Abstract
Amphisomes are intermediate/hybrid organelles produced through the fusion of endosomes with autophagosomes within cells. Amphisome formation is an essential step during a sequential maturation process of autophagosomes before their ultimate fusion with lysosomes for cargo degradation. This process is highly regulated with multiple protein machineries, such as SNAREs, Rab GTPases, tethering complexes, and ESCRTs, are involved to facilitate autophagic flux to proceed. In neurons, autophagosomes are robustly generated in axonal terminals and then rapidly fuse with late endosomes to form amphisomes. This fusion event allows newly generated autophagosomes to gain retrograde transport motility and move toward the soma, where proteolytically active lysosomes are predominantly located. Amphisomes are not only the products of autophagosome maturation but also the intersection of the autophagy and endo-lysosomal pathways. Importantly, amphisomes can also participate in non-canonical functions, such as retrograde neurotrophic signaling or autophagy-based unconventional secretion by fusion with the plasma membrane. In this review, we provide an updated overview of the recent discoveries and advancements on the molecular and cellular mechanisms underlying amphisome biogenesis and the emerging roles of amphisomes. We discuss recent developments towards the understanding of amphisome regulation as well as the implications in the context of major neurodegenerative diseases, with a comparative focus on Alzheimer’s disease and Parkinson’s disease.
Introduction
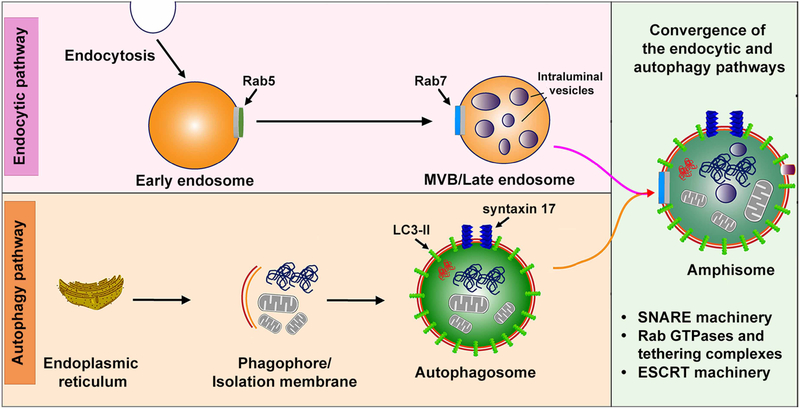
Macroautophagy, hereafter referred to as autophagy, is a major cytosolic degradative system involving sequestration of damaged cellular components and dysfunctional organelles within autophagosomes for subsequent lysosomal clearance [1]. This mechanism relies on dedicated autophagy regulators — the autophagy-related proteins — and can be induced by various stress stimuli such as nutrient deprivation, energy loss, redox condition, hypoxia, as well as the presence of protein aggregates and intracellular pathogens. Upon autophagy activation, an initial step is to form a pre-autophagosomal membrane called phagophore or isolation membrane that is elongated to facilitate cargo engulfment and finally enclosed to form autophagosomes. The outer membrane of autophagosomes thus fuses with lysosomes to generate autolysosomes so that the sequestered cargoes are degraded within autolysosomes through the activity of lysosomal hydrolases. In the past twenty years, numerous studies have demonstrated that the maturation of autophagosomes is a more complex process, which requires successive fusion events with the endo-lysosomal compartment [2]. Before ultimately fusing with lysosomes, autophagosomes can fuse with late endosomes (LEs)/multivesicular bodies (MVBs) to generate an intermediate/hybrid organelle — amphisome. The formation of amphisomes facilitates autophagosomes to further mature, which supplies autophagosomes with several key molecules such as SNAREs, Rab GTPases, and tethering complexes that are necessary for subsequent fusion with lysosomes to produce autolysosomes.
Although the autophagy system has been extensively studied in non-neuronal cells, the function of this system in neurons is still far from being clear. Autophagy failure has been indicated as a major concern, relevant to the development of the pathologies in major neurodegenerative diseases [3]. Neurons are highly polarized cells and characterized by a complex dendritic arbor and a very long axon that emerges from the soma and bridges vast distances that can extend more than a meter in the human body. Given the post-mitotic nature of neurons, sophisticated mechanisms that assure the timely disposal of damaged proteins and organelles are essential for neuronal health and function. Autophagy serves as a key quality control mechanism that ensures the physical and functional integrity of proteins and organelles and thus plays a pivotal role in the maintenance of neuronal homeostasis. Autophagosomes are continuously generated in the distal axons of neurons and thus neurons face unique challenges to remove these autophagosomes from axonal terminals for their clearance in the soma, where mature lysosomes are predominantly located. Fusion with LEs/MVBs to form amphisomes is necessary for nascent autophagosomes to gain retrograde transport motility so that amphisomes function as a transporter to mediate the delivery of autophagic cargoes to the soma for lysosomal degradation, which is critical for autophagy function in neurons.
In addition, amphisomes provide a crossroad for the intersection of the autophagic and endocytic pathways. In recent years, while significant efforts have been taken to advance our detailed understanding of the autophagy mechanism in non-neuronal and neuronal cells, the latest studies have unveiled potential roles of amphisomes beyond autophagy-mediated cargo elimination within cells. In this review, we summarize the progress that has been made in the elucidation of the molecular and cellular mechanisms underlying the autophagosome to LE fusion events during autophagosome maturation, and discuss the non-canonical functions of amphisomes implicated in multiple physiological processes. Finally, we review recent findings of the potential roles of amphisomes under pathophysiological conditions, with a focus on neurodegenerative diseases.
Molecular machinery in amphisome biogenesis
Amphisomes have been defined as degradative compartments within cells, which are produced through the fusion of autophagosomes with LEs/MVBs [4]. Achieved knowledge about the biogenesis of amphisomes is mostly from the studies conducted in non-neuronal cells [5, 6]. Here, we discuss several key molecules that have been indicated to drive amphisome generation, including soluble N-ethylmaleimide-sensitive factor activating protein receptors (SNAREs), Rab GTPases, tethering complexes, and endosomal sorting complex required for transport (ESCRT) proteins.
SNARE machinery
Like many other organelles in cells, membrane fusion events involve the SNARE proteins [7]. The vesicle-localized (v)- and target-membrane-bound (t)-SNARE proteins are localized respectively on two different vesicles and interact progressively to assemble a quadruple helix called the trans-SNARE. This trans-SNARE forms a zipper, which brings two opposing membranes closer and generates a pulling force on both membranes allowing them to fuse. SNARE proteins also participate in the key steps of autophagosome formation and subsequent maturation. Most v-SNAREs have an arginine residue in the center of the SNARE domain (R-SNAREs), whereas a glutamine (or aspartate) residue is found in syntaxins and SNAP-25-like proteins (Q-SNAREs) of the t-SNAREs [8, 9]. The R/v-SNARE protein VAMP7 and its partners — syntaxin 7 (Qa/t-SNARE) and syntaxin 8 (Qc/t-SNARE) were demonstrated to direct the formation of autophagosomes by mediating fusion of Atg16 vesicles with phagophores. Thus, the organelles can reach a proper size critical for their maturation into autophagosomes [10]. These SNAREs are also required for autophagosome biogenesis in yeast [11]. The maturation of autophagosomes into amphisomes requires syntaxin 17, an autophagosomal Q/t-SNARE, which exclusively localizes to the outer membrane of completed autophagosomes and is necessary for the autophagosome to LE fusion events [12, 13]. Importantly, syntaxin 17 interacts with synaptosomal-associated protein 29 (SNAP29) to form the syntaxin 17/SNAP29 complex, allowing their binding to VAMP8, the R-SNARE localized to the endosomal and lysosomal membranes. Syntaxin 17 is known to be recruited to autophagosomes through its LC3-interacting region (LIR) and its autophagosomal localization can be regulated by the lysosomal-associated membrane protein 2 (LAMP-2), LC3/GABA type A receptor-associated protein (GABARAP), and immunity-related GTPase M (IRGM) [13–15]. Loss of LAMP-2 or the silencing IRGM disrupts autophagic flux due to a mislocalization of syntaxin 17 [14, 15]. In neurons, we have provided direct evidence that syntaxin 17 mediates autophagosome fusion with LEs to create amphisome hybrid organelle [16, 17]. Syntaxin 17 RNAi in primary neurons significantly decreases the number of amphisomes coupled with abnormal accumulation of autophagosomes, suggesting that autophagosome maturation into amphisomes is halted as the result of the impaired fusion between autophagosomes and LEs [16, 17]. So far, syntaxin 17 is the only confirmed SNARE protein driving the formation of amphisomes in neurons.
In addition to syntaxin 17, other SNARE proteins have been identified to participate in the autophagosome/amphisome to lysosome fusion. The SNARE Vti1B, which localizes to LEs and lysosomes, and its interacting partners — syntaxin 6 and VAMP3/Cellubrevin — were reported to have a role in the fusion of autophagosomes with LEs or lysosomes [18, 19]. In contrast, data from another study concluded that Vti1B played no role in autophagic fusion [13]. The conflicting observations are likely attributed to differential regulation of the binding partners upon autophagy induction in response to distinct stimuli. Additionally, VAMP3/Cellubrevin has been indicated to play a role in membrane trafficking in non-neuronal cells. Studies in K562 reticulocytes, a cell line from human blood, have demonstrated that VAMP3/Cellubrevin and VAMP7 are another R-SNAREs that steer autophagosome-LE fusion to produce amphisomes [20]. VAMP3/Cellubrevin is highly abundant in the brain [21]. However, its role in amphisome biogenesis in neurons is poorly understood. In a recent study, YKT6 was proposed as a novel autophagosomal SNARE protein that forms a SNARE complex with SNAP29 and lysosomal syntaxin 7 in HeLa cells, which is required for autophagosomal fusion independent of syntaxin 17 [22]. Interestingly, in Drosophila, YKT6 was shown to localize to lysosomes and autolysosomes and form a SNARE complex with syntaxin 17 and SNAP29, which can be outcompeted from this SNARE complex by VAMP7 [23]. These findings suggest that YKT6 acts as a non-conventional, regulatory SNARE in this process. More investigations are needed to address whether YKT6 is also involved in the formation of amphisomes.
Rab GTPases and tethering complexes
While SNAREs are essential for the fusion of membranes, these SNARE proteins alone are not sufficient and require other factors to complete the process, particularly Rab GTPases and two major tethering complexes — the homotypic fusion and protein sorting complex (HOPS) and class C core vacuole/endosome tethering complex (CORVET). CORVET serves as a Rab5 GTPase effector complex, whereas HOPS is an evolutionarily conserved membrane tethering complex for membranes containing Rab7 GTPase and is critical for the autophagosome to LE/lysosome fusion events [24–26]. The interaction of Rab proteins with these tethering complexes brings membranes into contact, appearing to be a key event in endosomal fusion, which is further enhanced by SNAREs present on both membranes. Rab5 is the predominant GTPase on early endosomes (EEs), whereas Rab7 is abundant on LEs/MVBs [27, 28]. Most cargo sorting takes place in EEs that need to mature into LEs/MVBs, a process characterized by increased luminal acidification and the switch from Rab5 to Rab7, so-called ‘Rab conversion’. Such a mechanism is critical for the cargoes sorted into intraluminal vesicles to be degraded upon fusion with catalytically active lysosomes [29]. Interestingly, both Rab5 and Rab7 were reported to be present on autophagic membranes [30, 31]. A role of Rab5 in the early steps of the autophagy pathway is to regulate the removal of defined targets by inducing autophagy [32]. However, Rab5 is dispensable for autophagosome maturation [33], and indeed the exchange of Rab5 to Rab7 is essential for the formation of amphisomes/autolysosomes [19, 31]. Rab7 is associated with autophagosomes prior to autophagosomal fusion with lysosomes, which is activated by the guanine nucleotide exchange factor (GEF) complex, Mon1-Ccz1 [30, 34]. By binding to Atg8 in yeast and Atg8a in Drosophila, the GEF complex is recruited to autophagosomes and enhances Atg8-dependent activation of Rab7, thereby promoting autophagosome-endolysosome fusion. The HOPS complex was proposed to play a role in autophagy maturation by directly interacting with syntaxin 17 to facilitate the proper assembly of the SNARE complex [35, 36]. Moreover, UV radiation resistance-associated gene (UVRAG) can interact with the HOPS core complex and facilitate the fusion of autophagosomes with LEs/lysosomes by stimulating Rab7 activity [37]. Some studies have suggested that biogenesis of lysosome-related organelles complex 1 (BLOC-1)-related complex (BORC), Atg14, and BIRC6 function as tethers and thereby regulate autophagosome maturation [38–40]. In particular, BORC recruits the HOPS tethering complex to lysosomal membranes and facilitates the assembly of the syntaxin 17/VAMP8/SNAP29 complex, which is involved in amphisome formation. Atg14 binds to the syntaxin 17/SNAP29 complex on autophagosomes and primes this complex for VAMP8 interaction to promote autophagosome-endolysosome fusion. BIRC6 is an E2/E3 ubiquitin-conjugating enzyme and interacts with two Atg8 members GABARAP and GABARAPL1 as well as with syntaxin 17, acting as tethering components that regulate autophagosome-LE/lysosome fusion. Therefore, these observations support the notion that Rab GTPases along with the tethering complexes play a crucial role in the regulation of progressive maturation of autophagosomes. However, whether these key molecules regulate amphisome biogenesis in neurons remains largely unknown.
ESCRT machinery
Endosomal sorting complexes required for transport (ESCRT) machinery are known to mediate the maturation of LEs/MVBs. Several reports have established that ESCRTs are also involved in the regulation of autophagic flux whereas defects in ESCRTs interfere with autophagosome maturation [41–44]. The ESCRT machinery is connected to autophagy by driving autophagosome-LE/MVB fusion [45]. Studies have provided strong evidence that mutations in ESCRTs disrupt the formation of amphisomes and impact autophagy function. For example, ESCRT-associated AAA-ATPase Vps4/SKD1 is found to dissociate the ESCRTs from the endosomal membrane, a critical step for endosomal maturation. Interestingly, overexpression of the dominant-negative form Vps4/SKD1E235Q leads to an increase in autophagosomes but a decrease in autolysosomes [46]. In line with this data, lysobisphosphatidic acid (LBPA), a lipid found in LEs, fails to be delivered to autophagosomes in Vps4/SKD1E235Q cells under starvation conditions. These findings consistently suggest that impaired fusion of autophagosomes with LEs/MVBs halts the delivery of endocytic cargoes to autophagosomes in the mutant cells [46]. Similar observations were obtained in D. melanogaster ovarian follicular cells and fat bodies, in which expressing vps4 dominant-negative mutants increased autophagosomal accumulation [44]. Mutant cells for vps28 (ESCRT-I), vps25 (ESCRT-II), or vps32 (ESCRT-III) exhibit an increase in autophagosomes while amphisomes or autolysosomes are reduced. Defects in autophagosome maturation and fusion with lysosomes were also reported in ESCRT-0 VPS27/HRS depleted HeLa cells and MEFs [47]. Loss of VPS32/SNF7 (ESCRT-III) or expression of a mutant form CHMP2Bintron5 in neurons led to an increase in the number of autophagosomes [42]. Moreover, the ESCRT-1 subunit TSG101 was found to participate in the regulation of the amphisome-lysosome fusion event [48]. The ESCRT-0 Tom1 protein was reported to promote the formation of amphisomes through its interaction with myosin VI and the autophagy adaptors NDP52, T6BF, and optineurin [49]. Unlike almost all other myosins, myosin VI moves toward the minus end of actin filaments and functions in a variety of intracellular processes including sorting in the endocytic pathway [50]. Myosin VI localizes to autophagosomes and the interaction of myosin VI with the ESCRT-0 Tom1 in endocytic compartments facilitates the trafficking of endocytic cargo to autophagosomes. In the cells lacking myosin VI or the ESCRT-0 TOM1, abnormal accumulation of immature autophagosomes and impaired fusion with lysosomes were observed, accompanied by a significant reduction in the clearance of protein aggregates. This study has suggested that myosin VI likely docks or tethers the ESCRT-0 TOM1-associated endosomes to autophagosomes, thereby enhancing the autophagosome to endosome fusion events during autophagosome maturation [49]. Collectively, these findings suggest that ESCRTs are critical for autophagosome maturation, which can promote the fusion of endosomes and autophagosomes to form amphisomes and act as a hub-like system driving the final maturation of both LEs/MVBs and autophagosomes. Thus, alteration of LEs/MVBs can cause defects in autophagosome maturation and then the fusion with lysosomes.
The intersection of the autophagy and endocytosis pathways at amphisomes
Mounting evidence indicates that the autophagy and endocytic pathways are strongly interconnected in non-neuronal cells and neurons and share multiple common machineries. Amphisomes are formed upon the fusion of LEs/MVBs with autophagosomes, thus providing a crossroad for these two pathways (Figure 1). Defects in the biogenesis and maturation of endosomes can have an impact on amphisome generation and thereby compromise autophagy function. Several endocytic regulators, such as Rab7 and ESCRTs, essential for the proper function of the endocytic pathway, have been shown to directly regulate autophagy or the fusion of autophagosomes with LEs/lysosomes as discussed above [6, 51]. Atg9 is known to contribute the membrane to the phagophore assembly site during the biogenesis of autophagosomes. Interestingly, the endocytic pathway was reported to control the proper function of Atg9. Studies have revealed that this transmembrane protein colocalizes with transferrin-, Rab11-, Rab7-, and Rab9-associated endosomes [52, 53]. The trafficking of Atg9 was disrupted in cells in the absence of the components of clathrin-mediated endocytosis, including clathrin and the assembly protein (AP) complex 2 (AP-2) or AP-4 [54, 55]. One work has uncovered that the clathrin and AP-2 complex are also relevant to Atg16 trafficking and the formation of pre-autophagosomal structures [56]. In addition, studies from C. elegans, Drosophila, and mammals have demonstrated that the ESCRT machinery is involved in autophagy regulation [57, 58]. Genetic disruptions of ESCRTs cause autophagosome accumulation and trigger neurodegeneration [42–44]. On the other hand, the autophagy machinery was reported to play a role in the function of the endocytic pathway. A recent study showed that cells lacking Atg7 or Atg16L1 displayed abnormal early endosomes, which resulted in disturbed trafficking of epidermal growth factor receptors [59]. Vps34, a mammalian phosphatidylinositol (PI) 3-kinase, drives the biogenesis of phosphatidylinositol 3-phosphate (PI3P), a lipid required for autophagosome formation and the proper function of the autophagy system [60]. Vps34 is widely distributed in the brain and deletion of Vps34 in neurons leads to impaired axonal pruning, synapse loss, and progressive neurodegeneration [61–63]. Importantly, these defects are attributed to a disruption of the endocytic pathway rather than autophagy dysfunction [62, 63]. However, whether Vps34 plays a role in the biogenesis of amphisomes is currently clear. Collectively, these studies have established the direct involvement of the endocytic system and its players in the regulation of autophagosome biogenesis and maturation, but the knowledge on the role of autophagy in endocytosis is still limited. Also, whether these mechanisms have an impact on amphisome formation needs to be addressed.
Figure 1. Amphisomes in autophagosome maturation.
Autophagosomes undergo a progressive maturation process by interacting with multi-vesicular bodies (MVBs)/late endosomes (LEs) to generate intermediate/hybrid compartments — amphisomes, which are the intersection of the autophagy and endocytic pathways. Several key molecules such as SNAREs, Rab GTPases, tethering complexes, and ESCRTs are involved in the autophagosome-MVB/LE fusion event.
Amphisome retrograde transport in the axons of neurons
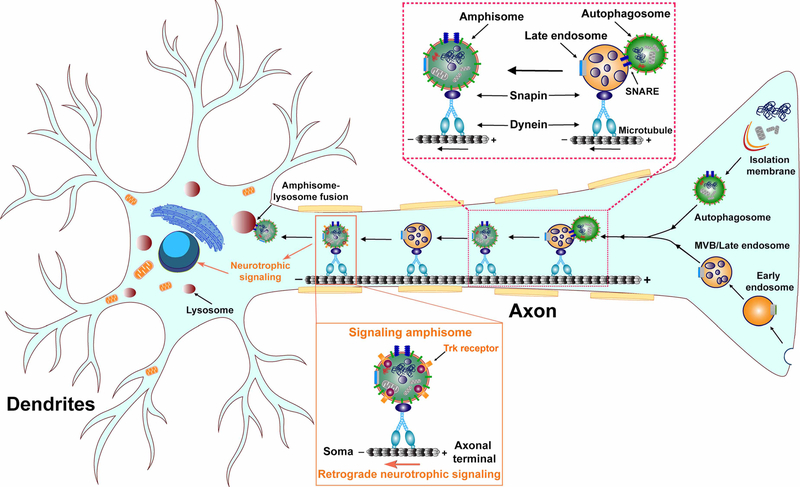
In non-neuronal cells, the autophagosome-LE fusion that produces amphisomes has a transient nature and is quickly transformed into autolysosomes for degradation. In neurons, autophagosomes are continuously generated in axonal terminals and need to move back to the soma for the clearance of autophagic cargoes within autolysosomes [64–71]. Retrograde axonal transport, driven by microtubule-dependent minus-end-directed dynein motors, is a common feature of both endosomes and autophagosomes through which targeted materials and autophagic cargoes from distal axons can be delivered to the soma for lysosomal degradation. Mutation or inhibition of the motor activity in dynein motors limits autophagic clearance [72, 73]. In addition to dynein motors, this mechanism involves the adaptors that attach dynein motors to targeted cargoes and thereby enable cargo retrograde movement. Multiple motor adaptors for retrograde axonal transport of endosomes have been described. Intriguingly, the function of these motor adaptors is not restricted to the transport of endosomes, and they also drive the transport of autophagosomes [74–79], suggesting a common mechanism for retrograde transport of both endosomes and autophagosomes.
Our previous studies have established that Snapin functions as a dynein motor adaptor to mediate retrograde transport of LEs in the axons of neurons [64, 80]. Through its direct interaction with the dynein intermediate chain (DIC), Snapin recruits dynein motors to the membrane of LEs, enabling LE retrograde movement toward the soma. Moreover, we have demonstrated that newly generated autophagosomes in distal axons rapidly fuse with LEs to form amphisomes through which autophagosomes are loaded with the dynein-Snapin motor-adaptor transport machinery and thus gain retrograde transport motility [16, 17]. Such a mechanism is required for the removal of autophagic cargoes from axonal terminals to facilitate their clearance within autolysosomes after fusion with lysosomes in the soma, which reduces autophagic stress in distal axons (Figure 2). Our recent studies have further uncovered that the dynein-Snapin-mediated retrograde transport coordinates with Ras homolog enriched in brain (RHEB)-dependent mitophagy to promote the elimination of damaged mitochondria at synaptic terminals, a mechanism critical for the maintenance of mitochondrial homeostasis and synaptic integrity [81, 82]. Apart from the dynein-Snapin transport complex, AP-2 was also reported to regulate axonal transport of autophagosomes/amphisomes, which involves the association of AP-2αA, a large brain-specific subunit of AP-2 with LC3 and of AP-2β with the p150Glued subunit of the dynein motor cofactor — the dynactin complex [76]. Neurons in the absence of AP-2 exhibit impaired retrograde transport of autophagosomes/amphisomes and autophagy defects. Interestingly, the involvement of AP-2 in autophagosome transport in axons could be independent of its established role in endocytosis. However, it is unclear how the dynein-Snapin and AP-2-dynactin transport machineries are coordinated to regulate retrograde transport of amphisomes in the axons of neurons.
Figure 2. Amphisome retrograde transport in the axons of neurons.
Formation of amphisomes through nascent autophagosome fusion with LEs in distal axons enables the dynein-Snapin motor-adaptor complex to drive retrograde transport of amphisomes for lysosomal degradation in the soma of neurons. In addition, signaling amphisomes, comprising a subset of amphisomes, mediate retrograde movement of p75NTR or BDNF-activated TrkB receptors from axonal terminals toward the soma, participating in the long-range retrograde neurotrophic signaling in neurons.
Amphisomes in retrograde neurotrophic signaling
In neurons, autophagy also plays a non-degradative role in the regulation of neurotrophic signaling. Neurotrophins are a family of growth factors including the brain-derived neurotrophic factor (BDNF) with an essential role in the retrograde control of critical neuronal functions such as axonal outgrowth, synaptogenesis, and synaptic plasticity [83]. The signaling process is initiated by neurotrophin binding to and activating the tyrosine receptor kinase (Trk) receptor. The ligand-receptor complex is then internalized into intracellular signaling compartments called signaling endosomes that undergo long-distance retrograde transport in axons, which allows the retrograde propagation of the signal [83]. Through the isolation of these carriers, earlier studies uncovered the presence of activated Trk receptors and the dynein motor transport machinery [84]. Importantly, the endosomal markers Rab5 and Rab7 were also found to be associated with these signaling compartments, thus termed signaling endosomes, which represent a subset of endosomes [74, 85]. Using Botulinum neurotoxin (BoNT) that travels in signaling endosomes containing the neurotrophin receptors p75NTR, studies have revealed that autophagosomes carry BoNT as cargo and mediate BoNT retrograde movement in axons [86, 87]. Importantly, cargoes sequestered within these autophagosomes are partially provided by the endocytic pathway, suggesting that these compartments represent amphisomes in nature following fusion with LEs/MVBs.
It is known that retrograde trafficking of BDNF-activated TrkB receptors in signaling endosomes constitutes an important long-distance signaling mechanism that conveys information from nerve terminals to the soma [88]. Recent studies suggest that BDNF/TrkB receptors are transported by amphisomes in which the incorporation of activated TrkB receptors from LEs confers signaling capabilities [75, 76]. Our earlier studies provided evidence that the dynein-Snapin-mediated retrograde transport participates in the regulation of BDNF/TrkB signaling in neurons [74]. Interestingly, SIPA1L2, a RapGAP protein, was proposed to function as a motor adaptor of TrkB-amphisomes to dynein motors by directly interacting with TrkB and Snapin, thereby temporally and spatially modulating long-range and local TrkB signaling [75]. Disruption of amphisome retrograde transport leads to defects in BDNF/TrkB signaling, as evidenced by a severe impairment of neuronal arborization and a reduction in BDNF levels, a major target of TrkB [76]. This work has also shown that prevention of autophagosome biogenesis mimics defective neuronal arborization, indicating that long-distance retrograde transport of TrkB receptors in amphisomes is required for BDNF-TrkB-mediated regulation of gene expression. Another study has further demonstrated that TrkB-amphisomes not only are essential for long-range BDNF/TrkB signaling but also regulate TrkB signaling at single presynaptic terminals [75]. This temporal and spatial control of TrkB signaling is achieved by modulating retrograde transport of TrkB-amphisomes. Interestingly, stopover of the transport complex at single synaptic terminals results in local activation of extracellular signal-regulated protein kinase 1/2 (ERK1/2) signaling at synaptic boutons along with enhancement of synaptic transmission. Defects in this mechanism trigger impairment in presynaptic long-term plasticity at hippocampal mossy fiber terminals and defective spatial separation, suggesting its relevance to presynaptic plasticity. Collectively, neurons have utilized the spatial segregation of autophagosomes/amphisomes and lysosomes to integrate retrograde neurotrophic signaling and ensure the long-range signaling capabilities of receptors (Figure 2). However, it remains unclear what the molecular and biochemical properties of signaling amphisomes are, whether signaling amphisomes contain engulfed cargoes targeted for clearance, and how the distally activated neurotrophin receptors escape from lysosomal degradation upon their arrival to the soma. Further investigations including ultrastructural analysis are required to advance our understanding of the biogenesis of signaling amphisomes.
Secretory amphisomes
Amphisomes have been defined as degradative organelles in cells. However, several studies have suggested that an amphisome population with non-degradative function — secretory amphisomes — constitutes an unconventional secretion mechanism through autophagy-based fusion with the plasma membrane. In goblet cells of the intestinal epithelium, amphisomes were shown to promote the secretion of mucus granules, which is required for providing the barrier of the mucus that protects against intestinal pathogens [89]. Another study reported secretory functions of amphisomes in interferon (IFN)-γ-stimulated lung epithelial cells [90]. IFN-γ treatment activates autophagy in lung epithelial cells, which triggers the extracellular secretion of annexin A2 (ANXA2), a phospholipid-binding protein. This unconventional secretion takes place likely through ANXA2 containing amphisomes that are positive for LC3B and CD63, and also depends on Atg5, Rab11, Rab8A, and Rab27A. These observations suggest that the formation of LEs/MVBs and autophagosomes and the subsequent fusion with the plasma membrane are relevant processes for ANXA2 secretion. However, further studies are needed to distinguish autophagy-mediated unconventional secretion from the release of exosomes. Several lines of studies have demonstrated that LE/MVB formation is also needed for autophagy-dependent secretion of interleukin (IL)-1β, a pro-inflammatory cytokine [91]. Importantly, IL-1β secretion is unaffected by downregulation of autophagosome/amphisome fusion with lysosomes as well as cargo degradation [92], suggesting that IL-1β carriers can fuse directly with the plasma membrane. These findings consistently indicate that the extensive crosstalk between the autophagy and endocytic systems promotes autophagy-based unconventional secretion. Of note, Rab8A plays a role in both IFN-γ-stimulated ANXA2 secretion [90] and autophagy-mediated IL-1β secretion [93]. Amphisome fusion with the plasma membrane was also proposed to be relevant to membrane remodeling essential for the final stage of reticulocyte maturation [94]. In particular, large glycophorin A-containing vesicles formed at the cytosolic face of the plasma membrane can be internalized and fuse with autophagosomes before their exocytosis to expel the contents within autophagosomes. The mechanism of cargo selection and the common molecular machinery that drives the fusion of secretory amphisomes with the plasma membrane remain to be elucidated, and further studies are also needed to delineate the possible connections between autophagy-associated conventional secretion and exosome release.
Amphisomes in neurodegenerative diseases
Neurodegenerative diseases are characterized by progressive degeneration and loss of neurons, structures, and functions of the nervous system. The common feature of these diseases is a progressive accumulation of misfolded proteins, protein aggregates, or fibrils in neurons. Autophagy constituents the key quality control mechanism for the elimination of misfolded and aggregation-prone proteins and dysfunctional organelles within neurons. However, current data suggest that alterations in the autophagy and endocytic pathways underlie the earliest events in neurodegenerative diseases preceding the appearance of neuropathology [3]. Abnormal accumulation of autophagosomes is a prominent feature in the brains of patients with Alzheimer’s disease (AD), Parkinson’s disease (PD), Amyotrophic Lateral Sclerosis (ALS), or Huntington’s disease (HD), but the underlying mechanisms remain largely unknown. Importantly, the misfolded proteins or aggregates formed by these proteins can be transmitted from affected neurons to healthy neurons. This neuron-to-neuron transmission of secreted pathologically misfolded proteins or amyloid has been proposed as the molecular basis of propagation of protein malconformation cytopathology and disease progression in neurogenerative diseases. While amphisomes and LEs normally fuse with lysosomes for degradation, these prelysosomal compartments may fuse with the plasma membrane to release any contained amyloid under the conditions of perturbed membrane trafficking and/or lysosomal deficiency. Enormous interest in the unconventional secretion of amyloids from neurons has led to the rapid growth of new findings in this field. Here we discuss the potential roles of amphisomes in the pathogenesis of major neurodegenerative diseases (Figure 3).
Figure 3. Amphisomes in neurodegenerative diseases.
Abnormal accumulation of amphisomes is a feature of autophagic stress in major neurodegenerative diseases. In Alzheimer’s disease (AD), defective amphisome retrograde transport leads to amphisome retention in axons as a consequence of the dynein-Snapin uncoupling due to the interaction of oligomeric amyloid β (Aβ)42 with dynein motors. Such a defect impairs autophagic clearance and augments Aβ production by promoting amphisome-enriched β-site APP cleaving enzyme 1 (BACE1) cleavage of amyloid precursor protein (APP). AP-2 reduction also contributes to impeded retrograde movement of amphisomes in AD axons. Autophagic stress might trigger the amphisome-mediated extracellular secretion of Aβ and tau in AD and other tauopathy diseases. In Parkinson’s disease (PD), autophagy-based unconventional secretion through amphisomes enhances the extracellular release of α-synuclein and participates in the transmission of α-synucleinopathy. In Amyotrophic Lateral Sclerosis (ALS) and Huntington’s disease (HD), aberrant accumulation of enlarged amphisomes is attributed to lysosomal deficiency in ALS motor neurons or the altered endocytic system in HD neurons. In contrast, amphisome formation is disrupted in Niemann-Pick type C1 (NPC1) disease.
Alzheimer’s disease
AD is the most common form of neurodegenerative disease and a leading cause of dementia in the aging populations [95]. The disease progression involves cognitive decline, memory loss, and neuronal death in the cerebral cortex and subcortical regions. AD patient brains are characterized by extracellular amyloid plaque deposits, composed of agglomerated amyloid β (Aβ) peptides, as well as intracellular accumulation of neurofibrillary tangles (NFTs), consisting of hyperphosphorylated tau protein. The increase in amyloidogenic processing of amyloid precursor protein (APP), which leads to Aβ overproduction, is a key feature underlying the pathogenesis of AD [96–98]. Strong evidence indicates a link between alterations in the autophagy and endolysosomal systems and early AD pathophysiology. Massive accumulation of autophagic vacuoles (AVs) along with endosomes were observed in induced pluripotent stem cells (iPSCs)-derived neurons and post-mortem brains of both familial and sporadic AD patients [65, 99–105]. However, the cause of such autophagosomal and endosomal proliferations in AD brains remains poorly understood.
We and others have demonstrated that AVs drastically accumulate within the dystrophic neurites and synaptic terminals of AD brains [3, 69]. This raises a fundamental question as to whether defects in the removal of newly generated autophagosomes from distal axons through retrograde transport disrupt autophagic clearance and thus trigger autophagic stress in AD neurons. Our work has revealed aberrant accumulation of amphisomes at presynaptic terminals in the brains of AD patients and AD-related mutant human APP (hAPP) transgenic (Tg) mice. Importantly, such a defect is attributed to impaired retrograde transport of amphisomes [69]. Furthermore, soluble Aβ42 oligomers enriched in axons interact with dynein motors. This interaction interferes with the coupling of the dynein motor with its adaptor Snapin, interrupting the attachment of dynein motors to amphisomes. As a result, dynein-Snapin-driven retrograde transport of amphisomes is hampered, thereby trapping amphisomes in distal axons and impairing their degradation within lysosomes in the soma of AD neurons [69, 106]. In agreement with these findings, deletion of snapin in mice phenocopies AD-linked synaptic autophagic stress, whereas overexpression of Snapin in mutant hAPP neurons decreases autophagic accumulation at presynaptic terminals by enhancing retrograde transport of amphisomes. Our studies have further shown that β-site APP cleaving enzyme 1 (BACE1), a rate-limiting enzyme for APP amyloidogenic processing and Aβ generation, is concentrated within LEs/amphisomes and the dynein-Snapin transport machinery-loaded LEs/amphisomes promote BACE1 retrograde trafficking to lysosomes for degradation in the soma [107–109]. Such a mechanism is critical for the modulation of BACE1 turnover and its β secretase activity, thus controlling BACE1 cleavage of APP and Aβ production. In addition to the dynein-Snapin-driven retrograde transport-mediated control of BACE1 trafficking and turnover, a recent study reported that AP-2, originally proposed to mediate the reformation of synaptic vesicles and the retrograde transport of amphisomes containing BDNF/TrkB receptors [76, 110], was also involved in the regulation of BACE1 trafficking and degradation [111]. iPSC-derived neurons from patients with late-onset AD displayed a decrease in AP-2 levels. Moreover, deletion of AP-2 in mouse brains increases BACE1 accumulation within LEs/amphisomes coupled with elevated Aβ generation. Therefore, these studies consistently suggest that defects in retrograde transport of amphisomes exacerbate autophagy failure and halt BACE1 trafficking toward lysosomes for proper degradation, aggravating amyloid pathology in AD.
In AD brains, a significant amount of APP is processed at the plasma membrane and then Aβ is released to the extracellular space, contributing to the formation of amyloid plaques. In addition to its direct release from the plasma membrane, Aβ can be generated from the inside of neurons and secreted through unconventional mechanisms. Of note, several studies indicate that intracellular APP and Aβ localize to LEs/MVBs and exosomes in the vulnerable neurons of AD brains, and the prevention of LE/MVB fusion with lysosomes can enhance the release of Aβ [112–115]. These observations suggest that Aβ can be produced and deposited into the lumen of LEs followed by the release through the fusion of LEs/MVBs with the plasma membrane. It is known that pathogenic forms of misfolded proteins and protein aggregates are the substrates of the autophagy pathway [3]. Studies using mouse models and cell culture have shown that AVs are enriched in Aβ-generating machinery where Aβ can be produced [101, 116–118]. The cellular levels of APP and Aβ were reported to be partially regulated by autophagy [119]. Interestingly, Atg7 deficiency in AD mouse brains leads to intracellular accumulation of Aβ, accompanied by a significant reduction in extracellular amyloid plaque burden [117]. Thus, these results support an important role for autophagy in the regulation of Aβ secretion and amyloidogenesis. It is conceivable that retrograde transport impairment-induced massive accumulation of AVs, especially amphisomes, may promote the release of Aβ at the axonal terminals of AD neurons. However, direct evidence of autophagy-based Aβ secretion in AD neurons is still lacking and whether Aβ is released through autophagosomes or amphisomes remains undefined.
Tauopathies are characterized by abnormal accumulation of hyperphosphorylated tau proteins in the cytoplasm that results in the formation of tau aggregates and fibrils along with NFTs, a pathogenic hallmark of tauopathy diseases, including AD. A growing body of evidence suggests that amphisomes are involved in autophagy-dependent tau secretion. Studies have shown that membranous organelle-based unconventional secretion leads to the extracellular release of tau either in a free form or bound to a vesicle. Autophagy stress and the presence of tau within AVs are characteristics in the brains of AD and other tauopathies [120]. These tau-enriched AVs could be redirected for secretion by directly fusing with the plasma membrane. It was reported that the release of tau is enhanced upon autophagy induction by starvation or pharmacological agents or upon lysosomal deficiency, but is decreased under the condition of autophagy inhibition [121–124]. Through electron microscopy analysis at the ultrastructural level, a study from neuroblastoma cells has provided a piece of direct evidence showing that AVs possibly containing tau were approaching the plasma membrane and perhaps releasing tau [125]. Moreover, autophagy induction upon the oxygen-glucose deprivation (OGD) was shown to promote the release of free tau along with tau within microvesicles (MVs) positive for LC3, suggesting the possibility of tau secretion through amphisomes [123]. Given the fact that autophagy induction directs the fusion of LEs/MVBs with AVs to form amphisomes [126], tau targeted for the extracellular release likely arrives in amphisomes through LEs/MVBs rather than autophagosomes. A close link has been established between autophagy and other unconventional secretion pathways [127]. Thus, more studies are required to advance our understanding of whether and how tau utilizes AVs as a carrier in the process of secretion, and whether autophagy-mediated tau release plays a key role in driving the propagation of tau pathologies in tauopathy diseases.
Parkinson’s disease
PD is the second most common neurodegenerative disease and is characterized by the formation of Lewy bodies and progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta [128, 129]. α-synuclein, the most abundant protein in Lewy body inclusions of PD [130], is the substrate of the autophagy pathway and can be removed and degraded through autophagy activity. On the other hand, α-synuclein aggregates have also been indicated to inhibit autophagy function [131–133]. Aberrant autophagy function and autophagosome accumulation are also observed in PD patient brains and have been implicated in the pathogenesis of PD [3]. Also, α-synuclein has been documented to undergo unconventional secretion from cultured nerve cells [134–140]. Interneuronal transmission of endogenously produced and secreted α-synuclein has been demonstrated both in vitro and in vivo [134, 141–146]. These results highlight a critical role of α-synuclein secretion and uptake in the transmission of α-synucleinopathy with Lewy body diseases. Earlier studies have suggested endosomes as the secretory organelles for the dissemination of α-synuclein by exocytosis [137, 138]. Prior to exocytosis, cytosolic α-synuclein is imported into intraluminal vesicles of LEs/MVBs dependent and independent of the ESCRT complex [147, 148]. Amphisomes were shown to undergo physiological exocytosis, which can be enhanced by stressful or pathological conditions [89]. In nerve growth factor (NGF)-differentiated PC12 catecholaminergic nerve cells, expressing PD-related protein TPPP/p25α induces lysosomal deficiency by blocking fusion of amphisomes with lysosomes. In line with this finding, these cells exhibit an increase in the amphisome-mediated release of α-synuclein monomer and aggregates to the extracellular surroundings [136, 149]. The secretion of α-synuclein was found to be upregulated by Rab8, which was previously reported to be involved in vesicle docking to the acceptor membrane upstream of the SNARE machinery in membrane fusion events [150]. Recent work provided additional evidence that α-synuclein was released and transferred via amphisome-like structures upon inhibition of the autophagy-lysosomal pathway, suggesting that amphisomes could be an alternative candidate for α-synuclein secretion besides LEs/MVBs [151]. More work is needed to elucidate detailed mechanisms underlying α-synuclein secretion through autophagy-based unconventional secretion and its relevance to the development of PD pathologies.
Other neurodegenerative diseases
The mechanisms underlying autophagy dysregulation in other neurodegenerative diseases have been understudied. ALS is characterized by progressive degeneration of motor neurons in the brain and the spinal cord, leading to muscle weakness, atrophy, and paralysis [152]. While 5%–10% of ALS patients suffer from familial ALS, mutations in superoxide dismutase 1 (SOD1) account for 2% of total disease cases. Similar to Aβ, tau, and α-synuclein, several lines of evidence have demonstrated prion-like propagation of mutant SOD1 misfolding via exosome-dependent and exosome-independent mechanisms in neuronal cells [153–155]. Defective autophagy has been indicated in ALS, but distinct underlying mechanisms were proposed [156–160]. A mutant form of ALS2, an activator of Rab5, was proposed to disrupt ALS2-dependent activation of Rab5, leading to defects in the formation of amphisomes in this familial form of ALS [157]. In a mouse model of ALS expressing mutant human SOD1, lysosomal defects have been found to result in abnormal accumulation of amphisome-like structures [66]. However, whether amphisomes are directly involved in SOD1 secretion and interneuron transmission in ALS remains largely unknown. Niemann-Pick type C1 (NPC1) disease is a lipid-storage disorder associated with neurodegeneration and liver function and is characterized by cholesterol accumulation within LEs/lysosomes resulting from disease-causing mutations in the NPC1 protein [161, 162]. A study reported that defects in amphisome formation caused by a failure in SNARE machinery hinder the maturation of autophagosomes and thus impair autophagy function in NPC1 disease-linked cells [163]. Interestingly, stimulation of autophagy or expression of functional NPC1 protein restores the function of this pathway. HD is a fatal neurodegenerative disorder caused by an expansion of a polyglutamine tract in the huntingtin (htt) protein that mediates the formation of intracellular protein aggregates. Accumulation of enlarged amphisomes was reported in cultured neurons expressing a mutant htt fragment, which is attributed to a disruption of the endocytic system that triggers neurodegeneration [164].
Conclusions and perspectives
By interacting with endosomes to form amphisomes, autophagosomes undergo a sequential maturation process before ultimate fusion with lysosomes, eventually resulting in the degradation of sequestrated autophagic cargoes [45, 165–167]. Many membrane fusion machineries have been identified to be involved in the fusion between autophagosomes and endosomes. These proteins need to be delivered to the surface of autophagosomes to avoid premature fusion with lysosomes. Thus, autophagosome-endosome fusion to generate amphisomes could be a critical step in controlling the progressive maturation of autophagosomes. Despite evidence of the importance of this intermediate step in the autophagy pathway, it is largely overlooked. Many studies in the field have been focusing on autophagosome-lysosome fusion but with very limited data describing amphisome formation and the fusion of amphisomes to lysosomes. Importantly, the mechanism underlying amphisome biogenesis in neurons remains poorly understood. Amphisomes also play a non-degradative role in the regulation of retrograde neurotrophic signaling in neurons. However, how the biogenesis of signaling amphisomes occurs and whether cargoes sequestered within signaling amphisomes are also targets for autophagic clearance are still largely unknown. Autophagy is crucial for memory formation and age-related cognitive decline has been linked to autophagy dysfunction. Thus, whether autophagy deficits impair neurotrophic signaling and whether the absence of BDNF/TrkB signaling as a result of the dysregulation of signaling amphisomes contributes to age-associated cognitive impairment are critical questions for future studies. Moreover, autophagy failure has been implicated in the pathogenesis of neurodegenerative diseases [3]. Detailed elucidation of amphisome biogenesis is necessary to advance our understanding of this middle step in autophagosome maturation and to clarify whether and how the amphisome stage is affected as well as its impact on disease pathologies. A better understanding of this pathway would highlight a potential avenue for biomarkers. Given the rapid development in this field in recent years, these important questions need to be addressed in a timely manner.
The autophagy and endolysosomal pathways directly intersect at amphisomes, the contents of which have multiple fates, including lysosomal degradation or extracellular release. Physiological exocytosis of amphisomes through autophagy-based unconventional secretion has been demonstrated to participate in multiple biological processes and can be facilitated by the crosstalk between autophagy and the endolysosomal system. Under the conditions of disturbed membrane trafficking and/or lysosomal deficiency, amphisomes accumulate within cells, which either leads to cell death or promotes the exocytosis of the accumulating amphisomes, as a last resort, to release contents to the surroundings. In neurodegenerative diseases, some studies indicate that enhanced exocytosis of amphisomes can mitigate the toxicity induced by the aberrant accumulation of disease-causing protein aggregates/amyloid [136]. However, direct evidence of autophagy-based secretion in diseased neurons is still lacking and it is also unclear whether amphisome-dependent amyloid release cooperates with the exosomal pathway. More work is required to elucidate detailed mechanisms and their relevance to the development of neurodegeneration. On the other hand, amphisomes-mediated autophagy-dependent amyloid secretion has been proposed as a mechanism underlying the propagation of pathologies associated with neurodegenerative diseases. Thus, there is a suspicion that the manipulations of amphisome secretion through membrane trafficking pathways would be a promising strategy for therapeutic developments to enhance amyloid clearance and thus combat disease pathologies. However, from the propagation perspective, not all secreted amyloids are the same. Amphisomes likely release both monomeric amyloid and soluble amyloid in the form of proteotoxic oligomers and aggregates [168]. Therefore, it is important to determine which of these species are most relevant for disease propagation to develop focused therapeutic strategies in the future.
Acknowledgements
The authors thank Y. Zuo and J. Yoon for editing and other members of the Cai laboratory for their assistance and discussion.
Funding
This work was supported by National Institutes of Health grants R01NS089737 and R01GM135326 (to Q.C.)
Abbreviations
- AD
Alzheimer’s disease
- ALS
Amyotrophic Lateral Sclerosis
- ANXA2
annexin A2
- AP
assembly protein (AP)
- APP
Amyloid precursor protein
- AVs
autophagic vacuoles
- Aβ
amyloid β
- BACE-1
β-site APP cleaving enzyme 1
- BDNF
brain-derived neurotrophic factor
- BoNT
Botulinum neurotoxin
- BROC
biogenesis of lysosome-related organelles complex 1 (BLOC-1)-related complex
- CORVET
class C core vacuole/endosome tethering complex
- DIC
dynein intermediate chain
- EE
early endosome
- ERK1/2
extracellular signal-regulated protein kinase 1/2
- ESCRT
endosomal sorting complexes required for transport
- GABARAP
GABA type A receptor-associated protein
- GEF
guanine nucleotide exchange factor
- HD
Huntington’s disease
- HOPS
homotypic fusion and protein sorting complex
- htt
Huntingtin protein
- IFN
interferon
- IL
interleukin
- iPSCs
induced pluripotent stem cells
- IRGM
immunity-related GTPase M
- LAMP-2
lysosomal-associated membrane protein 2
- LBPA
lysobisphosphatidic acid
- LC3
microtubule-associated proteins 1A/1B light chain 3
- LE
late endosomes
- LIR
LC3-interacting region
- MVB
multivesicular body
- MVs
microvesicles
- NFTs
neurofibrillary tangles
- NGF
nerve growth factor
- NPC1
Niemann-Pick type C1
- OGD
oxygen-glucose deprivation
- PD
Parkinson’s diseases
- PI3P
phosphatidylinositol 3-phosphate
- RHEB
Ras homolog enriched in brain
- SNAP29
synaptosomal-associated protein 29
- SNAREs
soluble N-ethylmaleimide-sensitive factor activating protein receptors
- SOD1
superoxide dismutase 1
- Tg
transgenic
- Trk
tyrosine receptor kinase
- UVRAG
UV radiation resistance-associated gene
- VAMP
vesicle associated membrane proteins
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Ktistakis NT and Tooze SA (2016) Digesting the expanding mechanisms of autophagy. Trends Cell Biol. 26, 624–635 10.1016/j.tcb.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 2.Zhao YG and Zhang H (2019) Autophagosome maturation: an epic journey from the ER to lysosomes. J. Cell Biol. 218, 757–770 10.1083/jcb.201810099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nixon RA (2013) The role of autophagy in neurodegenerative disease. Nat. Med. 19, 983–997 10.1038/nm.3232 [DOI] [PubMed] [Google Scholar]
- 4.Gordon PB, Høyvik H and Seglen PO (1992) Prelysosomal and lysosomal connections between autophagy and endocytosis. Biochem. J. 283, 361–369 10.1042/bj2830361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liou W, Geuze HJ, Geelen MJH and Slot JW (1997) The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J. Cell Biol. 136, 61–70 10.1083/jcb.136.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corona AK, Saulsbery HM, Velazquez AFC and Jackson WT (2018) Enteroviruses remodel autophagic trafficking through regulation of host SNARE proteins to promote virus replication and cell exit. Cell Rep. 22, 3304–3314 10.1016/j.celrep.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Südhof TC and Rothman JE (2009) Membrane fusion: grappling with SNARE and SM proteins. Science 323, 474–477 10.1126/science.1161748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weimbs T, Low SH, Chapin SJ, Mostov KE, Bucher P and Hofmann K (1997) A conserved domain is present in different families of vesicular fusion proteins: a new superfamily. Proc. Natl Acad. Sci. U.S.A. 94, 3046–3051 10.1073/pnas.94.7.3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutton RB, Fasshauer D, Jahn R and Brunger AT (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 395, 347–353 10.1038/26412 [DOI] [PubMed] [Google Scholar]
- 10.Moreau K, Ravikumar B, Renna M, Puri C and Rubinsztein DC (2011) Autophagosome precursor maturation requires homotypic fusion. Cell 146, 303–317 10.1016/j.cell.2011.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair U, Jotwani A, Geng J, Gammoh N, Richerson D, Yen W-L et al. (2011) SNARE proteins are required for macroautophagy. Cell 146, 290–302 10.1016/j.cell.2011.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takáts S, Nagy P, Varga Á, Pircs K, Kárpáti M, Varga K et al. (2013) Autophagosomal Syntaxin17-dependent lysosomal degradation maintains neuronal function in Drosophila. J. Cell Biol. 201, 531–539 10.1083/jcb.201211160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itakura E, Kishi-Itakura C and Mizushima N (2012) The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151, 1256–1269 10.1016/j.cell.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 14.Hubert V, Peschel A, Langer B, Gröger M, Rees A and Kain R (2016) LAMP-2 is required for incorporating syntaxin-17 into autophagosomes and for their fusion with lysosomes. Biol. Open 5, 1516–1529 10.1242/bio.018648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Jain A, Farzam F, Jia J, Gu Y, Choi SW et al. (2018) Mechanism of Stx17 recruitment to autophagosomes via IRGM and mammalian Atg8 proteins. J. Cell Biol. 217, 997–1013 10.1083/jcb.201708039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng X-T, Zhou B, Lin M-Y, Cai Q and Sheng Z-H (2015) Axonal autophagosomes use the ride-on service for retrograde transport toward the soma. Autophagy 11, 1434–1436 10.1080/15548627.2015.1062203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng X-T, Zhou B, Lin M-Y, Cai Q and Sheng Z-H (2015) Axonal autophagosomes recruit dynein for retrograde transport through fusion with late endosomes. J. Cell Biol. 209, 377–386 10.1083/jcb.201412046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuta N, Fujita N, Noda T, Yoshimori T and Amano A (2010) Combinational soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins VAMP8 and Vti1b mediate fusion of antimicrobial and canonical autophagosomes with lysosomes. Mol. Biol. Cell 21, 1001–1010 10.1091/mbc.e09-08-0693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minowa-Nozawa A, Nozawa T, Okamoto-Furuta K, Kohda H and Nakagawa I (2017) Rab35 GTPase recruits NDP52 to autophagy targets. EMBO J. 36, 2790–2807 10.15252/embj.201796463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fader CM, Sánchez DG, Mestre MB and Colombo MI (2009) TI-VAMP/VAMP7 and VAMP3/cellubrevin: two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways. Biochim. Biophys. Acta Mol. Cell Res. 1793, 1901–1916 10.1016/j.bbamcr.2009.09.011 [DOI] [PubMed] [Google Scholar]
- 21.McMahon HT, Ushkaryov YA, Edelmann L, Link E, Binz T, Niemann H et al. (1993) Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature 364, 346–349 10.1038/364346a0 [DOI] [PubMed] [Google Scholar]
- 22.Matsui T, Jiang P, Nakano S, Sakamaki Y, Yamamoto H and Mizushima N (2018) Autophagosomal YKT6 is required for fusion with lysosomes independently of syntaxin 17. J. Cell Biol. 217, 2633–2645 10.1083/jcb.201712058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takáts S, Glatz G, Szenci G, Boda A, Horváth GV, Hegedűs K et al. (2018) Non-canonical role of the SNARE protein Ykt6 in autophagosome-lysosome fusion. PLoS Genet. 14, e1007359 10.1371/journal.pgen.1007359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darsow T, Rieder SE and Emr SD (1997) A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J. Cell Biol. 138, 517–529 10.1083/jcb.138.3.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gissen P, Johnson CA, Gentle D, Hurst LD, Doherty AJ, O’Kane CJ et al. (2005) Comparative evolutionary analysis of VPS33 homologues: genetic and functional insights. Hum. Mol. Genet. 14, 1261–1270 10.1093/hmg/ddi137 [DOI] [PubMed] [Google Scholar]
- 26.Takáts S, Pircs K, Nagy P, Varga Á, Kárpáti M, Hegedűs K et al. (2014) Interaction of the HOPS complex with syntaxin 17 mediates autophagosome clearance in Drosophila. Mol. Biol. Cell 25, 1338–1354 10.1091/mbc.e13-08-0449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chavrier P, Parton RG, Hauri HP, Simons K and Zerial M (1990) Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell 62, 317–329 10.1016/0092-8674(90)90369-P [DOI] [PubMed] [Google Scholar]
- 28.Jäger S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P et al. (2004) Role for Rab7 in maturation of late autophagic vacuoles. J. Cell Sci. 117, 4837–4848 10.1242/jcs.01370 [DOI] [PubMed] [Google Scholar]
- 29.Wandinger-Ness A and Zerial M (2014) Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb. Perspect. Biol. 6, a022616–a022616 10.1101/cshperspect.a022616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutierrez MG, Munafó DB, Berón W and Colombo MI (2004) Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J. Cell Sci. 117, 2687–2697 10.1242/jcs.01114 [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Miao G, Xue X, Guo X, Yuan C, Wang Z et al. (2016) The vici syndrome protein EPG5 is a Rab7 effector that determines the fusion specificity of autophagosomes with late endosomes/lysosomes. Mol. Cell 63, 781–795 10.1016/j.molcel.2016.08.021 [DOI] [PubMed] [Google Scholar]
- 32.Ravikumar B, Imarisio S, Sarkar S, O’Kane CJ and Rubinsztein DC (2008) Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of huntington disease. J. Cell Sci. 121, 1649–1660 10.1242/jcs.025726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hegedűs K, Takáts S, Boda A, Jipa A, Nagy P, Varga K et al. (2016) The Ccz1-Mon1-Rab7 module and Rab5 control distinct steps of autophagy. Mol. Biol. Cell 27, 3132–3142 10.1091/mbc.e16-03-0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao J, Langemeyer L, Kümmel D, Reggiori F and Ungermann C (2018) Molecular mechanism to target the endosomal Mon1-Ccz1 GEF complex to the pre-autophagosomal structure. eLife 7, e31145 10.7554/eLife.31145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang P, Nishimura T, Sakamaki Y, Itakura E, Hatta T, Natsume T et al. (2014) The HOPS complex mediates autophagosome-lysosome fusion through interaction with syntaxin 17. Mol. Biol. Cell 25, 1327–1337 10.1091/mbc.E13-08-0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orr A, Song H, Rusin SF, Kettenbach AN and Wickner W (2017) HOPS catalyzes the interdependent assembly of each vacuolar SNARE into a SNARE complex. Mol. Biol. Cell 28, 975–983 10.1091/mbc.e16-10-0743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang C, Lee J, Inn K-S, Gack MU, Li Q, Roberts EA et al. (2008) Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat. Cell Biol. 10, 776–787 10.1038/ncb1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia R, Guardia CM, Pu J, Chen Y and Bonifacino JS (2017) BORC coordinates encounter and fusion of lysosomes with autophagosomes. Autophagy 13, 1648–1663 10.1080/15548627.2017.1343768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diao J, Liu R, Rong Y, Zhao M, Zhang J, Lai Y et al. (2015) ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature 520, 563–566 10.1038/nature14147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebner P, Poetsch I, Deszcz L, Hoffmann T, Zuber J and Ikeda F (2018) The IAP family member BRUCE regulates autophagosome–lysosome fusion. Nat. Commun. 9, 599 10.1038/s41467-018-02823-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roudier N, Lefebvre C and Legouis R (2005) CeVPS-27 is an endosomal protein required for the molting and the endocytic trafficking of the low-density lipoprotein receptor-related protein 1 in Caenorhabditis elegans. Traffic 6, 695–705 10.1111/j.1600-0854.2005.00309.x [DOI] [PubMed] [Google Scholar]
- 42.Lee J-A, Beigneux A, Ahmad ST, Young SG and Gao F-B (2007) ESCRT-III dysfunction causes autophagosome accumulation and neurodegeneration. Curr. Biol. 17, 1561–1567 10.1016/j.cub.2007.07.029 [DOI] [PubMed] [Google Scholar]
- 43.Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerød L, Fisher EMC et al. (2007) Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J. Cell Biol. 179, 485–500 10.1083/jcb.200702115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rusten TE, Vaccari T, Lindmo K, Rodahl LMW, Nezis IP, Sem-Jacobsen C et al. (2007) ESCRTs and Fab1 regulate distinct steps of autophagy. Curr. Biol. 17, 1817–1825 10.1016/j.cub.2007.09.032 [DOI] [PubMed] [Google Scholar]
- 45.Berg TO, Fengsrud M, Strømhaug PE, Berg T and Seglen PO (1998) Isolation and characterization of Rat liver amphisomes: evidence for fusion of autophagosomes with both early and late endosomes. J. Biol. Chem. 273, 21883–21892 10.1074/jbc.273.34.21883 [DOI] [PubMed] [Google Scholar]
- 46.Nara A, Mizushima N, Yamamoto A, Kabeya Y, Ohsumi Y and Yoshimori T (2002) SKD1 AAA ATPase-Dependent endosomal transport is involved in autolysosome formation. Cell Struct. Funct. 27, 29–37 10.1247/csf.27.29 [DOI] [PubMed] [Google Scholar]
- 47.Tamai K, Tanaka N, Nara A, Yamamoto A, Nakagawa I, Yoshimori T et al. (2007) Role of Hrs in maturation of autophagosomes in mammalian cells. Biochem. Biophys. Res. Commun. 360, 721–727 10.1016/j.bbrc.2007.06.105 [DOI] [PubMed] [Google Scholar]
- 48.Majumder P and Chakrabarti O (2015) Mahogunin regulates fusion between amphisomes/MVBs and lysosomes via ubiquitination of TSG101. Cell Death Dis. 6, e1970–e1970 10.1038/cddis.2015.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tumbarello DA, Waxse BJ, Arden SD, Bright NA, Kendrick-Jones J and Buss F (2012) Autophagy receptors link myosin VI to autophagosomes to mediate Tom1-dependent autophagosome maturation and fusion with the lysosome. Nat. Cell Biol. 14, 1024–1035 10.1038/ncb2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buss F, Spudich G and Kendrick-Jones J (2004) MYOSIN VI: cellular functions and motor properties. Annu. Rev. Cell Dev. Biol. 20, 649–676 10.1146/annurev.cellbio.20.012103.094243 [DOI] [PubMed] [Google Scholar]
- 51.Lefebvre C, Legouis R and Culetto E (2018) ESCRT and autophagies: endosomal functions and beyond. Semin. Cell Dev. Biol. 74, 21–28 10.1016/j.semcdb.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 52.Longatti A, Lamb CA, Razi M, Yoshimura S, Barr FA and Tooze SA (2012) TBC1D14 regulates autophagosome formation via Rab11- and ULK1-positive recycling endosomes. J. Cell Biol. 197, 659–675 10.1083/jcb.201111079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orsi A, Razi M, Dooley HC, Robinson D, Weston AE, Collinson LM et al. (2012) Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy. Mol. Biol. Cell 23, 1860–1873 10.1091/mbc.E11-09-0746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Popovic D and Dikic I (2014) TBC1D5 and the AP2 complex regulate ATG9 trafficking and initiation of autophagy. EMBO Rep. 15, 392–401 10.1002/embr.201337995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Pace R, Skirzewski M, Damme M, Mattera R, Mercurio J, Foster AM et al. (2018) Altered distribution of ATG9A and accumulation of axonal aggregates in neurons from a mouse model of AP-4 deficiency syndrome. PLoS Genet. 14, e1007363 10.1371/journal.pgen.1007363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ravikumar B, Moreau K, Jahreiss L, Puri C and Rubinsztein DC (2010) Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat. Cell Biol. 12, 747–757 10.1038/ncb2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rusten TE and Stenmark H (2009) How do ESCRT proteins control autophagy? J. Cell Sci. 122, 2179–2183 10.1242/jcs.050021 [DOI] [PubMed] [Google Scholar]
- 58.Kaul Z and Chakrabarti O (2018) Endosomal sorting complexes required for ESCRTing cells toward death during neurogenesis, neurodevelopment and neurodegeneration. Traffic 19, 485–495 10.1111/tra.12569 [DOI] [PubMed] [Google Scholar]
- 59.Fraser J, Simpson J, Fontana R, Kishi-Itakura C, Ktistakis NT and Gammoh N (2019) Targeting of early endosomes by autophagy facilitates EGFR recycling and signalling. EMBO Rep. 20, e47734 10.15252/embr.201947734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marat AL and Haucke V (2016) Phosphatidylinositol 3-phosphates-at the interface between cell signalling and membrane traffic. EMBO J. 35, 561–579 10.15252/embj.201593564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Issman-Zecharya N and Schuldiner O (2014) The PI3K class III complex promotes axon pruning by downregulating a Ptc-derived signal via endosome-lysosomal degradation. Dev. Cell 31, 461–473 10.1016/j.devcel.2014.10.013 [DOI] [PubMed] [Google Scholar]
- 62.Wang L, Budolfson K and Wang F (2011) Pik3c3 deletion in pyramidal neurons results in loss of synapses, extensive gliosis and progressive neurodegeneration. Neuroscience 172, 427–442 10.1016/j.neuroscience.2010.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou X, Wang L, Hasegawa H, Amin P, Han B-X, Kaneko S et al. (2010) Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proc. Natl Acad. Sci. U.S.A. 107, 9424–9429 10.1073/pnas.0914725107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai Q, Lu L, Tian J-H, Zhu Y-B, Qiao H and Sheng Z-H (2010) Snapin-regulated late endosomal transport is critical for efficient autophagy-lysosomal function in neurons. Neuron 68, 73–86 10.1016/j.neuron.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng X-T, Xie Y-X, Zhou B, Huang N, Farfel-Becker T and Sheng Z-H (2018) Characterization of LAMP1-labeled nondegradative lysosomal and endocytic compartments in neurons. J. Cell Biol. 217, 3127–3139 10.1083/jcb.201711083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gowrishankar S, Yuan P, Wu Y, Schrag M, Paradise S, Grutzendler J et al. (2015) Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer’s disease amyloid plaques. Proc. Natl Acad. Sci. U.S.A. 112, E3699–E3708 10.1073/pnas.1510329112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee J-H, Rao MV, Yang D-S, Stavrides P, Im E, Pensalfini A et al. (2019) Transgenic expression of a ratiometric autophagy probe specifically in neurons enables the interrogation of brain autophagy in vivo. Autophagy 15, 543–557 10.1080/15548627.2018.1528812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maday S and Holzbaur ELF (2016) Compartment-specific regulation of autophagy in primary neurons. J. Neurosci. 36, 5933–5945 10.1523/JNEUROSCI.4401-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tammineni P, Ye X, Feng T, Aikal D and Cai Q (2017) Impaired retrograde transport of axonal autophagosomes contributes to autophagic stress in Alzheimer’s disease neurons. Elife 6, e21776 10.7554/eLife.21776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie Y, Zhou B, Lin M-Y, Wang S, Foust KD and Sheng Z-H (2015) Endolysosomal deficits augment mitochondria pathology in spinal motor neurons of asymptomatic fALS mice. Neuron 87, 355–370 10.1016/j.neuron.2015.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yap CC, Digilio L, McMahon LP, Garcia ADR and Winckler B (2018) Degradation of dendritic cargos requires Rab7-dependent transport to somatic lysosomes. J. Cell Biol. 217, 3141–3159 10.1083/jcb.201711039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ravikumar B, Acevedo-Arozena A, Imarisio S, Berger Z, Vacher C, O’Kane CJ et al. (2005) Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat. Genet. 37, 771–776 10.1038/ng1591 [DOI] [PubMed] [Google Scholar]
- 73.Katsumata K, Nishiyama J, Inoue T, Mizushima N, Takeda J and Yuzaki M (2010) Dynein- and activity-dependent retrograde transport of autophagosomes in neuronal axons. Autophagy 6, 378–385 10.4161/auto.6.3.11262 [DOI] [PubMed] [Google Scholar]
- 74.Zhou B, Cai Q, Xie Y and Sheng Z-H (2012) Snapin recruits dynein to BDNF-TrkB signaling endosomes for retrograde axonal transport and is essential for dendrite growth of cortical neurons. Cell Rep. 2, 42–51 10.1016/j.celrep.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andres-Alonso M, Ammar MR, Butnaru I, Gomes GM, Acuña Sanhueza G, Raman R et al. (2019) SIPA1L2 controls trafficking and local signaling of trkB-containing amphisomes at presynaptic terminals. Nat. Commun. 10, 5448 10.1038/s41467-019-13224-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kononenko NL, Claßen GA, Kuijpers M, Puchkov D, Maritzen T, Tempes A et al. (2017) Retrograde transport of trkB-containing autophagosomes via the adaptor AP-2 mediates neuronal complexity and prevents neurodegeneration. Nat. Commun. 8, 14819 10.1038/ncomms14819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fu M and Holzbaur ELF (2014) Integrated regulation of motor-driven organelle transport by scaffolding proteins. Trends Cell Biol. 24, 564–574 10.1016/j.tcb.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khobrekar NV, Quintremil S, Dantas TJ and Vallee RB (2020) The dynein adaptor RILP controls neuronal autophagosome biogenesis, transport, and clearance. Dev. Cell 53, 141–153.e4 10.1016/j.devcel.2020.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wong YC and Holzbaur ELF (2014) The regulation of autophagosome dynamics by Huntingtin and HAP1 Is disrupted by expression of mutant Huntingtin, leading to defective cargo degradation. J. Neurosci. 34, 1293–1305 10.1523/JNEUROSCI.1870-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheng Z-H and Cai Q (2011) Uncovering the role of snapin in regulating autophagy-lysosomal function. Autophagy 7, 445–447 10.1038/nrn3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han S, Jeong YY, Sheshadri P, Su X and Cai Q (2020) Mitophagy regulates integrity of mitochondria at synapses and is critical for synaptic maintenance. EMBO Rep. 21, e49801 10.15252/embr.201949801F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han S, Jeong YY, Sheshadri P and Cai Q (2020) Mitophagy coordination with retrograde transport ensures the integrity of synaptic mitochondria. Autophagy 16, 1925–1927 10.1080/15548627.2020.1810919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang EJ and Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 10.1146/annurev.neuro.24.1.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Delcroix J-D, Valletta JS, Wu C, Hunt SJ, Kowal AS and Mobley WC (2003) NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron 39, 69–84 10.1016/S0896-6273(03)00397-0 [DOI] [PubMed] [Google Scholar]
- 85.Ye M, Lehigh KM and Ginty DD (2018) Multivesicular bodies mediate long-range retrograde NGF-TrkA signaling. eLife 7, e33012 10.7554/eLife.33012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang T, Martin S, Papadopulos A, Harper CB, Mavlyutov TA, Niranjan D et al. (2015) Control of autophagosome axonal retrograde flux by presynaptic activity unveiled using botulinum neurotoxin type A. J. Neurosci. 35, 6179–6194 10.1523/JNEUROSCI.3757-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang T, Martin S, Nguyen TH, Harper CB, Gormal RS, Martínez-Mármol R et al. (2016) Corrigendum: flux of signalling endosomes undergoing axonal retrograde transport is encoded by presynaptic activity and trkB. Nat. Commun. 7, 13768 10.1038/ncomms13768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barford K, Deppmann C and Winckler B (2017) The neurotrophin receptor signaling endosome: where trafficking meets signaling. Dev. Neurobiol. 77, 405–418 10.1002/dneu.22427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patel KK, Miyoshi H, Beatty WL, Head RD, Malvin NP, Cadwell K et al. (2013) Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. EMBO J. 32, 3130–3144 10.1038/emboj.2013.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y-D, Fang Y-T, Cheng Y-L, Lin C-F, Hsu L-J, Wang S-Y et al. (2017) Exophagy of annexin A2 via RAB11, RAB8A and RAB27A in IFN-γ-stimulated lung epithelial cells. Sci. Rep. 7, 5676 10.1038/s41598-017-06076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang M, Kenny SJ, Ge L, Xu K and Schekman R (2015) Translocation of interleukin-1β into a vesicle intermediate in autophagy-mediated secretion. eLife 4, e11205 10.7554/eLife.11205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kimura T, Jia J, Kumar S, Choi SW, Gu Y, Mudd M et al. (2017) Dedicated SNAREs and specialized TRIM cargo receptors mediate secretory autophagy. EMBO J. 36, 42–60 10.15252/embj.201695081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dupont N, Jiang S, Pilli M, Ornatowski W, Bhattacharya D and Deretic V (2011) Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. EMBO J. 30, 4701–4711 10.1038/emboj.2011.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Griffiths RE, Kupzig S, Cogan N, Mankelow TJ, Betin VMS, Trakarnsanga K et al. (2012) Maturing reticulocytes internalize plasma membrane in glycophorin A-containing vesicles that fuse with autophagosomes before exocytosis. Blood 119, 6296–6306 10.1182/blood-2011-09-376475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Long JM and Holtzman DM (2019) Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179, 312–339 10.1016/j.cell.2019.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haass C and Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 8, 101–112 10.1038/nrm2101 [DOI] [PubMed] [Google Scholar]
- 97.Hardy J and Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356 10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- 98.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S et al. (2012) A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488, 96–99 10.1038/nature11283 [DOI] [PubMed] [Google Scholar]
- 99.Nixon RA (2007) Autophagy, amyloidogenesis and Alzheimer disease. J. Cell Sci. 120, 4081–4091 10.1242/jcs.019265 [DOI] [PubMed] [Google Scholar]
- 100.Nixon RA and Yang D-S (2011) Autophagy failure in Alzheimer’s disease–locating the primary defect. Neurobiol. Dis. 43, 38–45 10.1016/j.nbd.2011.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee J-H et al. (2005) Macroautophagy–a novel β-amyloid peptide-generating pathway activated in Alzheimer’s disease. J. Cell Biol. 171, 87–98 10.1083/jcb.200505082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cataldo AM, Barnett JL, Pieroni C and Nixon RA (1997) Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer’s disease: neuropathologic evidence for a mechanism of increased β-amyloidogenesis. J. Neurosci. 17, 6142–6151 10.1523/JNEUROSCI.17-16-06142.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT and Nixon RA (2000) Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and down syndrome: differential effects of APOE genotype and presenilin mutations. Am. J. Pathol. 157, 277–286 10.1016/s0002-9440(10)64538-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C et al. (2012) Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 482, 216–220 10.1038/nature10821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A et al. (2005) Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J. Neuropathol. Exp. Neurol. 64, 113–122 10.1093/jnen/64.2.113 [DOI] [PubMed] [Google Scholar]
- 106.Tammineni P and Cai Q (2017) Defective retrograde transport impairs autophagic clearance in Alzheimer disease neurons. Autophagy 13, 982–984 10.1080/15548627.2017.1291114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ye X and Cai Q (2014) Snapin-mediated BACE1 retrograde transport is essential for its degradation in lysosomes and regulation of app processing in neurons. Cell Rep. 6, 24–31 10.1016/j.celrep.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ye X, Feng T, Tammineni P, Chang Q, Jeong YY, Margolis DJ et al. (2017) Regulation of synaptic amyloid-β generation through BACE1 retrograde transport in a mouse model of Alzheimer’s disease. J. Neurosci. 37, 2639–2655 10.1523/JNEUROSCI.2851-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Feng T, Tammineni P, Agrawal C, Jeong YY and Cai Q (2017) Autophagy-mediated regulation of BACE1 protein trafficking and degradation. J. Biol. Chem. 292, 1679–1690 10.1074/jbc.M116.766584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kononenko NL, Puchkov D, Classen GA, Walter AM, Pechstein A, Sawade L et al. (2014) Clathrin/AP-2 mediate synaptic vesicle reformation from endosome-like vacuoles but are not essential for membrane retrieval at central synapses. Neuron 82, 981–988 10.1016/j.neuron.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 111.Bera S, Camblor-Perujo S, Calleja Barca E, Negrete-Hurtado A, Racho J, De Bruyckere E et al. (2020) AP-2 reduces amyloidogenesis by promoting BACE1 trafficking and degradation in neurons. EMBO Rep. 21, e47954 10.15252/embr.201947954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sharples RA, Vella LJ, Nisbet RM, Naylor R, Perez K, Barnham KJ et al. (2008) Inhibition of γ-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J. 22, 1469–1478 10.1096/fj.07-9357com [DOI] [PubMed] [Google Scholar]
- 113.Vingtdeux V, Sergeant N and Buée L (2012) Potential contribution of exosomes to the prion-like propagation of lesions in Alzheimer’s disease. Front. Physiol. 3, 229 10.3389/fphys.2012.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P et al. (2006) Alzheimer’s disease β-amyloid peptides are released in association with exosomes. Proc. Natl Acad. Sci. U.S.A. 103, 11172–11177 10.1073/pnas.0603838103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H et al. (2002) Intraneuronal Alzheimer aβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am. J. Pathol. 161, 1869–1879 10.1016/S0002-9440(10)64463-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jaeger PA, Pickford F, Sun C-H, Lucin KM, Masliah E and Wyss-Coray T (2010) Regulation of amyloid precursor protein processing by the beclin 1 complex. PLoS ONE 5, e11102 10.1371/journal.pone.0011102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nilsson P, Loganathan K, Sekiguchi M, Matsuba Y, Hui K, Tsubuki S et al. (2013) Aβ secretion and plaque formation depend on autophagy. Cell Rep. 5, 61–69 10.1016/j.celrep.2013.08.042 [DOI] [PubMed] [Google Scholar]
- 118.Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA et al. (2008) The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J. Clin. Invest. 118, 2190–2199 10.1172/JCI33585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tian Y, Bustos V, Flajolet M and Greengard P (2011) A small-molecule enhancer of autophagy decreases levels of abeta and APP-CTF via Atg5-dependent autophagy pathway. FASEB J. 25, 1934–1942 10.1096/fj.10-175158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Piras A, Collin L, Grüninger F, Graff C and Rönnbäck A (2016) Autophagic and lysosomal defects in human tauopathies: analysis of post-mortem brain from patients with familial Alzheimer disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. Commun. 4, 22 10.1186/s40478-016-0292-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen X, Li Y, Wang C, Tang Y, Mok S-A, Tsai RM et al. (2020) Promoting tau secretion and propagation by hyperactive p300/CBP via autophagy-lysosomal pathway in tauopathy. Mol. Neurodegener. 15, 2 10.1186/s13024-019-0354-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kang S, Son SM, Baik SH, Yang J and Mook-Jung I (2019) Autophagy-mediated secretory pathway is responsible for both normal and pathological tau in neurons. J. Alzheimer’s Dis. 70, 667–680 10.3233/JAD-190180 [DOI] [PubMed] [Google Scholar]
- 123.Lonati E, Sala G, Tresoldi V, Coco S, Salerno D, Milani C et al. (2018) Ischemic conditions affect rerouting of tau protein levels: evidences for alteration in tau processing and secretion in hippocampal neurons. J. Mol. Neurosci. 66, 604–616 10.1007/s12031-018-1199-7 [DOI] [PubMed] [Google Scholar]
- 124.Mohamed N-V, Plouffe V, Rémillard-Labrosse G, Planel E and Leclerc N (2014) Starvation and inhibition of lysosomal function increased tau secretion by primary cortical neurons. Sci. Rep. 4, 5715 10.1038/srep05715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tang Z, Ioja E, Bereczki E, Hultenby K, Li C, Guan Z et al. (2015) Mtor mediates tau localization and secretion: implication for Alzheimer’s disease. Biochim. Biophys. Acta Mol. Cell Res. 1853, 1646–1657 10.1016/j.bbamcr.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 126.Fader CM, Sánchez D, Furlán M and Colombo MI (2008) Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in K562 cells. Traffic 9, 230–250 10.1111/j.1600-0854.2007.00677.x [DOI] [PubMed] [Google Scholar]
- 127.Xu J, Camfield R and Gorski SM (2018) The interplay between exosomes and autophagy – partners in crime. J. Cell Sci. 131, jcs215210 10.1242/jcs.215210 [DOI] [PubMed] [Google Scholar]
- 128.Lotharius J and Brundin P (2002) Impaired dopamine storage resulting from α-synuclein mutations may contribute to the pathogenesis of Parkinson’s disease. Hum. Mol. Genet. 11, 2395–2407 10.1093/hmg/11.20.2395 [DOI] [PubMed] [Google Scholar]
- 129.Parkkinen L, O’Sullivan SS, Collins C, Petrie A, Holton JL, Revesz T et al. (2011) Disentangling the relationship between Lewy bodies and nigral neuronal loss in Parkinson’s disease. J. Parkinsons. Dis. 1, 277–286 10.3233/JPD-2011-11046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jucker M and Walker LC (2013) Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501, 45–51 10.1038/nature12481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sarkar S, Davies JE, Huang Z, Tunnacliffe A and Rubinsztein DC (2007) Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant Huntingtin and α-Synuclein. J. Biol. Chem. 282, 5641–5652 10.1074/jbc.M609532200 [DOI] [PubMed] [Google Scholar]
- 132.Winslow AR, Chen C-W, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA et al. (2010) α-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J. Cell Biol. 190, 1023–1037 10.1083/jcb.201003122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tanik SA, Schultheiss CE, Volpicelli-Daley LA, Brunden KR and Lee VMY (2013) Lewy body-like α-synuclein aggregates resist degradation and impair macroautophagy. J. Biol. Chem. 288, 15194–15210 10.1074/jbc.M113.457408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S and Wood MJA (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 10.1038/nbt.1807 [DOI] [PubMed] [Google Scholar]
- 135.Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L et al. (2012) Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol. Neurodegener. 7, 42 10.1186/1750-1326-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ejlerskov P, Rasmussen I, Nielsen TT, Bergström A-L, Tohyama Y, Jensen PH et al. (2013) Tubulin polymerization-promoting protein (TPPP/p25α) promotes unconventional secretion of α-synuclein through exophagy by impairing autophagosome-lysosome fusion. J. Biol. Chem. 288, 17313–17335 10.1074/jbc.M112.401174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH et al. (2010) Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 30, 6838–6851 10.1523/JNEUROSCI.5699-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hasegawa T, Konno M, Baba T, Sugeno N, Kikuchi A, Kobayashi M et al. (2011) The AAA-ATPase VPS4 regulates extracellular secretion and lysosomal targeting of α-synuclein. PLoS ONE 6, e29460 10.1371/journal.pone.0029460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jang A, Lee H-J, Suk J-E, Jung J-W, Kim K-P and Lee S-J (2010) Non-classical exocytosis of α-synuclein is sensitive to folding states and promoted under stress conditions. J. Neurochem. 113, 1263–1274 10.1111/j.1471-4159.2010.06695.x [DOI] [PubMed] [Google Scholar]
- 140.Lee H-J, Patel S and Lee S-J (2005) Intravesicular localization and exocytosis of α-synuclein and its aggregates. J. Neurosci. 25, 6016–6024 10.1523/JNEUROSCI.0692-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Desplats P, Lee H-J, Bae E-J, Patrick C, Rockenstein E, Crews L et al. (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc. Natl Acad. Sci. U.S.A. 106, 13010–13015 10.1073/pnas.0903691106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hansen C, Angot E, Bergström A-L, Steiner JA, Pieri L, Paul G et al. (2011) α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Invest. 121, 715–725 10.1172/JCI43366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A et al. (2011) Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71 10.1016/j.neuron.2011.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li J-Y, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ et al. (2008) Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 14, 501–503 10.1038/nm1746 [DOI] [PubMed] [Google Scholar]
- 145.Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ et al. (2012) Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 10.1126/science.1227157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ and Lee VMY (2012) Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 209, 975–986 10.1084/jem.20112457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tofaris GK, Kim HT, Hourez R, Jung J-W, Kim KP and Goldberg AL (2011) Ubiquitin ligase Nedd4 promotes α-synuclein degradation by the endosomal–lysosomal pathway. Proc. Natl Acad. Sci. U.S.A. 108, 17004–17009 10.1073/pnas.1109356108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Davies SE, Hallett PJ, Moens T, Smith G, Mangano E, Kim HT et al. (2014) Enhanced ubiquitin-dependent degradation by Nedd4 protects against α-synuclein accumulation and toxicity in animal models of Parkinson’s disease. Neurobiol. Dis. 64, 79–87 10.1016/j.nbd.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lindersson E, Lundvig D, Petersen C, Madsen P, Nyengaard JR, Højrup P et al. (2005) P25alpha stimulates alpha-synuclein aggregation and is co-localized with aggregated alpha-synucleinin alpha-synucleinopathies. J. Biol. Chem. 280, 5703–5715 10.1074/jbc.M410409200 [DOI] [PubMed] [Google Scholar]
- 150.Grigoriev I, Yu KL, Martinez-Sanchez E, Serra-Marques A, Smal I, Meijering E et al. (2011) Rab6, Rab8, and MICAL3 cooperate in controlling docking and fusion of exocytotic carriers. Curr. Biol. 21, 967–974 10.1016/j.cub.2011.04.030 [DOI] [PubMed] [Google Scholar]
- 151.Minakaki G, Menges S, Kittel A, Emmanouilidou E, Schaeffner I, Barkovits K et al. (2018) Autophagy inhibition promotes SNCA/alpha-synuclein release and transfer via extracellular vesicles with a hybrid autophagosome-exosome-like phenotype. Autophagy 14, 98–119 10.1080/15548627.2017.1395992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zarei S, Carr K, Reiley L, Diaz K, Guerra O, Altamirano PF et al. (2015) A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 6, 171 10.4103/2152-7806.169561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gomes C, Keller S, Altevogt P and Costa J (2007) Evidence for secretion of Cu, Zn superoxide dismutase via exosomes from a cell model of amyotrophic lateral sclerosis. Neurosci. Lett. 428, 43–46 10.1016/j.neulet.2007.09.024 [DOI] [PubMed] [Google Scholar]
- 154.Grad LI, Yerbury JJ, Turner BJ, Guest WC, Pokrishevsky E, O’Neill MA et al. (2014) Intercellular propagated misfolding of wild-type Cu/Zn superoxide dismutase occurs via exosome-dependent and -independent mechanisms. Proc. Natl Acad. Sci. U.S.A. 111, 3620–3625 10.1073/pnas.1312245111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Münch C, O’Brien J and Bertolotti A (2011) Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc. Natl Acad. Sci. U.S.A. 108, 3548–3553 10.1073/pnas.1017275108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pasquali L, Ruffoli R, Fulceri F, Pietracupa S, Siciliano G, Paparelli A et al. (2010) The role of autophagy: what can be learned from the genetic forms of amyotrophic lateral sclerosis. CNS Neurol. Disord. Drug Targets 9, 268–278 10.2174/187152710791292594 [DOI] [PubMed] [Google Scholar]
- 157.Laird FM, Farah MH, Ackerley S, Hoke A, Maragakis N, Rothstein JD et al. (2008) Motor neuron disease occurring in a mutant dynactin mouse model is characterized by defects in vesicular trafficking. J. Neurosci. 28, 1997–2005 10.1523/JNEUROSCI.4231-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sasaki S (2011) Autophagy in spinal cord motor neurons in sporadic amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 70, 349–359 10.1097/NEN.0b013e3182160690 [DOI] [PubMed] [Google Scholar]
- 159.Otomo T, Higaki K, Nanba E, Ozono K and Sakai N (2011) Lysosomal storage causes cellular dysfunction in mucolipidosis II skin fibroblasts. J. Biol. Chem. 286, 35283–35290 10.1074/jbc.M111.267930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hadano S, Otomo A, Kunita R, Suzuki-Utsunomiya K, Akatsuka A, Koike M et al. (2010) Loss of ALS2/Alsin exacerbates motor dysfunction in a SOD1-expressing mouse ALS model by disturbing endolysosomal trafficking. PLoS ONE 5, e9805 10.1371/journal.pone.0009805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Karten B, Peake KB and Vance JE (2009) Mechanisms and consequences of impaired lipid trafficking in niemann–Pick type C1-deficient mammalian cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1791, 659–670 10.1016/j.bbalip.2009.01.025 [DOI] [PubMed] [Google Scholar]
- 162.Rosenbaum AI and Maxfield FR (2011) Niemann-Pick type C disease: molecular mechanisms and potential therapeutic approaches. J. Neurochem. 116, 789–795 10.1111/j.1471-4159.2010.06976.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sarkar S, Carroll B, Buganim Y, Maetzel D, Ng AHM, Cassady JP et al. (2013) Impaired autophagy in the lipid-storage disorder Niemann-Pick type C1 disease. Cell Rep. 5, 1302–1315 10.1016/j.celrep.2013.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Richards P, Didszun C, Campesan S, Simpson A, Horley B, Young KW et al. (2011) Dendritic spine loss and neurodegeneration is rescued by Rab11 in models of Huntington’s disease. Cell Death Differ. 18, 191–200 10.1038/cdd.2010.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gordon PB and Seglen PO (1988) Prelysosomal convergence of autophagic and endocytic pathways. Biochem. Biophys. Res. Commun. 151, 40–47 10.1016/0006-291x(88)90556-6 [DOI] [PubMed] [Google Scholar]
- 166.Lamb CA, Yoshimori T and Tooze SA (2013) The autophagosome: origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 14, 759–774 10.1038/nrm3696 [DOI] [PubMed] [Google Scholar]
- 167.Shen H-M and Mizushima N (2014) At the end of the autophagic road: an emerging understanding of lysosomal functions in autophagy. Trends Biochem. Sci. 39, 61–71 10.1016/j.tibs.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 168.Langer F, Eisele YS, Fritschi SK, Staufenbiel M, Walker LC and Jucker M (2011) Soluble Aβ seeds are potent inducers of cerebral β-amyloid deposition. J. Neurosci. 31, 14488–14495 10.1523/JNEUROSCI.3088-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]