plan that detailed procedures such as fast-track approval of pandemic vaccines, and post-authorization follow-up [21]. The fast-track approval approach, which was used in H1N1 influenza vaccine development [22], encompasses submission of a ‘core pandemic dossier’ and authorization of a ‘mock-up’ vaccine, was designed to expedite regulatory assessment of pandemic influenza vaccines, and allow licensure of a vaccine prior to the peak phase of the pandemic [23]. The use of ‘mock-up’ vaccines involves preparing non-clinical, safety, and immunogenicity data with an index influenza virus that may possibly arise in the future and is produced with the same method as intended for the actual vaccine but, does not contain antigens from the viral strain. Although this approach was not utilized with COVID-19 vaccine approvals, a similar principal is now established to expedite the approval of potential SARS-CoV-2 variant-specific vaccines (see Future Outlook section).
In 2010, shortly after the H1N1 outbreak, the FDA set up the Medical Countermeasures Initiative to enhance future pandemic responses and ensure safety and effectiveness of drugs, vaccines, and diagnostic tests [24]. Experience with these previous pandemics highlighted the lack of harmonization between regulatory authorities to expedite the dossier preparation and review process, the need for continual industry-regulatory interactions and flexibility, as well as for efficient data sharing and collaboration. These learnings were fundamental in shaping the response to COVID-19.
2.3. COVID-19 vaccine development: an accelerated evolution
The development program of the Ebola vaccine Ervebo® [25] took 5 years; with its Phase I trial starting in 2013 [26] and its first approval by the FDA in 2019 [27], 18 months after the peak period of the outbreak [25]. While this was fast compared to standard timelines, it would not be sufficient to tackle the scale and speed of the COVID-19 pandemic, and is in stark contrast to the approval of the first COVID-19 vaccine that took less than 10 months.
The reasons for this short turnaround during COVID-19 were multifactorial and include at-risk investment by manufacturers, the higher public health need, novel platform technologies, and optimal use of the procedural systems for the development of emergency vaccines (Figure 2). Adoption of large-scale, innovative clinical trial designs, so-called ‘seamless trials,’ where certain phases of vaccine development are consolidated into a single protocol, which is continually adapted as needed, e.g. Phase I/II or Phase I/II/III, also shortened timelines (Figure 3) [28]. Additionally, in contrast to Ebola, COVID-19 case numbers were considerably higher [25] allowing clinical trial efficacy endpoints to be met sooner.
Figure 2.
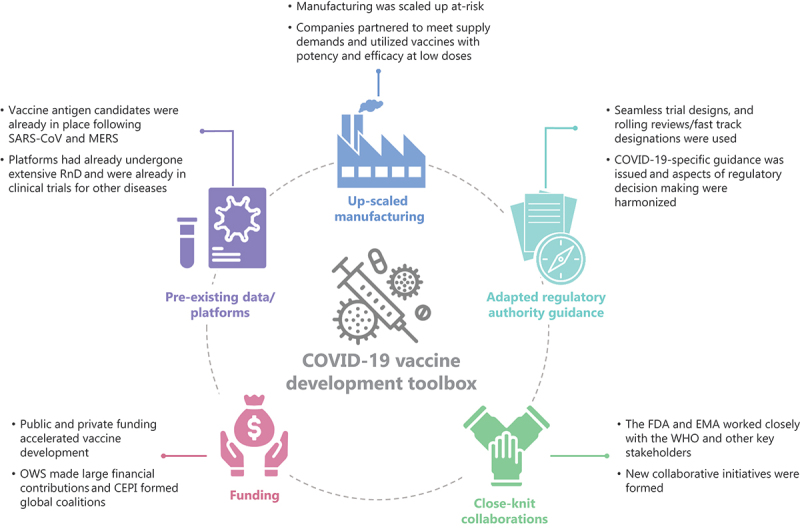
Key factors in expediting COVID-19 vaccine development and approval.
Figure 3.
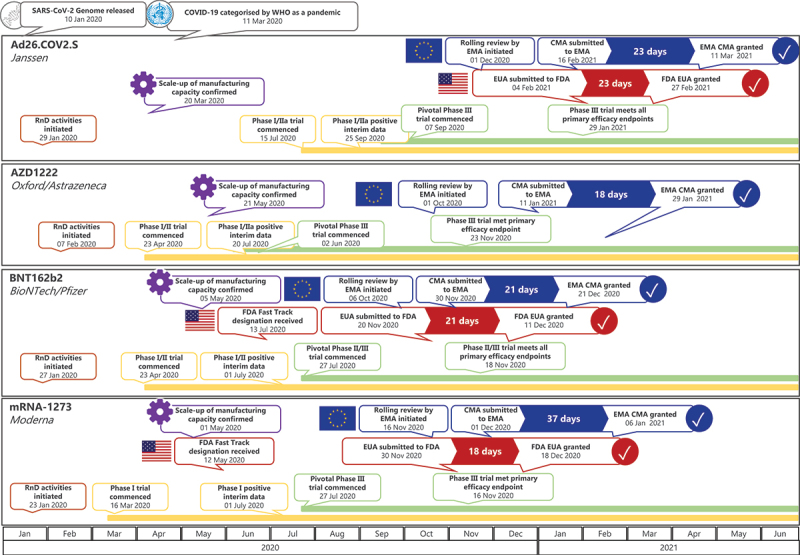
Timeline of the development of COVID-19 vaccines from the start of clinical development through to regulatory approval1.
ACT, Access to COVID-19 Tools; CEPI, Coalition for Epidemic Preparedness Innovations; COVID-19, coronavirus disease 19; EMA, European Medicines Agency; FDA, US Food and Drug Administration; ICMRA, International Coalition of Medicines Regulatory Authorities; MERS, Middle East Respiratory Syndrome coronavirus; OWS, Operation Warp Speed; RnD, research and development; SARS-CoV, Severe Acute Respiratory Syndrome coronavirus-2; WHO, World Health Organization. References:[12], [20], [29], [35], [36], [50], [53], [54], [59], [61-63].
CMA, conditional marketing authorisation; EMA, European Medicines Agency; EUA, emergency use authorisation; EUL, emergency use license; RnD, research and development; WHO, World Health Organization.
1Dates/ review periods sourced from Clinicaltrials.gov, EMA and FDA websites, and BioNTech, Pfizer, Astrazeneca, University of Oxford and Moderna press releases.
2.3.1. Regulatory authority approval process optimization
Emergency Use pathways have proven to be critical in several countries for the approval of COVID-19 vaccines. Both the FDA and European regulators supported seamless trial development where key steps were carried out in parallel, rather than sequentially, and allowed the sponsors to implement rapid decision-making based on emerging data (Figure 1). Additionally, they also issued various COVID-19-specific guidance to scaffold and facilitate regulatory submissions. Rolling reviews by the EMA allowed a head start in assessing data from the clinical trials enabling companies to submit further data as and when it was generated. Upon CMA submission, the review time is a maximum of 20 days (as opposed to 40–70 days previously). Dossier scope can be agreed case-by-case prior to submission and must show a favorable benefit-risk profile and that it meets quality, safety, and efficacy standards [29]. The COVID-19 EMA Pandemic Task Force was set up to further bolster the management of vaccine development and surveillance [30]. National authorities, e.g. the Paul-Ehrlich-Institute, also implemented rolling reviews during clinical trial authorizations and rapid scientific advice procedures [31] as well as allowing vaccine candidates to be investigated simultaneously within the same trial (e.g. NCT04380701). The European Parliament approved a temporary derogation of the environmental risk assessment requirements to help ensure that those clinical trials with genetically modified organisms began as soon as possible [32]. The FDA issued special guidance on EUA for COVID-19 vaccines, outlining criteria and considerations for issuing an EUA, and requests that sponsors would continue to assimilate data in ongoing trials following submission of the EUA [33].
Long-term safety monitoring was a key aspect defined by the FDA and EMA; a pharmacovigilance plan at the time of BLA and post-marketing safety studies is required by the FDA [34] and, similarly, the EMA issued guidance instructing that applicants provide monthly reports with all known safety and efficacy data [35].
Commitment from regulatory authorities in terms of alignment on regulatory requirements was reinforced during COVID-19. In order to streamline applications, the EMA and FDA began to harmonize certain procedures, e.g. a joint EMA-FDA pediatric investigation plan is now accepted for COVID-19 [36,37]. Both the EMA and FDA facilitated continuous, real-time dialogs with manufacturers over the course of the pandemic, as well as issuing supplemental Q&A documents for new guidance and early development schemes (e.g. EMA’s PRIority MEdicines [PRIME] scheme) [17]. To support COVID-19 vaccine development, target product profiles were created by the FDA and EMA to provide a clear guide for developers and detail requirements for vaccines, an aspect that was not implemented prior to the pandemic [34,38].
2.3.2. Collaborative response
COVID-19 necessitated stronger, multilateral global collaboration and work sharing between regulatory authorities, government, and the private sector. The International Coalition of Medicines Regulatory Authorities (ICMRA) is a voluntary organization set up to provide a foundation for global strategic partnership and information sharing among regulatory bodies with the aim of cultivating a collective response toward the development and availability of COVID-19 vaccines [39].
The EMA and FDA have had a longstanding partnership to leverage each other’s expertise but further collaboration between them was driven by COVID-19 under the umbrella of ICMRA, e.g. in March 2020 they convened to discuss the pre-clinical data requirements and possibility of regulatory convergence [40]. In order to help execute and expedite its pandemic response, the EMA operates closely with the European Commission, European Center for Disease Prevention and Control, Health Security Committee as well as the WHO [41] and the unfamiliar challenges of the COVID-19 pandemic required adaptation of those operating models. The COVID-19 Evidence Accelerator was founded to provide a platform for government, research institutes, and health systems to allow for systematized, efficient information sharing [42]. The Access to COVID-19 Tools Accelerator is a collaboration set up amongst governments, scientists, businesses, global health organizations (e.g. Coalition for Epidemic Preparedness Innovations [CEPI] and the WHO) and others to accelerate development of and access to COVID-19 vaccines [43].
The pandemic also highlighted the power of fusing academic expertise in research and early development with the global development and manufacturing capabilities of the pharmaceutical industry. Indeed, the collaboration between the Jenner Institute and Oxford Vaccine Group (University of Oxford) and AstraZeneca, where the University of Oxford drove the initial vaccine candidate development and AstraZeneca provided expertise in large-scale drug development and manufacturing, proved pivotal in the rapid advancement of AZD1222 [45]. Moderna’s collaboration with the National Institute of Health, fast-tracking the development of mRNA-1273, is another testament to the power of academic-industry partnerships in addressing a public health emergency [46]. BioNTech benefitted from its deeply academically rooted model of operating its research and translation. Additionally, innovative industry–industry partnerships allowed expedited vaccine development by sharing expertise, data and technology transfer, funding, and up-scaled manufacture [44]. Following preclinical development of BNT162b2 by BioNTech, BioNTech and Pfizer established a partnership in March 2020 to leverage Pfizer’s vaccine development, commercial-scale manufacturing, and regulatory experience [47]. BioNTech led Phase I trials in Germany from April 2020 and subsequently BioNTech/Pfizer jointly entered co-development with global Phase I to III trials [48]. In the same period, a collaboration between BioNTech and Fosun was initiated to support vaccine development in China [49].
2.3.3. Preexisting data and platforms
Preexisting non-clinical data from research during previous coronavirus outbreaks allowed the early stages of vaccine design to be almost bypassed [12]. The full-length spike glycoprotein of SARS-CoV-2 was chosen as the vaccine antigen primarily due to experience with SARS-CoV and MERS, which illustrated that the vaccines provide viral protection [50]. Importantly, clinical research has already been conducted with the platforms that comprise the basis of the vaccines that are now approved for COVID-19. Viral vector vaccines have been extensively studied in the last 30 years and had previously been shown to have the capacity to induce strong, long-lasting immune responses in other infectious diseases and cancer [51]. AD26.COV.2 leveraged Janssen’s preexisting AdVac® technology that was used in the development of the EMA-approved Ebola vaccine (Zabdeno/Mvabea) [52]. Messenger ribonucleic acid (mRNA) technologies had also undergone extensive basic research and development for more than 20 years prior to gaining visibility during the COVID-19 pandemic [53–55], and the first-in-human trial with mRNA was initiated in 2009 [56]. Clinical trials in various cancer indications investigating personalized vaccines using mRNA technology had been conducted previously (e.g. NCT04486378, NCT03815058). As this involved swift, on-demand design and manufacturing of vaccines tailored to each individual patient, the proficiency of mRNA technology for a platform approach in regulatory terms had already been pursued [57]. This prior experience enabled early discussions with regulators on the use of platform technology approaches to develop vaccines and led to adoption of these principles during COVID-19, allowing the extrapolation of safety information across different vaccine candidates and implementation of a platform-based non-clinical toxicology program in support of first-in-human trials [58].
2.3.4. Funding
Funding was a key element that catalyzed the development of COVID-19 vaccines; without economic support, many companies would have been unable to take the financial risk of overlapping the steps of vaccine development. Operation Warp Speed is a US private-public initiative that involves a collaboration of key stakeholders (e.g. the FDA), as well as private firms and other federal agencies. It was initially funded with $10 billion from the Coronavirus Aid, Relief, and Economic Security Act and had already made significant investments into several vaccine candidates by the first quarter of 2020, e.g. $456 million for Moderna’s vaccine (mRNA-1273) and $1.2 billion for the Oxford/AstraZeneca vaccine (AZD1222) [59]. Other organizations, e.g. research funding bodies such as the German Federal Ministry of Education and Research (BMBF), also financially supported pharmaceutical companies early on in the pandemic [60]. CEPI is a global coalition between public and private organizations, and pledges to act as a pillar of global health security by providing funding and facilitation for innovative vaccine platforms [61]. It founded an international network of centralized laboratories to enable testing and the comparison of immunological COVID-19 vaccine responses and has supported nine vaccines thus far, including those manufactured by Moderna and University of Oxford and AstraZeneca [61].
2.3.5. Manufacturing and production
Pharmaceutical companies set about to prepare for large-scale vaccine manufacture early on in the pandemic and vaccine production was initiated at-risk, prior to late-stage clinical trial results (Figure 3) [12]. In order to produce a sufficient supply of vaccines to meet the envisaged demand, a number of companies formed partnerships with contract manufacturers, e.g. Moderna united with Lonza in May 2020 with plans to provide 1 billion vaccine doses per year [62].
Rapid, high-throughput vaccine production also hinges on the type of vaccine being made and how amenable it is to larger scale production. A salient feature of mRNA platforms is the ability to up-scale their production quickly and their potency and efficacy at low doses, the latter lowering the volume required and thus the time and cost of manufacture [53,63]. Post-manufacturing storage and delivery is another important consideration; adenoviral vaccines are stable at room temperature short-term which is advantageous for vaccine deployment in countries where cold chain storage is limited [64]. Prior to the pandemic, aside from some frameworks, e.g. the European Joint Procurement Framework [65], there was limited guidance or contingency plans from regulatory authorities to ensure supply of medicinal products in a public health emergency. In April 2020, the EMA, European Commission, and national competent authorities issued joint COVID-19-specific adaptations to the regulatory framework to reduce risks of vaccine shortages while ensuring high standards of quality, safety, and efficacy [66]. For example, where justified, temporary changes could apply to deferral of certain routine procedures such as stability testing, in order to focus resources on release testing [66].
3. Approved COVID-19 vaccines
As of 28 January 2022 there are 140 vaccines in clinical development, 194 in pre-clinical development [67], and a number of vaccines are now approved for human use [4–10,68]. Figure 3 shows those that have been approved by the FDA and/or EMA. Two of the first COVID-19 vaccines authorized for use were mRNA vaccines: mRNA-1273 (Moderna) [5,9], initially recommended for those aged ≥18 years, and BNT162b2 (BioNTech/Pfizer) [4,7], initially recommended for those aged ≥16 years (both by the FDA and EMA). A third COVID-19 viral vector vaccine, AZD1222 (Oxford/AstraZeneca), was granted EMA CMA in January 2021 for use in people aged ≥18 years [8]. Ad26.COV2.S (Janssen/Johnson and Johnson), also a viral vector vaccine, was granted FDA EUA and EMA CMA in late February/early March 2021 [6,10]. The initial CMA/EUA indications have since been extended for some vaccines, e.g. for use in children aged 5 to 15 years [4,7,9,69], and, in some countries, heterologous vaccine regimens are used [70]. Figure 3 also depicts the overlapping stages of development of these vaccines and timings between submission and approval. As detailed in the sections above, clinical trials did not have to take place sequentially, using integrated approaches, and fast-track designations by the FDA and rolling submissions by the EMA could start well before the end of the pivotal late-stage trials. Evidence from longer term post-marketing studies or trials and real-world evidence are continuously generated following CMAs and EUAs, which further informs on vaccine effectiveness and safety [34,35,37].
Outside of the US and the European Union, multiple other vaccines have been approved at the national level. Sputnik V (Gam-COVID-Vac, Gamaleya Research Institute) was one of the first COVID-19 vaccines to be registered for use in any country, approved by the Russian Ministry of Health in August 2020 [71]. China’s CoronaVac (Sinovac) vaccine, an inactivated whole-virus vaccine was approved for emergency use in China in July 2020 [72]. Unlike the vaccines approved by the EMA and FDA, these approvals adopted different fast-tracked regulatory processes according to country-specific needs; however, there were major concerns among both the scientific community and the public given the paucity of data prior to their approval [73]. At the time of its first approval, Sputnik V had only been administered to 76 subjects in early-stage trials and neither safety nor efficacy data had been generated from a large-scale Phase III trial [73]. Similarly, the CoronaVac vaccine lacked transparent and comprehensive Phase III documentation prior to approval, with data primarily being reported in press releases or government media reports [74]. This was in stark contrast to the EMA- and FDA-approved vaccines, where primary safety and efficacy endpoints from Phase III trials had been met at the time of respective CMAs and EUAs. Although vaccines such as these have undoubtedly significantly contributed to vaccine access worldwide (particularly in countries where global demand surpassed supply) with Sputnik V now approved in 70 countries and CoronaVac received EUL by the WHO in June 2021 [75], the negative press surrounding their approval likely fueled public uncertainty and mistrust with COVID-19 vaccines in general, including those that had undergone more rigorous scrutinization by regulatory authorities prior to approval. This emphasizes the critical importance of thorough investigation of vaccines, even in a public health emergency, as well as a need for harmonization of regulatory approval standards not just between the EMA and FDA but at a global level, to circumvent public confusion and mistrust, which can ultimately lead to vaccine hesitancy.
4. Conclusions
COVID-19 continues to be one of the most pressing challenges of this generation and has sparked unanticipated upheaval of the vaccine development process. Learnings from previous pandemics, as well as the use of existing pre-clinical data and vaccine platforms, leveraging existing regulatory tools, early and real-time dialogs between regulators and manufacturers, access to funding, parallel, and ‘seamless’ trial designs and at-risk manufacture, enabled compressed vaccine development timelines.
5. Expert opinion
The COVID-19 pandemic has not gone through its full life cycle and new challenges occur which require tightly aligned joint learning cycles of vaccine developers, regulators, and policymakers to shape a path forward which provides the proper response to the pandemic and can also serve as blueprint for future pandemics. Challenges now relate to waning vaccine effectiveness [76] and the emerging SARS-CoV-2 variants [77], some of which are known to cause more serious clinical severity or an increase in infectivity (for a recent review, see Harvey et al. 2021 [78]). Existing approved COVID-19 vaccines have been shown to be efficacious against some variants, although data with some vaccines show this is to a lesser degree compared with the original strain [79–84]. Trials are ongoing to either investigate modified or variant-specific vaccines using the same platforms [85] or booster vaccines (an additional dose of the original vaccine) to restore protection [86–88]. Owing to their immunogenicity, which is equivalent or higher than after the primary schedule, and safety profile [7,9], booster doses of BNT162b2 and mRNA-1273 have now been approved by the FDA and/or EMA. Furthermore, a third dose has been approved as part of the primary series for immunocompromised patients [7,9,89]. Regulators had previously reacted to the challenge of variants and waning immunity by holding consultations or creating guidance on how these modified vaccines targeting variants of concern may be assessed [90–92]. However, a globally harmonized regulatory path or decision-making model for transitioning to a variant adapted vaccine has not been established yet. Given that more escape variants may arise that are more transmissible and/or associated with higher disease severity, a key challenge will be conducting placebo-controlled trials now that a large proportion of the population is vaccinated. Randomized trials with authorized vaccines as the comparator or observational studies could be options, although these both have ethical and logistical drawbacks [90,91]. ‘Correlates of protection,’ where biomarkers such as antibody responses are used to indicate immune response/protection, may negate the need for large, randomized trials and expedite vaccine development [93].
A workshop held by ICMRA amongst key regulators discussed how second-generation vaccines may be developed; the consensus was that correlates of immunity (immunobridging) trials may be necessary if clinical endpoint efficacy trials are not feasible [94]. Some regulatory authorities (e.g. Health Canada, SwissMedic) have now issued statements aligning with this proposal [95], although the reliability of such studies is still to be determined [96]. Both the EMA and FDA have supplied guidance on changes to vaccine composition (i.e. vaccines targeted toward variants) in a pandemic situation. The EMA allows a ‘type II variation’ to the CMA and the FDA can grant an amendment to the EUA provided the technological platform of the vaccine remains similar to the original. Large-scale safety and efficacy trials may not be required and efficacy should be demonstrated in immunogenicity trials [33,97].
Additionally, there are inequalities in global vaccine access, with a significantly lower number of vaccines being distributed in low-income countries. mRNA and viral vector vaccines have primarily been implemented in high-income countries whilst in low- to middle-income countries, a combination of mRNA, inactivated, and viral vector vaccines have had a greater presence. Recombinant vaccines have been presented in both developed and lesser developed markets [98]. Mass distribution of COVID-19 vaccines to low- and middle-income countries which are low cost, provide long-term immunity (i.e. do not require frequent booster vaccinations), can be manufactured locally, and delivered easily at a large scale, is also key to ensuring global immunity [99].
Looking even further ahead, with future pandemics in mind, from a regulatory and vaccine development perspective, the pandemic has highlighted the need for close-knit cooperation between regulatory authorities, vaccine developers and manufacturers, and those who are responsible for policies and public health decisions. Further global harmonization of regulatory guidance where applicants would conform to an agreed set of international requirements, as well as, for example, adoption of standard clinical trial endpoints for vaccine efficacy to allow a harmonized evaluation of benefit and risk and potential to pool data for analyses of immunologic endpoints, could help toward shortening dossier preparation and vaccine approval times. Platform technology approaches with known safety and efficacy profiles that allow for reactive vaccine design should continue to be used to expedite access to vaccines. A vaccine Platform Technology Master File (PTMF) could be utilized in future, which would act as a stand-alone part of the dossier and remain unchanged regardless of the sequence change to the platform [58].
In conclusion, to respond optimally in future disease outbreaks, development of regulatory strategies that are tailored to cope with emergency situations should continue and international collaborations between manufacturers, health and regulatory authorities are paramount and should be built upon. Detailed evaluation of the regulatory approaches used during COVID-19 may help to identify those that had the most meaningful impacts or those that could be enhanced in future to further expedite vaccine approval pathways.
Acknowledgments
The authors would like to thank Ugur Sahin (BioNTech SE) for his support in developing the outline of this manuscript. Medical writing support was provided by Cindy Cheung, PhD, from Scion (development of the initial outline and literature search) and Camilla West, PhD, from BioNTech SE (assisting authors with the development of the initial draft and incorporation of comments and revisions) according to Good Publication Practice guidelines.
Funding Statement
BioNTech SE provided funding to the Medical Communications agency Scion for the development of the initial outline and literature search.
Article highlights
Vaccine development usually takes anywhere between 10 and 15 years and, whilst learnings were made through previous pandemics and accelerated emergency approval mechanisms were in place to allow rapid access to vaccines, the urgency of the COVID-19 pandemic meant vaccine development needed fundamental optimization.
Regulatory authorities quickly reacted to the pandemic by optimizing their processes, such as accelerated review timelines, issuing COVID-19 vaccine-specific guidance and facilitating real-time dialogue with manufacturers.
Multilateral global collaboration and work sharing between regulatory authorities, government and the private sector facilitated expedited vaccine development.
Pre-existing data were available owing to previous coronavirus outbreaks, and the mRNA- and adenovector platforms that were used for the first approved COVID-19 vaccines were already being used in clinical trials or other marketed vaccines (Zabdeno/Mvabea vaccine for Ebola).
Funding was another aspect that expedited development; private-public initiatives, such as Operation Warp Speed and the Coalition for Epidemic Preparedness Innovations provided scaffolds for manufacturers to drive development of their candidates forward.
Vaccine developers started manufacturing their vaccines well before commencing late-stage trials, allowing them to be poised and prepared for mass supply.
These factors led to some vaccines gaining emergency approval in major highly regulated markets less than 10 months after the start of Phase I trials and positive clinical trial results are now reflected in global real-world data.
Challenges lie with vaccine variants and waning immunity; vaccine developers and vaccine policymakers must adopt effective strategies to swiftly and in a coordinated manner react as the pandemic evolves.
Regulators, manufacturers, and policymakers can learn from COVID-19 for the future; they must continue to work harmoniously together and adopt new strategies to respond even more rapidly and effectively in the case of other outbreak situations.
Author contributions
All authors substantially contributed to the writing, intellectual revisions, and preparation of this review. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article and have given their approval for this version to be published.
Declaration of interests
D Barouch is co-inventor on provisional vaccine patents (63/121,482; 63/133,969; 63/135,182). P Neels provides consultancy services for COVID-19 dossiers for the following: BioNTech SE, ACMBio, Icosavac, Curevac, Univercells, Moderna, Egypt authorities: EDA, Saudi Arabia FDA. Ö Türeci is employed by BioNTech SE, has issued patents with BioNTech SE and TRON Translational Oncology Mainz, has a leadership role with The Association for Cancer Immunotherapy (CIMT), is co-founder of and holds shares with BioNTech SE. R Rizzi, E Lagkadinou, and S Pather are employed by BioNTech SE. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.World Health Organization . Coronavirus Disease (COVID-19). Press Conference, 28 February 2020 [Internet]. [cited 2022 Feb 10]. Available from: https://www.who.int/docs/default-source/coronaviruse/transcripts/who-audio-emergencies-coronavirus-press-conference-full-28feb2020.pdf?sfvrsn=13eeb6a4_2
- 2.World Health Organization . WHO director-general’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [Internet]. [cited 2022 Feb 10]. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020
- 3.Le TT, Cramer JP, Chen R, et al. Evolution of the COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:667–668. [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration . Emergency Use Authorization (EUA) for an unapproved product review memorandum. Application 27034 [Internet]. [cited 2022 Feb 10]. Available from: https://www.fda.gov/media/144416/download
- 5.US Food and Drug Administration . Emergency Use Authorization (EUA) for an unapproved product review memorandum. Application 27073 [Internet]. [cited 2022 Feb 10]. Available from: https://fda.report/media/144673/Moderna+COVID-19+Vaccine+review+memo.pdf
- 6.US Food and Drug Administration . Emergency Use Authorization (EUA) for an unapproved product review memorandum. Application 27205 [Internet]. [cited 2022 Feb 10]. Available from: https://www.fda.gov/media/146338/download
- 7.Comirnaty SmPC . BioNTech manufacturing GmbH. Comirnaty SmPC. European medicines agency website [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf
- 8.Vaxzevria SmPC. AstraZeneca AB. Vaxzevria SmPC . European medicines agency website [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_en.pdf
- 9.Spikevax SmPC. Moderna Biotech Spain S.L. Spikevax SmPC . European medicines agency website [Internet] [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/product-information/spikevax-previously-covid-19-vaccine-moderna-epar-product-information_en.pdf
- 10.COVID-19 Vaccine Janssen SmPC . Janssen-Cilag International NV. COVID-19 vaccine Janssen SmPC. European medicines agency website [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-janssen-epar-product-information_en.pdf
- 11.World Health Organization . WHO coronavirus (COVID-19) dashboard [Internet]. [cited 2022 Feb 10]. Available from: https://covid19.who.int/?gclid=CjwKCAjwo4mIBhBsEiwAKgzXOFGg4woTngtSuJSNB_cgi19enDLeCPhDJ6h_EmBvLeuAAknOhLICixoCrS4QAvD_BwE
- 12.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. [DOI] [PubMed] [Google Scholar]
- 13.Ball P. The lightning-fast quest for COVID vaccines - and what it means for other diseases. Nature. 2021;589:16–18. [DOI] [PubMed] [Google Scholar]
- 14.S-B S, Chang H-L, Chen K-T. Current status of mumps virus infection: epidemiology, pathogenesis, and vaccine. Int J Environ Res Public Health. 2020;17. DOI: 10.3390/ijerph17051686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piret J, Boivin G. Pandemics throughout history. Front Microbiol. 2020;11:631736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration . Fast Track [Internet]. [cited 2022 Feb 10]. Available from: https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/fast-track
- 17.European Medicines Agency . EMA initiatives for acceleration of development support and evaluation procedures for COVID-19 treatments and vaccines [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/other/ema-initiatives-acceleration-development-support-evaluation-procedures-covid-19-treatments-vaccines_en.pdf
- 18.US Food and Drug Administration . Emergency use authorization of medical products and related authorities [Internet]. [cited 2022 Feb 10. Available from: https://www.fda.gov/media/97321/download
- 19.European Medicines Agency . Conditional marketing authorization, report on ten years of experience at the European Medicines Agency [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/report/conditional-marketing-authorisation-report-ten-years-experience-european-medicines-agency_en.pdf
- 20.US Food and Drug Administration . Emergency use authorization for vaccines to prevent COVID-19 - Guidance for Industry [Internet]. [cited 2022 Feb 10]. Available from 2022 Feb 10: https://www.fda.gov/media/142749/download
- 21.European Medicines Agency . EMEA pandemic influenza crisis management plan for the evaluation and maintenance of pandemic influenza vaccines and antivirals [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/other/european-medicines-agency-pandemic-influenza-crisis-management-plan-evaluation-maintenance-pandemic_en.pdf
- 22.Pandemrix SmPC. GlaxoSmithKline Biologicals . European Medicines Agency website [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/product-information/pandemrix-epar-product-information_en.pdf
- 23.European Medicines Agency . Pandemic report and lessons learned [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/report/pandemic-report-lessons-learned-outcome-european-medicines-agencys-activities-during-2009-h1n1-flu_en.pdf
- 24.US Food and Drug Administration . Medical countermeasures initiative [Internet]. [cited 2022 Feb 10]. Available from: https://www.fda.gov/emergency-preparedness-and-response/medical-countermeasures-initiative-mcmi/about-mcmi
- 25.Excler J-L, Saville M, Berkley S, et al. Vaccine development for emerging infectious diseases. Nat Med. 2021;27:591–600. [DOI] [PubMed] [Google Scholar]
- 26.ClinicalTrials.gov . Evaluation of the safety and immunogenicity of three consistency lots and a high-dose lot of rVSV-ZEBOV-GP (V920 Ebola Vaccine) in healthy adults (V920-012) [Internet]. [cited 2022 Feb 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT02503202
- 27.US Food and Drug Administration . ERVEBO. BLA clinical review memorandum. [Internet]. [cited 2022 Feb 10]. Available from: https://www.fda.gov/media/134270/download
- 28.Jiang Z, Wang X, Xia J. Considerations on the clinical development of COVID-19 vaccine from trial design perspectives. Hum Vaccin Immunother. 2021;17:656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.European Medicines Agency . COVID-19 guidance: research and development [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/guidance-developers-companies/covid-19-guidance-research-development; • Reason: This provides vaccine developers with guidance and support during the development process for EMA submission.
- 30.European Medicines Agency . Mandate, objectives and rules of procedure of the COVID-19 EMA pandemic Task Force (COVID-ETF) [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/other/mandate-objectives-rules-procedure-covid-19-ema-pandemic-task-force-covid-etf_en.pdf
- 31.Paul-Ehrlich-Institut . Accelerated procedures and processes in the COVID-19 pandemic [Internet]. [cited 2022 Feb 10]. Available from: https://www.pei.de/EN/newsroom/hp-news/2020/201118-bah-pei-dialogue-accelerated-procedures-covid-19.html?nn=164440.
- 32.Iglesias-Lopez C. Temporary derogation of European environmental legislation for clinical trials of genetically modified organisms (GMOs) for COVID-19. Cytotherapy. 2021;23(1):10–11. DOI: 10.1016/j.jcyt.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Food and Drug Administration . Emergency use authorization for vaccines to prevent COVID-19 [Internet]. [cited 2022 Feb 10]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/emergency-use-authorization-vaccines-prevent-covid-19; • Reason: This provides vaccine developers with guidance and support during the development process for US submission.
- 34.US Food and Drug Administration . Development and licensure of vaccines to prevent COVID-19 [Internet]. [cited 2022 Feb 10]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-and-licensure-vaccines-prevent-covid-19
- 35.European Medicines Agency . Pharmacovigilance plan of the EU regulatory network for COVID-19 vaccines [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/other/pharmacovigilance-plan-eu-regulatory-network-covid-19-vaccines_en.pdf; • Reason: This reference describes the activities European regulators will conduct once the vaccine are authorized
- 36.European Medicines Agency . FDA/EMA common commentary on submitting an initial Pediatric Study Plan (iPSP) and Paediatric Investigation Plan (PIP) for the prevention and treatment of COVID-19 [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/other/fda/ema-common-commentary-submitting-initial-paediatric-study-plan-ipsp-paediatric-investigation-plan_en.pdf
- 37.European Medicines Agency . Advancing international collaboration on COVID-19 real-world evidence and observational studies [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/news/advancing-international-collaboration-covid-19-real-world-evidence-observational-studies
- 38.European Medicines Agency . EMA considerations on COVID-19 vaccine approval [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/other/ema-considerations-covid-19-vaccine-approval_en.pdf
- 39.International Coalition of Medicines Regulatory Authorities . Statement on COVID-19 [Internet]. [cited 2022 Feb 10]. Available from: http://www.icmra.info/drupal/en/covid-19
- 40.US Food and Drug Administration . Summary of FDA & EMA global regulators meeting on data requirements supporting first-in-human clinical trials with SARS-CoV-2 vaccines [Internet].s [cited 2022 Feb 10]. Available from: https://www.fda.gov/news-events/fda-meetings-conferences-and-workshops/summary-fda-ema-global-regulators-meeting-data-requirements-supporting-first-human-clinical-trials
- 41.European Medicines Agency . EMA’s governance during COVID-19 pandemic [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/emas-governance-during-covid-19-pandemic#working-with-eu-and-international-partners-section
- 42.Friends of Cancer Research . COVID-19 evidence accelerator [Internet]. [cited 2022 Feb 10]. Available from: https://friendsofcancerresearch.org/covid19
- 43.World Health Organization . What is the ACT-accelerator [Internet]. [cited 2022 Feb 10]. Available from: https://www.who.int/initiatives/act-accelerator/about
- 44.International Federation of Pharmaceutical Manufacturers and Associations . COVID-19 R&D-based pharma industry’s innovative partnership allowed expedited vaccine development by sharing expertise, data and technology transfer, funding, and up-scaled manufacture to meet urgent global supply needs [Internet]. [cited 2022 Feb 10]. Available from: https://www.ifpma.org/wp-content/uploads/2021/03/IFPMA_Industrys-collaborations-on-COVID-vaccines-and-therapeutics-23.03.2021_V02.pdf
- 45.Astrazeneca Press Releases . AstraZeneca and Oxford University announce landmark agreement for COVID-19 vaccine[Internet]. [cited 2022 Feb 10]. Available from: https://www.astrazeneca.com/media-centre/press-releases/2020/astrazeneca-and-oxford-university-announce-landmark-agreement-for-covid-19-vaccine.html
- 46.Moderna Press Releases . Moderna announces first participant dosed in NIH-led phase 1 study of mRNA vaccine (mRNA-1273) against novel coronavirus [Internet]. [cited 2022 Feb 10]. Available from: https://investors.modernatx.com/news/news-details/2020/Moderna-Announces-First-Participant-Dosed-in-NIH-led-Phase-1-Study-of-mRNA-Vaccine-mRNA-1273-Against-Novel-Coronavirus-03-16-2020/default.aspx
- 47.Pfizer Press Release . Pfizer and BioNTech to co-develop potential COVID-19 vaccine [Internet]. [cited 2022 Feb 10]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-further-details-collaboration
- 48.Pfizer Press Release . Pfizer and BioNTech announce Phase 3 trial data showing high efficacy of a booster dose of their COVID-19 vaccine [Internet]. [cited 2022 Feb 10]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-phase-3-trial-data-showing
- 49.BioNTech Press Releases . BioNTech and fosun pharma form COVID-19 vaccine strategic alliance in China [Internet]. [cited 2022 Feb 10]. Available from: https://investors.biontech.de/news-releases/news-release-details/biontech-and-fosun-pharma-form-covid-19-vaccine-strategic
- 50.Yong CY, Ong HK, Yeap SK, et al. Recent advances in the vaccine development against middle east respiratory syndrome-coronavirus. Front Microbiol. 2019;10:1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendonça SA, Lorincz R, Boucher P, et al. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines. 2021;6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.European Medicines Agency . Zabeno Summary of Product Characteristics [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/product-information/zabdeno-epar-product-information_en.pdf
- 53.Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sahin U, Karikó K, Ö T. mRNA-based therapeutics—developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. [DOI] [PubMed] [Google Scholar]
- 55.Hinz T, Kallen K, Britten CM, et al. The european regulatory environment of RNA-based vaccines. Methods Mol Biol. 2017;1499:203–222. [DOI] [PubMed] [Google Scholar]
- 56.Weide B, Pascolo S, Scheel B, et al. Direct injection of protamine-protected mRNA: results of a phase ½ vaccination trial in metastatic melanoma patients. J Immunother. 2009;32:498–507. [DOI] [PubMed] [Google Scholar]
- 57.Britten CM, Singh-Jasuja H, Flamion B, et al. The regulatory landscape for actively personalized cancer immunotherapies. Nat Biotechnol. 2013;31:880–882. [DOI] [PubMed] [Google Scholar]
- 58.Vandeputte J, Saville M, and Cavaleri M, et al. IABS/CEPI platform technology webinar: is it possible to reduce the vaccine development time? Biologicals. 2021;71:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Importance of the platform approach to developing COVID-19 vaccines.
- 59.Department of Health and Human Services, USA . Explaining operation warp speed [Internet]. [cited 2022 Feb 10]. Available from: https://health.mo.gov/living/healthcondiseases/communicable/novel-coronavirus-lpha/pdf/fact-sheet-operation-warp-speed.pdf
- 60.The German Federal Ministry of Education and Research . German government funds three vaccine developers [Internet]. [cited 2022 Feb 10]. Available from: https://www.bundesregierung.de/breg-de/themen/buerokratieabbau/impfstofffoerderung-1772846
- 61.The Coalition for Epidemic Preparedness Innovations . CEPI - Our Mission [Internet]. [cited 2022 Feb 10]. Available from: https://cepi.net/about/whyweexist/
- 62.Lonza Press Releases . Moderna and Lonza announce worldwide strategic collaboration to manufacture moderna’s vaccine (mRNA-1273) against novel coronavirus [Internet]. [cited 2022 Feb 10]. Available from: https://www.lonza.com/news/2020-05-01-04-50
- 63.Jackson NAC, Kester KE, Casimiro D, et al. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines. 2020;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhan W, Muhuri M, Tai PWL, et al. Vectored immunotherapeutics for infectious diseases: can rAAVs be the game changers for fighting transmissible pathogens? Front Immunol. 2021;12:673699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.European Commission . Memo. Framework contracts for pandemic influenza vaccines [Internet]. cited 2022 Feb 10]. Available from: https://ec.europa.eu/health/system/files/2019-03/ev_20190328_memo_en_0.pdf
- 66.European Medicines Agency . Questions and answers on regulatory expectations for medicinal products for human use during the COVID-19 pandemic [Internet]. [cited 2022 Feb 10]. Available from: https://ec.europa.eu/health/sites/default/files/human-use/docs/guidance_regulatory_covid19_en.pdf
- 67.World Health Organization . COVID-19 vaccine tracker and landscape [Internet]. [cited 2022 Feb 10]. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 68.World Health Organization . Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process [Internet]. [cited 2022 Feb 10]. Available from: https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_15July2021.pdf
- 69.US Food and Drug Administration . FDA briefing document. EUA amendment request for Pfizer-BioNTech COVID-19 Vaccine for use in children 5 through 11 years of age [Internet]. [cited 2022 Feb 10]. Available from: https://www.fda.gov/media/153447/download
- 70.Paul-Ehrlich Institute . COVID-19 vaccine Vaxzevria (AstraZeneca) – review on scientific questions performed at EU level [Internet]. [cited 2022 Feb 10]. Available from: https://www.pei.de/EN/newsroom/hp-news/2021/210415-eu-covid-19-vaccine-vaxzevria-review-scientific-questions.html
- 71.Science . Russia’s approval of a COVID-19 vaccine is less than meets the press release [Internet]. [cited 2022 Feb 10]. Available from: https://www.science.org/content/article/russia-s-approval-covid-19-vaccine-less-meets-press-release
- 72.Kyriakidis NC, López-Cortés A, González EV, et al. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Callaway E. Russia’s fast-track coronavirus vaccine draws outrage over safety. Nature. 2020;584:334–335. [DOI] [PubMed] [Google Scholar]
- 74.Tanveer S, Rowhani-Farid A, Hong K, et al. Transparency of COVID-19 vaccine trials: decisions without data. BMJ Evid Based Med. 2021. DOI: 10.1136/bmjebm-2021-111735. [DOI] [PubMed] [Google Scholar]
- 75.World Health Organization . WHO validates Sinovac COVID-19 vaccine for emergency use and issues interim policy recommendations [Internet]. [cited 2022 Feb 10]. Available from: https://www.who.int/news/item/01-06-2021-who-validates-sinovac-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations
- 76.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Centers for Disease Control and Prevention . SARS-CoV-2 variant classifications and definitions [Internet]. [cited 2022. Feb 10]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html
- 78.Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Emary KRW, Golubchik T, Aley PK, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wall EC, Wu M, Harvey R, et al. AZD1222-induced neutralising antibody activity against SARS-CoV-2 delta VOC. Lancet. 2021;398:207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wall EC, Wu M, Harvey R, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moderna Press Releases . Moderna provides a clinical update on the neutralizing activity of its COVID-19 vaccine on emerging variants including the delta variant first identified in India [Internet]. [cited 2022. Feb 10]. Available from: https://investors.modernatx.com/news/news-details/2021/Moderna-Provides-a-Clinical-Update-on-the-Neutralizing-Activity-of-its-COVID-19-Vaccine-on-Emerging-Variants-Including-the-Delta-Variant-First-Identified-in-India-06-29-2021/default.aspx
- 83.Johnson and Johnson Press Releases . Positive New Data for Johnson & Johnson single-shot COVID-19 vaccine on activity against delta variant and long-lasting durability of response [Internet]. [cited 2022. Feb 10]. Available from: https://www.jnj.com/positive-new-data-for-johnson-johnson-single-shot-covid-19-vaccine-on-activity-against-delta-variant-and-long-lasting-durability-of-response
- 84.Ikegame S, Siddiquey M, Hung C-T, et al. Neutralizing activity of Sputnik V vaccine sera against SARS-CoV-2 variants. Res Sq. 2021. DOI: 10.21203/rs.3.rs-400230/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.ClinicalTrials.gov . Phase II/III study of AZD2816, for the prevention of COVID-19 in adults (AZD2816) [Internet]. [cited 2022. Feb 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT04973449?term=AZD2816&draw=2&rank=1
- 86.ClinicalTrials.gov . A phase 3 study to evaluate the safety, tolerability, and immunogenicity of multiple production lots and dose levels of BNT162b2 RNA-Based COVID-19 vaccines against COVID-19 in healthy participants; 2021.
- 87.ClinicalTrials.gov . A study to evaluate the immunogenicity and safety of mRNA-1273.211 vaccine for COVID-19 variants [Internet]. [cited 2022. Feb 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT04927065?term=NCT04927065&rank=1
- 88.ClinicalTrials.gov . Delayed heterologous SARS-CoV-2 vaccine dosing (boost) after receipt of EUA vaccines [Internet]. [cited 2022. Feb 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT04889209
- 89.US Food and Drug Administration . FDA authorizes booster dose of Pfizer-BioNTech COVID-19 vaccine for certain populations [Internet]. [cited 2022 Feb 10]. Available from: https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-covid-19-vaccine-certain-populations
- 90.World Health Organization . WHO ad hoc consultation COVID vaccines methodological approaches to assess variants effect on vaccine efficacy, effectiveness and impact [Internet]. [cited 2022 Feb 10]. Available from: https://cdn.who.int/media/docs/default-source/documents/r-d-blueprint-meetings/blueprint-covid-vaccines-and-variants-research-methods.pdf?sfvrsn=27feead4_1&download=true
- 91.US Food and Drug Administration . Coronavirus (COVID-19) update: FDA issues policies to guide medical product developers addressing virus variants[Internet]. [cited 2022 Feb 10]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-policies-guide-medical-product-developers-addressing-virus
- 92.European Medicines Agency . Reflection paper on the regulatory requirements for vaccines intended to provide protection against variant strain(s) of SARS-CoV-2 [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-regulatory-requirements-vaccines-intended-provide-protection-against-variant_en.pdf; • This reflection paper outlines the quality, nonclinical and clinical data that would be required to support approval of a variant vaccine.
- 93.Koup RA, Donis RO, Gilbert PB, et al. A government-led effort to identify correlates of protection for COVID-19 vaccines. Nat Med. 2021;27:1493–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.International Coalition of Medicines and Regulatory Authorities . ICMRA COVID-19 vaccine development: future steps Workshop [Internet]. [cited 2022 Feb 10]. Available from: http://www.icmra.info/drupal/covid-19/24june2021
- 95.Australian Government Department of Health . Access consortium: alignment with ICMRA consensus on immunobridging for authorising new COVID-19 vaccines [Internet]. [cited 2022 Feb 10]. Available from: https://www.tga.gov.au/access-consortium-alignment-icmra-consensus-immunobridging-authorising-new-covid-19-vaccines
- 96.Krause PR, Fleming TR, Longini IM, et al. SARS-CoV-2 variants and vaccines. N Engl J Med. 2021;385:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.European Medicines Agency . Procedural guidance for variant strain(s) update to vaccines intended for protection against human coronavirus [Internet]. [cited 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/procedural-guidance-variant-strains-update-vaccines-intended-protection-against-human-coronavirus_en.pdf
- 98.Maxmen A. The fight to manufacture COVID vaccines in lower-income countries. Nature. 2021;597:455–457. [DOI] [PubMed] [Google Scholar]
- 99.European Commission . An U.S.-EU agenda for beating the global pandemic: vaccinating the world, saving lives now, and building back better health security [Internet]. [cited 2022 Feb 10. Available from: https://ec.europa.eu/commission/presscorner/detail/en/statement_21_4846


