Abstract
Background
A considerable proportion of individuals report persistent, debilitating and disparate symptoms despite resolution of acute COVID-19 infection (i.e. long COVID). Numerous registered clinical trials investigating treatment of long COVID are expected to be completed in 2021–2022. The aim of this review is to provide a scope of the candidate treatments for long COVID. A synthesis of ongoing long COVID clinical trials can inform methodologic approaches for future studies and identify key research vistas.
Methods
Scoping searches were conducted on multiple national and international clinical trial registries. Interventional trials testing treatments for long COVID were selected. The search timeline was from database inception to 28 July 2021.
Results
This scoping review included 59 clinical trial registration records from 22 countries with a total projected enrolment of 6718. Considerable heterogeneity was exhibited amongst component records with respect to the characterization of long COVID (i.e. name, symptoms- including frequency, intensity, trajectory and duration- mode of ascertainment, and definition of acute phase). In addition, the majority of proposed interventions were non-pharmacological and either targeted multiple long COVID symptoms simultaneously, or focussed on treatment of respiratory/pulmonary sequelae. Multiple interventions targeted inflammation, as well as tissue oxygenation and cellular recovery, and several interventions were repurposed from analogous conditions.
Conclusions
The results of this scoping review investigating ongoing clinical trials testing candidate treatments for long COVID suggest that a greater degree of definitional stringency and homogeneity is needed insofar as the characterization of long COVID and inclusion/exclusion criteria.
Keywords: Long COVID, post-COVID-19 condition, fatigue, dyspnoea, treatment, rehabilitation
Introduction
As the portrait of COVID-19 comes into focus, the scientific and public emphasis has turned to the sequelae of acute infection. An estimated 10–30% of individuals experience persistent symptoms following resolution of acute COVID-19 (https://www.sciencedirect.com/science/article/pii/S0889159121006516) [1–4], a phenomenon colloquially termed ‘long COVID’. The diverse clinical spectrum of long COVID includes respiratory (e.g. dyspnoea and cough), physical (e.g. myalgia and arthralgia), neurocognitive (e.g. cognitive impairment, brain fog, fatigue, and anosmia) and affective (e.g. depression) symptoms [3].
Multiple definitions of long COVID exist. In October 2021, the World Health Organization (WHO) proposed the moniker ‘post COVID-19 condition’, defined as persistent symptoms usually occurring 3 months from onset in individuals with past confirmed or probable SARS-CoV-2 infection and persisting for at least 2 months which cannot be explained by an alternative diagnosis [4]. The Centres for Disease Control and Prevention (CDC) has operationalized a definition of ‘post-COVID conditions’ as symptoms persisting 4 or more weeks after infection [5]. The National Institutes of Health (NIH) has referred to symptoms persisting beyond the initial stages of COVID-19 as ‘Post-Acute Sequelae of SARS-CoV-2 infection (PASC)’ [6]. In addition, the National Institute for Health and Care Excellence (NICE) defines ‘post-COVID-19 syndrome’ (PCS) as a constellation of symptoms which develop during or following COVID-19 infection, persist for more than 12 weeks, and are not sufficiently explained by alternative diagnoses [7]. Symptoms enduring for 4–12 weeks are termed ‘ongoing symptomatic COVID-19’ [7].
Individuals with long COVID report significant functional impairment, quality of life decrement, and socioeconomic burdens associated with the foregoing persistent symptoms [2,3,8]. There is consensus that an estimate of over two billion persons may have been infected with SARS-CoV-2 [9–11], suggesting that long COVID is a global public health priority. The burden associated with long COVID exists alongside a separate body of literature affirming that rates of stress-related mental disorders (e.g. major depressive disorder) have been increasing during the pandemic, adding further complexity to diagnostic considerations [12]. Notwithstanding the rapid emergence of long COVID clinics for multidisciplinary rehabilitation [13], there is no established and/or effective treatment for long COVID syndrome. However, clinical research efforts investigating potential interventions for long COVID are underway, targeting putative underlying mechanisms including, but not limited to, dysregulation of the immune-inflammatory axis, autonomic dysfunction, systemic and central endotheliopathy, and residual organ pathology (e.g. lung fibrosis).
The high estimated global case rate of COVID-19 provides the impetus to scope candidate treatments for long COVID. The results of this review are intended to highlight treatments which may soon become available, research timelines, possible mechanisms subserving disparate long COVID symptoms and applicable biological targets, as well as key limitations of current trials and unmet needs, in order to guide future research.
Methods
Search strategy
The protocol pertaining to this scoping review was registered on the Open Science Framework (OSF) Registries (https://osf.io/gjhp6). The Joanna Briggs Institute (JBI) framework for scoping reviews [14] was used to guide reporting, as well as previously established methodology from our group for conducting reviews of ongoing clinical trials [15,16]. In accordance with the 2018 Preferred Reporting Items for Systematic Reviews and Meta Analyses guidelines: scoping review extension (PRISMA-ScR) [17], a scoping search was conducted from database inception to 28 July 2021.
The following databases were independently searched by two reviewers (FC and AL): NIH clinicaltrails.gov, Health Canada Clinical Trials Database, WHO International Clinical Trials Registry Platform (ICTRP), European Union Clinical Trials Register (EU CTR), and the Australia New Zealand Clinical Trials Registry (ANZCTR). No publication date or language restrictions were imposed. The search string implemented was: ‘long covid’ OR ‘persistent covid’ OR ‘post covid’ OR ‘post-acute sequelae of SARS-CoV-2 PASC’ OR ‘enduring COVID-19 sequelae’ OR ‘long-haul covid’ OR ‘long-tail covid’ OR ‘post-COVID-19 syndrome’. Only ‘COVID-19’ was searched on the Health Canada Clinical Trials Database as the foregoing query generated zero results.
Titles and summaries were independently screened by two reviewers (FC and AL). Trial registration records identified as potentially relevant by at least one reviewer were retrieved and duplicates were removed. Full-text registrations were independently screened by two reviewers (FC and AL), with discrepancies resolved through discussion.
Inclusion and exclusion criteria
We sought clinical trial registration records proposing interventions intended to treat long COVID, variably defined as sequelae of COVID-19 infection persisting beyond resolution of the acute phase of disease. Inclusion criteria were established prior to record review and were as follows:
Treatment group comprises individuals of any sex, ethnicity, or age with long COVID (i.e. experiencing persistent symptoms reasonably attributable to prior SARS-CoV-2 infection, as ascertained by investigators).
Experimental study design; controlled or non-controlled, open label or masked, and any status except ‘withdrawn/no longer available’.
Intervention is intended to ameliorate long COVID (defined previously).
Exclusion criteria were:
Non-experimental study design (e.g. no intervention/exposure assigned by investigator, symptoms are only characterized/profiled).
Study population solely comprises healthy volunteers.
Persistent symptoms are not reasonably attributable to prior SARS-CoV-2 infection.
Trial status is ‘withdrawn’ or ‘no longer available’.
Intervention is not intended to treat long COVID; including treatment of acute COVID-19, symptoms in the immediate post-discharge period (i.e. deconditioning rehabilitation), or symptoms resulting from severe complications of COVID-19 (e.g. post-intensive care syndrome, lung fibrosis due to acute respiratory distress syndrome [ARDS]).
Pre- or post-exposure prophylaxis for COVID-19, or prevention of long COVID symptoms (e.g. by administration of the intervention during the acute phase of disease).
Insufficient information provided to ascertain inclusion or exclusion.
Data extraction and synthesis
Data from component records were extracted by four reviewers (FC, AL, MYJ, and MY) using a piloted data extraction form, with each record independently extracted by a minimum of two reviewers. Data were then corroborated, with discrepancies resolved through discussion. Information to be extracted was established a priori and included registration number, country of recruitment, sponsor, estimated/actual end date, study design (allocation, masking model, controlled/non-controlled), duration and phase, age range of participants, estimated/actual enrolment, long COVID characterization, trial aim, long COVID symptom targeted, intervention (description, dosage, frequency and duration), rationale/mechanism of action applicable to long COVID and primary outcome measure(s).
Included records underwent qualitative analysis via narrative synthesis. Methodological quality and risk of bias were not assessed owing to the succinct and preliminary nature of clinical trial registration records.
Results
Overview of trials
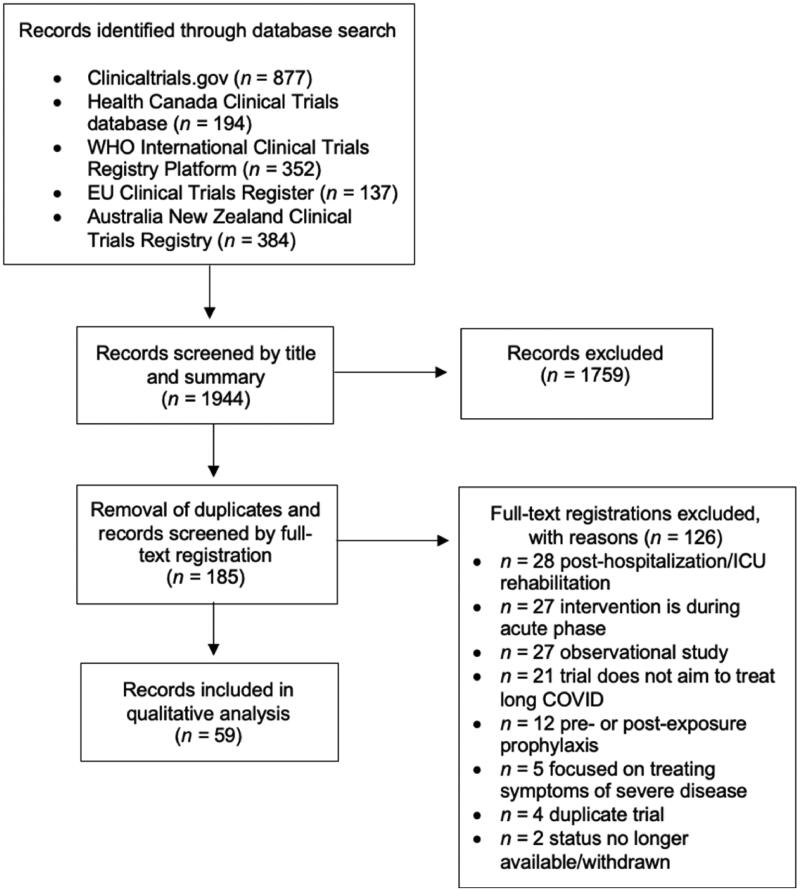
The database searches yielded a total of 1945 registration records, all of which were screened by title and summary. Following removal of duplicates, 185 records were screened by review of full-text, resulting in further exclusion of 126 records. Details of record selection are provided in Figure 1. In total, 59 clinical trial registration records were included in the review: 44 (74.6%) randomized controlled trials and 15 (25.4.1%) non-randomized and/or non-controlled trials. Thirty-four (57.6%) trial designs involved masking whereas 25 (43.4%) trials were open label. Of 24 (40.7%) trial registrations which specified a phase, 8 were Phase II, 6 were Phase III, 3 were Phase IV, 2 were Phase 0/I and Phase II/III, and 1 was Phase I. No trials had posted results at the time of writing.
Figure 1.
Flow diagram of registration record selection.
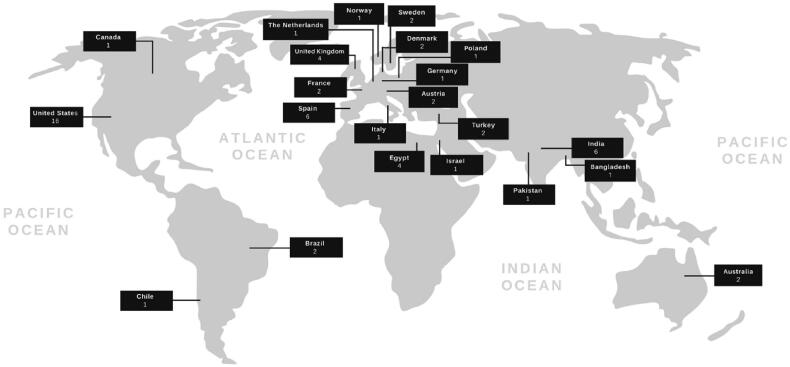
The global distribution of trials is depicted in Figure 2. Total projected enrolment was 6718 persons, estimated/actual recruitment ranged from 5 to 1500, and the mean sample size was 113.9 (SD: 214.5) persons. A majority of trials specified adult participants (i.e. 18+ years), with one trial including individuals of all ages and another including persons aged 8–88 years. Most trials are expected to be completed in 2021–2022. Trial durations ranged from 2 to 78 weeks, with a mean duration of 13.5 weeks (SD: 14.9). Table 1 in the Supplement provides detailed characteristics for all 59 component records. All trial registrations were included in qualitative analysis via narrative synthesis.
Figure 2.
Global distribution of included trials (n = 59).
Table 1.
Interventions organized by treatment category.
| Pharmacotherapy | Biologic | Dietary supplement | Homeopathic/alternative medicine | Treatment/procedure | Rehabilitation programme/therapy |
|---|---|---|---|---|---|
|
|
|
|
|
|
Subcategories are not mutually exclusive; for example, under the ‘Rehabilitation Program/Therapy’ category, one trial may have been counted as part of multiple sub-groups (e.g. both exercise-based rehabilitation programme and rehabilitation programme with virtual component) if both components were specified in the program description.
CBD: cannabidiol
Long COVID characterization
Component records exhibited considerable heterogeneity regarding the syndrome of persistent post-infection symptoms (i.e. the condition under investigation), which was differentially termed ‘long COVID’, ‘post-COVID-19 syndrome (PCS)’, post-acute sequelae of COVID-19 (PACS) and ‘SARS-CoV-2 post-viral fatigue syndrome’. A majority of trials specified a requirement for a prior confirmed positive diagnosis of COVID-19 (i.e. by reverse transcription-polymerase chain reaction, serology, and/or antigen testing) in addition to symptoms persisting beyond resolution of the acute phase of disease or for several weeks following infection. Although most trials did not specify how resolution of the acute phase would be determined, seven trials included a requirement for at least one negative COVID-19 test prior to enrolment, thus operationalizing the resolution of the acute phase as the lack of viral shedding.
Thirty-two trials (54.2%) did not specify a required symptom duration, however, amongst records which specified the foregoing criteria, the minimum qualifying duration of symptom persistence varied between 3 weeks and 3 months. The most frequently listed durations were 4 and 12 weeks, concordant with the CDC and NICE definitions, respectively. Only one study required participants to be formally diagnosed with post-COVID-19 condition as per ICD-10 U09.9.
Frequently listed persistent symptoms were: respiratory/pulmonary sequelae (i.e. dyspnoea, pulmonary fibrosis and cough), fatigue, decreased exercise tolerance, cognitive impairment, headache, anxiety, depression, insomnia and myalgias or arthralgias. A majority of trials assessed persistent symptoms using a validated scale (e.g. Chalder fatigue scale [CFQ], modified Borg scale) or test (e.g. 6-min walk test [6MWT]), although a few trials ascertained symptoms via self-report. Although most trials did not specify symptom intensity, several trials required qualifying symptoms to meet scale cut-offs (e.g. score ≥ 3 on the Somatic Symptom Scale-8 [SSS-8], FACIT fatigue score ≤ 29) or physiological testing limits (e.g. abnormal diffusion capacity for carbon monoxide [DLCO]). In addition, one study specified that persistent symptoms must be present for a minimum of 4 d a week, and two trials stated that symptoms must have resulted in reduced physical functionality and/or disability. Characterizations of long COVID by each component record are listed in Supplementary Table 1.
Overview of interventions
Most candidate interventions were non-pharmacological. Twenty-three trials involved rehabilitation/therapy programmes, 12 of which included a virtual/telemedicine component. Rehabilitation programmes were mostly based on physiotherapeutic protocols designed to improve multiple long COVID physical and cardiorespiratory symptoms concurrently, or solely targeted to respiratory symptoms. Within the context of the WHO international classification of function (ICF) biopsychosocial model of disability [18], the majority of interventions were designed to address impairments/limitations in body functions and structures (e.g. improve lung function through breathing exercises), and/or impairments/limitations in activities and participation (e.g. improve cognitive impairment via cognitive training). A few interventions addressed personal factors (e.g. via cognitive behavioural therapy).
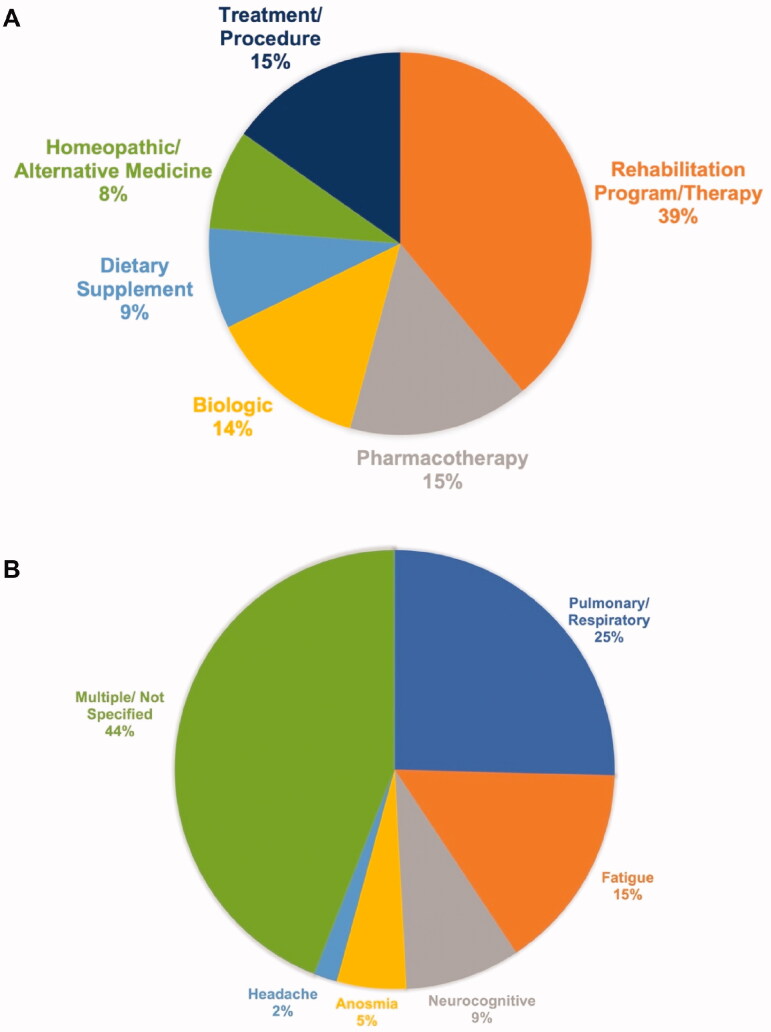
Nine trials proposed pharmacotherapies (both FDA-approved and experimental), eight biological treatments (e.g. autologous cell therapies and monoclonal antibodies), five dietary supplements, five homeopathic/alternative medicine regimes, and nine trials involved various treatments/procedures (e.g. monopolar radio frequency and hyperbaric oxygen therapy [HBOT]). In some instances, multiple trials proposed the same intervention (e.g. transcranial Direct Current Stimulation [tDCS], HBOT) for different long COVID symptoms. Interventions categorized by type are listed in Table 1, and the relative proportions of different types of interventions are depicted in Figure 3(A).
Figure 3.
(A) Proportions of types of interventions. Intervention categories are mutually exclusive (n = 59 trials). (B) Proportions of long COVID symptoms under investigation. Symptom categories are mutually exclusive (n = 59 trials).
Long COVID symptoms targeted
Most interventions intended to treat multiple symptoms of long COVID simultaneously (e.g. fatigue, pain, and sleeping disorders), or did not specify which symptoms were targeted. Of trials which targeted a specific symptom/symptom category, the majority (15 trials) focussed on respiratory or pulmonary sequelae. Moreover, nine trials endeavoured to treat fatigue, five to treat neurocognitive symptoms, three to treat anosmia and one to treat headache. A summary of interventions stratified by the long COVID symptom category targeted is provided in Table 2, and the relative proportions of long COVID symptom categories targeted are depicted in Figure 3(B).
Table 2.
Interventions organized by long COVID symptom(s) targeted.
| Pulmonary/respiratory | Fatigue | Neurocognitive | Anosmia | Headache | Multiple symptoms/not specified |
|---|---|---|---|---|---|
|
|
|
|
|
|
Subcategories are not mutually exclusive; for example one trial may have been counted as part of multiple sub-categories (e.g. as part of both ‘Inspiratory muscle training (IMT)’ and ‘telerehabilitation/physiotherapy’) if both components were included in the description of the intervention.
CBD: cannabidiol
Primary outcome measures largely consisted of changes across validated scales or physiological tests assessing for the presence, intensity, and functional outcomes associated with long COVID symptoms. Commonly implemented scales included the CFQ for fatigue, post-COVID functional status (PCFS) scale for multiple long COVID symptoms, and modified Borg scale for dyspnoea. In addition, fatigue, dyspnoea, and other respiratory sequelae were frequently assessed using the 6MWT, and exercise capacity was measured via the cardiopulmonary exercise test (CPET) and maximal oxygen consumption (V̇O2 max). Quality of life and functional outcomes were assessed by scales including, but not limited to, the 36-Item Short Form Survey (SF-36) and European Quality of Life 5 dimension 5 level (EQ-5D-5L). Primary outcome measures for the included records are specified in Supplementary Table 1.
Putative mechanisms subserving long COVID
Interventions predominantly focussed on reducing activated inflammatory processes (e.g. modulating inflammatory cytokine levels), enhancing oxygenation of tissues, and promoting cellular recovery. In addition, interventions designed to target respiratory sequelae included bronchodilators which improve mucus clearance (e.g. S-1126, Montelukast), and exercises to strengthen respiratory muscles (e.g. inspiratory muscle training).
Multiple candidate treatments were repurposed from similar conditions; for example, exercise-based rehabilitation programmes previously effective in treating cancer-associated fatigue syndrome (NCT04841759), tDCS effective for treating fatigue in patients with neurological disorders (NCT04876417), and a ‘mind-body program’ originally developed for chronic back pain (NCT04854772). Half of all trials did not provide a rationale or mechanism of action for the intervention and of those provided, many were non-specific (e.g. ‘demonstrates anti-inflammatory properties’). Few trials provided a targeted mechanism of action in accordance with existing theories regarding the biology subserving long COVID (e.g. digestion of ribonucleic acid contained in autoantibodies and immune complexes, in accordance with theories postulating the involvement of autoimmunity in long COVID) [19].
Discussion
Herein we provided a scope of the candidate treatments for long COVID. Considerable heterogeneity was exhibited amongst component records with respect to the characterization of long COVID (i.e. symptoms – including frequency, intensity, trajectory and duration – mode of ascertainment, and definition of acute phase). In addition, most candidate interventions were non-pharmacological and either intended to treat multiple long COVID symptoms simultaneously or focussed on respiratory/pulmonary sequelae. Multiple interventions targeted inflammation, tissue oxygenation, and cellular recovery, and several interventions were repurposed from analogous conditions.
Persistent symptoms are not uncommon following infection with viral or bacterial agents. For example, outbreaks of fatigue syndrome (including general malaise and neurological abnormalities) were documented following the Spanish influenza of 1918 [20] and the 2002–2004 severe acute respiratory syndrome (SARS) epidemic [21,22]. Previous psychoneuroimmunological research has established links between pro-inflammatory cytokines and neuropsychiatric symptoms including depression and cognitive impairment [23–25]. However, despite their long history, treatments with robust efficacy for post-viral syndromes and related conditions, such as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), have not been developed. Chronic ‘unexplained’ (± post-infectious) symptoms remain a controversial and poorly understood topic in medicine. A spectrum of sub-populations may exist, and some patients’ symptoms may root from complex brain network dysfunction seen in functional neurological disorders and/or somatic symptom disorders [26]. The pervasiveness of COVID-19 provides the exigency to advance our understanding of post-infectious syndromes and related conditions.
Recommendations for future research
The component trials investigated herein can be used to identify key research objectives for future long COVID research. First, a greater degree of definitional stringency is needed insofar as the characterization of long COVID and inclusion/exclusion criteria. The discrepancies between the WHO, CDC, NIH, and NICE definitions of long COVID should be resolved to increase homogeneity. A consensus definition of long COVID, both in terms of specific symptoms, symptom intensity, frequency, duration since onset and trajectory, should be founded on data from replicated studies which include diverse populations. Data science methodology (e.g. machine learning) can be leveraged to identify characteristic symptom patterns amongst large samples. In addition, explicit differential diagnoses should be devised to determine whether certain presentations of long COVID are forme fruste of mental illness (e.g. major depressive disorder).
National registries should be established to monitor individuals with COVID-19 long-term. Stratifying long COVID into several sub-syndromes (e.g. by symptom, severity, duration or trajectory) may be useful for diagnosis, prevention, treatment, and healthcare reimbursement. In keeping with this thought, interventional trials should specify which dimensions of long COVID are targeted, as one treatment is unlikely to address disparate symptoms due to divergence in pathophysiology. Ideally, individuals participating in long COVID trials should be formally diagnosed with post-COVID-19 condition (i.e. ICD-10 U09.9).
In consideration of the non-specific and multifaceted nature of many long COVID symptoms (e.g. fatigue and myalgia), investigators should establish that symptoms are not pre-existent (i.e. prior to COVID-19 infection), attributable to other conditions (e.g. dementia, major depressive disorder, chronic obstructive pulmonary disease, and/or medication-induced adverse effects), or a consequence of severe COVID-19 (e.g. fatigue due to deconditioning in the ICU). Investigators must also confirm prior COVID-19 infection in participants either via documentation of a prior positive test or a presumptive prior infection ascertained via clinical interview. Moreover, relative susceptibility to long COVID in populations with pre-existing conditions, psychosomatic influences, varying acute episode severity, and vaccination status should be investigated, along with interventions administered during the acute phase which can prevent persistent symptoms, and the differential efficacy of candidate treatments amongst the foregoing subpopulations. Symptoms and functional outcomes should be assessed objectively at multiple time points using appropriate, sensitive, and validated scales and physiological tests, along with concerted thresholds for clinical significance. For example, the PCFS, fatigue severity scale (FSS), modified Borg dyspnoea scale, EQ-5D-5L, spirometry, and functional neuroimaging techniques may be appropriate [27].
Second, more pharmacological interventions should be investigated for the treatment of long COVID. Notably, further research is warranted to evaluate the effects of psychotropic medications (e.g. antidepressants) in alleviating pro-inflammatory cytokine responses and psychiatric symptoms in long COVID [28]. A majority of component trials proposed rehabilitation programmes or procedures, which are resource-intensive, inefficient and not likely to be scalable. Internet-based psychological interventions (e.g. internet cognitive behaviour therapy) can provide a cost-effective approach to targeting specific physical and psychiatric symptoms while avoiding risk of COVID-19 transmission [29,30]. In consideration of the high estimated global prevalence of long COVID, treatments which are inexpensive, widely accessible, convenient, and have favourable safety and tolerability profiles are needed. In keeping with this thought, the efficacy and feasibility of lifestyle interventions (e.g. regular exercise and dietary changes) should also be investigated.
A pattern exhibited amongst component trials was the repurposing of treatments for analogous conditions, including but not limited to ME/CFS, neurological disorders, dyspnoea in cystic fibrosis, asthma, allergic rhinitis, and chronic back pain. Indeed there is evidence of similar clinical presentation and overlapping biological substrates between symptoms of long COVID and conditions such as ME/CFS [31]. In addition to the foregoing conditions, treatments for depression, neuromuscular disorders, endothelial dysfunction, autoimmune diseases, Alzheimer’s, and dementia may prove beneficial for patients with long COVID due to potential transdiagnostic processes.
The majority of candidate treatments primarily targeted respiratory sequelae or multiple symptoms (see Figure 3(B)); more targeted interventions are needed for fatigue, cognitive impairment, and neuropsychiatric sequelae, which are the most common persistent symptoms [1,3]. In addition, only 2 of 59 trials (3.4%) included persons under the age of 18; although long COVID is reportedly less common in children compared to adults, preliminary evidence has demonstrated that approximately 15% of children may experience persistent symptoms attributable to COVID-19 [32]. As such, it is imperative to establish the safety and tolerability of long COVID treatments in individuals under 18 years.
Basic research to determine the underlying biology of disparate long COVID symptoms, including pleiotropic mechanisms of action, is needed to inform future interventional trials. Persistent symptoms arising from different SARS-CoV-2 variants should be monitored. Moreover, investigation of biomarkers (e.g. pro-inflammatory cytokine levels and autoantibodies), including how such parameters change in response to treatments, and imaging of organs, such as the brain and lungs, may accelerate our understanding of the molecular mechanisms subserving long COVID. Future research should also aim to evaluate whether long COVID increases the risk of mental illness and/or suicide. The foregoing question is contextualized by a separate body of research which has established that mood disorders increase the risk of hospitalization and mortality due to COVID-19 [33]. Taken together, a multidisciplinary and multidimensional approach is warranted to assess the prevalence of complications associated with an overlap of COVID-19 and other mental and medical disorders.
Whereas research into treatments should focus on disparate symptoms, the implementation of interventions for long COVID requires an interdisciplinary approach, integrating tailored rehabilitation (including virtual services) and/or pharmacotherapies. As COVID-19 can affect any organ system, specialist care across diverse disciplines may be required, as well as long-term follow up. Establishing the infrastructure to deliver individualized treatment, with the proviso of sufficient resources to support high volumes of patients, is a primary objective for healthcare systems.
Limitations
The nature of trial registration records limits the strength of our conclusions. Foremost, we cannot comment on the efficacy of the candidate treatments as no trials have published results at the time of manuscript writing. An additional limitation is that we may have omitted some relevant studies as we did not search every available clinical trial registry. Furthermore, trial registrations are not peer-reviewed, which is especially pertinent to information regarding mechanisms of action. Furthermore, registrations are preliminary and may not accurately or comprehensively reflect the methodology actually carried out. The records provided insufficient information to facilitate bias assessment, and the amount of detail regarding rationale, condition, methodology and intervention were highly variable between trials.
Conclusion
In this review of 59 trial registration records, we provided a scope of the candidate treatments for long COVID. Moreover, we investigated the characterization of long COVID, types of treatments, symptoms addressed and putative mechanisms subserving long COVID symptoms. Key limitations and unmet needs were the inconsistent and frequently vague characterization of long COVID, lack of trials addressing long COVID in children, and non-specific as well as resource-intensive nature of most candidate treatments. Future research should endeavour to establish a consensus characterization of long COVID, greater homogeneity in terms of inclusion/exclusion criteria, evaluate safe and effective pharmacotherapies (some of which may be repurposed from similar conditions), and parse out the underlying pathophysiology of long COVID.
Supplementary Material
Funding Statement
This research did not receive any grants or funding.
Disclosure statement
FC and RSM conceptualized and designed study. FC and AL performed study search and selection. FC, AL, MYJ and MY performed data extraction. FC and AL performed data synthesis. FC performed data analyses and drafted the manuscript. All authors revised and approved the final version of the manuscript.
RSM has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie. RSM is a CEO of Braxia Scientific Corp. JDR is the medical director of Braxia Health (formally known as the Canadian Rapid Treatment Centre of Excellence and is a fully owned subsidiary of Braxia Scientific Corp) which provides ketamine and esketamine treatment for depression; he has received research grant support from the American Psychiatric Association, the American Society of Psychopharmacology, the Canadian Cancer Society, the Canadian Psychiatric Association, the Joseph M. West Family Memorial Fund, the Timeposters Fellowship, the University Health Network Centre for Mental Health, and the University of Toronto and speaking, consultation, or research fees from Allergan, COMPASS, Janssen, Lundbeck and Sunovion. YL has received personal fees from Braxia Scientific Corp. LMWL has received personal fees from Braxia Scientific Corp and honoraria Medscape. All other authors declare no conflicts of interest or financial disclosures.
References
- 1.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and Meta-analysis. Sci Rep. 2021;11(1):16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michelen M, Cheng V, Manoharan L, et al. Characterising long term Covid-19: a living systematic review. bioRxiv. 2020. Available from: http://medrxiv.org/lookup/doi/10.1101/2020.12.08.20246025
- 3.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2021. DOI: 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed]
- 5.CDC . Post-COVID conditions. 2021. [cited 2021 Jul 22]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects.html
- 6.NIH launches new initiative to study “long COVID.” 2021. [cited 2021 Sep 2]. Available from: https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid
- 7.Overview | COVID-19 rapid guideline: managing the long-term effects of COVID-19 | Guidance | NICE. 2021 [cited 2021 May 19]. Available from: https://www.nice.org.uk/guidance/ng188
- 8.Report: what does COVID-19 recovery actually look like? - patient led research collaborative. 2020. [cited 2021 Aug 26]. Available from: https://patientresearchcovid19.com/research/report-1/
- 9.Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, march 23-may 12, 2020. JAMA Intern Med. 2020;180(12):1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu SL, Mertens AN, Crider YS, et al. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat Commun. 2020;11(1):4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aizenman N. Why the pandemic is 10 times worse than you think. NPR. 2021. [cited 2021 May 19]. Available from: https://www.npr.org/sections/health-shots/2021/02/06/964527835/why-the-pandemic-is-10-times-worse-than-you-think
- 12.Xiong J, Lipsitz O, Nasri F, et al. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J Affect Disord. 2020; 277:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.“Bad brain fog”: patients who had covid-19 reveal new phenomenon - CNN Video. CNN. 2021. [cited 2021 Jul 22]. Available from: https://www.cnn.com/videos/health/2021/02/19/post-covid-symptoms-gupta-vpx.cnn
- 14.Chapter 11: Scoping reviews. 2020 [cited 2021 Aug 25]. Available from: https://wiki.jbi.global/display/MANUAL/Chapter + 11%3A+Scoping+reviews
- 15.Peyrovian B, McIntyre RS, Phan L, et al. Registered clinical trials investigating ketamine for psychiatric disorders. J Psychiatr Res. 2020;127:1–12. [DOI] [PubMed] [Google Scholar]
- 16.Siegel AN, Meshkat S, Benitah K, et al. Registered clinical studies investigating psychedelic drugs for psychiatric disorders. J Psychiatr Res. 2021; 139:71–81. [DOI] [PubMed] [Google Scholar]
- 17.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. [DOI] [PubMed] [Google Scholar]
- 18.Chan F, Gelman JS, Ditchman N, et al. The world health organization ICF model as a conceptual framework of disability. In: Chan F, editor. Understanding psychosocial adjustment to chronic illness and disability: a handbook for evidence-based practitioners in rehabilitation. New York (NY): Springer Publishing Co.; 2009. p. 23–50. [Google Scholar]
- 19.Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595(7866):283–288. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman LA, Vilensky JA.. Encephalitis lethargica: 100 years after the epidemic. Brain. 2017;140(8):2246–2251. [DOI] [PubMed] [Google Scholar]
- 21.Chrousos GP, Kaltsas G.. Post-SARS sickness syndrome manifestations and endocrinopathy: how, why, and so what? Clin Endocrinol (Oxf). 2005;63(4):363–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moldofsky H, Patcai J.. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Ho RCM, Mak A.. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a Meta-analysis and Meta-regression. J Affect Disord. 2012;139(3):230–239. [DOI] [PubMed] [Google Scholar]
- 24.Ng A, Tam WW, Zhang MW, et al. IL-1β, IL-6, TNF- α and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci Rep. 2018;8(1):12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee Y, Mansur RB, Brietzke E, et al. Peripheral inflammatory biomarkers define biotypes of bipolar depression. Mol Psychiatry. 2021;26(7):3395–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burke MJ, Del Rio C.. Long COVID has exposed medicine’s blind-spot. Lancet Infect Dis. 2021;21(8):1062–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho RC, Sharma VK, Tan BYQ, et al. Comparison of brain activation patterns during olfactory stimuli between recovered COVID-19 patients and healthy controls: a functional near-Infrared spectroscopy (fNIRS) study. Brain Sci. 2021;11(8):968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Y, Ho CS, Liu X, et al. Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PLoS One. 2017;12(10):e0186700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang MWB, Ho RCM.. Moodle: the cost effective solution for internet cognitive behavioral therapy (I-CBT) interventions. Technol Health Care. 2017;25(1):163–165. [DOI] [PubMed] [Google Scholar]
- 30.Soh HL, Ho RC, Ho CS, et al. Efficacy of digital cognitive behavioural therapy for insomnia: a meta-analysis of randomised controlled trials. Sleep Med. 2020;75:315–325. [DOI] [PubMed] [Google Scholar]
- 31.Wong TL, Weitzer DJ.. Long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-a systemic review and comparison of clinical presentation and symptomatology. Medicina. 2021;57(5):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buonsenso D, Munblit D, De Rose C, et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110(7):2208–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ceban F, Nogo D, Carvalho IP, et al. Association between mood disorders and risk of COVID-19 infection, hospitalization, and death: a systematic review and Meta-analysis. JAMA Psychiatry. 2021;78(10):1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.