Abstract
Background:
Talimogene laherparepvec (TVEC) is an injectable attenuated oncolytic herpes simplex virus (HSV-1) used in the treatment of loco regionally metastatic melanoma. Lesions managed by TVEC are generally considered unresectable at time of initiation of intralesional therapy; however, a subset of patients go on to have surgical resection of loco regionally controlled disease. We sought to review our experience with surgical excision of treated lesions to offer an insight into the radiologic correlate, treatment effect, and pathological findings of intralesional TVEC therapy.
Methods:
This is a retrospective descriptive case series of patients who underwent TVEC injection at Mayo Clinic, Rochester, MN, between October 2016 and July 2020. Institutional Institutional Review Board approval was obtained.
Results:
Twenty-one patients underwent intralesional TVEC, met inclusion criteria, and were included in this series. Seven went on to surgical excision of the injected lesions after an initial course of TVEC. Of those 7 patients, 4 had residual melanoma in the specimen on final pathology, while 3 had a complete pathologic response. All 3 patients who had no residual disease on pathology continued to have fluorodeoxyglucose (FDG) avidity on preoperative positron emission tomography scan of the excised lesions.
Discussion:
Despite ongoing FDG avidity on PET scan, patients who have well-controlled disease and stability over time of the injected lesions may benefit from surgical excision following a course of TVEC. This may render the patient clinically disease free and/or allow them a reprieve from TVEC treatment.
Keywords: melanoma, general surgery, surgical oncology
Introduction
Treatment options for loco regionally metastatic melanoma have evolved significantly in recent years, with advancements including effective systemic therapy as well as intralesional therapy.1,2 One such option is talimogene laherparepvec (TVEC), an intralesional oncolytic viral therapy which is Food and Drug Administration (FDA) approved for adults with unresectable melanoma.3 Talimogene laherparepvec is a live attenuated herpes simplex virus (HSV-1) which is engineered to replicate in tumor cells and eradicates melanoma by causing local tumor destruction within the injected lesion and also by stimulating a bystander immune response in non-injected lesions.3–5 Although curative resection is not an option for many patients with loco regionally recurrent melanoma, a proportion of patients are able to undergo excision of the lesions after obtaining durable local disease control and demonstrating no evidence of distant progression with TVEC and systemic therapy.6 There are currently no algorithmic guidelines to determine which patients should undergo surgical resection after TVEC, but overall this has been limited to patients who have demonstrated disease control over the time period of the intralesional therapies, indicating a relatively quiescent biological process. In a subset of patients, final surgical pathology has shown no residual melanoma at the time of resection.7,8 This descriptive case series reviews our single-center experience with selective resection of in-transit lesions following a course of TVEC.
Methods
This study was approved by the Mayo Clinic Institutional Review Board. This is a retrospective case series of patients who underwent TVEC therapy at Mayo Clinic, Rochester, Minnesota, between October 2016 and July 2020. Talimogene laherparepvec was FDA approved in October 2015 and was integrated into our institution’s oncology practice 1 year later. Data points were abstracted from the electronic medical record, including patient demographics, oncologic history, prior treatment approaches, disease outcomes, details of TVEC treatment, and disease status at last follow-up. For patients who underwent excision after a course of TVEC, imaging, including ultrasound and positron emission tomography (PET) scans, was sequentially reviewed in addition to surgical pathology. The indication for surgical resection was also retrieved from the medical record. Due to the small number of patients who have undergone surgical excision after TVEC at our institution, no formal statistical analysis was performed and results have been reported descriptively.
Results
A total of 21 patients underwent TVEC intralesional therapy for melanoma at our institution over the time period of the study. Of these patients, 7 went on to surgical excision of at least 1 injected lesion. All 7 patients were being treated for recurrent metastatic melanoma with in-transit disease or distant skin/soft tissue metastasis. Prior to resection, all 7 of these patients had well-controlled loco regional disease and either no distant metastases or well-controlled oligometastatic disease. Pathological analysis of the excised lesions indicated that 3 patients had a complete response, while 4 patients had residual disease in the resected specimen. It should be noted that one of the patients who had residual melanoma underwent palliative resection for an enlarging, symptomatic lesion with otherwise stable disease, and therefore, viable disease on pathology was expected.
Demographic characteristics and treatment course of the 7 patients who underwent surgical excision are described in Tables 1 and 2. The 3 patients who had no residual disease at the time of resection had continued fluorodeoxyglucose (FDG) activity in the excised lesions immediately prior to surgery as described in Table 2 and demonstrated in Figure 1. Additionally, these 3 patients showed improvement in lesion size but had persistent mass lesions on ultrasound leading up to the resection (Figure 2). Final pathology at the time of resection for each of the 3 patients is described in Table 2.
Table 1.
Demographics and Details of Treatment Course for the 7 Patients Who Underwent Surgical Excision of One or More Lesions Following a Course of TVEC Therapy.
| Age at initial melanoma diagnosis + sex (male/female) | Number of TVEC cycles | Number of lesions injected | Number of lesions resected after TVEC | Systemic therapy while on TVEC |
|---|---|---|---|---|
| 74 M | 27 | 18 | 1 | Pembrolizumab |
| 47 M | 7 | 6 | 1 | Pembrolizumab |
| 60 M | 16 | 14 | 1 | Nivolumab |
| 39 M | 16 | 2 | 2 | Pembrolizumab |
| 56 F | 15 | 3 | 3 | Nivolumab |
| 69 M | 12 | 4 | 3 | Nivolumab |
| 44 F | 13 | 9 | 10 | None |
Abbreviation: TVEC, talimogene laherparepvec.
Table 2.
Details of Surgical Management of the Same 7 Patients Who Underwent Resection After TVEC Therapy, Including Clinical Response to TVEC Therapy, Imaging Findings, Final Pathology and Most Current Disease Status.
| Patient Age at melanoma diagnosis (years) & Sex | Initial size of resected lesion(s) (cm) | Size of lesion(s) at time of resection (cm) | FDG avid on pre-op PET? | FDG avidity (SUV max) of resected lesions on pre-op PET | Goal of resection | Surgical pathology | Time from excision at most recent follow-up and current disease status |
|---|---|---|---|---|---|---|---|
| 74 M | 9.0 × 6.0 | 1.5 × 0.9 | Yes | 4.7 | Render NED | Residual melanoma | 16 months-NED |
| 47 M | 2.5 × 1.5 | 15 × 11 | Yes | 9.5 | Palliation | Residual melanoma | Deceased 1 year post-op |
| 60 M | 1.8 | 1.0 | No | None | Diagnostic + stop TVEC-cost and travel burden | Residual melanoma | Deceased 1 year 8 months post-op due to complications from distant disease |
| 39 M | #1: 1.0 × 0.7 | #1: 0.3 × 0.4 | Yes | 2.5 | Stop TVEC-cost and travel burden | No residual melanoma-2 benign lymph nodes w/follicular and paracortical lymphoid hyperplasia | 2 years, 5 months-alive with distant disease |
| #2: 0.7 × 0.5 | #2: 0.7 × 0.5 | ||||||
| 56 F | #1: 0.9 × 0.6 | #1: 0.6 × 0.6 | Yes | #1: 3.1 | Render NED | No residual melanoma-necrosis with inflammation including granulomatous inflammation compatible with treatment-related changes | 1 year-NED |
| #2: 0.9 | #2: 0.9 × 0.9 | #2: 3.8 | |||||
| #3: 2 cm | #3: 0.7 × 0.9 cm | #3: 1.7 | |||||
| 69 M | #1: 0.8 × 0.7 | #1: 0.4 | Yes | 4.4 | Render NED | Tumoral melanosis with no viable disease identified | <1 month-NED |
| #2: 1.3 × 1.5 | #2: 0.8 | ||||||
| #3: 0.4 × 0.5 | #3: 0.9 | ||||||
| 44 F | #1: 3.7 × 2.5 | #1: 2.5 | Yes | “Increased” | Render NED | Residual melanoma | Continued in-transit disease-undergoing systemic and radiation therapy |
| #2: 0.7 | #2: 1.0 | ||||||
| #3: 0.6 | #3: CR | ||||||
| #4: 0.3 | #4: CR | ||||||
| #5: 2.1 × 0.8 | #5: 0.4 | ||||||
| #6: 0.5 | #6: 0.3 | ||||||
| #7: 0.7 | #7: 0.4 | ||||||
| #8: 0.9 | #8: 0.8 | ||||||
| #9: 2.4 × 1.5 | #9: 1.5 |
Abbreviations: CR, complete response; F, female; FDG, fluorodeoxyglucose; M, male; NED, no evidence of disease; PET, positron emission tomography; SUV, standardized uptake value; TVEC, talimogene laherparepvec.
Figure 1.
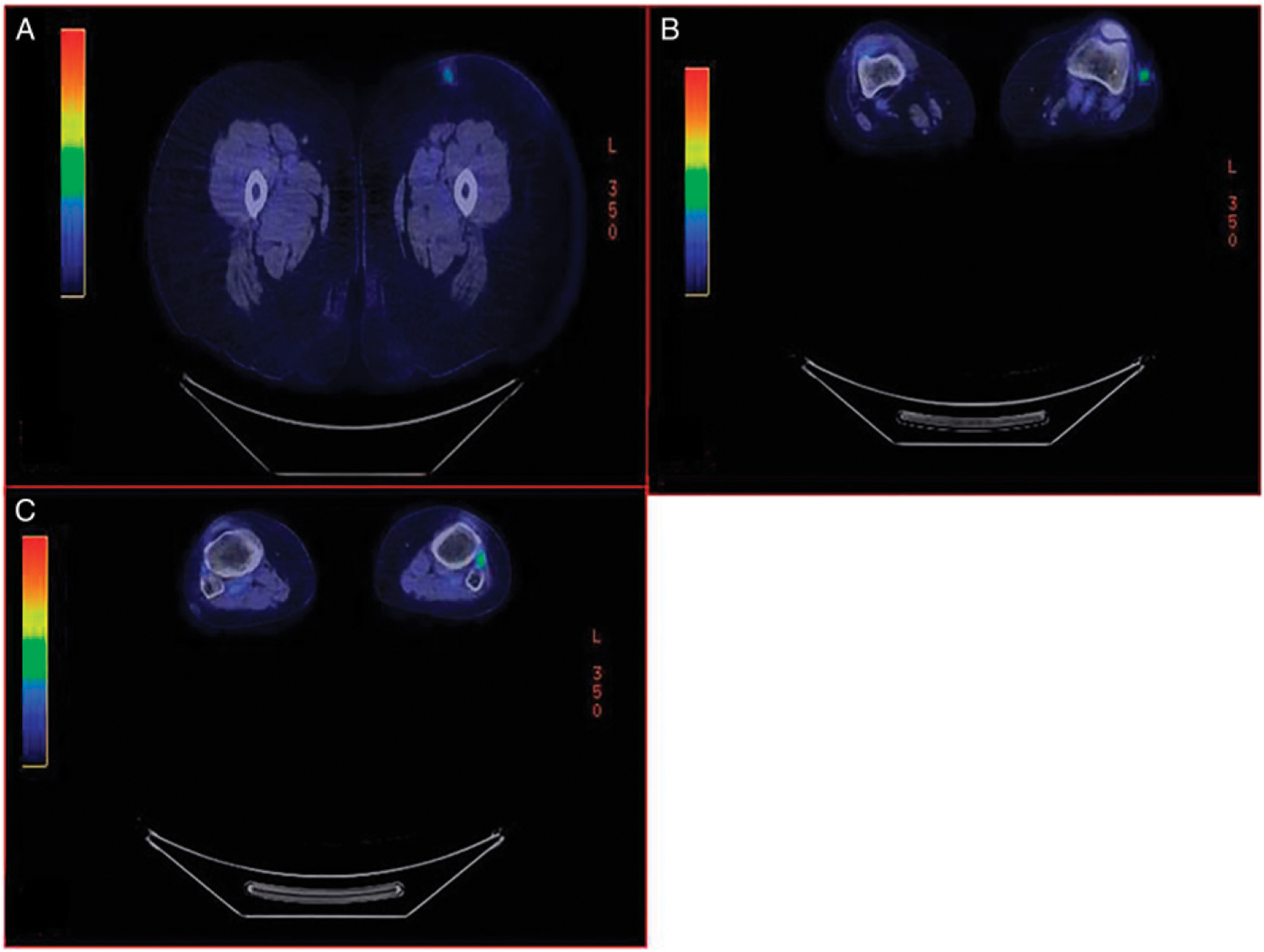
Preoperative PET imaging of a 56 -year-old female patient showing 3 FDG avid lesions of the left lower extremity (A), (B), (C). All 3 lesions were resected with no residual melanoma. (A) Lesion #1: SUV max 3.1 compared to 4.3 on PET CT 3 months prior. (B) Lesion # 2: SUV max 3.8 increased from 2.5 on PET CT 3 months prior. (C) Lesion # 3: SUV max 1.7 from 2.0 on PET CT 3 months prior. FDG, fluorodeoxyglucose; PET, positron emission tomography; SUV, standardized uptake value.
Figure 2.
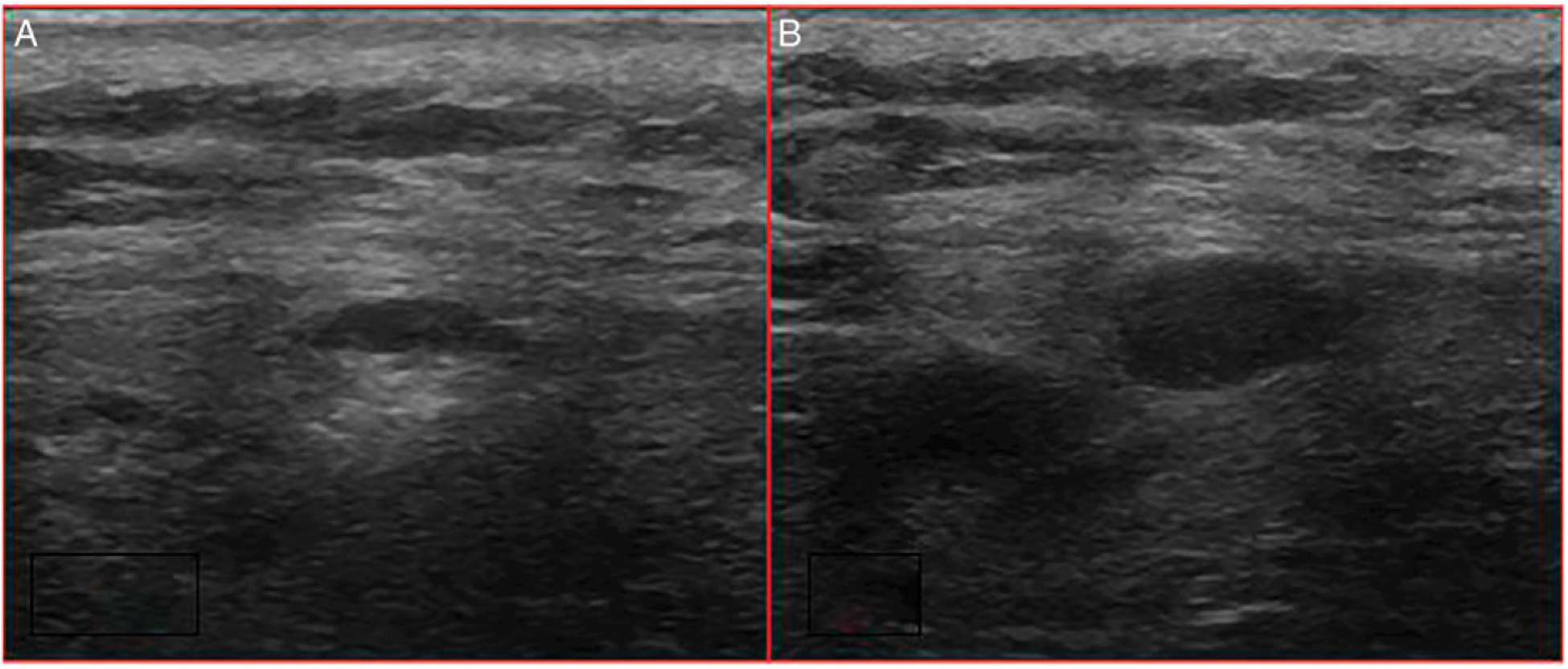
Ultrasound findings of a 39-year-old male patient with 2 lesions (A), (B) on the upper back prior to surgical excision. This is the most recent ultrasound obtained prior to surgery—2 months prior to resection. At the time of surgical resection, intraoperative ultrasound was utilized to localize the lesions; although images from the surgery were not saved in the electronic medical record, the operative dictation describes 2 discrete subcutaneous hypoechoic masses measuring approximately 4 mm and 1 cm in greatest diameter, respectively, as seen in the images. These lesions were also FDG avid on PET. Final pathology showed 2 benign lymph nodes with follicular and paracortical hyperplasia. FDG, fluorodeoxyglucose; PET, positron emission tomography.
Of the 4 patients that had residual melanoma at the time of excision, one showed no FDG activity on the preoperative PETscan, while the other 3 had PETavid lesions prior to resection. Three of the 4 patients showed improvement in lesion size on ultrasound throughout the course of TVEC therapy, with the exception being the patient that underwent palliative resection of a single symptomatic, progressing lesion.
For the 3 patients who had complete pathologic response, the goal of resection was to render them with no evidence of disease (NED). By completing active treatment of the patients’ measureable disease, this would also alleviate the financial and logistical burden of travel and inconvenience required from frequent clinic visits associated with TVEC treatment. For the 4 patients that had residual disease at the time of resection, the goals were to render patients NED, allowing a treatment holiday (2), for diagnostic purposes (1), and for an isolated symptomatic progressing lesion in the setting of otherwise stable disease (1). Resection was successful in 1 patient, allowing a treatment holiday, and in another, distant stage IV disease required further systemic treatment. The patient who underwent diagnostic excision had a necrotic lesion that remained the sole site of disease, he had already undergone multiple indeterminate punch biopsies, and ultimately excision enabled completion of TVEC therapy following prolonged treatment. Current disease status at most recent follow-up for all patients who underwent resection is summarized in Table 2.
Of note, 3 additional patients that underwent TVEC therapy at our institution had complete clinical and radiographic response, including ultrasound and PET scan, of all injected lesions. These 3 patients were observed without surgical intervention and are separate from the 7 described in this series, though within our cohort of 21 TVEC patients. These 3 patients with a clinical/radiographic complete response remain NED, between 4 months and 3 years after the completion of TVEC therapy. Two of these patients continued on systemic therapy with single agent nivolumab or pembrolizumab for the duration of their TVEC treatments, while one was on no systemic agents due to intolerance of multiple prior treatments.
In total, we describe seven of 21 (33.3%) patients who underwent surgical excision following a prolonged period of intralesional TVEC therapy, 3 who had evidence of persistent disease on PET scan and ultrasound and demonstrated a complete pathologic response at time of surgical excision.
Discussion
Intralesional therapy with TVEC represents one of the recent advances in the management of metastatic melanoma and has changed treatment practice at many centers over the last 5 years. As a novel therapy, its uses continue to be refined with experience. This case series describes a single center’s experience with surgical resection of radiographically stable lesions following TVEC, which is presently an evolving area of practice. Of the 7 patients that underwent surgical excision following a course of TVEC, 6 continued to have FDG avid lesions at the time of resection. Despite this, 3 of those patients had no residual disease on final pathology, while the 1 patient that did not have any FDG activity prior to excision had residual melanoma in the resected specimen. Additionally, all patients that underwent excision had persistent hypoechoic masses on ultrasound at the time of surgical resection. As such, when considering resection of lesions following a course of TVEC, a preoperative PET scan showing activity in the target lesion does not assure residual viable disease. Furthermore, a quiescent PET does not rule out viable disease.
An initial flare of injected lesions on PET and/or clinical pseudo-progression of seemingly new lesions following initiation of TVEC therapy is well described and likely represents an active inflammatory infiltrate rendering small clinically occult lesions detectable.9 Persistent PET activity despite a complete pathologic response to TVEC has not been previously described. In one of the patients who had complete pathologic response at the time of resection, final pathology showed necrosis with inflammation including granulomatous inflammation, compatible with treatment-related changes, likely explaining the pre-op PET. In the second patient with complete pathologic response, final pathology showed 2 benign lymph nodes with follicular and paracortical lymphoid hyperplasia, which would explain an ongoing hypoechoic mass though does not necessarily account for the FDG activity. The third patient with complete pathologic response had pathology showing tumor melanosis, which similarly does not account for the ongoing FDG avidity on PET.
The goals of resection were different in each patient. As a quaternary care center in a rural area, many of our patients travel significant distance to reach the clinic. Presenting to clinic every other week for months of TVEC injections can be a hardship involving significant cost and time burden away from work and family. After demonstrating durable control and isolated disease, selective resection to allow a treatment holiday can offer benefit from a financial and quality of life standpoint. Our experience suggests that the decision to proceed with surgical resection following a course of TVEC is a shared decision between the health care team and patient, based on the patient’s goals, prognosis, and future treatment plans.
Although this is the first report of a complete pathologic response despite ongoing FDG avidity on PET scan following selective resection of TVEC-treated lesions, this is a small case series and thus has limitations. As a result of the small number of patients in this series, we do not have power for meaningful formal statistical analysis. As our TVEC program initiated in 2016, we do not have significant long-term follow-up on local disease recurrence for these patients. Given that TVEC is an evolving therapy, this case series offers our first insight into the merit of resection of these lesions after stable loco regional disease is established and highlights the limitations of relying on PET scans alone for determining measureable malignancy. As our oncology practice continues to evolve, with intralesional therapy being a more integral part of the treatment plan for many patients, we aim for this and other studies to help establish guidelines for surgical resection following intralesional treatment.
The role of intralesional therapy in the armamentarium of treatment options for advanced melanoma continues to evolve. Disease free and overall survival are the main measures of oncologic outcomes; however, relieving burden of care, minimizing costs and toxicity, and partnering with patients to optimize their goals and quality of life are critical aspects of management for patients with advanced disease. For select patients that have demonstrated well controlled or no distant metastatic disease, as well as durable well-controlled loco regional disease on TVEC, surgical excision of remaining injected lesions presents an attractive option to alleviate patients of continued frequent treatments and potentially render them NED. Although PET CT is helpful to assist with following these patients’ disease status, ongoing FDG activity does not necessarily indicate active melanoma in all actively treated lesions, and a cold PET did not guarantee no residual melanoma, as shown in this series. Unfortunately, no single imaging modality can reliably determine if viable disease is present. In select patients with stable disease, surgical intervention of remaining clinical lesions can offer patients respite from laborious medical interventions and on occasion reveal an unexpected complete pathologic response. Further studies are needed to help delineate which patients may achieve the most benefit from excision of lesions that have been responding well to intralesional therapy both in terms of disease status and quality of life metrics.
Key Takeaways.
Patients undergoing TVEC therapy for metastatic melanoma may benefit from surgical resection of selected lesions in the setting of otherwise well controlled, stable disease.
Ongoing fluorodeoxyglucose avidity on positron emission tomography (PET) scan does not necessarily indicate active melanoma in all injected lesions during TVEC treatment.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Heart, Lung, and Blood Institute (TAM, grant number: R38HL150086).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Gimbel MI, Delman KA, Zager JS. Therapy for unresectable recurrent and in-transit extremity melanoma. Cancer Control. 2008;15(3):225–232. [DOI] [PubMed] [Google Scholar]
- 2.Racz JM, Block MS, Baum CL, Jakub JW. Management of local or regional non-nodal disease. J Surg Oncol. 2019. Jan; 119(2):187–199. [DOI] [PubMed] [Google Scholar]
- 3.Oglesby A, Algazi AP, Daud AI. Intratumoral and combination therapy in melanoma and other skin cancers. Am J Clin Dermatol. 2019;20:781–796. [DOI] [PubMed] [Google Scholar]
- 4.Perez MC, Zager JS, Amatruda T, et al. Observational study of talimogene laherparepvec use for melanoma in clinical practice in the United States (COSMUS-1). Melanoma Manag. 2019;6(2):MMT19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conry RM, Westbrook B, McKee S, Norwood TG. Talimogene laherparepvec: First in class oncolytic virotherapy. Hum Vaccin Immunother. 2018;14(4):839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puzanov I, Milhem MM, Minor D, et al. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol. 2016;34(22):2619–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louie RJ, Perez MC, Jajja MR, et al. Real-world outcomes of talimogene laherparepvec therapy: A multi-institutional experience. J Am Coll Surg. 2019;228(4):644–649. [DOI] [PubMed] [Google Scholar]
- 8.Andtbacka RH, Ross M, Puzanov I, et al. Patterns of clinical response with talimogene laherparepvec (T-VEC) in patients with melanoma treated in the OPTiM phase III clinical trial. Ann Surg Oncol. 2016;23(13):4169–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayan CY, Lopez AT, Gartrell RD, et al. The role of oncolytic viruses in the treatment of melanoma. Curr Oncol Rep. 2018; 20; 80; Erratum in. Curr Oncol Rep. 2012;10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]


