ABSTRACT
Introduction
Due to the vaccine’s short supply and the efficacy of a single dose of the ChAdOx1 (AZD1222) vaccine, many governments delayed the interval between prime and boost dose from 4 to 8–12 weeks. However, the waning of immune response in this period is a concern. This study evaluated the durability, contributing factors of anti-RBD antibody concentration, and reactogenicities after the single dose of AZD1222 vaccine in the Thai population.
Methods
This was a single-center, prospective cohort study at Chulabhorn Hospital, Bangkok, Thailand. Individuals 18 years or older who were negative for anti-SARS-CoV-2 antibody were eligible. Anti- receptor-binding domain antibody concentrations were tested at least three weeks after the first vaccination and immediately before the second dose of vaccine. Information on reactogenicities was obtained via a questionnaire sent by a short message service.
Results
Anti-RBD Antibody concentration at 2 and 3 months post-vaccination were significantly higher than at 1 months post-vaccination (20.14 BAU/mL (95%CI; 16.37, 24.77) at 1 month, 48.08 BAU/mL (95%CI; 42.76, 54.08) at 2 month, and 65.01 BAU/mL (95%CI; 58.88,71.61) at 3 month). Adverse events occurred in approximately 60% of participants. Factors influencing vaccine immunogenicity include age, sex, the time elapsed from the first dose of vaccine, and underlying disease with diabetes and hematologic disease.
Conclusion
A single dose of AZD1222 could elicit immune responses that did not decline within three months in Thai individuals. These data support the public health strategy of a delay between the prime and boost dose of AZD1222 of 4 to 12 weeks.
KEYWORDS: ChAdOx1, AZD1222, COVID-19 vaccine, single-dose vaccine, immunogenicity, safety
Introduction
All three arms of adaptive immunity, including antibody responses, CD4+ T cell responses, and CD8+ T cell responses, are required to prevent and control the COVID-19.1 Antibodies targeting the receptor-binding domain (RBD) of the spike protein of SARS-CoV-2 can potently neutralize the virus.2 The ChAdOx1 (AZD1222) vaccine developed by Oxford University and AstraZeneca Biopharmaceuticals consists of a replication-deficit simian adenoviral vector containing the full-length DNA sequence of the spike protein. Clinical studies of the ChAdOx1 (AZD1222) vaccine in the United Kingdom, South Africa, and Brazil showed excellent efficacy in preventing symptomatic SARS-CoV-2 infection and severe COVID-19 after two doses of the vaccine spaced at least four weeks apart.3,4 Moreover, three weeks after a single dose of the ChAdOx1 (AZD1222) vaccine, recipients were protected from symptomatic SARS-CoV-2 infection for 12 weeks.5 In addition to the clinical study results, neutralizing antibody levels also predict vaccine efficacy. Higher neutralizing antibody titers are generally associated with increased vaccine efficacy.6 Salazar et al. found that anti-RBD immunoglobulin titers correlated with in vitro virus neutralization.7 Due to the vaccine’s short supply and the efficacy of a single dose of the ChAdOx1 (AZD1222) vaccine, many governments, including Thailand, delayed the interval between prime and boost dose from 4 to 8–12 weeks. However, the waning of immune response in this period is a concern.8
This study aims to evaluate the durability, contributing factors of anti-RBD antibody concentration, and reactogenicities after a single dose of AZD1222 vaccine in the Thai population.
Materials and methods
Study design and participants
This was a single-center, prospective cohort study designed to evaluate the durability, influence factors of anti-RBD antibodies concentration, and reactogenicities after a single dose of the ChAdOx1 (AZD1222) vaccine in Thai health care workers at Chulabhorn Hospital, Bangkok, Thailand. The health care workers in our institute who plan to vaccinate with AZD1222 vaccine and are interested in joint this study were recruited. Participants were recruited between March 31, 2021, and May 5, 2021. Individuals 18 years or older who were negative for anti-SARS-CoV-2 antibody were eligible. Individuals who had previously received COVID-19 vaccines, pregnant or lactating women, patients receiving steroids or immunosuppressive agents, and patients with active underlying diseases were excluded. Written informed consent was obtained from each participant before enrollment. The study protocol, case records form, and consent form were reviewed and approved by the Ethics Committee for Human Research, Chulabhorn Research Institute (reference number: 022/2564).
Procedures
Participants were tested for baseline anti-RBD immunoglobulin titers following enrollment in the study using a 6 mL serum sample. Sera were sent to the central laboratory department of the Chulabhorn hospital and processed within 6 hours. Identical procedures were repeated three weeks after the first dose of the vaccine and before the second dose of the vaccine. The ChAdOx1 (AZD1222) vaccine used in this study was manufactured and vialed following Good Manufacturing Practices and was approved by the regulatory agency of Thailand. All vaccine doses contained 5–6.5 × 1010 viral particles. The registered nurses administered the vaccine by intramuscular injection into the deltoid muscle.
Safety
After 30 minutes of observation to monitor the immediate adverse event, participants could go home. On days 1 and 7 post-vaccinations, participants were queried regarding reactogenicities via a questionnaire sent by a short message service. The severity of adverse events was graded by participants (mild vs. moderate vs. severe severity).
Measurement of anti-RBD immunoglobulin titers
Serum levels of anti-RBD immunoglobulin were measured using the Elecsys Anti-SARS-CoV-2 S (Elecsys-S) kit (Roche Diagnostics, Mannheim, Germany), an automated electrochemiluminescence immunoassay (ECLIA). The measurement and validation were performed according to the manufacturer's instruction. The Elecsys-S uses the double-antigen sandwich principle to detect anti-S protein antibodies. The measurement range was 0.4 to 2,500 U/mL. The manufacturer’s suggested cutoff value for a positive result was >0.8 U/mL. Based on the international standard for anti-SARS-CoV-2 immunoglobulin titers developed by the WHO,9 Elecsys-S U was converted to binding antibody units (BAU) using the equation: Elecsys-S U = 0.972 × BAU. The threshold using Elecsys-S for detection of neutralizing antibodies (1:20) following natural infection was 133 BAU/mL (2.12 log10). Using this cut-point value, the specificity and sensitivity were 84% and 74.5%, respectively. However, the Elecsys-S assay cannot differentiate between high and low neutralizing antibody titers.10
Primary endpoints
The primary endpoint was the geometric mean concentration (GMC) of anti-RBD antibody at 2 and 3 months after a single dose of AZD1222 compared with the GMC at the first month. One month was defined as four weeks.
The secondary endpoints were the contributing factor that correlated with the anti-RBD antibody concentration and the reactogenicities within seven days following vaccination.
Statistical analysis
Summary statistics were presented as medians and interquartile ranges (IQRs) as well as geometric means and 95% confidence intervals (CIs). Levels of anti-RBD immunoglobulin among different time points were compared using multiple linear regression, using the first month as the reference group. Correlation between anti-RBD immunoglobulin levels and contributing factors was assessed using multiple linear regression. Statistical analyses were performed using IBM SPSS statistic version 26 and GraphPad Prism version 9. Values of P < .05 were considered statistically significant.
Results
Participants
A total of 864 participants were recruited. However, we excluded 70 participants from this analysis. Nine participants had no baseline anti-RBD antibody level, 3 participants with prior seropositive before vaccination, and 58 participants with no anti-RBD antibody data after vaccination. Finally, there were 796 participants in this study.
Approximately two-thirds were female (517, 64.9%). The median age was 40 years (IQR 30–57 years). Demographic data and anti-RBD antibody titers are summarized in Table 1. We planned to measure anti-RBD antibodies two times, the first time at 3 weeks after the first dose and the second time before the second dose. Some participants did not convenient for anti-RBD antibodies measurement at 3 weeks after vaccination. Therefore, we allowed them to measure anti-RBD antibodies within 7 weeks. One thousand one hundred fifty-five serum samples measured anti-RBD antibodies after the first dose and before the second dose of the vaccines. Three hundred sixty-one participants were measured anti-RBD antibodies two times, and 433 were measured one time. The interval between the first and second dose of vaccine in the initial study protocol was 12 weeks. However, during the study period, outbreaks of the delta variant of SARS-CoV-2 occurred in Thailand. The Thai national COVID-19 vaccine guideline-recommended shortening the interval between the first and second dose of the AZD1222 vaccine to 8 weeks. So, the participants in this study have received the second dose at an 8–11 week interval (Table 2).
Table 1.
Demographic characteristics of study participants
| All participants (N = 794) | |
|---|---|
| Female, n (%) | 517 (65.1%) |
| Age (years), median (IQR) | 40 (30, 57) |
| Underlying comorbidities | |
| Cardiovascular disease | 5 (0.63%) |
| Diabetes | 11 (1.39%) |
| Obese | 1 (0.13%) |
| Hypertension | 26 (3.27%) |
| Dyslipidemia | 27 (3.40%) |
| Hematologic disease | 8 (1.00%) |
IQR, interquartile range. Obese; body mass index > 25 kg/m2.
Table 2.
The geometric mean concentration (GMC) of anti-RBD antibody after a single dose of the AZD1222 vaccine (weeks)
| Time elapsed from first dose of AZD1222 (weeks) | Sample (N) | Geometric mean concentration of anti-SARS-CoV-2 antibody concentration (95%CI) (BAU/mL) | Geometric mean ratio (95%CI)* |
|---|---|---|---|
| Baseline level | 794 | < 0.4 | |
| 3 | 118 | 14.80 (10.81, 20.23) | Reference |
| 4 | 120 | 27.23 (20.84,35.56) | 1.79 (1.30, 2.48) |
| 5 | 123 | 46.56 (37.07,58.34) | 3.05 (2.22, 4.18) |
| 6 | 251 | 48.87 (41.50, 57.54) | 3.79 (2.86, 5.01) |
| 7 | 63 | 47.97 (37.41, 61.52) | 4.78 (3.21, 7.11) |
| 8 | 1 | 65.48 | 4.28 (0.36, 50.58) |
| 9 | 66 | 56.75 (42.46, 75.86) | 4.20 (2.88, 6.12) |
| 10 | 382 | 65.16 (58.48, 72.44) | 4.47 (3.43, 5.81) |
| 11 | 31 | 83.75 (55.98, 125.31) | 5.58 (3.41, 9.16) |
*adjust with age, sex, underlying disease, GMR; geometric mean ratio, 95%CI; 95% confidence interval.
Anti-RBD antibodies
Anti-RBD antibodies were detectable as early as three weeks after a single dose of the ChAdOx1 (AZD1222) vaccine. At three weeks, the geometric mean concentration was 14.80 (95% CI, 10.81, 20.23) BAU/mL. The concentration of anti-RBD antibodies reached high levels at five weeks post-vaccination (GMC, 46.56 (95%CI, 37.07–58.34) BAU/mL) and persisted or slightly increased through 11 weeks post-vaccination (Figure 1 and Table 2). Anti-RBD Antibody concentration at 2 and 3 months post-vaccination were significantly higher than at 1 months post-vaccination (20.14 BAU/mL (95%CI; 16.37, 24.77) at 1 month, 48.08 BAU/mL (95%CI; 42.76, 54.08) at 2 month, and 65.01 BAU/mL (95%CI; 58.88,71.61) at 3 month). The GMR was 2.72 (95%CI; 2.21–3.33) for two months and 3.33 (95%CI; 2.73–4.05) for three months compared with the first month (Figure 2 and Table 3). Based on multiple regression modeling, sex, age, underlying comorbidity with diabetes or hematologic disease, and time elapsed from first vaccination were correlated with anti-RBD antibody concentration. (Table 4). Men had 29% (95%CI; 16%-39%) lower anti-RBD antibodies concentration than women. Time elapsed from the first dose through week-11 post-vaccination was also correlated with anti-RBD antibody levels. The anti-RBD antibody level increased on average by 16% (95%CI; 13%-20%) every week from the first dose through 11 weeks post-vaccination. Age was also correlated with anti-RBD antibody levels. For every 10-year increase in age, the mean anti-RBD antibody level decreased by 15% (95%CI; 10%-20%). Participants with diabetes or hematologic disease comorbidity had 55% (95%CI; 23%-84%) and 56% (95%CI; 3%-80%) lower anti-RBD antibodies concentration. (Table 4).
Figure 1.
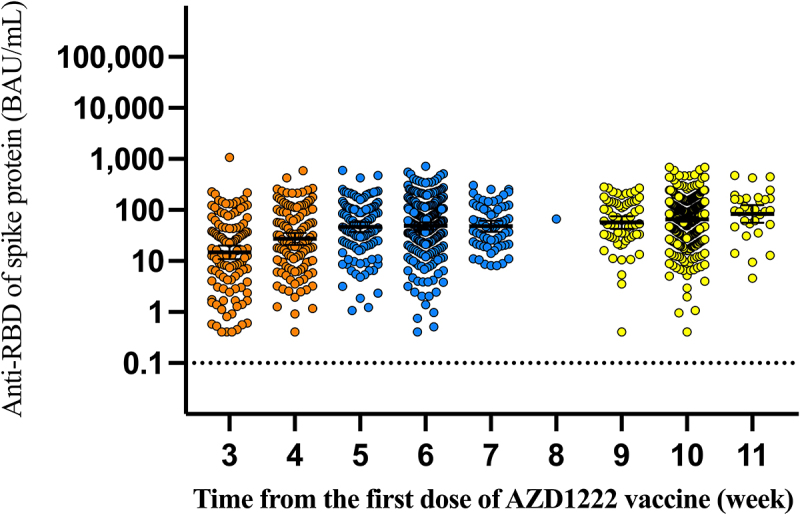
Anti-RBD antibody levels over 11 weeks following the first dose of the AZD1222 vaccine.
Figure 2.
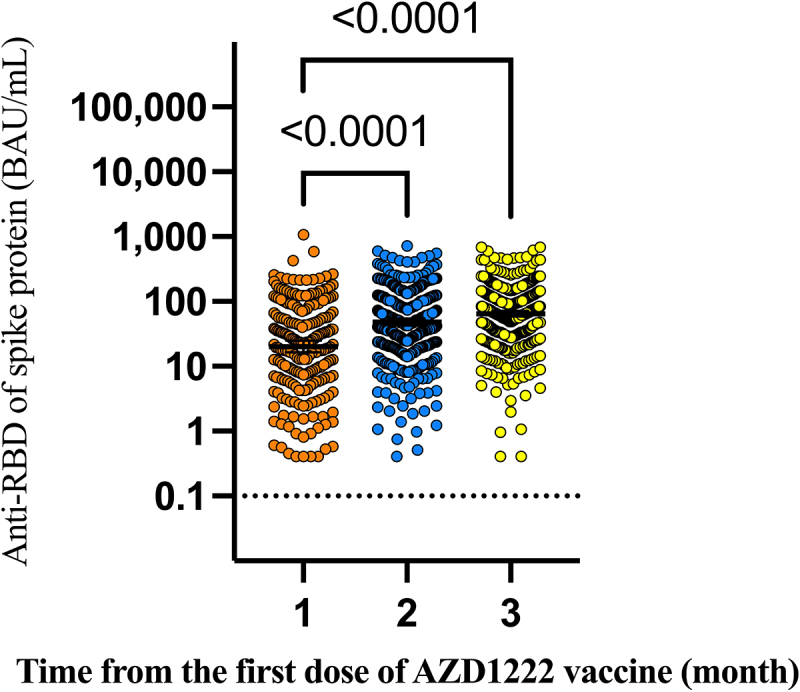
Anti-RBD antibody levels over 3 months following the first dose of the AZD1222 vaccine.
Table 3.
The geometric mean concentration (GMC) of anti-RBD antibody after a single dose of the AZD1222 vaccine (months)
| Time elapsed from the first dose of AZD1222 (month) | Sample (N) | GMC of anti-SARS-CoV-2 antibody (95%CI) (BAU/mL) | GMR (95%CI)* |
|---|---|---|---|
| Baseline level | 794 | 0.4 | |
| 1 | 238 | 20.14 (16.37, 24.77) | reference |
| 2 | 438 | 48.08 (42.76, 54.08) | 2.72 (2.21–3.33) |
| 3 | 479 | 65.01 (58.88,71.61) | 3.33 (2.73–4.05) |
*adjust with age, sex, underlying disease, GMR; geometric mean ratio, 95%CI; 95% confidence interval.
Table 4.
Linear regression analysis of contributing factors of anti-RBD antibody concentration (antilogarithm)
| Predictor | Adjusteda (95%CI) |
|---|---|
| R2 | 0.132 |
| Sex | |
| Female | reference |
| Male | 0.71 (0.61–0.84) |
| Age (decades) | 0.85 (0.80–0.90) |
| Time elapsed from first dose of AZD1222 (weeks) | 1.16 (1.13–1.20) |
| Underlying comorbidity | |
| Cardiovascular disease | 0.11 (0.39–1.65) |
| Diabetes | 0.45 (0.26–0.77)* |
| Obese | 0.48 (0.04–5.79) |
| Hypertension | 0.90 (0.58–1.39) |
| Dyslipidemia | 0.64 (0.40–1.04) |
| End-stage kidney disease | 0.20 (0.02–2.32) |
| Hematologic disease | 0.44 (0.20–0.97)* |
aadjust with age, sex, underlying comorbidity, and time elapsed after the first dose. 95%CI; 95% confidence interval. * p-value <.05.
Safety
The reactogenicities were common after a single dose of the ChAdOx1 (AZD1222) vaccine. Overall, 322 of 538 participants (59.9%) reported adverse events who responded to the questionnaire. Most adverse events developed within 24 hours following vaccination. Adverse events were mild to moderate in most participants. Systemic adverse events were more common than local reactions. The most common adverse events that developed within 24 hours were myalgia (50.56%), fever (48.51%), headache (43.31%), fatigue (40.15%), and injection site reactions (24.35%) (Figure 3).
Figure 3.
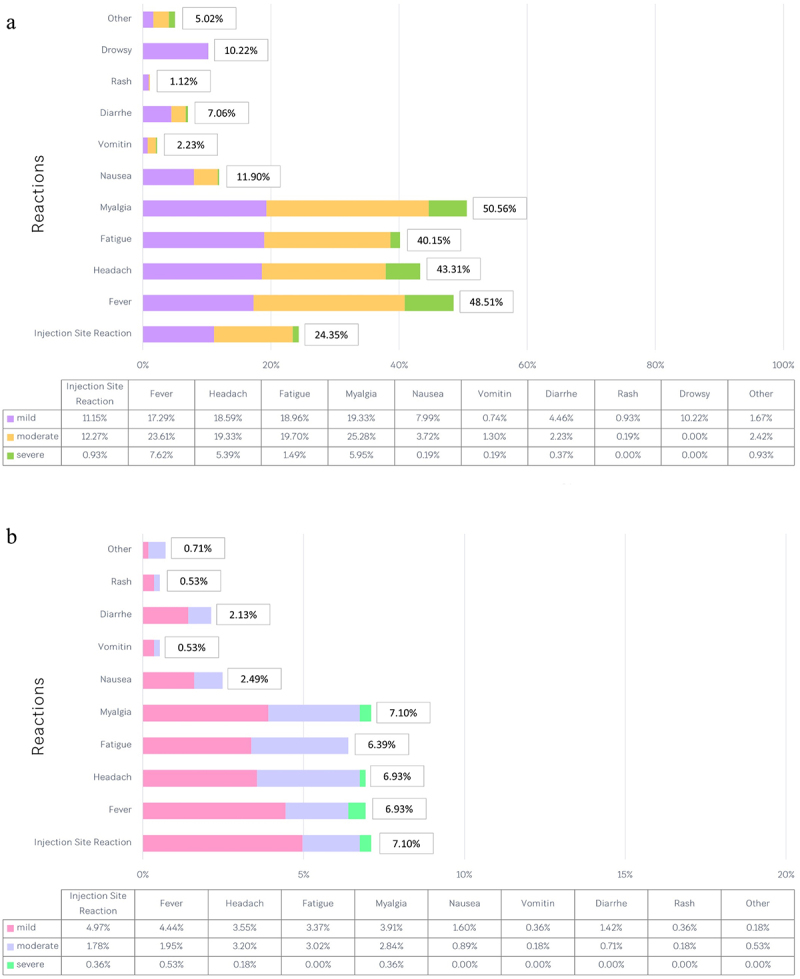
Reactogenicities after a first dose of AZD1222 vaccine within 7 days.
Discussion
The results of this study indicate that a single dose of the ChAdOx1 (AZD1222) vaccine can elicit antibody responses as early as three weeks post-vaccination and persist through at least three months without declining. Female participants had significantly higher anti-RBD antibody concentrations compared with male participants. Participants with older age had a lower immune response. Diabetes and hematologic disease reduce the immune response after vaccination. The reactogenicities after vaccination were common. However, most participants had mild to moderate degrees of severity.
This finding contrasts with the results of Flaxman et al., showing that a single dose of ChAdOx1 (AZD1222) stimulated immune responses that declined over 320 days after a single dose of the AZD1222 vaccine. This study enrolled 480 participants with an 18–55 year age range, median age 37.2 years (IQR; 29.0–47.0). The GMR of anti-spike protein at day 180 was 0.51 (95%CI; 0.45–0.57) compared with day 28 post-vaccination.8 The participants’ age in this study was similar to ours. The different demographic data were ethnicity and sex, half of the participants (49.4%) were female, and most of the participants (90.6%) were white. The median body mass index (BMI) of participants in the Flaxman et al. study was 24.9 (IQR; 22.4–27.8). Our results also contrast with those of immune responses to mRNA vaccination studies. The mRNA-1273 vaccine-elicited peak neutralizing antibody levels two weeks after the second dose of the vaccine declined slowly with a half-life of 2 to 3 months.11 This finding supported the public health strategy of a delay between the first and second dose of ChAdOx1 (AZD1222) of 4 to 12 weeks5 when vaccine supply is limiting, especially in developing countries.
The reason why our finding differences from those of other studies was unknown. There are a few hypotheses that expand our study result. First, the antigen carried by the adenoviral vector can persist for a long time. A mice model study showed that the transgene sequence of a viral vector could be detected for up to 1 year in the liver, spleen, and inoculated muscle after inoculum with the viral vector vaccine. Moreover, the protein produced by the transgene was detected at least 5 weeks after vaccination. The transgene product-specific memory T cells were also detected 405 days after vaccination.12 The sustain of memory T cells may reflect the prolonged transgene expression of the viral vector vaccine.13 The persistence of the vector genome and transgene protein was also demonstrated in nonhuman primates (NPH) for up to 22 months.14 The clinical study also demonstrated the persistence of the immune response after the adenoviral vector vaccination. After a single dose of the ChAd3-EBO-Z vaccine in phase 1/2a clinical, the IgG antibodies against glycoprotein were peaked on day 28 and persisted through day 180 with an approximate 6 months half-life.15 Moreover, the long half-life of antibody response was found after a single dose of the ChAdOx1 (AZD1222) vaccine.8 Contrast with the mRNA vaccine platform, which has a half-life of antibody 2 to 3 months.11 Second, the bodyweight of each individual may affect the vaccine response. The lower body weight individuals tend to have more immune response with the same dose of vaccine. For example, the study of the BNT162b2 vaccine in adolescents aged 12–15 years compared with those aged 16–25 years using 30 µg of the BNT162b2. The result found that the adolescent group had more immune response with the GMR of neutralizing titer 1.76 (95%CI, 1.47–2.10).16 However, this effect was not seen after the mRNA1273 vaccination in adolescents.17 The participants in South-East Asia had significantly lower body mass index compared with North-West Europe.18 Third, the different immune responses may be due to different ethnicities. The polymorphism in major histocompatibility complex (MHC) genes, pattern recognition receptors (PRRs), or single-nucleotide polymorphisms (SNPs) have been demonstrated to be associated with variations in an immune response.19 For example, during the COVID-19 pandemic, evidence supports those Black and South Asian individuals had a higher hospitalization rate, ICU admission, and death than White individuals.20 For the influenza A vaccine, the polymorphism of the IGHV-1 69 gene modulates anti-influenza repertoire expression. Moreover, the distribution of the IGHV-1 69 alleles was impacted by ethnicity.21 Further research is needed to establish the impact of the polymorphism in the COVID-19 vaccine.
To our knowledge, this is the first study that showed a single dose of the AZD1222 vaccine could elicit a long-lasting immune response without decline within three months.
Limitations of this study included 1) drop-out of participants who did not complete two times of anti-RBD antibody measurement. This is the major confounding factor that interferes with the study result. 2) Timing of anti-RBD antibodies measurement in each participant was different in the majority of participants. This factor also interferes with the result of the study.
In conclusion, we demonstrated the persistence without a decline of antibody responses for 11 weeks after a single dose of the ChAdOx1 (AZD1222) vaccine in a Thai population with mild to moderate reactogenicities. Factors influencing vaccine immunogenicity were age, sex, time following the first dose of vaccine, and diabetes or hematologic disease comorbidity.
Acknowledgments
We thank the clinical research management unit for managing this project. We also thank the central laboratory center of Chulabhorn Hospital for anti-RBD antibody testing. Dr. Chirayu Auewarakul for supporting this project. We gratefully acknowledge funding from Chulabhorn Royal Academy. We thank Edanz (www.edanz.com/ac) for editing a draft of this manuscript.
Funding Statement
This study was supported by funding from Chulabhorn Royal Academy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, Belanger S, Abbott RK, Kim C, Choi J, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers TF, Zhao F, Huang D, Beutler N, Burns A, He WT, Limbo O, Smith C, Song G, Woehl J, et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369:956–6. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–11. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 7.Salazar E, Kuchipudi SV, Christensen PA, Eagar TN, Yi X, Zhao P, Jin Z, Long SW, Olsen RJ, Chen J, et al. Relationship between anti-spike protein antibody titers and SARS-CoV-2 in vitro virus neutralization in convalescent plasma. bioRxiv. 2020;2020.06.08.138990. doi: 10.1101/2020.06.08.138990. [DOI] [Google Scholar]
- 8.Flaxman A, Marchevsky N, Jenkin D, Aboagye J, Aley PK, Angus BJ, Belij-Rammerstorfer S, Bibi S, Bittaye M, Cappuccini F, et al. Tolerability and immunogenicity after a late second dose or a third dose of ChAdOx1 (AZD1222) nCoV-19 (AZD1222). SSRN. 2021. https://ssrn.com/abstract=3873839 or doi: 10.2139/ssrn.3873839. [DOI] [Google Scholar]
- 9.Kristiansen PA, Page M, Bernasconi V, Mattiuzzo G, Dull P, Makar K, Plotkin S, Knezevic I.. WHO international standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397:1347–48. doi: 10.1016/S0140-6736(21)00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Resman Rus K, Korva M, Knap N, Avsic Zupanc T, Poljak M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J Clin Virol. 2021;139:104820. doi: 10.1016/j.jcv.2021.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doria-Rose N, Suthar MS, Makowski M, O’Connell S, McDermott AB, Flach B, Ledgerwood JE, Mascola JR, Graham BS, Lin BC, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for COVID-19. N Engl J Med. 2021;384:2259–61. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-mccoy KC, Hensley SE, Zhou D, Lin S-W, Bian A, Xiang ZQ, Iparraguirre A, et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood. 2007. Sept 15;110(6):1916–23. doi: 10.1182/blood-2007-02-062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finn JD, Bassett J, Millar JB, Grinshtein N, Yang TC, Parsons R, Evelegh C, Wan Y, Parks RJ, Bramson JL, et al. Persistence of transgene expression influences CD8+ T-cell expansion and maintenance following immunization with recombinant adenovirus. J Virol. 2009. Dec;83(23):12027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penaud-Budloo M, Le Guinea C, Nowrouzi A, Toromanoff A, Cherel Y, Chenuaud P, Schmidt M, von Kalle C, Rolling F, Moullier P, et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J Virol. 2008. Aug;82(16):7875–85. doi: 10.1128/JVI.00649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Santis O, Audran R, Pothin E, Warpelin-Decrausaz L, Vallotton L, Wuerzner G, Cochet C, Estoppey D, Steiner-Monard V, Lonchampt S, et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis. 2016. Mar;16(3):311–20. doi: 10.1016/S1473-3099(15)00486-7. [DOI] [PubMed] [Google Scholar]
- 16.Frenck RW Jr., Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, Perez JL, Walter EB, Senders S, Bailey R, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021. July 15;385(3):239–50. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali K, Berman G, Zhou H, Deng W, Faughnan V, Coronado-Voges M, Ding B, Dooley J, Girard B, Hillebrand W, et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med. 2021. Dec 9;385(24):2241–51. doi: 10.1056/NEJMoa2109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menigoz K, Nathan A, Turrell G. Ethnic differences in overweight and obesity and the influence of acculturation on immigrant bodyweight: evidence from a national sample of Australian adults. BMC Public Health. 2016. Sept 5;16:932. doi: 10.1186/s12889-016-3608-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jethwa H, Wong R, Abraham S. Covid-19 vaccine trials: ethnic diversity and immunogenicity. Vaccine. 2021. June 16;39(27):3541–43. doi: 10.1016/j.vaccine.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathur R, Rentsch CT, Morton CE, Hulme WJ, Schultze A, MacKenna B, Eggo RM, Bhaskaran K, Wong AYS, Williamson EJ, et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet. 2021. May 8;397(10286):1711–24. doi: 10.1016/S0140-6736(21)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avnir Y, Watson CT, Glanville J, Peterson EC, Tallarico AS, Bennett AS, Qin K, Fu Y, Huang C-Y, Beigel JH, et al. IGHV1-69 polymorphism modulates anti-influenza antibody repertoires, correlates with IGHV utilization shifts and varies by ethnicity. Sci Rep. 2016. Feb 16;6:20842. doi: 10.1038/srep20842. [DOI] [PMC free article] [PubMed] [Google Scholar]


