Abstract
The mammalian dentition is a serially homogeneous structure that exhibits wide numerical and morphological variation among multiple different species. Patterning of the dentition is achieved through complex reiterative molecular signaling interactions that occur throughout the process of odontogenesis. The secreted signaling molecule Sonic hedgehog (Shh) plays a key role in this process, and the Shh coreceptor growth arrest-specific 1 (Gas1) is expressed in odontogenic mesenchyme and epithelium during multiple stages of tooth development. We show that mice engineered with Gas1 loss-of-function mutation have variation in number, morphology, and size of teeth within their molar dentition. Specifically, supernumerary teeth with variable morphology are present mesial to the first molar with high penetrance, while molar teeth are characterized by the presence of both additional and absent cusps, combined with reduced dimensions and exacerbated by the presence of a supernumerary tooth. We demonstrate that the supernumerary tooth in Gas1 mutant mice arises through proliferation and survival of vestigial tooth germs and that Gas1 function in cranial neural crest cells is essential for the regulation of tooth number, acting to restrict Wnt and downstream FGF signaling in odontogenic epithelium through facilitation of Shh signal transduction. Moreover, regulation of tooth number is independent of the additional Hedgehog coreceptors Cdon and Boc, which are also expressed in multiple regions of the developing tooth germ. Interestingly, further reduction of Hedgehog pathway activity in Shhtm6Amc hypomorphic mice leads to fusion of the molar field and reduced prevalence of supernumerary teeth in a Gas1 mutant background. Finally, we demonstrate defective coronal morphology and reduced coronal dimensions in the molar dentition of human subjects identified with pathogenic mutations in GAS1 and SHH/GAS1, suggesting that regulation of Hedgehog signaling through GAS1 is also essential for normal patterning of the human dentition.
Keywords: supernumerary tooth, vestigial, molar cusp, molar size, molecular signaling, WNT
Introduction
The mammalian dentition is a serially homologous structure defined by species-specific numerical and morphological variation achieved through reiterative molecular signaling during multiple stages of odontogenesis (Cobourne and Sharpe 2010; Jernvall and Thesleff 2012; Yu and Klein 2020). Specifically, signaling between oral ectoderm and cranial neural crest cell (CNCC)–derived (ecto)mesenchyme generates an ectodermal thickening that develops into a bud-stage tooth germ, which rapidly converts into cap and bell stages to establish and refine coronal shape (Jernvall and Thesleff 2000, 2012). The molecular interactions that drive odontogenesis are dominated by Wnt, bone morphogenetic protein, fibroblast growth factor (FGF), and Hedgehog signaling (Lan et al. 2014).
The modern human dental formula is reduced, having lost an incisor and 2 premolars during evolution (O’Leary et al. 2013), while the mouse dentition is highly reduced, being monophyodont and consisting of 1 incisor and 3 molars separated by an edentulous diastema (Prochazka et al. 2010). Detailed analysis of this diastema region has revealed paired vestigial tooth primordia appearing sequentially mesial to the first molar (M1) in both jaws from embryonic day (E)13.5 (Viriot et al. 2000; Peterková et al. 2002) (R1, R2; MS, R2 in maxilla and mandible, respectively) before disappearing at the bud stage through apoptosis (Peterková et al. 2003). R2 survival has been identified as the origin of supernumerary teeth present in the diastema of multiple mouse mutants (Kassai et al. 2005; Peterková et al. 2005; Klein et al. 2006; Ohazama et al. 2009; Ahn et al. 2010). A network of feedback inhibition restricts Wnt and downstream FGF activity in R2, preventing development beyond the bud stage and maintaining a reduced murine dental formula (Cobourne and Sharpe 2010; Lan et al. 2014). Specifically, canonical Lrp5/6-dependent Wnt signaling induces the secreted Wnt inhibitor Sostdc1 (Wise, Ectodin, or USAG-1) in dental mesenchyme to establish negative feedback in R2 (Ahn et al. 2010), while FGF signaling is restricted in epithelium and mesenchyme by Sprouty2/4 FGF inhibitor activity, respectively (Klein et al. 2006). Sonic hedgehog (Shh) is a target of Sostdc1-mediated Wnt signaling, contributing to Dkk1-mediated negative feedback acting to further inhibit R2 Wnt activity (Ahn et al. 2010). The precise role of Shh within this model is not fully understood, but a reaction-diffusion mechanism has been suggested, where temporospatial balance between Wnt and Hedgehog is responsible for tooth phenotype (Ahn et al. 2010; Cho et al. 2011).
Shh is a versatile signaling molecule mediating pathway activity through ligand binding to the Patched1 (Ptch1) receptor (Stone et al. 1996) facilitated by coreceptors including the GPI-anchored membrane protein Gas1 (growth arrest-specific 1) (Allen et al. 2007; Martinelli and Fan 2007) and immunoglobulin superfamily transmembrane proteins Cdon and Boc (Kang et al. 1997, 2002). These coreceptors can bind Shh (Okada et al. 2006; Tenzen et al. 2006; Martinelli and Fan 2007; McLellan et al. 2008) and Ptch1 (Bae et al. 2011; Izzi et al. 2011) and are collectively essential for vertebrate Hedgehog signaling (Allen et al. 2011).
Here we show that the Gas1–/– molar dentition has abnormal coronal morphology and supernumerary teeth arising through R2 survival. Gas1 function in CNCC inhibits Wnt signaling through facilitation of Shh to restrict tooth number. We also demonstrate defective coronal morphology in the molars of human subjects carrying missense mutations in GAS1, suggesting that regulation of SHH through GAS1 is also essential for patterning the human dentition.
Materials and Methods
Experimental materials and methods are provided in the supplementary appendix material.
Results
Multiple Anomalies in the Dentition of Gas1–/– Mice
We undertook Gas1 expression analysis in the developing molar dentition within the context of Hedgehog activity (Appendix Fig. 1A–O). Gas1 was initially expressed within mesenchyme peripheral to the molar tooth germ, progressively localizing in odontogenic mesenchyme adjacent to the regressing R2. By late cap stage, transcripts were also present in epithelium of the dental lamina and oral surface of the outer enamel epithelium. In addition, the mesenchymal expression was now localized to a region between the oral epithelium and oral surface of the tooth germ, extending to posterior regions of the buccal (vestibular) oral cavity. These expression domains were suggestive of a role in patterning and morphogenesis of the developing dentition.
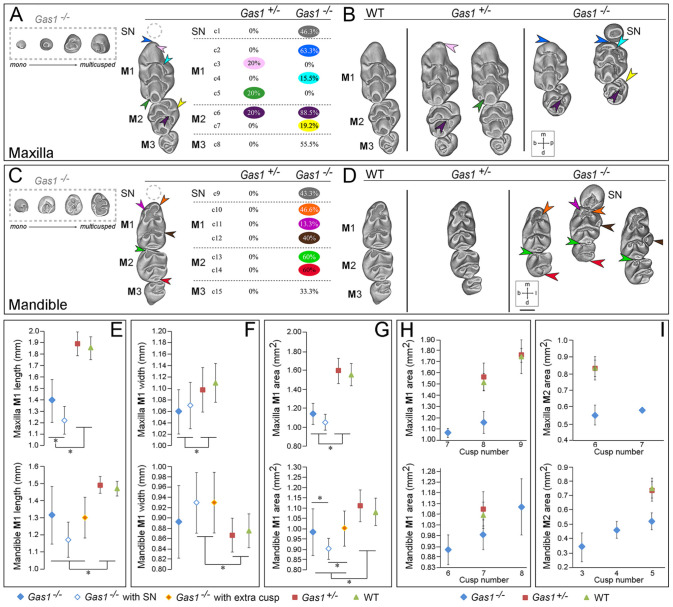
To investigate the role of Gas1 during dental development, we compared arrangement and shape of molar tooth rows in maxilla and mandible of wild-type (WT) and Gas1 mutant mice (Fig. 1A–D). In our sample, 80% of Gas1–/– mice had a supernumerary tooth mesial to M1 (maxilla 46.3%; mandible 43.3%), which exhibited wide morphological variation (Fig. 1A, C; gray hatched box). However, 55.5% of mutants had absence of at least 1 M3, 55.5% and 33.3% were missing in the maxillary and mandibular molar tooth rows, respectively. In the maxilla, 50% of supernumerary teeth were associated with a missing M3 in the same row, while in the mandible, this was 37.5%.
Figure 1.
Dental character matrix of Gas1 mutant mice. (A, C) The first column displays molar tooth rows with supernumerary tooth morphology variation shown within a gray hatched box and colored arrowheads localizing the most frequent defects in mutant backgrounds. The second column lists each character with the observed occurrence frequency in mutant mice. (A) Maxillary dental row (c1–8 are defects of the mutant maxillary molar dentition); c1, supernumerary tooth (50% of supernumerary teeth were associated with a missing M3 in the same row); c2, straight M1 mesial cusp with a vertical tilt of the mesial component (blue arrow); c3, M1 extra mesial cusp (pink arrow); c4, absence of M1 buccal cusp connection from the first chevron (turquoise arrow); c5, connection pinching to disconnection of the M1 third chevron cusps (dark green arrow); c6, disconnection of the M2 mesiolingual cusp from the first chevron (deep purple arrow); c7, abnormal connection between the 2 M2 lingual cusps (yellow arrow); c8, absence of M3. (C) Mandibular dental row (c9–15 are defects of the mutant mandibular molar dentition); c9 supernumerary tooth (37.5% of supernumerary teeth were associated with a missing M3 in the same row); c10, absence of the M1 first lingual cusp (orange arrow); c11, absence of the M1 first buccal cusp (light purple arrow); c12, presence of an extra M1 first lingual cusp (brown arrow); c13, absence of the M3 mesiolingual cingulum (light green arrow); c14, absence of the M2 distal-most cusp (red arrow); c15, absence of M3. Molar phenotype of wild-type (WT), Gas1+/–, and Gas1–/– mice. (B, D) WT, Gas1+/–, and Gas1–/– molar tooth rows are shown in the 3 columns (respectively from left to right). (B) For the maxillary molar dentition: straight M1 mesial cusp associated with a vertical tilt of the mesial component (blue arrow), M1 extra mesial cusp (pink arrow), absence of the M1 palatal cusp connection from the first chevron (turquoise arrow), connection pinching to disconnection of the M1 third chevron cusps (dark green arrow), disconnection of the M2 mesiopalatal cusp from the first chevron (deep purple arrow), and abnormal connection between the 2 M2 palatal cusps (yellow arrow). (D) For the mandibular molar dentition: absence of the M1 first lingual cusp (orange arrow), absence of the M1 first buccal cusp (light purple arrow), presence of an extra M1 first lingual cusp (brown arrow), absence of the M2 first chevron cusp (light green arrow), and absence of the M2 most distal step (red arrow). (E–G) Maxillary and mandibular molar dimensions in WT, Gas1+/- and Gas1–/– mice. (E) M1 mesiodistal length (mm). (F) M1 buccal-lingual width (mm). (G) M1 coronal surface area (mm2). (H, I) Maxillary and mandibular M1 and M2 coronal surface area and cusp number. (H) M1. (I) M2. For (E) to (I), maxillary teeth are shown in the upper part of the panel and mandibular in the lower. Scale bar in D = 0.45 mm for (A) to (D). M1, M2, M3, first, second, third molar, respectively; SN, supernumerary tooth. b, buccal; d, distal; l, lingual (mandible); m, mesial; p, palatal (maxilla). Blue solid diamond, Gas1–/–; blue outline diamond, Gas1–/– with supernumerary tooth; green triangle, WT; orange diamond, Gas1–/– with extra cusp; red square, Gas1+/–. *Indicates significant difference (P < 0.05).
All Gas1–/– molar dentitions displayed anomalies in occlusal morphology, with less severe variation in heterozygotes. In the Gas1–/– maxilla, the M1 mesiocentral cusp tilt was subvertical (63.3% overall, all M1 associated with a supernumerary) (Fig. 1A, B; blue arrows), and a palatal cusp connection within the first chevron was absent (15.5%) (Fig. 1A, B; turquoise arrows). In heterozygotes, 20% of maxillary M1 had an extra mesial cusp (Fig. 1A, B; pink arrows) as well as a connection anomaly between both cusps of the third chevron (Fig. 1A, B; dark green arrows). The maxillary M2 in both Gas1–/– and heterozygous mice had a frequent disconnection of the mesiopalatal cusp from the first chevron (88.5% mutant; 20% heterozygote) (Fig. 1A, B; deep purple arrows) sometimes associated with an abnormal connection between the 2 palatal cusps (Fig. 1A, B, yellow arrows). In the mandible, heterozygous molars were essentially normal (Fig. 1C, D), but the Gas1–/– M1 had a missing buccal cusp in 13%, as well as an extra lingual cusp in 40% (Fig. 1C, D; light purple and brown arrows, respectively), and lacked the mesiolingual cusp (46.6% overall, 92.3% associated with a supernumerary) (Fig. 1C, D; orange arrows) and, more rarely, the mesiobuccal cusp (13.3% overall, 7.7% associated with a supernumerary). In addition, 60% of Gas1–/– mandibular M2 lacked the mesiobuccal cingulum and distal-most cusp (Fig. 1C, D; light green and red arrows, respectively).
Dimensional variations between M1 of WT and Gas1–/– mice were also present. In both maxilla and mandible M1, length, width, and tooth surface area was reduced in mutants compared to heterozygote and WT, with M1 length further reduced in the presence of a supernumerary (Fig. 1E–G). M1 dimensions in Gas1–/– were 25% and 9% smaller than WT in the maxilla and mandible, respectively, while M2 was 34% and 37% smaller. Interestingly, cusp number increased with tooth occlusal surface area regardless of genotype. However, comparing WT and Gas1–/– teeth demonstrated that very different surface areas could contain the same number of cusps (Fig. 1H, I).
Supernumerary Teeth in Gas1–/– Mice Are a Product of the R2 Vestigial Tooth Bud
We next investigated developmental origins of supernumerary teeth in Gas1–/– mice using 3-dimensional (3D) reconstruction (Fig. 2A–X). At E13.5, vestigial primordia were visible mesial to the bud-stage M1 with no obvious morphological differences between WT and mutant (Fig. 2A–D, E–H). At E14.5, R2 was still identifiable anterior to the cap-stage M1 in WT, although some incorporation into the M1 cap was evident in the mandible (Prochazka et al. 2010; Viriot et al. 2000) (Fig. 2I, J–L). In Gas1–/– mice, R2 development continued toward an independent rudimentary cap stage in both arches, accompanied by delayed M1 cap formation (Fig. 2M–P). At E15.5, the diastema buds were no longer distinct entities in WT, particularly in the mandible, where R2 was now a component of the M1 cap (Fig. 2Q–T). However, cap-stage supernumerary tooth germs were present in Gas1–/– embryos, anterior to a diminutive M1 cap-stage tooth germ in both arches (Fig. 2U–X). These findings suggested the developmental basis of Gas1–/– supernumerary teeth was survival of R2, a finding confirmed by comparison of Shh expression in 3D M1 reconstructions (Appendix Fig. 2A–F) and analysis of proliferation and cell death. At E13.5, there was significantly more proliferation in the mutant R2 epithelium compared to WT, which continued in the epithelium and mesenchyme at E14.5. In addition, increased cell death was identified in the WT R2 epithelium compared to the mutant at E14.5 (Appendix Fig. 3A-J).
Figure 2.
Supernumerary teeth in Gas1–/– mice are a product of the R2 vestigial tooth germ. 3D reconstructions of the epithelial component and serial parasagittal histology of the left maxillary and right mandibular M1 in wild-type (WT) and Gas1–/– mice. For the maxilla, (A, E, I, M, Q, U) show 3D reconstruction of M1 from the palatal aspect, while (B, F, J, N, R, V) are orientated from below. For the mandible, (C, G, K, O, S, W) show 3D reconstruction from the buccal aspect, while (D, H, L, P, T, X) are viewed from below. Serial histology is orientated from palatal through mid to buccal aspects (i, ii, iii; respectively) for the left maxillary M1 and from buccal through mid to lingual aspects (i, ii, iii; respectively) for the right mandibular M1. All images are orientated with mesial to the right. It can be seen that R1 and MS degenerate in the maxillary and mandibular M1 of WT and Gas1–/– mice. However, while R2 degenerates in the WT maxillary and mandibular M1, in the Gas1–/–, this vestigial tooth bud survives and goes on to form a supernumerary tooth in the maxilla and mandible. Scale bar in X = 150 µm for (A) to (X) and in (X) iii (lingual) = 100 µm for all histological sections. ek, primary enamel knot; M1, first molar; SN, supernumerary tooth. a, aboral; b, buccal; d, distal; m, mesial; o, oral; p, palatal. Green arrowhead, MS (maxilla) and R1 (mandible); red circle highlights developing R2 in the mutant tooth germ; yellow arrowhead, R2 (maxilla and mandible).
Gas1 Regulates Tooth Number through the Shh Pathway
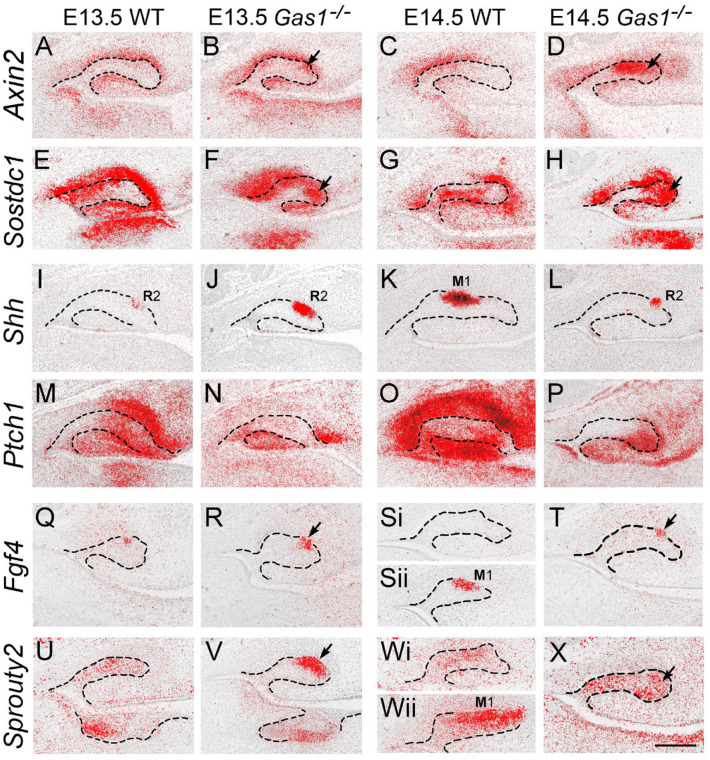
We next investigated Wnt, Hedgehog, and FGF signaling in WT and Gas1–/– maxillary M1 between E13.5 and 14.5. In WT, Axin2 was largely restricted to odontogenic mesenchyme, while Sostdc1 was present in epithelium and mesenchyme (Fig. 3A, C, E, G). However, both these Wnt targets were ectopically expressed in the mutant R2 epithelium at both stages (Fig. 3B, D, F, H arrowed). Significantly, increased Wnt activity in the mutant R2 was associated with reduced Shh signal transduction. Despite sustained and increased Shh transcription in the mutant R2 compared to WT (Fig. 3I–L), Ptch1 was reduced (Fig. 3M–P). At E13.5, increased Fgf4 expression was also present in the mutant R2 compared to WT, while at E14.5, expression was lost in WT (although now present in M1) and retained in the mutant R2 (Fig. 3Q, R, S, T arrowed). A similar picture was seen in expression of the FGF target Sprouty2, which was also increased in the mutant R2 at E13.5 and increased at E14.5 in contrast to WT (Fig. 3U, V, W, X arrowed). An absence of Gas1 coreceptor function in mediating Shh signaling in odontogenic mesenchyme resulted in increased Wnt and FGF signaling in R2.
Figure 3.
Increased WNT signaling is associated with reduced Sonic hedgehog (Shh) transduction and increased fibroblast growth factor (FGF) signaling in the developing R2 of Gas1–/– mice. 35S radiolabeled in situ hybridization on parasagittal sections through the developing maxillary molar tooth germs at E13.5 and E14.5. (A–D) Axin2. (E–H) Sostdc1. (I–L) Shh. (M–P) Ptch1. (Q–T) Fgf4. (U–X) Sprouty2. At E13.5 and E14.5, there was increased expression of Axin2 and Sostdc1 in the mutant R2 and proximate mesenchyme (A, B, C, D and E, F, G, H) (black arrows indicate increased expression in R2). In contrast, despite sustained transcription of Shh in the mutant R2 (I, J, K, L), Ptch1 expression was reduced in the mutant (M, N, O, P). Consistent with a picture of increased Wnt signaling through reduced Shh inhibitory activity, the downstream Wnt targets Fgf4 and Sprouty2 were also increased in the mutant R2 compared to wild type (WT) at these stages, respectively (Q, R, S, T) and (U, V, W, X) (black arrows indicate increased expression in R2). Note a lack of Fgf4 and Sprouty2 expression in the WT R2 at E14.5 (Si and Wi, respectively) but expression of both genes in more lingual regions of the tooth germ in association with the M1 enamel knot (Sii and Wii, respectively). Hatched black line represents epithelial boundary. Black arrows indicate increased WNT signal transduction in R2. Scale bar in (X) = 100 µm for (A) to (X). Representative sections through WT and mutant maxillary tooth germs from n = 4 animals (n = 8 molars). M1, first molar enamel knot.
Gas1 Regulates Tooth Number Independently through CNCC
Given the early expression of Gas1 in odontogenic mesenchyme localizing around R2 (see Appendix Fig. 1K–M), we investigated whether Gas1 function was essential in this tissue for regulation of tooth number. Specifically, we analyzed Wnt1-Cre;Gas1fl/fl conditional mutant mice, among which all exhibited supernumerary teeth mesial to M1 (83% maxilla; 67% mandible) (Fig. 4A, B), demonstrating that Gas1 is essential in murine CNCC for regulation of tooth number.
Figure 4.
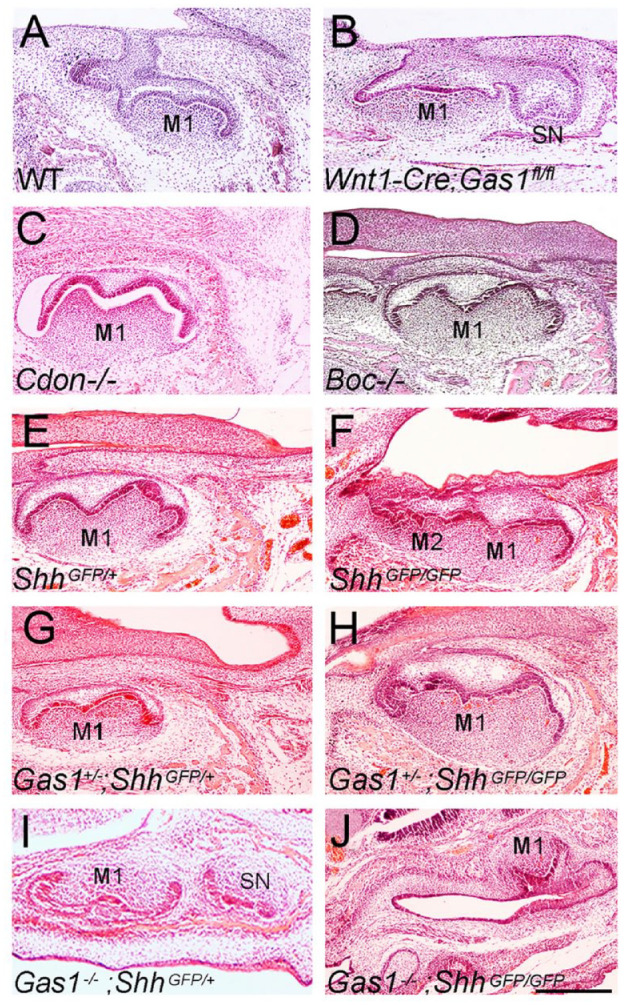
Molar phenotype of Hedgehog pathway mutant mice. (A–D) E16.5 (A, B) and E17.5 (C, D) mice lacking Hedgehog coreceptor function: (A) wild type (WT); (B) Wnt1-Cre;Gas1fl/fl (n = 3); (C) Cdon–/–(n = 6); (D) Boc–/– (n = 9). (E–J) E17.5 (E) ShhGFP/+; (F) ShhGFP/GFP (n = 8); (G) Gas1+/–;ShhGFP/+ (n = 8); (H) Gas1+/–; ShhGFP/GFP (n = 8); (I) Gas1–/–;ShhGFP/+ (n = 4); and (J) Gas1–/–; ShhGFP/GFP (n = 1). Loss of a single Gas1 allele does not affect molar phenotype in a ShhGFP/+ background or alter molar fusion in a ShhGFP/GFP background, while Gas1–/–; ShhGFP/+ mice have supernumerary teeth with reduced penetrance. Gas1–/–;ShhGFP/GFP embryos are early embryonic lethal, with a lack of mandibular molar development and only a poorly formed molar tooth identifiable in the anterior maxilla. Scale bar in J = 250 µm for (A) to (J).
Gas1 is known to interact with the Hedgehog coreceptors Cdon and Boc in different developmental contexts (Allen et al. 2011), and all 3 are expressed in the developing tooth (Appendix Fig. 4M-T). We investigated the effect of individual loss of coreceptor function on tooth development. Notably, at E18.5, there was no evidence of supernumerary tooth formation in either Cdon–/– or Boc–/– single mutants (Fig. 4C, D).
Given the independent role of Gas1 and reduced levels of Shh transduction observed in R2 of Gas1 mutants (see Fig. 3M–P), we investigated the consequences of further reducing Hedgehog signal levels in this mutant background. Gas1+/–; Shh+/– mice are normal, but loss of both Shh alleles in a Gas1–/– background leads to gross craniofacial defects (Seppala et al. 2007). We therefore used the Shhtm6Amc allele (ShhGFP), which encodes a bioactive Shh–green fluorescent protein (Shh::gfp)–tagged protein (Chamberlain et al. 2008). ShhGFP/+ mice are normal, but ShhGFP/GFP mice are hypomorphic, and demonstrated M1–M2 fusion with complete penetrance but no supernumerary teeth (Fig. 4E, F). Gas1+/–;ShhGFP/+ mice had normal molar development while Gas1+/–;ShhGFP/GFP maintained the molar fusion phenotype (Fig. 4G, H). However, Gas1–/–;ShhGFP/+ mice had supernumerary teeth with reduced penetrance (38%) in comparison to Gas1–/–, while Gas1–/–;ShhGFP/GFP embryos were early embryonic lethal, with only 1 developing sufficiently to identify a lack of mandibular molars and only a poorly formed single molar in the anterior maxilla (Fig. 4I, J).
Loss of Function in Hedgehog Signaling Affects Human Dental Development
We next investigated the potential influence of GAS1-mediated Hedgehog signaling during human dental development by examining the permanent dentition of 3 subjects identified with pathogenic mutations at the GAS1 (n = 2) or SHH/GAS1 loci (n = 1) and features within the clinical spectrum of Holoprosencephaly (HPE), comparing them to population-matched controls (n = 4) (Ribeiro et al. 2010). The human M1 phenotype was characterized by significant mesiodistal shortening and an increased coronal width/length ratio (Appendix Fig. 5A–C). Moreover, there was absence of specific cusps and modification of interconnections between cusps when compared to population controls (Fig. 5A, B). Specifically, the mandibular M1 had absence of the distobuccal cusp (Hld, hypoconulid) in all subjects, while the maxillary M1 distopalatal cusp (Hy, hypocone) was reduced or absent in 1 subject (GAS1 c.775G > A). The maxillary M1 in 2 of 3 subjects had a large marked groove separating the distobuccal (Mc, metacone) from the mesiopalatal cusp (Pr, protocone), which removed the enamel bridge usually present between these 2 cusps. There was also evidence of shoveling affecting the maxillary incisor crowns in 1 subject, taurodontism in 2 subjects, and generalized root shortening but no evidence of supernumerary teeth in the permanent dentition (data not shown).
Figure 5.
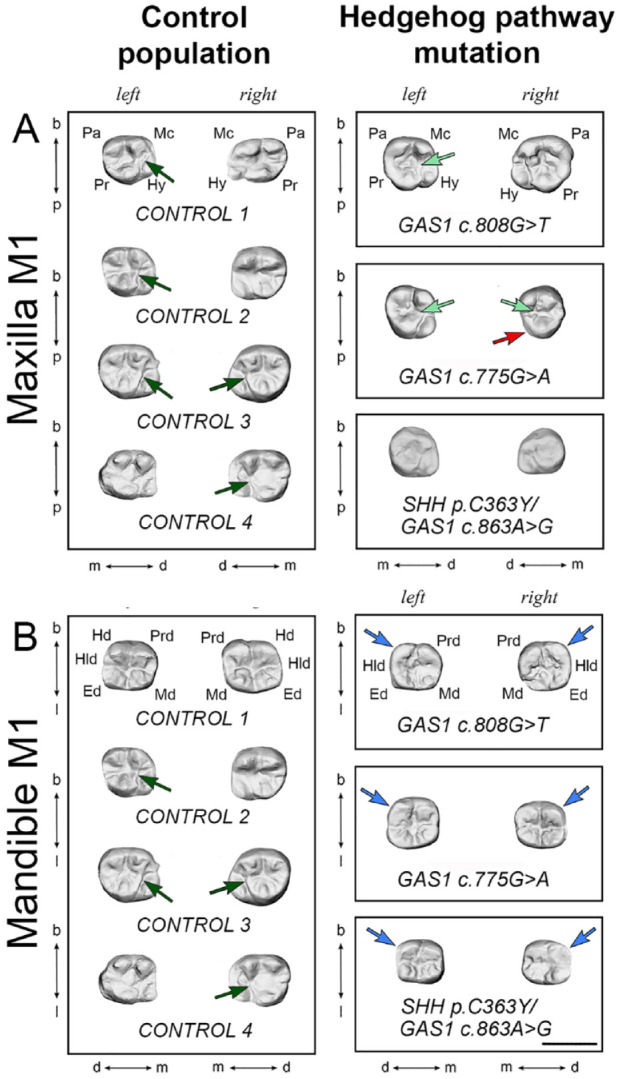
First permanent molar phenotype of human subjects with Hedgehog pathway mutation. (A) Maxillary and (B) mandibular left and right first permanent molars (M1) of representative control population (left panels) and human subjects with mutation in GAS1 or SHH/GAS1 (right panels). In the control population maxillary M1, a bridge separated the metacone and protocone in 5 of 8 teeth (dark green arrows), which was replaced by a groove in 3 of 6 subjects with mutations (light green arrows). In addition, the hypocone was absent in the right maxillary M1 in the subject with GAS1 c.775G > A mutation (red arrow). In the mandible, the M1 hypoconulid was absent in the left and right M1 of all subjects with mutations (blue arrows). Scale bar in B = 1.0 cm for (A) and (B). Ed, entoconid; Hd, hypoconid; Hld, hypoconulid; Hy, hypocone; Mc, metacone; Md, metaconid; Pa, paracone; Pr, protocone; Prd, protoconid. B, buccal; d, distal; l, lingual (mandible); m, mesial; p, palatal (maxilla).
Discussion
We have identified morphological variation in the molar dentition of mice lacking function of the Hedgehog coreceptor Gas1 consistent with a model of altered Shh transduction. This included supernumerary tooth formation, altered cusp pattern, and size variation. Significantly, we have also have found cusp pattern and size defects in M1 of human subjects identified with pathogenic mutations in GAS1 and SHH. These findings suggest some conservation of developmental pathways during patterning of the dentition.
Supernumerary Tooth Formation in Gas1–/– Mice
Gas1–/– mice with the most severe craniofacial defects have supernumerary teeth in the molar dentition of both jaws with high penetrance (Seppala et al. 2007; Ohazama et al. 2009), while those surviving beyond birth have a prevalence of around 50% in both jaws. Among ERK-MAPK pathway mutants, Sprouty2–/–, Sprouty4–/–, and Rsk2–/Y mice all have supernumerary teeth mesial to M1. Sprouty2–/– have <5% in maxilla but >90% in mandible (Klein et al. 2006); Sprouty4–/– and Rsk2–/Y have 17% and 14%, respectively, in maxilla and 3% and 14%, respectively, in mandible (Klein et al. 2006; Marangoni et al. 2015). Among EDA pathway mutants, supernumerary teeth are present in 8% and 7% of molar rows in Tabby/+ and Edardl–j mice, respectively (Charles et al. 2009). Supernumerary teeth have also been reported in Ectodin–/– and Sostdc1–/– mutants with frequencies of 60% in both jaws reported for Sostdc1–/– mice (Kassai et al. 2005). Gas1–/– mice therefore represent mutants in which the frequencies of supernumerary tooth occurrence in the molar dentition are among the highest.
We have shown the developmental origin of Gas1–/– supernumerary teeth to be R2 survival. Under normal circumstances, Wnt signaling establishes negative feedback in R2 through induction of Sostdc1 in dental mesenchyme (Ahn et al. 2010). Shh is a key target of Sostdc1-mediated Wnt signaling, contributing to this negative feedback through Wnt inhibition in R2 (Ahn et al. 2010). Wnt and Hedgehog activity is finely balanced in R2, which ultimately dictates developmental fate (Ahn et al. 2010; Cho et al. 2011). In the absence of Gas1, Shh transduction is reduced in M1 mesenchyme, and Wnt signaling in R2 elevates beyond the threshold required for survival. Indeed, maternal injection of the Shh-blocking antibody 5E1 or Shh downregulation in PCS1-MRCS1Δ/Δ enhancer mutants also produces supernumerary teeth in the molar dentition (Cho et al. 2011; Seo et al. 2018; Kim et al. 2019). Interestingly, Gas1–/– embryos demonstrate increased levels of Sostdc1 activity in R2 epithelium compared to reporter activity seen in Sostdc1 mutants (Ahn et al. 2010). In this model, relative levels of signaling within and around R2 are crucial and carefully regulated through multiple levels of negative feedback involving Wnt, FGF, and Hedgehog.
The importance of thresholds has been demonstrated through analysis of Sostdc1 and ShhGFP/Cre loss-of-function mice, in which individual heterozygotes are normal, but double heterozygotes have supernumerary teeth through increased Wnt and R2 survival (Ahn et al. 2010). In Gas1–/– mice, Wnt signaling is increased because an absence of Gas1 reduces Shh signaling in odontogenic mesenchyme. However, the relationship with Shh signal transduction is complex because the transcriptional targets Ptch1 and Ptch2 negatively regulate signaling (Marigo et al. 1996; Chuang and McMahon 1999), while Gas1, Cdon, and Boc act positively but are negatively regulated by pathway activation (Tenzen et al. 2006; Martinelli and Fan 2007). Gas1 can also bind Ptch2 (Kim et al. 2020), but Ptch2 expression is restricted to the enamel knot in the developing tooth germ (Motoyama et al. 1998). Cdon and Boc are individually redundant for molar tooth number regulation.
Molecular Interactions Regulating Cusp Pattern
Shh has been shown to restrict cusp formation by regulating cusp spacing (Cho et al. 2011; Harjunmaa et al. 2012; Kim et al. 2019) and is also required for spatial patterning between individual molars (Cho et al. 2011). Embryos exposed to 5E1 have supernumerary cusps predominantly affecting maxillary M1 and mandibular M2 (Cho et al. 2011; Kim et al. 2019). Gas1 heterozygotes demonstrated an additional maxillary M1 mesial cusp, while the mutant mandibular M1 had an additional lingual cusp. However, the mutant also had absence of some cusps, including the mandibular M1 mesiolingual and M2 first chevron buccal and distal-most cusps. This suggests a potential role for Gas1 during evolutionary modulation of cusp morphology. The most severe molar pattern defects are associated with Shh inhibition from E14.5 (Cho et al. 2011; Kim et al. 2019). In Gas1–/– mice, while there is a reduction in Shh signaling within the tooth germ, transduction is not completely lost, and therefore phenotypes might be expected to be more subtle.
The presence of a supernumerary tooth in Gas1–/– mice directly affected the shape of the M1 mesial extremity in both jaws, with a corresponding size decrease in M1 through R2 tissue normally incorporated into this tooth contributing to an independent supernumerary. Shape diversity of the supernumerary was high in Gas1–/– mice, ranging from tiny rounded and pointed teeth to larger, more complex crown forms that included alternately arranged cusps linked by a zigzag crest. This pattern variation has also been described in ERK-MAPK pathway mutants (Marangoni et al. 2015); therefore, no specific supernumerary tooth shape signature can be ascribed to Gas1 mutants. However, a clear phenotypic signature in the molar dentition of Gas1–/– mice was the presence of additional lingual cusps on the mandibular M1, which are never present in Sprouty and Rsk mutant mice (Charles et al. 2009; Marangoni et al. 2015) and are not known in the dentition of any living Murinae.
Are the supernumerary teeth seen in Gas1–/– mice true premolars? The rodent molar dentition has a unity of shape, cuspal morphology, and arrangement that constitutes a morphological signature type. In rodents who have retained a premolar dentition (dormice, squirrels, porcupines), these teeth have a shape and cusp arrangement that very closely resemble that of molars. Direct comparison of a molar supernumerary from a mutant mouse is probably nonsensical because the mutant tooth has a shape and cusp arrangement that make it a supernumerary tooth of Murinae, and wild Murinae do not have premolars.
GAS1 Function in Human Dental Development
The human subjects with mutation in GAS1 or GAS1/SHH have an HPE craniofacial phenotype characterized by a flat face, maxillary and nasal hypoplasia, absent columella, and bilateral cleft lip/palate (Ribeiro et al. 2010). There was a general reduction in length of M1 in these subjects and absence of the mandibular M1 hypoconulid. This is a relatively rare phenotype in modern humans, representing around 0% to 10% of individuals with a maximum frequency of 20% in the populations of western Eurasia. The hypoconulid appeared relatively late in the evolution of mammals and was probably one of the last cusps to be established (Scott and Turner 1997). The maxillary hypocone was absent in 1 subject (GAS1 c.775G > A). This is 1 of the 4 major cusps that compose the quadrangular permanent maxillary M1 and the last major cusp addition to the M1 crown during the evolution of primates. The absence of a M1 hypocone is therefore an extremely rare phenotype in humans, although this cusp may be reduced in some individuals (Scott and Turner 1997). Interestingly, reduction of the M1 hypocone and hypoconulid resembles the essential cusp pattern and shape of M2. These cusps are naturally reduced in M2 and may indicate a role for GAS1 in mediating this process in humans.
Author Contributions
M. Seppala, B. Thivichon-Prince, L. Viriot, M.T. Cobourne, contributed to conception, design, data acquisition, analysis, or interpretation, drafted and critically revised the manuscript; G.M. Xavier, contributed to conception, design, data acquisition, analysis, or interpretation, drafted the manuscript; N. Shaffie, I. Sangani, A.A. Birjandi, J. Rooney, J.N.S. Lau, R. Dhaliwal, O. Rossi, M.A. Riaz, D. Stonehouse-Smith, Y. Wang, S.N. Papageorgiou, contributed to data acquisition, analysis, or interpretation, drafted the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-pdf-1-jdr-10.1177_00220345211049403 for Gas1 Regulates Patterning of the Murine and Human Dentitions through Sonic Hedgehog by M. Seppala, B. Thivichon-Prince, G.M. Xavier, N. Shaffie, I. Sangani, A.A. Birjandi, J. Rooney, J.N.S. Lau, R. Dhaliwal, O. Rossi, M.A. Riaz, D. Stonehouse-Smith, Y. Wang, S.N. Papageorgiou, L. Viriot and M.T. Cobourne in Journal of Dental Research
Acknowledgments
We are indebted to the late Antonio Richieri-Costa (1946-2019), a geneticist at the Hospital for Rehabilitation of Craniofacial Anomalies, University of São Paulo, who kindly provided the human dental records. He sadly passed away before the completion of this investigation. We thank Mathilde Bouchet for her help in the production of 3D reconstructions of dental rows using the Nanotom within the ANIRA-IMMOS platform of SFR Biosciences and Paul Tafforeau for his help in use of the Synchrotron X-ray microtomography at the ESRF in Grenoble. We are grateful to the following investigators for complementary DNA: Andrew McMahon, Shh; Matthew Scott, Ptch1; Chen-ming Fan, Gas1; Robert Krauss, Cdon, Boc; and Atsushi Ohazama, Sostdc1, Axin2. We thank Paul Sharpe and Cynthia Andoniadou for helpful comments relating to the manuscript. Finally, we thank Chen-Ming Fan for long-term support and generosity in providing mutant embryos.
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant to M. Seppala and M.T. Cobourne from the European Orthodontic Society. G.M. Xavier was supported by the Academy of Medical Sciences (Wellcome Trust, British Heart Foundation, Arthritis Research UK) and was the recipient of National Institute of Health Research (NIHR) UK Clinical Lectureship; D. Stonehouse-Smith is a NIHR Academic Clinical Fellow in Orthodontics.
ORCID iDs: J. Rooney  https://orcid.org/0000-0003-2860-2284
https://orcid.org/0000-0003-2860-2284
O. Rossi  https://orcid.org/0000-0002-6116-9892
https://orcid.org/0000-0002-6116-9892
D. Stonehouse-Smith  https://orcid.org/0000-0002-1096-5012
https://orcid.org/0000-0002-1096-5012
S.N. Papageorgiou  https://orcid.org/0000-0003-1968-3326
https://orcid.org/0000-0003-1968-3326
M.T. Cobourne  https://orcid.org/0000-0003-2857-0315
https://orcid.org/0000-0003-2857-0315
References
- Ahn Y, Sanderson BW, Klein OD, Krumlauf R. 2010. Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development. 137(19):3221–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BL, Song JY, Izzi L, Althaus IW, Kang JS, Charron F, Krauss RS, McMahon AP. 2011. Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Dev Cell. 20(6):775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BL, Tenzen T, McMahon AP. 2007. The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes Dev. 21(10):1244–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae GU, Domene S, Roessler E, Schachter K, Kang JS, Muenke M, Krauss RS. 2011. Mutations in CDON, encoding a hedgehog receptor, result in holoprosencephaly and defective interactions with other hedgehog receptors. Am J Hum Genet. 89(2):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain CE, Jeong J, Guo C, Allen BL, McMahon AP. 2008. Notochord-derived Shh concentrates in close association with the apically positioned basal body in neural target cells and forms a dynamic gradient during neural patterning. Development. 135(6):1097–1106. [DOI] [PubMed] [Google Scholar]
- Charles C, Pantalacci S, Tafforeau P, Headon D, Laudet V, Viriot L. 2009. Distinct impacts of Eda and Edar loss of function on the mouse dentition. PLoS One. 4(4):e4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SW, Kwak S, Woolley TE, Lee MJ, Kim EJ, Baker RE, Kim HJ, Shin JS, Tickle C, Maini PK, et al. 2011. Interactions between Shh, Sostdc1 and Wnt signaling and a new feedback loop for spatial patterning of the teeth. Development. 138(9):1807–1816. [DOI] [PubMed] [Google Scholar]
- Chuang PT, McMahon AP. 1999. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 397(6720):617–621. [DOI] [PubMed] [Google Scholar]
- Cobourne MT, Sharpe PT. 2010. Making up the numbers: the molecular control of mammalian dental formula. Semin Cell Dev Biol. 21(3):314–324. [DOI] [PubMed] [Google Scholar]
- Harjunmaa E, Kallonen A, Voutilainen M, Hamalainen K, Mikkola ML, Jernvall J. 2012. On the difficulty of increasing dental complexity. Nature. 483(7389):324–327. [DOI] [PubMed] [Google Scholar]
- Izzi L, Levesque M, Morin S, Laniel D, Wilkes BC, Mille F, Krauss RS, McMahon AP, Allen BL, Charron F. 2011. Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation. Dev Cell. 20(6):788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. 2000. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 92(1):19–29. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. 2012. Tooth shape formation and tooth renewal: evolving with the same signals. Development. 139(19):3487–3497. [DOI] [PubMed] [Google Scholar]
- Kang JS, Gao M, Feinleib JL, Cotter PD, Guadagno SN, Krauss RS. 1997. Cdo: An oncogene-, serum-, and anchorage-regulated member of the Ig/fibronectin type III repeat family. J Cell Biol. 138(1):203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Mulieri PJ, Hu Y, Taliana L, Krauss RS. 2002. Boc, an Ig superfamily member, associates with Cdo to positively regulate myogenic differentiation. EMBO J. 21(1–2):114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassai Y, Munne P, Hotta Y, Penttila E, Kavanagh K, Ohbayashi N, Takada S, Thesleff I, Jernvall J, Itoh N. 2005. Regulation of mammalian tooth cusp patterning by ectodin. Science. 309(5743):2067–2070. [DOI] [PubMed] [Google Scholar]
- Kim J, Ahn Y, Adasooriya D, Woo EJ, Kim HJ, Hu KS, Krumlauf R, Cho SW. 2019. Shh plays an inhibitory role in cusp patterning by regulation of Sostdc1. J Dent Res. 98(1):98–106. [DOI] [PubMed] [Google Scholar]
- Kim Y, Lee J, Seppala M, Cobourne MT, Kim SH. 2020. Ptch2/Gas1 and Ptch1/Boc differentially regulate Hedgehog signalling in murine primordial germ cell migration. Nature communications. 11(1):1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein OD, Minowada G, Peterkova R, Kangas A, Yu BD, Lesot H, Peterka M, Jernvall J, Martin GR. 2006. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev Cell. 11(2):181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Jia S, Jiang R. 2014. Molecular patterning of the mammalian dentition. Semin Cell Dev Biol. 25–26:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan JS, Zheng X, Hauk G, Ghirlando R, Beachy PA, Leahy DJ. 2008. The mode of Hedgehog binding to Ihog homologues is not conserved across different phyla. Nature. 455(7215):979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangoni P, Charles C, Tafforeau P, Laugel-Haushalter V, Joo A, Bloch-Zupan A, Klein OD, Viriot L. 2015. Phenotypic and evolutionary implications of modulating the ERK-MAPK cascade using the dentition as a model. Sci Rep. 5:11658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. 1996. Biochemical evidence that patched is the Hedgehog receptor. Nature. 384(6605):176–179. [DOI] [PubMed] [Google Scholar]
- Martinelli DC, Fan CM. 2007. Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev. 21(10):1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama J, Takabatake T, Takeshima K, Hui C. 1998. Ptch2, a second mouse Patched gene is co-expressed with Sonic hedgehog. Nat Genet. 18(2):104–106. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Haycraft CJ, Seppala M, Blackburn J, Ghafoor S, Cobourne M, Martinelli DC, Fan CM, Peterkova R, Lesot H, et al. 2009. Primary cilia regulate Shh activity in the control of molar tooth number. Development. 136(6):897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, McConnell SK. 2006. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 444(7117):369–373. [DOI] [PubMed] [Google Scholar]
- O’Leary MA, Bloch JI, Flynn JJ, Gaudin TJ, Giallombardo A, Giannini NP, Goldberg SL, Kraatz BP, Luo ZX, Meng J, et al. 2013. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science. 339(6120):662–667. [DOI] [PubMed] [Google Scholar]
- Peterková R, Lesot H, Viriot L, Peterka M. 2005. The supernumerary cheek tooth in tabby/EDA mice—a reminiscence of the premolar in mouse ancestors. Arch Oral Biol. 50(2):219–225. [DOI] [PubMed] [Google Scholar]
- Peterková R, Peterka M, Lesot H. 2003. The developing mouse dentition: a new tool for apoptosis study. Ann N Y Acad Sci. 1010:453–466. [DOI] [PubMed] [Google Scholar]
- Peterková R, Peterka M, Viriot L, Lesot H. 2002. Development of the vestigial tooth primordia as part of mouse odontogenesis. Connect Tissue Res. 43(2–3):120–128. [DOI] [PubMed] [Google Scholar]
- Prochazka J, Pantalacci S, Churava S, Rothova M, Lambert A, Lesot H, Klein O, Peterka M, Laudet V, Peterkova R. 2010. Patterning by heritage in mouse molar row development. Proc Natl Acad Sci U S A. 107(35):15497–15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro LA, Quiezi RG, Nascimento A, Bertolacini CP, Richieri-Costa A. 2010. Holoprosencephaly and holoprosencephaly-like phenotype and GAS1 DNA sequence changes: report of four Brazilian patients. Am J Med Genet A. 152A(7):1688–1694. [DOI] [PubMed] [Google Scholar]
- Scott GR, Turner CG. 1997. Geographic variation in tooth crown and root morphology. In: Scott GR, Turner CG, editors. The anthropology of modern human teeth. New York (NY): Cambridge University Press. p. 165–242. [Google Scholar]
- Seo H, Amano T, Seki R, Sagai T, Kim J, Cho SW, Shiroishi T. 2018. Upstream enhancer elements of Shh regulate oral and dental patterning. J Dent Res. 97(9):1055–1063. [DOI] [PubMed] [Google Scholar]
- Seppala M, Depew MJ, Martinelli DC, Fan CM, Sharpe PT, Cobourne MT. 2007. Gas1 is a modifier for holoprosencephaly and genetically interacts with Sonic hedgehog. J Clin Invest. 117(6):1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, et al. 1996. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 384(6605):129–134. [DOI] [PubMed] [Google Scholar]
- Tenzen T, Allen BL, Cole F, Kang JS, Krauss RS, McMahon AP. 2006. The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signaling pathway and feedback network in mice. Dev Cell. 10(5):647–656. [DOI] [PubMed] [Google Scholar]
- Viriot L, Lesot H, Vonesch JL, Ruch JV, Peterka M, Peterková R. 2000. The presence of rudimentary odontogenic structures in the mouse embryonic mandible requires reinterpretation of developmental control of first lower molar histomorphogenesis. Int J Dev Biol. 44(2):233–240. [PubMed] [Google Scholar]
- Yu T, Klein OD. 2020. Molecular and cellular mechanisms of tooth development, homeostasis and repair. Development. 147(2):dev184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jdr-10.1177_00220345211049403 for Gas1 Regulates Patterning of the Murine and Human Dentitions through Sonic Hedgehog by M. Seppala, B. Thivichon-Prince, G.M. Xavier, N. Shaffie, I. Sangani, A.A. Birjandi, J. Rooney, J.N.S. Lau, R. Dhaliwal, O. Rossi, M.A. Riaz, D. Stonehouse-Smith, Y. Wang, S.N. Papageorgiou, L. Viriot and M.T. Cobourne in Journal of Dental Research