Abstract
Background:
This is the first-in-human study of icenticaftor, an oral potentiator of the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) channel. Restoration of CFTR activity has shown significant clinical benefits, but more studies are needed to address all CFTR mutations.
Methods:
Safety, pharmacodynamics/pharmacokinetics of icenticaftor were evaluated in a randomized, double-blind, placebo-controlled study in healthy volunteers. Efficacy was assessed in adult CF patients with ≥1 pre-specified CFTR Class III or IV mutation (150 and 450 mg bid), or homozygous for F508del mutation (450 mg bid). Primary efficacy endpoint was change from baseline in lung clearance index (LCI2.5). Secondary endpoints included %predicted FEV1 and sweat chloride level.
Results:
Class IV mutations were present in 22 patients, Class III in 2 (both S549N), and 25 were homozygous for F508del. Icenticaftor was well-tolerated in healthy and CF subjects with no unexpected events or discontinuations in the CF groups. The most frequent study-drug related adverse events in CF patients were nausea (12.2%), headache (10.2%), and fatigue (6.1%). Icenticaftor 450 mg bid for 14 days showed significant improvements in all endpoints versus placebo in patients with Class III and IV mutations; mean %predicted FEV1 increased by 6.46%, LCI2.5 decreased by 1.13 points and sweat chloride decreased by 8.36 mmol/L. No significant efficacy was observed in patients homozygous for a single F508del.
Conclusions:
Icenticaftor was safe and well-tolerated in healthy volunteers and CF patients, and demonstrated clinically meaningful changes in lung function and sweat chloride level in CF patients with Class III and IV CFTR mutations.
Keywords: Cystic fibrosis, Icenticaftor, Lung clearance index, Homozygous F508del mutation, Cystic fibrosis questionnaire
1. Background
Cystic fibrosis (CF) is the most common and life shortening recessive disease, affecting about 80,000 children and adults worldwide with an annual mortality rate of 1.5% and median age at death of 30.6 years [1]. The cystic fibrosis transmembrane conductance regulator (CFTR) proteins are chloride channels resident on the surface of epithelial cells in several body organs including the airways of the lungs. Mutations in the CFTR gene may result in the absence of or deficiencies in CFTR protein and cause CF [2,3]. Loss of functionality of CFTR in the airways results in decreased airway surface liquid volume, impaired mucociliary clearance, and chronic airway infection and inflammation leading to progressive lung disease [2]. More than 90% of the morbidity and mortality of CF result from respiratory disease [4].
Mutation-targeted CFTR modulators are becoming the cornerstone of CF management. CFTR potentiators help chloride flow through the CFTR protein channel at the cell surface. Monotherapy with the CFTR potentiator ivacaftor has demonstrated substantial improvements in lung function and reduced frequency of exacerbation by restoring CFTR activity in patients with surface-localized CFTR mutations (Class III and IV) [5,6]. The more common F508del mutation, the archetype Class II variant present in at least one copy in ~90% of the CF population, principally exhibits abnormal processing and trafficking, reducing levels at the plasma membrane [7]; when surface expression is partially restored by CFTR correctors, potentiators are needed to augment F508del CFTR activity as it also exhibits defective channel gating [7]. When used together with one or more CFTR correctors, CFTR potentiators provide clinical benefit [8], such as that seen with the triple combination therapy consisting of the CFTR corrector elexacaftor, with tezacaftor and ivacaftor, which resulted in improved clinical outcomes in patients with cystic fibrosis and F508del mutation [9]. When either corrector therapy or potentiator therapy was used as a single agent, bioactivity was observed by small reductions in sweat chloride, but this effect was insufficient to confer clinical benefit [10]. Although treatment with ivacaftor in combination with other CFTR correctors was beneficial for CF patients with the F508del mutation, ivacaftor also has a nonspecific destabilizing effect on F508del-CFTR, which is of uncertain clinical relevance [11]. In chronic exposure in in vitro laboratory studies, icenticaftor (QBW251), an orally administered CFTR potentiator, in combination with lumacaftor (a CFTR corrector) showed superiority in sustaining membrane expression and function of the mutant F508del-CFTR protein, compared with ivacaftor [12,13].
We hypothesized that icenticaftor would provide a favourable safety, tolerability, pharmacokinetic (PK), and pharmacodynamic profile as monotherapy in patients with specific Class III and IV mutations. As icenticaftor was optimized for the F508del mutation, and some patients with F508del exhibit partial retention of cell surface expression, the enrolment of CF patients homozygous for the F508del mutation in the study evaluated the effects of icenticaftor on safety, tolerability, and clinical benefit as monotherapy in the absence of a CFTR corrector.
In this manuscript, the results from the first-in-human study of icenticaftor monotherapy in healthy volunteers and CF patients are presented.
2. Methods
2.1. Study design
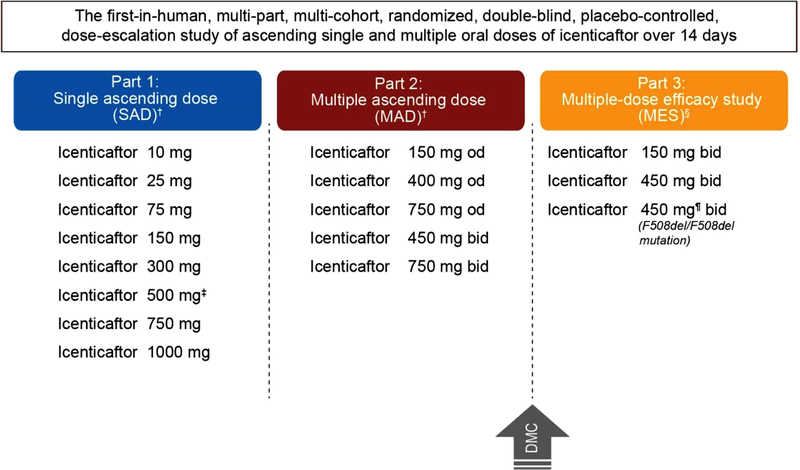
This was a first-in-human, multi-part, randomized, double-blind, placebo-controlled, dose-escalation study of single [SAD; Part 1] and multiple [MAD Part 2] ascending oral doses of icenticaftor over 14 days in healthy volunteers (EU Clinical Trials Register: 2011–005085-37) and multi-dose efficacy study [MES] in CF patients (Part 3; www.clinicaltrials.gov identifier: NCT02190604). Healthy volunteers and CF patients were randomized to receive icenticaftor or placebo (3:1 allocation ratio), across treatment groups in each part of the study (Fig. 1).
Fig. 1. Overall study overview and patient disposition.
† Healthy volunteers were randomized in to (3:1 ratio) for 14 days to icenticaftor (n = 6) or placebo (n = 2); ‡ Following completion of dosing, 5 out of 6 subjects received an additional dose for preliminary evaluation of effect of food on icenticaftor pharmacokinetics. Hence, the total number subjects remains 64; §icenticaftor was administered to patients with cystic fibrosis (Class III and IV or F508del/F508del mutation) for 14 days; icenticaftor 150 mg bid (n = 6), Placebo (n = 2); icenticaftor 450 mg bid(n = 12), Placebo (n = 4); icenticaftor 450 mg bid (F508del/F508del mutation, n = 19), Placebo (n = 6); ¶The planned enrolment in icenticaftor 450 mg bid was 32 patients who were homozygous for the F508del mutation; however, study enrolment was discontinued in this cohort based on results of interim analysis of 16 of 25 enrolled patients which suggested futility
bid, twice daily; DMC, data monitoring committee, MAD, multiple ascending dose; MES, multi-dose efficacy study; od, once daily; SAD, single ascending dose.
The SAD and MAD parts were performed at a single centre (Simbec Research Limited, Merthyr Tydfil CF48 4DR, UK) starting July 31, 2012, while the MES part was conducted at multiple sites in multiple countries from July 08, 2014, and the study was completed on Nov 30, 2015. In this study, icenticaftor in capsule formulation was used in all the treatment arms.
The study complied with the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines. Before the start of the study, the clinical trial protocol, patient information leaflet, informed consent form, and other locally required documents were reviewed and approved by the Independent Ethics Committees or Institutional Review Boards, or both, of the participating centres. An independent Data Monitoring Committee (DMC) formed by the North American CF Foundation Therapeutics Development Network provided safety oversight for CF patients.
2.2. Study populations
The SAD and MAD parts of the study recruited adult healthy volunteers and the MES part recruited patients with a confirmed diagnosis of CF as per the Cystic Fibrosis Foundation consensus guidelines [14] with at least one of 19 pre-specified Class III or IV mutations on one allele (mutations known or suspected to respond to monotherapy with a CFTR potentiator due to retained surface CFTR expression) or patients homozygous for the F508del mutation.
Other key inclusion criteria for CF patients were: FEV1 at screening of 40–100% of predicted normal (inclusive) and oxygen saturation at screening of ≥90% while breathing room air.
2.3. Objectives
The primary objective of the SAD and MAD parts of the study was to assess tolerability in healthy volunteers and to determine a tolerable dose of icenticaftor for the MES part. Efficacy of icenticaftor was tested in the MES part in patients with CF, where the primary endpoint was the change from baseline in lung clearance index (LCI2.5) on Day 15.
Other key endpoints of the MES part included changes in percent-predicted FEV1, sweat chloride levels in CF patients on Day 15, change in the Cystic Fibrosis Questionnaire-Revised patient-reported outcome (CFQ-R PRO) on Day 14, and the assessment of the PK of icenticaftor.
2.4. Assessments
Healthy volunteers were randomized into placebo and 8 dosing groups (icenticaftor 10–1000 mg in the SAD part. In the MAD part, healthy volunteers were randomized into 5 groups based on dose of icenticaftor including 150, 400 and 750 mg once daily [od]; 450 and 750 mg twice daily [bid]). In the MES, CF patients were randomized into 3 groups based on dose and type of mutation (all Class III and IV mutations: 150 and 450 mg bid; and F508del/F508del mutation: 450 mg bid). All three parts (SAD, MAD and MES) were placebo-controlled.
2.4.1. Safety
In both healthy volunteers and CF patients, safety assessments consisted of collecting all adverse events (AEs), serious AEs (SAEs), monitoring of hematology, blood chemistry and urine, pulse oximetry, vital signs, physical condition, and electrocardiograms.
2.4.2. Pharmacokinetics
PK assessments were performed in all parts (SAD, MAD, and MES) of the study. Icenticaftor levels in plasma and urine were analysed with the use of a validated high-performance liquid chromatography–mass spectrometry method with a lower limit of quantification of 1 ng/mL. The PK parameters of icenticaftor were determined by means of non-compartmental methods.
2.4.3. Efficacy
The multiple breath nitrogen washout technique was used to measure LCI2.5, an indicator of ventilation heterogeneity in patients with obstructive lung diseases. A decrease in LCI2.5 of 1 unit is considered clinically relevant [15]. All spirometry evaluations (FEV1) were performed as per American Thoracic Society/European Respiratory Society Task Force: Standardization of Spirometry guidelines [16]. The collection of sweat samples was performed using an approved Macroduct® sweat collection system. Sweat samples were collected in all 3 patient groups in the MES part at baseline and at Days 7, 14, 28, and 42. Patients completed the CFQ-R questionnaire prior to any other assessments being conducted at baseline and Days 14, 28, and 42. This questionnaire contains multiple domains (e.g. health-related quality of life, symptoms, and overall health perception); the key domain considered for this study was the respiratory domain. An increase in CFQ-R respiratory domain score of ≥4 indicates clinically meaningful improvement [17,20]. The MCID denotes to the respiratory domain only and not for the CFQ-R total score.
2.5. Statistical analyses
The sample sizes in the SAD and MAD parts were typical of dose-escalation studies and were appropriate to meet the objective of safety and tolerability assessment. The primary variable for the safety objective was occurrence of AEs and was counted by event and treatment received, and the corresponding percentages were calculated. Data from healthy volunteers were pooled across all groups. The number and percentage of patients with AEs were tabulated by body system, preferred term, and severity (mild, moderate, and severe). A reduction of 1.3 units in LCI2.5 corresponds to a 90% probability that the difference between icenticaftor and placebo is significant.
The sample sizes for the patient groups in the MES study were sufficiently powered, based on a Bayesian statistical model. A sample size of 32 patients was considered adequate to assess the change from baseline in LCI2.5, as compared to placebo. This provides 80% power to detect an effect of 1 unit assuming a standard deviation of 1.25, to evaluate the treatment effect for the primary endpoint, LCI2.5. The change from baseline after 2 weeks of therapy was analysed using a Bayesian model for repeated measures. The model included effects for baseline by time and treatment-by-time interactions. Non-informative priors were used to obtain the posterior estimates. Placebo data were pooled across patient groups to provide more robust estimates. The Bayesian approach was used to analyse all clinical endpoints, which enabled determination whether the probability that the drug is better than placebo was more than 90% for each clinical efficacy endpoint. Repeated measures for analysis of covariance were applied to the pharmacodynamic variables and CFQ-R patient-reported outcomes (PROs) to assess the sensitivity for the comparisons between icenticaftor and placebo, with and without stratification by genotype. Descriptive statistics were provided for the PK parameters by dose level. Time taken to reach the maximum concentration (Tmax) was evaluated by a nonparametric method with trough concentrations summarized by day and dose-level. Descriptive summary statistics are presented in terms of arithmetic mean, standard deviation (SD), coefficient of variance (CV). For the Bayesian analysis, data are presented as posterior mean change from baseline.
3. Results
3.1. Study population
Healthy volunteers were enrolled in the SAD (n = 64) and MAD (n = 40) parts, with all except 3 healthy volunteers in the MAD part completing the study as planned (2 healthy volunteers receiving placebo discontinued due to AEs and 1 volunteer was lost to follow-up). A summary of the demographics of participants in the SAD and MAD parts is provided in supplementary tables S1 and S2. All healthy volunteers and CF patients were male in the SAD and MAD parts.
In the MES part, 80 patients were screened across 26 sites in 7 countries, 49 of whom were randomized and 37 (76%) patients received icenticaftor therapy (supplementary figure S1). All randomised patients completed this part of the study. Mutations present in CF patients are presented in Table 1, and demographic features by mutation and dose groups are presented in supplementary table S3. Twenty two patients had at least a Class IV mutation, and two patients had the S549N Class III mutation, whereas 25 patients were homozygous for F508del. The total number of males (n = 30, 61%) was higher than females (n = 19, 39%) in the MES part. The mean (standard deviation [SD]) age of the study population was 30.1 (9.2) years; baseline values for efficacy endpoints (LCI2.5, FEV1, and sweat chloride) were comparable between the icenticaftor dose groups and placebo in patients with CF (Table 2 and supplementary table S3).
Table 1.
Summary by qualifying CFTR mutations by treatment and cohort (MES in CF patients).
| Mutation type | Treatment | Icenticaftor 150 mg bid N = 8 | Icenticaftor 450 mg bid N = 16 | Icenticaftor 450 mg bid F508del/F508del N = 25 |
|---|---|---|---|---|
|
| ||||
|
Class III mutation
*
S549N |
Icenticaftor 150 mg bid | 1 | – | – |
| Placebo | 1 | – | – | |
|
Class IV mutations
*
R117H, D1152H, R334W, R352Q, R347H |
Icenticaftor 150 mg bid | 5 | – | – |
| Icenticaftor 450 mg bid | – | 12 | – | |
| Placebo | 1 | 4 | – | |
| F508del/F508del | Icenticaftor 450 mg bid | – | – | 19 |
| Placebo | – | – | 6 | |
Mutations on the second allele varied among patients.bid, twice daily; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; MES, multi-dose efficacy study; N, total number of patients
Table 2.
Baseline visit characteristics for efficacy endpoints in CF patients (MES).
| Pooled Placebo* N = 12 | Icenticaftor 150 mg bid N = 6 | Icenticaftor 450 mg bid N = 12 | Icenticaftor 450 mg bid F508del/F508del N = 19 | ||
|---|---|---|---|---|---|
|
| |||||
| LCI2.5 | Mean (SD) | 16.35 (5.53) | 12.81 (4.06) | 14.78 (4.892) | 15.62 (4.17) |
| Range | 7.60 – 24.30 | 7.10 – 17.20 | 8.70 – 23.60 | 9.80 – 22.10 | |
| FEV1 % predicted | Mean (SD) | 64.48 (17.78) | 67.13 (11.69) | 66.02 (13.28) | 72.39 (17.66) |
| Range | 36.30 – 90.60 | 57.90 – 87.50 | 47.00 – 83.30 | 41.0 – 97.70 | |
| FEV1 (L) | Mean (SD) | 2.67 (0.92) | 2.57 (0.21) | 2.53 (0.66) | 2.76 (0.874) |
| Range | 1.10 – 4.30 | 2.30 – 2.90 | 1.60 – 3.60 | 1.50 – 4.20 | |
| Sweat chloride (mmol/L) | Mean (SD) | 92.50 (9.65) | 61.90 (31.77) | 67.65 (30.05) | 94.89 (13.28) |
| Range | 78.00 – 112.50 | 28.50 – 93.50 | 19.00 – 99.50 | 68.50 – 117.50 | |
Pooled placebo from all groupsbid, twice daily; CF, cystic fibrosis; FEV1, forced expiratory volume in one second; LCI2.5, Lung clearance index; MES, multi-dose efficacy study; SD, standard deviation.
3.2. Safety outcomes
Icenticaftor was well tolerated at all exposures tested up to 1000 mg following SAD and up to 750 mg bid following MAD in healthy volunteers. In the SAD and MAD part, headache (14%) and dizziness (8%), and headache (20%), flatulence (15%), cough (13%), and dizziness (8%), respectively, were the most commonly reported AEs across all doses (supplementary tables S4, S5 and S6).
In the MES part, 150 and 450 mg bid doses of icenticaftor (across all patient sub-groups) were well tolerated by CF patients with no unexpected events, deaths, or discontinuations. Three patients experienced serious AEs: sinusitis (n = 1) and pulmonary exacerbation of CF (n = 2); these events were not thought to be related to the study medication. The overall incidence of CF patients experiencing at least 1 AE was 82% across the treatment groups with 67% in the pooled placebo group. All AEs observed in these patients were mild-to-moderate in intensity and resolved by the end of the study (supplementary table S6). AEs suspected to be related to the study drug were reported in a total of 20 patients including 3 patients from the placebo group and 17 patients from the icenticaftor treatment groups. The most frequent AEs considered related to study drug were nausea (12.2%), headache (10.2%), and fatigue (6.1%); these events were observed in patients receiving icenticaftor 450 mg bid (supplementary table S7). Clinical biochemistry, hematology, urinalysis, electrocardiogram, vital signs, and pulse oximetry results did not indicate any clinically significant safety concerns.
3.3. Pharmacokinetics
In the SAD and MAD parts, the absorption of icenticaftor was moderate-to-rapid following the administration of od and bid doses. Results of PK analysis from SAD are presented in supplementary tables S8 and S9. In the MES part, absorption of icenticaftor was rapid-to-moderate with a median Tmax of ~2–3 h after administration of 150 or 450 mg bid doses. Following the 150 mg twice daily doses, the mean maximum serum concentration (Cmax) was 419 ng/mL after the first dose on Day 1 and 632 ng/mL on Day 14. After the 450 mg twice daily doses the mean Cmax was 1950 ng/mL on Day 1 and 4080 ng/mL on Day 14, respectively. Icenticaftor had over dose-proportional exposure. At steady-state, the mean Cmax and area under the curve (AUC) increased ~6– and 10–fold, respectively, with a 3-fold increase in dose. Icenticaftor exposure was moderately variable within patients after both single and multiple dosing (supplementary table S10)
3.4. Efficacy
3.4.1. Patients with Class III and IV mutations
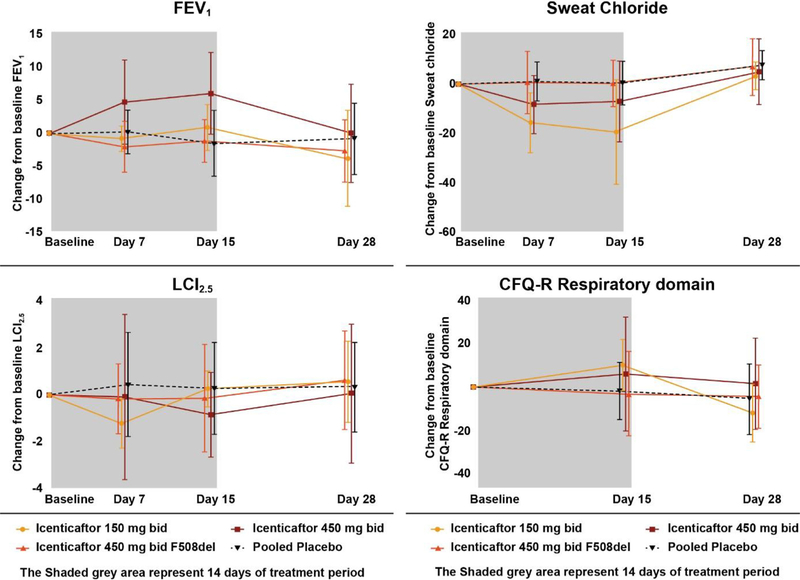
Since both Class III and IV mutations reside at the cell surface and respond robustly to icenticaftor in vitro, they were pooled for the efficacy analysis. In the icenticaftor 450 mg bid group, the difference in posterior mean change from baseline relative to pooled placebo for LCI2.5 was −1.13 (90% credible interval (CrI): −2.60, 0.28) and for % predicted FEV1 was 6.46 (90% CrI: 2.19, 10.68). These results indicate a beneficial treatment effect with icenticaftor as compared to placebo with a probability of 90.2% for LCI2.5 and 99.3% for FEV1. The difference in posterior mean change from baseline for sweat chloride relative to pooled placebo was −8.36 (90% CrI: −19.53, 2.76) mmol/L with icenticaftor 450 mg bid, indicating a beneficial treatment effect with icenticaftor compared to placebo, with a probability of 89.3% (Table 3). The 150 mg bid group showed a smaller effect on both endpoints. Clinically meaningful improvements in mean CFQ-R scores were observed in the respiratory domain in icenticaftor 450 mg bid group (6.06, range −66.7 to 33.3) compared with the pooled placebo group (−1.85, range −22.2 to 22.2) and known MCID of 4 (Fig. 2).
Table 3.
Bayesian analysis of change from baseline on Day 14/15 for efficacy end points in MES.
| N | Arithmetic mean (SD) | Posterior mean | Posterior distribution of treatment difference to placebo* | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Parameters | Icenticaftor/ Placebo* | Icenticaftor | Placebo* | Icenticaftor | Placebo* | Mean (90% CrI) | Probability better than placebo |
|
| |||||||
| Icenticaftor 450 mg bid (patients with Class III and IV mutation) | |||||||
| LCI2.5 | 11/11 | −0.85 (1.80) | 0.28 (1.96) | −0.85 | 0.28 | −1.13 (−2.60, 0.28) | 0.902 |
| FEV1% of predicted | 11/12 | 6.08 (6.25) | −1.53 (5.03) | 5.03 | −1.43 | 6.46 (2.19, 10.68) | 0.993 |
| Sweat Chloride | 9/11 | −7.11 (16.36) | 0.36 (8.86) | −7.86 | 0.50 | −8.36 (−19.53, 2.76) | 0.893 |
| CFQ-R | 11/12 | 6.06 (26.58) | −1.85 (13.26) | 5.68 | −1.53 | 7.21 (−5.53, 18.84) | 0.844 |
| Icenticaftor 450 mg bid (patients with F508del/F508del mutation) | |||||||
| LCI2.5 | 17/11 | −0.13 (2.28) | 0.28 (1.96) | −0.16 | 0.32 | 0.48 (−1.82, 0.86) | 0.726 |
| FEV1% of predicted | 16/12 | −1.10 (3.25) | −1.53 (5.03) | −0.86 | −1.39 | 0.53 (−2.19, 3.20) | 0.633 |
| Sweat Chloride | 17/11 | 0.26 (9.40) | 0.36 (8.86) | 1.35 | −0.07 | 1.43 (−4.86, 7.87) | 0.355 |
| CFQ-R | 19/12 | −3.22 (19.63) | −1.85 (13.26) | −2.61 | −2.85 | 0.24 (−7.52, 7.94) | 0.520 |
Pooled placebo from all groups. Posterior means from the statistical model and adjust for baseline Units for sweat chloride measurements are mmol/L bid, twice daily; CFQ-R, cystic fibrosis questionnaire-revised; CrI, credible interval; FEV1, forced expiratory volume in one second; LCI, lung clearance index; MES, multi-dose efficacy study; N, total number of patients; SD, standard deviation.
Fig. 2.
Arithmetic mean (SD) change from baseline for PD endpoints (LCI2.5, FEV1 % of predicted, sweat chloride mmol/L and respiratory domain score of the CFQ-R) at Day 14 or 15 by time point, groups and treatment in MES.
bid, twice daily; CFQ-R, cystic fibrosis questionnaire-revised; FEV1, forced expiratory volume in one second; LCI, lung clearance index; MAD, multiple ascending dose; MES, multi-dose efficacy study; PD, pharmacodynamics; SAD, single ascending dose; SD, standard deviation.
All patients returned to baseline for all efficacy endpoints at Day 28 following the end of treatment on Days 14/15 (supplementary figures S2 and S3).
3.4.2. Patients with homozygous F508del mutation
The icenticaftor 450 mg bid group with patients homozygous for the F508del mutation was stopped at an interim analysis because the posterior probability of demonstrating meaningful changes in any efficacy endpoint was low. The mean change in LCI2.5 was 0.48 (90% CrI: −1.82, 0.86) and for FEV1 was 0.53 (90% Crl: −2.19, 3.20). Other endpoints were similarly unaffected by icenticaftor therapy (Table 3, Fig. 2).
4. Discussion
In this first-in-human trial, icenticaftor was well tolerated in both healthy volunteers and CF patients. Icenticaftor was well tolerated at all exposures tested up to 750 mg bid in healthy volunteers and up to 450 mg bid in CF patients (with both Class III and IV or homozygous F508del mutations) with no unexpected AEs or deaths. Furthermore, the multi-dose efficacy study (MES) showed that monotherapy with icenticaftor was associated with clinically important improvements in lung function and overall health status and a reduction in sweat chloride in patients with Class III and IV mutations. However, initial clinical data from CF patients homozygous for the F508del mutation did not demonstrate evidence of efficacy with icenticaftor monotherapy.
Discovery and development of icenticaftor was driven by the objective of providing a CFTR potentiator that is effective in improving lung function and patient symptoms, as a monotherapy or as part of a combination CFTR modulator therapy for patients with CF. Patients with Class III and IV CFTR mutations are known to respond to monotherapy with a CFTR potentiator [5,18]; hence, CF patients with these mutations were recruited in this study and evaluated in dose response. We observed a clinically beneficial improvement in sweat chloride and in FEV1 and LCI2.5 in these patients compared with placebo. The results were comparable in similar populations tested with ivacaftor, although the magnitude did vary according to the CFTR mutation and its relative responsiveness [18,19,21]. Longer term studies would be needed to confirm these findings, but based on changes in sweat chloride and FEV1, we would expect similar long term clinical benefits with CFTR potentiation. This group also demonstrated clinically significant improvements in CFQ-R, thus substantiating the efficacy of icenticaftor in this subgroup of CF patients with the 450 mg bid regimen. Effect sizes observed in this group were similar to those demonstrated with ivacaftor in patients with Class IV mutations [19]. While the 150 mg bid regimen showed a lesser effect, the heterogeneity of the mutations across the two active cohorts complicates firm conclusions regarding dose response. Due to this genetic heterogeneity and the overall small sample size, further studies are needed to better describe the efficacy profile of icenticaftor in CF patients with Class III & IV mutations.
Icenticaftor did not demonstrate efficacy in patients homozygous for the more complex F508del mutation, which is associated with a processing and trafficking defect of the CFTR protein that reduces protein resident at the cell surface requisite for a pharmacological benefit. These observations suggest that, similar to ivacaftor, monotherapy with icenticaftor, is not clinically sufficient in this subgroup of patients [17,19,20] and that combination therapy of icenticaftor with a corrector is required to observe a clinical benefit [15]. Since icenticaftor demonstrated bioactivity in patients with Class III and IV mutations, icenticaftor has the potential to be useful when used in a combination therapeutic with CFTR correctors in patients.
In the PK analyses of the SAD and MAD parts of the study, following oral administration of icenticaftor, the median time taken to reach the maximum systemic concentration ranged from 0.8–4 h. Overall, the mean half-life of icenticaftor was 10–13 h in healthy volunteers. In the MES part, icenticaftor was absorbed rapidly after administration to CF patients. The PK profile for icenticaftor showed variability between single and multiple dosing in heterozygous (F508del mutation) CF patients with a gating or residual function mutation (Class III and IV).
This was a first-in-human study, which also explored proof-of-concept in the clinical development program of icenticaftor. The design enabled the assessment of icenticaftor tolerability and PK in healthy volunteers and a rapid transition into CF patients for the assessment of additional selected pharmacodynamic effects. However, the relatively mixed and small number of specific genotypes together with the limitation to only 2 weeks of treatment potentially affected the breadth of clinical experience gathered in this study. Therefore, results of this study need to be interpreted with caution and additional studies are required to fully appreciate the safety and efficacy profile of icenticaftor across multiple CF populations and possibly other indications that may benefit from the potentiation of the CFTR channel.
5. Conclusions
Icenticaftor, a potentiator of membrane-associated CFTR, was safe and well tolerated in healthy volunteers and patients with CF over a 2-week treatment period. Furthermore, icenticaftor demonstrated efficacy (improvement in FEV1, CFQ-R, LCI2.5, and decrease in sweat chloride) in CF patients heterozygous for Class IV mutations.
Supplementary Material
Acknowledgements
We would like to thank all the study volunteers and their families, site investigators, study personnel involved in our study, and the North American Cystic Fibrosis Foundation and the European Cystic Fibrosis Society for their invaluable contributions towards the success of this study. We would also like to thank Nancy Goodman and Gunilla Huledal for their efforts on the study. Editorial and writing support was provided by Santanu Bhadra, PhD (Novartis), Archana Jayaraman, PhD (Novartis) and Venkatesh Taadla (Novartis) in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Declaration of Competing Interest
IB, JM, BS, DR, IJ, KK, SM, LM, IMN, RV, JV, PSA, MSI are employees of Novartis. SK, JA, HD, MG and RS are past employees of Novartis. SMR reports grants, personal fees and non-financial support from Novartis, during the conduct of the study; grants, personal fees, non-financial support and other from Vertex Pharmaceuticals Inc., grants and personal fees from Bayer, grants from Translate Bio, non-financial support from Proteostasis, grants, personal fees and non-financial support from Galapagos/Abbvie, grants and personal fees from Synedgen/Synspira, grants from Eloxx, grants and personal fees from Celtaxsys, grants from Ionis, grants, personal fees and other from Renovion, outside the submitted work; in addition, SMR has a patent regarding the use of CFTR-directed therapies for diseases of mucus in which CFTR is not dysfunctional issued. NJS reports personal fees from Vertex, personal fees from Chiesi, personal fees from Raptor, personal fees from Pulmocide, personal fees from Novartis Pharmaceuticals Corporation, personal fees from Roche, personal fees from Teva, personal fees from Mylan, personal fees from Gilead, personal fees from Zambon, outside the submitted work. All other authors declare no competing interests.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcf.2020.11.002.
References
- [1].Annual Data Report 2017. Cystic Fibrosis Foundation Patient Registry. Available from: https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2017-Patient-Registry-Annual-Data-Report.pdf. Accessed 17 October 2019. [Google Scholar]
- [2].Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med 2007;261(1):5–16. [DOI] [PubMed] [Google Scholar]
- [3].O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet 2009;373(9678):1891–904. [DOI] [PubMed] [Google Scholar]
- [4].Welsh MJ RB, Accurso F, Cutting GR. Cystic fibrosis. In: Scriver CR BA, Valle D, Sly WS, Childs B, Kinzler KW, Vogelstein B, editors. The metabolic and molecular basis of inherited disease. New York: McGraw-Hill; 2001. p. 5121–89. [Google Scholar]
- [5].Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordonez C, Elborn JS. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365(18):1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sawicki GS, McKone EF, Pasta DJ, Millar SJ, Wagener JS, Johnson CA, Konstan MW. Sustained Benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med 2015;192(7):836–42. [DOI] [PubMed] [Google Scholar]
- [7].Quon BS, Rowe SM. New and emerging targeted therapies for cystic fibrosis. BMJ 2016;352:i859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 2015;373(3):220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Middleton PG, Mall MA, Dŕevínek P, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 2019;381(19):1809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Solomon GM, Marshall SG, Ramsey BW, Rowe SM. Breakthrough therapies: cystic fibrosis (CF) potentiators and correctors. Pediatr Pulmonol 2015;50(Suppl 40):S3–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chin S, Hung M, Won A, Wu YS, Ahmadi S, Yang D, Elmallah S, Toutah K, Hamilton CM, Young RN, Viirre RD, Yip CM, Bear CE. Lipophilicity of the cystic fibrosis drug, ivacaftor (VX-770), and its destabilizing effect on the major CF-causing mutation: F508del. Mol Pharmacol 2018;94(2):917–25. [DOI] [PubMed] [Google Scholar]
- [12].LeGrand D, Howsham C, Bala K, Williams G, Lock R, Nicholls I, et al. NVP-QBW251, a novel CFTR potentiator for the treatment of cystic fibrosis. Pediatric Pulmonol 2014;49(S38):S231–2. [Google Scholar]
- [13].Novartis data on file. [Google Scholar]
- [14].Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, Legrys VA, Massie J, Parad RB, Rock MJ, Campbell PW3rd. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr 2008;153(2):S4–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ratjen F, Hug C, Marigowda G, Tian S, Huang X, Stanojevic S, Milla CE, Robinson PD, Waltz D, Davies JC. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6–11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. Lancet Respir Med 2017;5(7):557–67. [DOI] [PubMed] [Google Scholar]
- [16].Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J 2005;26(2):319–38. [DOI] [PubMed] [Google Scholar]
- [17].Muhlebach MS, Clancy JP, Heltshe SL, Ziady A, Kelley T, Accurso F, Pilewski J, Mayer-Hamblett N, Joseloff E, Sagel SD. Biomarkers for cystic fibrosis drug development. J Cyst Fibros 2016;15(6):714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vertex Pharmaceuticals Incorporated. KalydecoTM (ivacaftor) tablets and oral granules, for oral use: US prescribing information. 2012, revised July, 2017. Accessed July 2019. [Google Scholar]
- [19].Rowe SM, Daines C, Ringshausen FC, Kerem E, Wilson J, Tullis E, Nair N, Simard C, Han L, Ingenito EP, McKee C, Lekstrom-Himes J, Davies JC. Tezacaftor-ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med 2017;377(21):2024–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important differences cores for the cystic fibrosis questionnaire-revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nick JA, St Clair C, Jones MC, Lan L, Higgins MVX12–770-113 Study Team. Ivacaftor in cystic fibrosis with residual function: lung function results from an N-of-1 study. J Cyst Fibros 2020;19(1):91–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.