Abstract
Evidence of synergism between combinations of vancomycin and β-lactam antibiotics against 59 isolates of methicillin-resistant staphylococci (Staphylococcus aureus, Staphylococcus epidermidis, and Staphylococcus haemolyticus) for which vancomycin MICs ranged from 1 to 16 μg/ml were tested by broth microdilution checkerboard, disk diffusion, agar dilution, and time-kill antimicrobial susceptibility tests. The combination of vancomycin and oxacillin demonstrated synergy by all test methods against 30 of 59 isolates; no antagonism was seen. Synergy with vancomycin was also found by modified disk diffusion testing for ceftriaxone, ceftazidime, cefpodoxime, and amoxicillin-clavulanate but not for aztreonam. Evidence of synergy correlated directly with vancomycin MICs. The efficacy of vancomycin given alone and in combination with nafcillin was tested in the rabbit model of experimental endocarditis caused by three clinical isolates of glycopeptide-intermediate-susceptible S. aureus (GISA) (isolates HIP5827, HIP5836, and MU50). Two of the GISA isolates (isolates MU50 and HIP5836) were extremely virulent in this model, with 27 of 42 (64%) animals dying during the 3-day trial. Therapy with either vancomycin or nafcillin given as a single agent was ineffective for animals infected with HIP5827 or MU50. However, the combination of vancomycin and nafcillin resulted in a mean reduction of 4.52 log10 CFU/g of aortic valvular vegetations per g compared to the reduction for controls for animals infected with HIP5827 and a reduction of 4.15 log10 CFU/g for animals infected with MU50. Renal abscesses caused by HIP5827 were sterilized significantly better with the combination of vancomycin and nafcillin than by either treatment alone. We conclude that the combination of vancomycin and β-lactams with antistaphylococcal activity is an effective regimen for the treatment of infections with clinical strains of staphylococci which demonstrate reduced susceptibility to glycopeptides.
Glycopeptides have been used successfully for the treatment of serious methicillin-resistant Staphylococcus aureus (MRSA) infections for the past 30 years. Clinical strains of S. aureus that demonstrate reduced susceptibility to glycopeptides have recently been described from geographically diverse sources (4, 5, 14, 22, 24, 33, 34). For these strains, known collectively as glycopeptide-intermediate-susceptible S. aureus (GISA), vancomycin MICs range from 8 to 16 μg/ml. Although the vancomycin MICs for these isolates remain below achievable levels in serum, clinical infections have responded poorly to the glycopeptides vancomycin and teicoplanin (32). Most patients have required a combination of therapeutic approaches for successful treatment, including surgery, prolonged vancomycin therapy, or the use of additional antistaphylococcal agents.
Although the first clinical isolate of GISA was described in 1997 (14), laboratory-derived strains of S. aureus which demonstrate reduced susceptibility to glycopeptides have been under investigation since the early 1990s (9, 17, 18, 26, 28, 29). These laboratory-derived strains are easily produced by step passage in the presence of increasing concentrations of glycopeptides. In one report of a laboratory-derived vancomycin-resistant isolate of S. aureus, Sieradzki and Tomasz (28) noted that this highly vancomycin resistant isolate became extremely sensitive to β-lactam antibiotics in comparison to its vancomycin-sensitive, methicillin-resistant parent. He termed this phenomenon “the seesaw effect” and attributed it to the alterations in cell wall composition required for vancomycin resistance. The previous report suggested that the alterations required for the optimal expression of vancomycin resistance affected the expression of methicillin resistance as well.
Investigators have also noted that combinations of glycopeptides and β-lactams can demonstrate additive or synergistic activity against MRSA isolates in in vitro tests, since they act as inhibitors at different stages of cell wall synthesis (2, 23, 30). These data, in combination with the observations of Sieradzki and Tomasz (28), raised the possibility that GISA strains may also respond synergistically to combinations of vancomycin and β-lactams.
In this report, we characterize the in vitro susceptibilities of staphylococcal isolates with a broad range of vancomycin MICs to combinations of vancomycin and β-lactams by the use of broth microdilution checkerboard testing and the disk diffusion, agar dilution, and time-kill methods. We then evaluated the effectiveness of combination vancomycin-nafcillin treatment of experimental aortic valve endocarditis caused by clinical GISA isolates in rabbits.
MATERIALS AND METHODS
Bacterial strains.
Fifty-nine methicillin-resistant staphylococcal isolates were examined. Forty-one methicillin-resistant staphylococcal strains (21 MRSA, 10 methicillin-resistant Staphylococcus epidermidis [MRSE], and 10 methicillin-resistant Staphylococcus haemolyticus [MRSH] strains) were taken from a collection of geographically diverse clinical strains maintained at the Medical College of Virginia Campus of Virginia Commonwealth University as described previously (7). Ten MRSE isolates collected between August 1998 and October 1998 were recovered from patients with infections of central venous catheters. Three MRSA isolates with reduced susceptibility to vancomycin (vancomycin MIC, 8 μg/ml; isolates HIP5827 [Michigan], HIP5836 [New Jersey] and MU50 [Japan]) and vancomycin-susceptible isolate MU3 (vancomycin MIC, 2 μg/ml) were kindly provided by Fred Tenover of the Centers for Disease Control and Prevention (34). Four staphylococcal strains with higher levels of vancomycin resistance were produced by step passage as described previously (8, 18). MRSA 27619VR (vancomycin MIC, 8 μg/ml) is an isogenic derivative of the vancomycin-susceptible parent S. aureus 27619 (7, 8). MRSA 5827HR (vancomycin MIC, 16 μg/ml) is an isogenic derivative of HIP5827. Finally, two MRSH isolates with higher levels of vancomycin resistance were isolated following successive overnight passages of S. haemolyticus 27280 in increasing concentrations of vancomycin. They were designated 27280-2 (vancomycin MIC, 8 μg/ml) and 27280-3 (vancomycin MIC, 16 μg/ml), respectively.
Antimicrobial susceptibility testing.
MICs were determined by the broth microdilution method in cation-adjusted Mueller-Hinton broth (Becton Dickinson, Cockeysville, Md.) according to the standards of the National Committee for Clinical Laboratory Standards (19). The MIC was the lowest concentration of antibiotic that yielded no visible growth after incubation at 37°C for 24 h.
Checkerboard synergy testing was performed by the microdilution method in microtiter trays with cation-adjusted Mueller-Hinton broth. Combinations of vancomycin and oxacillin were tested at concentrations of 0.25 to 16 and 0.125 to 128 μg/ml, respectively. Microtiter plates were incubated at 37°C and were read at 24 and 48 h. The fractional inhibitory concentration (FIC) index was calculated by adding the FICs (MIC of drug A in combination with drug B/MIC of drug A alone) of vancomycin and oxacillin. An FIC index of ≤0.5 was defined as synergy, an FIC index of >0.5 to 4.0 was defined as additive or indifferent, and an FIC index of >4.0 was defined as antagonistic. Checkerboard test results represented the average of duplicate tests.
Time-kill assays were performed in 20 ml of cation-adjusted Mueller-Hinton broth inoculated with the test organisms to a final concentration of from 5 × 106 to 5 × 107 CFU/ml. Vancomycin was tested at the MIC, 2× the MIC, and 4× the MIC. Oxacillin was used at a concentration of 6 μg/ml. Bacterial counts were taken at 0, 1, 4, and 24 h by plating 0.1-ml aliquots and serially diluting each onto Mueller-Hinton agar. Synergy between vancomycin and oxacillin was defined as a reduction in the initial inoculum of >2 log10 CFU/ml (99.9%) at 24 h.
Synergy between vancomycin and oxacillin was also determined by a modified disk diffusion method. Clinical isolates of GISA (HP5827, HP5836, and MU50) underwent Kirby-Bauer disk diffusion testing with commercially prepared brain heart infusion agar (BHIA) containing 6 μg of vancomycin (Remel, Lenexa, Kans.) per ml, and antimicrobial disks (BBL Sensi-Disc; Becton Dickinson) containing oxacillin (1 μg), amoxicillin-clavulanate (30 μg), ceftriaxone (30 μg), aztreonam (30 μg), and cefpodoxime (10 μg) according to the guidelines of the National Committee for Clinical Laboratory Standards (20). Synergy was defined as an enhancement in the zone of inhibition surrounding the antibiotic disk on vancomycin agar compared to the zone size around disks placed on BHIA containing no antibiotic.
Population analysis profiles were generated from overnight cultures of staphylococcal strains grown in Mueller-Hinton broth. Overnight cultures were diluted until the turbidity matched that of a 0.5 McFarland standard and serial dilutions were then plated on Mueller-Hinton agar plates containing vancomycin ranging in concentrations from 1 to 16 μg/ml. In tests for synergy, oxacillin at a fixed concentration of 6 μg/ml was also added to vancomycin-containing agar. The plates were incubated for 48 h, and the numbers of colonies were counted and plotted graphically.
Experimental infection.
The rabbit model of aortic valve endocarditis, which has been described previously (7, 8, 21), was used to evaluate the antibiotic treatment regimens. Seventy-two hours after transcarotid placement of a polyethylene catheter across the aortic valve, rabbits were injected intravenously through the marginal ear vein with 1 ml of an overnight culture containing 107 CFU of the test organism per ml. The test organisms included strains HIP5827, HIP5836, and MU50. Blood samples for culture were obtained 24 h later, and the rabbits were randomly assigned to one of the following treatment groups: vancomycin (Abbott Laboratories, Chicago, Ill.) given at 30 mg/kg of body weight intravenously every 12 h, nafcillin (Bristol-Meyer Squibb, Princeton, N.J.) given at 200 mg/kg intramuscularly every 8 h, and vancomycin given at 30 mg/kg intravenously every 12 h plus nafcillin given at 200 mg/kg intramuscularly every 8 h, or no treatment (control group). The surviving animals were killed with intravenous pentobarbital after a total of 3 days of antibiotic treatment. Rabbits with negative blood cultures at 24 h were excluded from subsequent analysis. To reduce the possibility of antibiotic carryover, rabbits were not killed until at least 18 h after administration of the last dose. The heart and kidneys were removed aseptically from each rabbit. Aortic valve vegetations were removed from each rabbit’s heart and weighed, and serial dilutions of vegetation homogenates were made. The kidneys were examined, and areas of abscess or infarct were removed, weighed, homogenized in saline, and serially diluted. Tissue homogenates were also plated onto Mueller-Hinton agar containing increasing concentrations of vancomycin in order to generate population analysis profiles. Cultures were read after 48 h. Titers of bacteria were expressed as log10 CFU per gram of vegetation or kidney tissue. Sterile vegetation and kidney cultures contained ≤2 and ≤1 log10 CFU/g, respectively (the limit of detection).
Inclusion criteria.
For the final analysis, data for animals that fulfilled the following criteria were included: (i) positive blood culture at 24 h, (ii) survival for at least 24 h of antibiotic treatment, (iii) proper placement of the catheter across the aortic valve at necropsy with macroscopic evidence of aortic valve endocarditis (visible vegetations), and (iv) aortic valve vegetation and kidney tissue yielding pure cultures of the test organism.
Statistical analysis.
The mean numbers of bacteria per gram of vegetation and kidney tissue in all treatment groups were compared by analysis of variance. Sterile aortic valve and kidney cultures were entered as 2 and 1 log10 CFU/g, respectively (the limit of detection). The Student-Newman-Keuls test was used to adjust for multiple comparisons. For analysis of the sterilization of tissue cultures, we used Fisher’s exact test. A P value of <0.05 was considered statistically significant for all tests.
RESULTS
Vancomycin–β-lactam synergy against methicillin-resistant staphylococcal strains.
A total of 59 methicillin-resistant staphylococci underwent antimicrobial susceptibility testing and testing for synergy between vancomycin and oxacillin by the broth microdilution checkerboard method. We included coagulase-negative staphylococcal species in our examination because the glycopeptide MICs for these isolates are increased compared to those for S. aureus. The results of these tests are presented in Table 1. Vancomycin-oxacillin combinations demonstrated synergy against all three clinical isolates of GISA as well as 4 of 10 MRSH and 18 of 20 MRSE isolates. Synergy was seen against all four step-passage isolates with higher levels of vancomycin resistance (isolates 27619VR, 5827HR, 27280-2, and 27280-3). No evidence of synergy was seen against 22 MRSA isolates for which vancomycin MICs were ≤2 μg/ml. No antagonism was noted against any of the isolates tested.
TABLE 1.
FIC indices of the combination of oxacillin and vancomycin for staphylococcal isolates
| Organism (no. of isolates) | Vancomycin MIC (μg/ml) | FIC index | Presence of synergyb |
|---|---|---|---|
| MRSA (22) | 1–2a | 0.51–2a | 0/22 |
| MRSE (20) | 1–4a | 0.08–0.56a | 18/20 |
| MRSH (10) | 2–4a | 0.19–1.5a | 4/10 |
| MRSA HIP5827 | 8 | 0.26 | 1 |
| MRSA HIP5836 | 8 | 0.09 | 1 |
| MRSA MU50 | 8 | 0.25 | 1 |
| S. haemolyticus 27280-2 | 8 | 0.25 | 1 |
| S. haemolyticus 27280-3 | 16 | 0.12 | 1 |
| S. aureus 27619VR | 8 | 0.19 | 1 |
| S. aureus 5827HR | 16 | 0.18 | 1 |
Values are ranges.
Data represent number of isolates against which synergy was observed/total number of isolates tested or number of isolates against which synergy was observed.
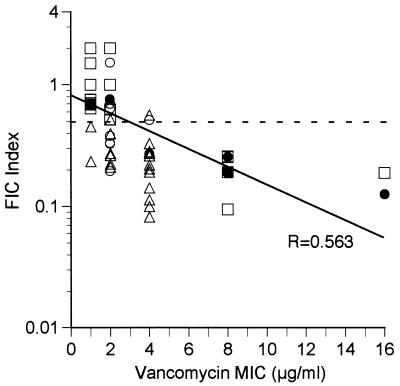
Among the staphylococcal isolates, the synergy of the combination of vancomycin and oxacillin was directly associated with the level of vancomycin resistance. The combination was more likely to exhibit synergism against isolates of staphylococci with higher levels of vancomycin resistance. The combination consistently demonstrated synergy against those isolates for which vancomycin MICs were ≥4 μg/ml. This is shown in Fig. 1, which plots the FIC index for isolates as a function of the vancomycin MIC. The FIC index for isolates is inversely correlated with the vancomycin MIC, indicating that higher levels of vancomycin resistance are associated with increasing synergy between the combination of vancomycin and β-lactams. This observation was also confirmed in the examination of the step-passage isolates: 27619VR (vancomycin MIC, 8 μg/ml), 27280-2 (vancomycin MIC, 8 μg/ml), and 27280-3 (vancomycin MIC, 16 μg/ml). In all three of these isolates the development of vancomycin resistance was associated with the development of synergism of the drug combination (FIC indices, 0.19190, 0.25195, and 0.1255 respectively), whereas synergism was not exhibited for either of the parent strains (strains 27619 and 27280; vancomycin MICs, 1 and 2 μg/ml, respectively; FIC indices, 0.6875 and 0.75, respectively).
FIG. 1.
Results of checkerboard testing of combinations of vancomycin and oxacillin against staphylococcal isolates with various degrees of vancomycin susceptibility. The FIC index is plotted against the MIC for each isolate of S. haemolyticus (○), S. epidermidis (▵), and S. aureus (□). Solid circles, S. haemolyticus isolates 27280, 27280-2, and 27280-3 (vancomycin MICs, 2, 8, and 16 μg/ml, respectively); solid squares, S. aureus isolates 27619 and 27619VR (vancomycin MICs, 1 and 8 μg/ml, respectively). The dashed line represents a FIC index of 0.5. Vancomycin and oxacillin demonstrated synergy against isolates whose data fall below this line. The solid line represents the best-fit line, with a corresponding correlation factor of 0.563.
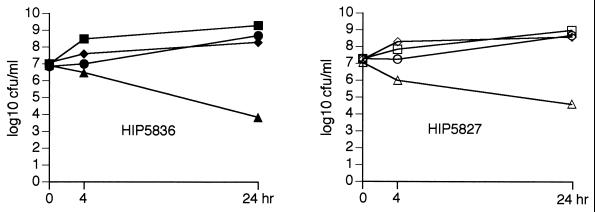
The synergistic activity of vancomycin in combination with oxacillin against GISA isolates was confirmed in time-kill experiments, the results of which are presented in Fig. 2. Vancomycin at concentrations up to 4 × the MIC showed no evidence of bactericidal activity. However, a bactericidal effect was seen with the combination of vancomycin (8 μg/ml) and oxacillin (6 μg/ml) at 24 h against two GISA isolates (isolates HIP5827 and HIP5836), with reductions in bacterial counts of 2.44 and 3.06 log10 CFU/ml, respectively.
FIG. 2.
Time-kill curves for GISA HIP5827 and HIP5836. Results for the following groups are represented; controls (squares), vancomycin at 8 μg/ml (circles), oxacillin at 6 μg/ml (diamonds), and vancomycin at 8 μg/ml in combination with oxacillin at 6 μg/ml (triangles).
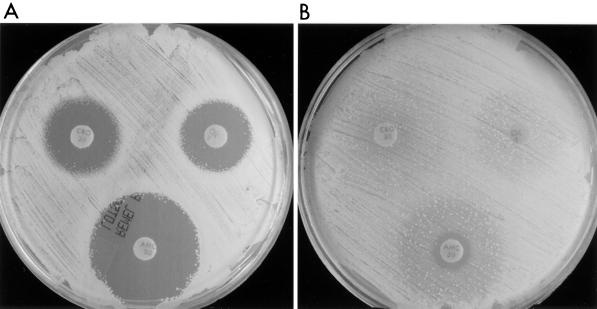
Synergism against GISA isolates could also be demonstrated by a modified disk diffusion method. For GISA isolates inoculated onto a BHIA plate containing vancomycin (6 μg/ml), an enhanced zone of inhibition surrounding the oxacillin (1 μg) disk was seen compared to that of disks on agar containing no antibiotic (no zone), indicating synergistic activity between the vancomycin contained within the agar and the oxacillin diffusion disk (Fig. 3). Enhanced zones of inhibition could be demonstrated with a number of β-lactams including ceftriaxone, ceftazidime, cepodoxime, and amoxicillin-clavulanate, but could not be demonstrated with aztreonam. The largest zones of inhibition were seen with amoxicillin-clavulanate. Similar synergism could be demonstrated by double-disk potentiation methods with oxacillin (1 μg) and vancomycin (30 μg) diffusion disks placed 15 to 20 mm apart (data not shown). However, the enhanced zones of inhibition surrounding the vancomycin disks were subtle and more difficult to interpret.
FIG. 3.
Modified Kirby-Bauer disk diffusion testing of a GISA isolate. HIP5827 was inoculated onto BHIA plates containing vancomycin at 6 μg/ml (A) and BHIA with no antibiotics (B), followed by the placement of antimicrobial disks. OX, oxacillin; CRO, ceftriaxone; and AMC, amoxicillin-clavulanic acid.
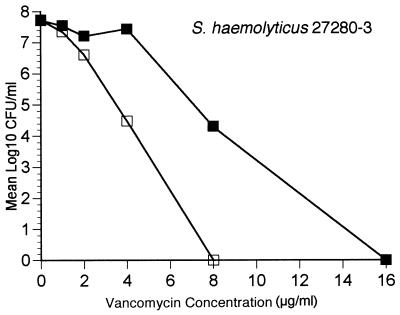
We were also interested in seeing whether synergism was related to suppression of subpopulations with a lower level of vancomycin resistance. To test this hypothesis, we performed population analysis with an MRSH isolate for which the vancomycin MIC was 16 μg/ml (isolate 27280-3). This isolate was produced following two overnight passages in increasing concentrations of vancomycin. Population analysis profiles confirmed the heterotypic nature of resistance among isolates with reduced vancomycin susceptibility compared to the nature of resistance among more vancomycin-susceptible isolates. Population analysis profiles were completed on vancomycin-containing agar as well as vancomycin-containing agar with a fixed concentration of oxacillin (6 μg/ml). These results are presented in Fig. 4 and demonstrate that synergy between vancomycin and oxacillin occurred, with suppression of the subpopulation with the highest level of vancomycin resistance.
FIG. 4.
Population analysis profiles of S. haemolyticus 27280-3. Overnight cultures were plated on media containing vancomycin (■) and vancomycin and oxacillin at 6 μg/ml (□).
Endocarditis.
Results of treatment of endocarditis caused by three clinical strains of GISA (strains HIP5827, HIP5836, and MU50) following a 3-day course of vancomycin (30 mg/kg twice a day) are presented in Table 2. Vancomycin treatment was ineffective against all three strains, with a <1-log10 CFU/g reduction in mean aortic valve vegetation counts compared to the counts for untreated controls. There was also high rate of mortality among both vancomycin-treated animals (5 of 12; 42%) and untreated control animals (12 of 12; 100%) infected with HP5836 and MU50, establishing the virulence of these strains. In contrast, none of the rabbits infected with HIP5827 and treated with vancomycin and only 42% of control animals died during the 4 day trial.
TABLE 2.
Outcome of 3-day treatment of experimental GISA aortic valve endocarditis with vancomycin-nafcillin
| Isolate | Treatment regimen | No. of rabbits surviving/no. treated | Organism count (mean ± SD log10 CFU/g)
|
|
|---|---|---|---|---|
| Aortic valve | Kidneya | |||
| HIP5827 | Controls | 6/11 | 10.3 ± 0.51 | 7.46 ± 0.06 (0/11) |
| Vancomycin | 9/9 | 9.66 ± 1.1 | 3.14 ± 1.39b (0/9) | |
| Nafcillin | 6/7 | 8.79 ± 0.82b | 3.13 ± 2.04b (2/7) | |
| Vancomycin + nafcillin | 9/9 | 5.77 ± 1.59c | 1.54 ± 1.63b (8/9)d | |
| MU50 | Controls | 0/8 | 10.31 ± 0.37 | 8.35 ± 0.97 (0/8) |
| Vancomycin | 4/6 | 9.43 ± 0.74 | 4.20 ± 3.26b (2/6) | |
| Nafcillin | 2/7 | 9.46 ± 1.14 | 5.52 ± 3.11b (1/7) | |
| Vancomycin + nafcillin | 6/7 | 6.25 ± 2.27c | 3.03 ± 2.80b (4/7)e | |
Values in parentheses are numbers of rabbits with sterile kidneys/number of rabbits treated.
P < 0.05 versus vancomycin, nafcillin, and control groups.
P < 0.05 for all regimens versus controls.
P = 0.0001 versus vancomycin and control groups; P = 0.035 versus nafcillin group by Fisher’s exact test.
P = 0.026 versus controls by Fisher’s exact test.
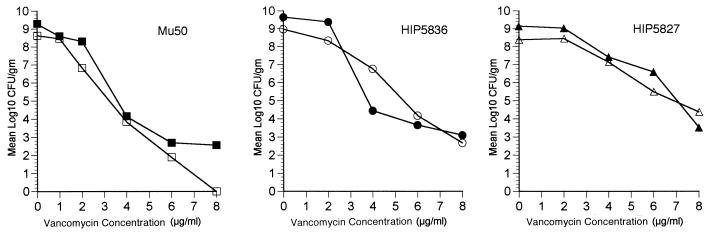
In order to determine whether selection of resistant subpopulations within vegetation material was a possible cause of the failure of vancomycin treatment, we determined the population analysis profiles of cultured vegetation material on vancomycin-containing agar. The profiles of all three clinical GISA isolates are presented in Fig. 5. In the population analysis profiles, vegetation material was serially diluted on agar plates containing increasing concentrations of vancomycin. The number of bacteria collected from untreated control animals was compared to the number of bacteria cultured from animals treated with vancomycin. The profiles of the vegetation material from control animals were nearly identical to those seen for the material from animals treated with vancomycin. No evidence of an increase in the proportion of cultured bacteria resistant to higher levels of vancomycin was detected. Thus, the selection of a highly vancomycin resistant subpopulation following vancomycin treatment did not occur.
FIG. 5.
Population analysis profiles of bacteria isolated from aortic valve vegetation material following 3 days of vancomycin treatment. Rabbits infected with MU50, HIP5827, and HIP5836 were treated with no antibiotics (solid symbols) or vancomycin at 30 mg/kg intravenously twice a day for 3 days (open symbols).
We next sought to confirm the in vitro data that indicated synergy between vancomycin and β-lactam antibiotics in the endcarditis model. Following the establishment of endocarditis with GISA HIP5827 and MU50, the animals were treated with either vancomycin, nafcillin, or the combination of the two antibiotics for 3 days. The results of these studies are presented in Table 2.
In the treatment of endocarditis due to HIP5827, a modest reduction in the aortic valve vegetation counts was seen when either vancomycin or nafcillin was given as a single agent: −0.64 and −1.51 log10 CFU/g, respectively. The combination of vancomycin and nafcillin was significantly better than either drug given alone, with a reduction in mean log aortic valve vegetation counts of −4.53 log10 CFU/g compared to the counts for untreated controls. Combination therapy also resulted in sterilization of kidney abscess tissue from 89% of animals, whereas sterilization of kidney abscesses occurred for only 12.5% of animals treated with either antibiotic alone.
The results seen for the treatment of clinical isolate GISA MU50 paralleled those seen for the treatment of HIP5827. Mean log aortic valve counts were reduced by 4.06 log10 CFU/g compared to the counts for untreated controls in rabbits treated with the combination. Again, therapy with either vancomycin or nafcillin as a single agent was ineffective, with a <1.0-log10 CFU/g reduction in mean aortic valve vegetation counts compared to the counts for untreated controls. The rate of kidney abscess sterilization was significantly higher among rabbits treated with the combination (57%) than among animals treated with either vancomycin (33%) or nafcillin (14%).
DISCUSSION
The emergence of decreasing levels of vancomycin susceptibility among clinical isolates of staphylococci has recently raised fears that effective antimicrobial treatment options for these isolates may soon be severely limited. Clinical data have suggested that patients infected with these isolates respond poorly to vancomycin monotherapy (5, 14). This poor clinical response has been supported by previous in vitro data and is confirmed in the rabbit model of experimental endcarditis as described in this report. We examined several properties of staphylococci with reduced susceptibility to vancomycin in an attempt to determine their contribution to the lack of effectiveness of vancomycin in the treatment of clinical GISA infections.
First, vancomycin resistance among staphylococci is expressed in a heterogeneous or heterotypic manner. This heterotypic expression may lead to difficulty in the detection of the phenotype by usual antimicrobial susceptibility testing and may be a partial explanation for the failure of antimicrobial therapy. Among methicillin-resistant staphylococci, the heterogeneous expression of methicillin resistance has been shown to be associated with the failure of β-lactams in experimental endocarditis models due to the selection of highly resistant subpopulations among vegetation material following exposure to β-lactams (6). One of the simplest means of demonstrating heterotypic expression is through the use of population analysis profiles. The population analysis profiles of clinical GISA isolates completed for this report demonstrate the heterotypic expression of vancomycin resistance, with a small proportion of cells expressing resistance to vancomycin at levels above the MIC. However, our data generated with rabbits with experimental endocarditis suggest that the failure of vancomycin therapy is not associated with the selection of highly resistant subpopulations among the entire heteroresistant population following treatment. In our endocarditis model, after treatment of infections caused by three clinical GISA isolates (isolates HIP5827, HIP5836, and MU50) with vancomycin, we found no significant change in the level of vancomycin resistance among cultured bacteria, as measured by population analysis profiles.
The second observed property of staphylococci associated with decreased susceptibility to vancomycin is the tendency to develop stepwise increases in resistance following short-term exposure to vancomycin or other glycopeptides. For laboratory isolates of both coagulase-negative and coagulase-positive staphylococci, increases in vancomycin MICs can be seen following a single overnight passage in the presence of subinhibitory concentrations of vancomycin (step passage). The development of vancomycin resistance following passage is particularly prominent among coagulase-negative staphylococci such as S. haemolyticus (26). In fact, the first vancomycin-resistant clinical staphylococcal isolate was an MRSH strain reported by Schwalbe et al. (25) in 1987. Additionally, vancomycin susceptibility as measured by MICs is lower among both MRSE and MRSH isolates in comparison to that among S. aureus isolates (1). Among clinical isolates, exposure to glycopeptides appears to be a prerequisite to the development of resistance because all current clinical GISA isolates have been isolated following vancomycin or teicoplanin treatment of patients. Although the in vitro development of vancomycin resistance occurs in a relatively short time frame, it is unclear over what time period in vivo selection of higher levels of vancomycin resistance occurs among S. aureus strains. In our endocarditis model, we did not observe in vivo selection of higher levels of vancomycin resistance following a 3-day treatment regimen. However, the isolation of clinical GISA isolates has followed relatively long periods of glycopeptide exposure ranging from 12 days to several months (5, 14, 22).
The third property of staphylococci relevant to decreased susceptibility to vancomycin is the poor bactericidal activity of this antibiotic. It has long been known that vancomycin is less rapidly bactericidal than β-lactams in the treatment of serious staphylococcal infections such as endocarditis (15). Vancomycin tolerance as an explanation for therapeutic failures has also been proposed by some investigators (13). Among GISA isolates, there was also a poor bactericidal response to vancomycin. Time-kill experiments demonstrated that vancomycin at concentrations up to 4× the MIC for GISA isolates had no bactericidal activity. This may be related to the fact that vancomycin does not display concentration-dependent killing (16). The current practice of intermittent dosing of vancomycin may hinder its action, since more effective killing may be achieved with continuous infusion, by which levels in serum are maintained above the vancomycin MIC. The lack of bactericidal activity of vancomycin was confirmed with the endocarditis model.
Although our report demonstrates that vancomycin montherapy is ineffective in the treatment of GISA infections, vancomycin still may have a role in the therapy of this emerging resistance phenotype. Our data indicate that vancomycin given in combination with antistaphylococcal β-lactams demonstrates synergistic activity against a variety of staphylococcal isolates with reduced susceptibility to glycopeptides. Synergy was demonstrated among both coagulase-negative and coagulase-positive staphylococci by the broth microdilution checkerboard, time-kill, disk diffusion, and agar dilution techniques. The synergy between vancomycin and a number of β-lactams with antistaphylococcal activity (resistance to staphylococcal β-lactamase) correlated with the glycopeptide resistance levels of the isolates. In fact, the development of vancomycin resistance in previously vancomycin-sensitive isolates was associated with the emergence of synergy. This was clearly demonstrated among step-passage isolates 27619VR, 27280-2, and 27280-3. Although the FIC indices for the vancomycin-susceptible parents 27619 and 27280 indicated only additive effects with the combination of vancomycin and oxacillin, for the step passage isolates the FIC indices were lower, indicating the presence of synergy between the same combinations of antibiotics (Fig. 1). Synergy between vancomycin and β-lactams was also associated with selective killing of the most vancomycin-resistant subpopulations (Fig. 3).
The observation that vancomycin and β-lactams may have synergistic activities against methicillin-resistant staphylococci has been made previously, as determined from the results of antimicrobial susceptibility testing (2, 12, 23, 31). Vancomycin has been demonstrated to have synergistic activity when it is combined with cephalothin (23), imipenem (2), cefazolin (31), and oxacillin (12) in in vitro tests. In addition, Shlaes et al. (26) noted that the combination of cefotaxime and teicoplanin showed synergism against the teicoplanin-resistant derivative S. aureus 12873. The data from our endocarditis model represent the first validation of these in vitro observations in an in vivo model of infection. For the treatment of experimental aortic valve endocarditis due to two separate strains of GISA (HIP5827 and MU50), the combination of vancomycin and nafcillin was significantly better than either drug given singly. Therapy with the combination of vancomycin and nafcillin also showed a higher rate of sterilization of kidney abscesses following treatment.
A detailed explanation for the observed synergism between vancomycin and β-lactams against staphylococcal isolates is confounded by an incomplete knowledge of the mechanisms responsible for vancomycin resistance, the expression of methicillin resistance, and the complex regulation of peptidoglycan synthesis in staphylococci. Glycopeptide-resistant isolates of staphylococci have been described to have the following characteristics: thicker cell walls (14, 27–29), slower growth (14, 28), decreased autolysis (27, 28), decreased peptidoglycan cross-linking following exposure to vancomycin, as evidenced by the lack of higher oligomer muropetide components of intact peptidoglycan cell wall by high-pressure liquid chromatographic analyses (28, 29), and the ability to trap vancomycin at the periphery of the cell wall (28, 29). These observations have supported the theory that resistance to the action of vancomycin involves the production of a thicker cell wall with an increased proportion of free pentapeptide d-Ala–d-Ala termini capable of binding to vancomycin. However, the ability to bind to vancomycin is not the sole explanation for resistance because the vancomycin binding capacity of glycopeptide-resistant step-passage isolates correlates poorly with the vancomycin MIC (29). Additional alterations of tertiary peptidoglycan structure, cell wall synthesis regulation, and cell wall porosity (29) are likely to contribute to vancomycin resistance.
The observed synergism between vancomycin and β-lactams against methicillin-resistant staphylococci may be related to the substrate specificity of PBP 2a. In the presence of β-lactams, PBP 2a is assumed to perform all cross-linking of the pentaglycine cross-bridges by transpeptidation of the terminal d-Ala. High-pressure liquid chromatographic analysis of methicillin-resistant staphylococci has shown that PBP 2a has a specific substrate specificity for monomeric disaccharide pentapeptides and is unable to cross-link to higher oligomeric muropeptides (3, 10, 11). Following exposure of GISA to both vancomycin and β-lactams, competition for monomeric muropeptide components of the developing peptidoglycan may be occurring. This mechanism is supported by the observation that a greater degree of synergy was seen against staphylococci with reduced vancomycin susceptibility and that synergy occurred only with higher concentrations of vancomycin, indicating concentration-dependent kinetics and a requirement for saturation of the available target.
The data presented in this report would indicate that future clinical isolates of GISA should undergo additional susceptibility testing for the presence of synergy between vancomycin and β-lactams. We conclude that combination therapy may be a reasonable alternative in the treatment of infections caused by staphylococcal isolates that demonstrate reduced susceptibility to glycopeptides.
ACKNOWLEDGMENTS
We thank Geri Hale-Cooper and Elizabeth Hanners for technical assistance.
REFERENCES
- 1.Archer G L, Climo M W. Antimicrobial susceptibility of coagulase-negative staphylococci. Antimicrob Agents Chemother. 1994;38:2231–2237. doi: 10.1128/aac.38.10.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barr J G, Smyth E T, Hogg G M. In vitro antimicrobial activity of imipenem in combination with vancomycin or teicoplanin against Staphylococcus aureus and Staphylococcus epidermidis. Eur J Clin Microbiol Infect Dis. 1990;9:804–809. doi: 10.1007/BF01967378. [DOI] [PubMed] [Google Scholar]
- 3.Billot-Klein D, Gutmann L, Bryant D, Bell D, van Heijenoort J, Grewal J, Shlaes D M. Peptidoglycan synthesis and structure in Staphylococcus haemolyticus expressing increasing levels of resistance to glycopeptide antibiotics. J Bacteriol. 1996;178:4696–4703. doi: 10.1128/jb.178.15.4696-4703.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyce J M, Medeiros A A. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Clinical isolates of methicillin-resistant Staphylococcus aureus from the United States with subpopulations of cells with reduced susceptibility to vancomycin, abstr. LB-15; p. 6. [Google Scholar]
- 5.Centers for Disease Control and Prevention. Update: Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morbid Mortal Weekly Rep. 1997;46:813–815. [PubMed] [Google Scholar]
- 6.Chambers H F, Hackbarth C J, Drake T A, Rusnak M G, Sande M A. Endocarditis due to methicillin-resistant Staphylococcus aureus in rabbits: expression of resistance to β-lactam antibiotics in vivo and in vitro. J Infect Dis. 1984;149:894–903. doi: 10.1093/infdis/149.6.894. [DOI] [PubMed] [Google Scholar]
- 7.Climo M W, Markowitz S M, Williams D S, Hale-Cooper C G, Archer G L. Comparison of in-vitro and in-vivo efficacy of FK037, vancomycin, imipenem and nafcillin against staphylococcal species. J Antimicrob Chemother. 1997;40:59–66. doi: 10.1093/jac/40.1.59. [DOI] [PubMed] [Google Scholar]
- 8.Climo M W, Patron R L, Goldstein B P, Archer G L. Lysostaphin treatment of experimental methicillin-resistant Staphylococcus aureus aortic valve endocarditis. Antimicrob Agents Chemother. 1998;42:1355–1360. doi: 10.1128/aac.42.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daum R S, Gupta S, Sabbagh R, Milewski W M. Characterization of Staphylococcus aureus isolates with decreased susceptibility to vancomcyin and teicoplanin: isolation and purification of a constitutively produced protein associated with decreased susceptibility. J Infect Dis. 1992;166:1066–1072. doi: 10.1093/infdis/166.5.1066. [DOI] [PubMed] [Google Scholar]
- 10.De Jonge B L, Tomasz A. Abnormal peptidoglycan produced in a methicillin-resistant strain of Staphylococcus aureus grown in the presence of methicillin: functional role for penicillin-binding protein 2a in cell wall synthesis. Antimicrob Agents Chemother. 1993;37:342–346. doi: 10.1128/aac.37.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Jonge B L M, Chang Y-S, Gage D, Tomasz A. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2a. J Biol Chem. 1992;267:11248–11254. [PubMed] [Google Scholar]
- 12.Domaracki B E, Evans A, Preston K E, Fraimov H, Venezia R A. Increased oxacillin activity associated with glycopeptides in coagulase-negative staphylococci. Eur J Clin Microbiol Infect Dis. 1998;17:143–150. doi: 10.1007/BF01691109. [DOI] [PubMed] [Google Scholar]
- 13.Gopal V, Bisno A L, Silverblatt F J. Failure of vancomycin treatment in Staphylococcus aureus endocarditis. In vivo and in vitro observations. JAMA. 1976;236:1604–1606. [PubMed] [Google Scholar]
- 14.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguru T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–146. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 15.Levine D P, Fromm B S, Reddy B R. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115:674–680. doi: 10.7326/0003-4819-115-9-674. [DOI] [PubMed] [Google Scholar]
- 16.Lowdin E, Odenholt I, Cars O. In vitro studies of pharmacodynamic properties of vancomycin against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1998;42:2739–2744. doi: 10.1128/aac.42.10.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mainardi J-L, Shlaes D M, Goering R V, Shlaes J H, Acar J F, Goldstein F W. Decreased teicoplanin susceptibility of methicillin-resistant strains of Staphylococcus aureus. J Infect Dis. 1995;171:1646–1650. doi: 10.1093/infdis/171.6.1646. [DOI] [PubMed] [Google Scholar]
- 18.Moreira B, Boyle-Vavra S, de Jonge B L M, Daum R S. Increased production of penicillin-binding protein 2, increased detection of other penicillin-binding proteins, and decreased coagulase activity associated with glycopeptide resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:1788–1793. doi: 10.1128/aac.41.8.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Approved standard M7-A4. 4th ed. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests, 6th ed. Approved standard M7-A6 (M100-S7). Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 21.Perlman B B, Freedman L R. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J Biol Med. 1971;44:206–213. [PMC free article] [PubMed] [Google Scholar]
- 22.Ploy M C, Grelaud C, Martin C, de Lumley L, Denis F. First clinical isolate of vancomycin-intermediate Staphylococcus aureus in a French hospital. Lancet. 1998;351:1212. doi: 10.1016/s0140-6736(05)79166-2. [DOI] [PubMed] [Google Scholar]
- 23.Portier H, Kazmierczak A, Lucht F, Tremeaux J C, Chavanet P, Duez J M. Cefotaxime in combinations with other antibiotics for the treatment of severe methicillin-resistant staphylococcal infections. Infection. 1985;13:S123–S128. doi: 10.1007/BF01644232. [DOI] [PubMed] [Google Scholar]
- 24.Robinson-Dunn B, Jennings G, Mitchell J, Ionescu M, Farnaz D, Donabedian S, Perri M B, Thal L A, Sunstrum J, Chow J W, Smith T, Zervos M J. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Characterization of a unique isolate of vancomycin-intermediate Staphylococcus aureus, abstr. LB-14; p. 6. [Google Scholar]
- 25.Schwalbe R S, Ritz W J, Verma P R, Barranco E A, Gilligan P H. Emergence of vancomycin resistance in Staphylococcus haemolyticus. J Infect Dis. 1987;161:45–51. doi: 10.1093/infdis/161.1.45. [DOI] [PubMed] [Google Scholar]
- 26.Shlaes D M, Shlaes J H, Vincent S, Etter L, Fey P D, Goering R V. Teicoplanin-resistant Staphylococcus aureus expresses a novel membrane protein and increases expression of penicillin-binding protein 2 complex. Antimicrob Agents Chemother. 1993;37:2432–2437. doi: 10.1128/aac.37.11.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sieradzki K, Villari P, Tomasz A. Decreased susceptibilities to teicoplanin and vancomycin among coagulase-negative methicillin-resistant clinical isolates of staphylococci. Antimicrob Agents Chemother. 1998;42:100–107. doi: 10.1128/aac.42.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieradzki K, Tomasz A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J Bacteriol. 1997;179:2557–2566. doi: 10.1128/jb.179.8.2557-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sieradzki K, Tomasz A. Suppression of glycopeptide resistance in a highly teicoplanin-resistant mutant of Staphylococcus aureus by transposon inactivation of genes involved in cell wall synthesis. Microb Drug Resist. 1998;4:159–168. doi: 10.1089/mdr.1998.4.159. [DOI] [PubMed] [Google Scholar]
- 30.Sieradzki K, Tomasz A. Suppression of beta-lactam antibiotic resistance in a methicillin-resistant Staphylococcus aureus through synergic action of early cell wall inhibitors and some other antibiotics. J Antimicrob Chemother. 1997;39(Suppl. A):47–51. doi: 10.1093/jac/39.suppl_1.47. [DOI] [PubMed] [Google Scholar]
- 31.Simon C, Simon M. In vitro activity of flomoxef and cefazolin in combination with vancomycin. Infection. 1991;19(Suppl. 5):S276–S278. doi: 10.1007/BF01645539. [DOI] [PubMed] [Google Scholar]
- 32.Small P M, Chambers H F. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother. 1990;34:1227–1231. doi: 10.1128/aac.34.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith T L, Pearson M, Wilcox K, Robinson-Dunn B, Hill B, Lancaster M, Rodgers G, Ruble C, Flegel E, McCallister S, Miller J M, Ellis H, Campbell C, Jarvis W R. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997. abstr. LB-16; p. 6. [Google Scholar]
- 34.Tenover F C, Lancaster M V, Hill B C, Steward C D, Stocker S A, Hancock G A, O’Hara C M, Clark N C, Hiramatsu K. Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J Clin Microbiol. 1998;36:1020–1027. doi: 10.1128/jcm.36.4.1020-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]