Abstract
Introduction
The synchronous development of a medullary and papillary carcinoma as two different tumors has only been reported very rarely. The aim of the current report is to describe an extremely rare occurrence of medullary carcinoma, papillary microcarcinoma, and Hashimoto thyroiditis.
Case report
A 53-year-old man presented with a right-sided neck mass. Ultrasound showed a well-defined nodule in the right mid third with microcalcification and increased nodular vascularity associated with multiple right-sided cervical lymphadenopathy. The histopathological examination showed multifocal medullary carcinoma with incidental finding of unifocal papillary microcarcinoma conventional type on the left side. Additional pathology of Hashimoto thyroiditis with a small intra-thyroidal parathyroid gland in the left thyroid gland. The procedure went perfectly and the patient was discharged home without any difficulties.
Discussion
Synchronous existence of these two neoplasms can occur in two forms: distinct medullary carcinoma and papillary carcinoma isolated by normal thyroid tissue, or mixed medullary and follicular-derived thyroid carcinoma, in which single or multiple lesions show morphology and immunoreactivity for both medullary carcinoma and follicular-derived carcinoma.
Conclusion
The synchronous coexistence of papillary microcarcinoma, medullary carcinoma, and Hashimoto's thyroiditis is an uncommon thyroid condition.
Keywords: Thyroid cancer, Papillary thyroid carcinoma, Follicular thyroid carcinoma, Medullary thyroid carcinoma, Hashimoto thyroiditis
Highlights
-
•
Thyroid malignancies are less common than other types of cancer.
-
•
The interactions between medullary and papillary carcinomas are of particular interest.
-
•
Synchronous development of medullary and papillary carcinoma has been reported very rarely.
-
•
In this paper, the occurrence of Hashimoto thyroiditis with papillary carcinoma and medullary carcinoma is reported.
1. Introduction
Thyroid malignancies are less common than other types of cancer, accounting for less than 1% of all cancers and less than 0.5% of all lethal cancers [1]. Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer, while medullary thyroid carcinoma (MTC) accounts for 5–10% of all cases [2]. Thyroid carcinomas are classified into two types based on their cellular origin: follicular and papillary carcinomas that arise from follicular cells, and medullary carcinomas that arise from parafollicular C-cells [3]. The interactions between medullary and papillary carcinomas are of particular interest [3]. Although there have been several cases of synchronous occurrences of both medullary and follicular cells, the synchronous development of a medullary and papillary carcinoma as two different tumors has only been reported very rarely [4].
Hashimoto thyroiditis (HT) is an autoimmune thyroid disease that is one of the types of chronic thyroiditis and the most prevalent non-iatrogenic cause of hypothyroidism [5]. According to epidemiological studies, the average concurrence rate between HT and PTC is around 23%. Although the mechanism behind this correlation is not fully understood, some experimental investigations have shown that the occurrence of HT and PTC at the same time reflects an immunological link [6]. However, the occurrence of MTC and papillary thyroid microcarcinoma (PTMC) at the same time with Hashimoto thyroiditis is extremely rare condition.
The aim of the current report is to describe an extremely rare occurrence of MTC, PTMC, and Hashimoto thyroiditis at the same time in a middle-aged male patient. The report has been arranged in line with SCARE 2020 guidelines and includes a brief literature review [7].
2. Case report
2.1. Patient information
A 53-year-old man presented with a right-sided neck small mass that had recently grown in size. He was taking thyroxine tab 100 μg 1 ∗ 1 after undergoing total thyroidectomy with no family history of thyroid cancer or illness of parathyroid, adrenal, or renal stones.
2.2. Clinical examination
On examination, there was a firm mobile lesion on the right side of the neck that was mobile but not tender, as well as redness over the skin of the mass.
2.3. Diagnostic assessment
On ultrasound (US), there was a well-defined nodule of 12 × 7.8 mm in the anterior aspect of the right mid third with microcalcification and increased peri and intra nodular vascularity associated with multiple right-sided pathological cervical lymphadenopathy. The largest was 42 × 12 mm in Group IV and 24 × 17 mm in Group III. Thyroid function was normal, but there was a high calcitonin level (>2000 pg/ml) and a carcinoembryonic antigen (CEA) level (251.6 ng/ml). A right-sided cervical fine needle aspiration biopsy with immunostaining revealed a high level of reactivity of neoplastic cells to chromogranin, synaptophysin, calcitonin, CEA, CKAE1/3, and CD45, all of which were associated with MTC.
2.4. Therapeutic intervention
After doing total thyroidectomy with right central neck dissection level VI, right lateral neck dissection levels (II, III, IV), and right mediastinal lymph node dissection. The histopathological examination showed multifocal MTC with the largest of 1.2 cm and an incidental finding of unifocal PTMC conventional type on the left side that measured about 0.2 cm. There was lympho-vascular and perineural invasion only on the right side of the MTC, and additional pathology of Hashimoto thyroiditis with a small intra-thyroidal parathyroid gland in the left thyroid gland (Fig. 1, Fig. 2, Fig. 3). 45 lymph nodes have been dissected, of them 9 lymph nodes have been involved (1 from right central, 7 from right lateral, and 1 mediastinal lymph node) with extra-nodal extension, largest metastatic deposit of 6 cm.
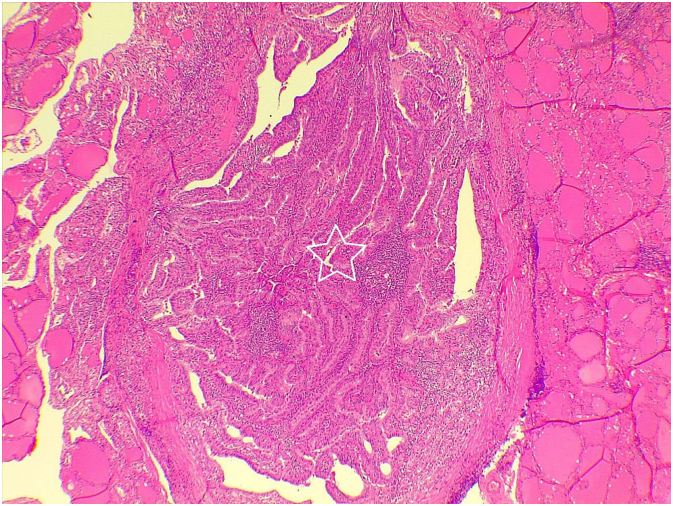
Fig. 1.
Section shows medullary thyroid carcinoma (star) arranged as dyscohesive plasmacytoid malignant cells in a stroma contains abundant amyloid (white arrow).
Fig. 2.
Papillary microcarcinoma (star) composes of complex papillary structures covered by follicular cells with classical features of papillary thyroid carcinoma.
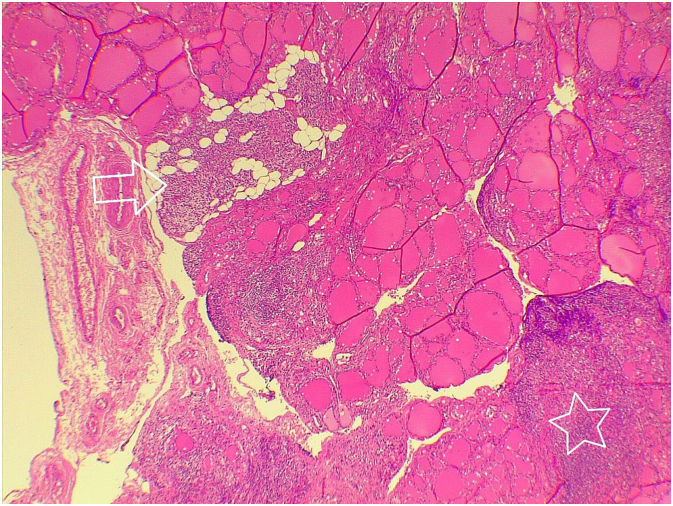
Fig. 3.
Intrathyroidal small parathyroid gland (arrow) inside the thyroid gland with lymphocytic infiltration forming lymphoid follicle (star).
2.5. Follow up
The procedure went perfectly and the patient was discharged home without any difficulties. Due to the small size of the left-sided PTMC, he was scheduled to receive radioactive iodine (RAI).
3. Discussion
Thyroid cancer is the most frequent endocrine malignancy, accounting for more than 90% of endocrine gland malignancies [8]. The most prevalent type of thyroid cancer is well-differentiated thyroid cancer, which includes papillary and follicular carcinomas, whereas medullary and anaplastic carcinomas account for 5–10% and 1.6% of cases, respectively [9]. 80% of thyroid cancers are PTCs. PTMC is defined by the World Health Organization (WHO) as a PTC with a diameter of 1 cm or less, and is a frequent accidental discovery in thyroid glands resected for other indications [10]. Radiation exposure, female sex, smoking, overweight, dietary iodine excess, alcohol, dietary nitrates, diabetes mellitus, and genetic factors have all been identified as risk factors for PTC [11]. MTCs are commonly non-inherited sporadic lesions that often occur in the fourth decade as a unifocal lesion with no concomitant endocrinopathies. Inherited MTC (25%) can have three forms: MEN 2A, MEN 2B, and familial MTC. Familial MTC develops as a solitary MTC without concomitant endocrinopathies; all hereditary types of MTC are autosomal dominant [4]. Pathological variations with follicular and papillary features are possible in MTC [4].
Regardless of the fact that the frequency of thyroid cancer has increased significantly in recent years, the concomitant occurrence of multiple tumors in a single thyroid gland is still a rare occurrence [12]. Synchronous existence of these two neoplasms can occur in two forms: distinct MTC and PTC isolated by normal thyroid tissue, or mixed medullary and follicular-derived thyroid carcinoma, in which single or multiple lesions show morphology and immunoreactivity for both MTC and follicular-derived carcinoma (PTC) [13]. The possibility of HT, PTC, and MTC coexisting is dependent on specific gene rearrangements. Researchers have discovered biomarkers that convert HT to PTC. Some of these biomarkers are p63 expression, PI3K/Akt expression, RET/PTC rearrangements, and BRAF mutation [11]. In addition to genetics, immune-mediated pathways combining PTC and HT were discovered. Immune cells may produce cellular mediators such as CD3+, CD4+, and Th17, which may result in thyroid cancer [14].
There are various concepts about the carcinogenesis of thyroid tumors with medullary and papillary carcinoma features [4]. The first is the “common stem cell theory,” which is based on the fact that follicular and parafollicular C-cells have common progenitor cells. According to this view, common stem cells initially undergo neoplastic transformation before being differentiated into the two separate cell subtypes [12]. Another hypothesis is the “field effect theory,” which claims that a shared oncogenic signal causes both follicular and parafollicular C-cell progenitor cells to change at the same time [12]. Other possible explanations for the simultaneous occurrence include the involvement of the RET proto-oncogene in both PTC and MTC, whether through a point mutation (in MTC) or a gene rearrangement (in PTC) [15].
A large number of small thyroid nodules or ‘incidentalomas’ are being discovered as a result of the increased usage of imaging techniques such as neck ultrasonography [16]. FNA cytology is recommended for nodules bigger than 1 cm, according to the American Thyroid Association's (ATA) standards [17]. Smaller nodules should be biopsied if ultrasonographic signs suggestive of cancer, such as calcification, enhanced intra-nodular vascularity as measured by Doppler flow, solid or hypoechoic appearance, irregular or blurred margins, or a taller than wide shape [18]. Thyroglobulin and thyroid transcription factor-1 (TTF-1) are both positive immunohistochemically in PTC. Calcitonin, CEA, and chromogranin are all negative in PTC cells. On the other hand, MTC cells stain positive for calcitonin, chromogranin, and CEA but negative for thyroglobulin [19].
When PTMC is diagnosed prior to surgery, the main approach is a total or near-total thyroidectomy to eliminate multifocal disease and lower the overall recurrence rate [17]. Neck dissection of the central compartment (Level VI) is likewise a controversial procedure. Recent studies have looked at the role of preventive central lymphadenectomy in the treatment of PTMC. One study supported prophylactic central neck dissection and suggested that clinicopathologic factors such as male sex, tumor multifocality, and extrathyroidal extension be taken into account while making the decision [20]. In most cases, the MTC component, which is considerably worse, determines the prognosis. As a result, therapy and follow-up should be arranged appropriately [21]. The procedure of choice in MTC is total thyroidectomy with central lymph node dissection, and depending on blood calcitonin levels and preoperative cervical US imaging, a more extensive operation with lateral neck dissection could be considered [22].
According to a number of studies, microcarcinomas have a better prognosis than larger tumors, with the risk of death often between 0% and 1%. Recurrent or persistent disease, on the other hand, is surprisingly common [16]. PTMCs were thought to have an indolent course with limited morbidity for decades. Ito et al. discovered that 70% of PTMC cases have stable tumors after a four-year observation period with no intervention. Despite the fact that these tumors are often regarded as clinically benign, some of them exhibit aggressive clinical behavior [23]. Age greater than 45 years, male sex, non-Caucasian race, extrathyroidal expansion, and both lymph node and distant metastases were all risk factors for recurrences [16]. Wong et al. reported that MTC/PTC collision tumors were diagnosed earlier in tumor progression than MTC alone, indicating a better prognosis [24]. Apart from these synchronous events, HT has been proposed as a preventive factor against PTC progression [25].
In conclusion, the synchronous coexistence of PTMC, MTC, and Hashimoto's thyroiditis is an uncommon thyroid condition.
Consent
Written informed consent was obtained from the patient's family for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Ethical approval
Approval is not necessary for case report (till 3 cases in single report) in our locality.
The family gave consent for the publication of the report.
Funding
None is found.
Guarantor
Fahmi Hussein Kakamad Fahmi.hussein@univsul.edu.iq.
Research registration number
Not applicable.
CRediT authorship contribution statement
Abdulwahid M. Salh: major contribution of the idea, literature review, final approval of the manuscript.
Abdulwahid M. Salh: Surgeon performing the operation, final approval of the manuscript.
Fahmi H. Kakamad: Writing the manuscript, literature review, final approval of the manuscript.
Rawa M. Ali, Karzan M. Salih, Karukh K. Mohammed, Shevan M. Mustafa: literature review, final approval of the manuscript.
Ari M. Abdullah: Histopathologist who made diagnosis, final approval of the manuscript.
Declaration of competing interest
None to be declared.
References
- 1.Baba H.O., Qaradakhy A.J., Abdullah A.M., Saliha A.M., Salih R.Q., Hussein H.M., et al. Synchronous occurrence of Hurthle cell carcinoma with Hodgkin's lymphoma; the first reported case with literature review. Ann.Med.Surg. 2021;69 doi: 10.1016/j.amsu.2021.102750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koudounarakis E., Karatzanis A., Chatzidakis A., Tzardi M., Velegrakis G. Synchronous multifocal medullary and papillary thyroid microcarcinoma detected by elastography. Int. J. Surg. Case Rep. 2014;5(1):5–7. doi: 10.1016/j.ijscr.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behrend M., Von Wasielewski R., Brabant G. Simultaneous medullary and papillary microcarcinoma of thyroid in a patient with secondary hyperparathyroidism. Endocr. Pathol. 2002;13(1):65–73. doi: 10.1385/ep:13:1:65. [DOI] [PubMed] [Google Scholar]
- 4.Giacomelli L., Guerriero G., Falvo L., Altomare V., Chiesa C., Ferri S., et al. Simultaneous occurrence of medullary carcinoma and papillary microcarcinoma of thyroid in a patient with MEN 2A syndrome.Report of a case. Tumori J. 2007;93(1):109–111. doi: 10.1177/030089160709300121. [DOI] [PubMed] [Google Scholar]
- 5.Konturek A., Barczyński M., Wierzchowski W., Stopa M., Nowak W. Coexistence of papillary thyroid cancer with Hashimoto thyroiditis. Langenbeck's Arch. Surg. 2013;398(3):389–394. doi: 10.1007/s00423-012-1021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon S., Chung H.S., Yu J.M., Yoo H.J., Park J.H., Kim D.S., et al. Associations between Hashimoto thyroiditis and clinical outcomes of papillary thyroid cancer: a meta-analysis of observational studies. Endocrinol. Metab. 2018;33(4):473–484. doi: 10.3803/EnM.2018.33.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Thoma A., et al. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Vanderpump M.P. The epidemiology of thyroid disease. Br. Med. Bull. 2011;99(1) doi: 10.1093/bmb/ldr030. [DOI] [PubMed] [Google Scholar]
- 9.Hundahl S.A., Cady B., Cunningham M.P., Mazzaferri E., McKee R.F., Rosai J., et al. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996: an American College of Surgeons Commission on Cancer Patient Care Evaluation Study. Cancer. 2000;89(1):202–217. doi: 10.1002/1097-0142(20000701)89:1<202::aid-cncr27>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Kösem M., Kotan Ç., Algün E., Topal C. Simultaneous occurrence of papillary intrafollicular and microcarcinomas with bilateral medullary microcarcinoma of the thyroid in a patient with multiple endocrine neoplasia type 2A: report of a case. Surg. Today. 2002;32(7):623–628. doi: 10.1007/s005950200112. [DOI] [PubMed] [Google Scholar]
- 11.Sulaieva O., Selezniov O., Shapochka D., Belemets N., Nechay O., Chereshneva Y., et al. Hashimoto's thyroiditis attenuates progression of papillary thyroid carcinoma: deciphering immunological links. Heliyon. 2020;6(1) doi: 10.1016/j.heliyon.2019.e03077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim K.J., Hong S.W., Kim S.M., Lee Y.S., Chang H.S., Park C.S. Synchronous occurrence of papillary, follicular, and medullary carcinoma in the same thyroid gland. Korean J.Endocr.Surg. 2014;14(3):167–170. [Google Scholar]
- 13.Takano A. Synchronous papillary thyroid carcinoma and medullary thyroid carcinoma–a pitfall waiting to happen. Malays.J.Pathol. 2017;39(2):171. [PubMed] [Google Scholar]
- 14.Degertekin C.K., Yilmaz B.A., Toruner F.B., Kalkanci A., Iyidir O.T., Fidan I., et al. Circulating Th17 cytokine levels are altered in Hashimoto's thyroiditis. Cytokine. 2016;80:13–17. doi: 10.1016/j.cyto.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson M., Williams D. On the origin of cells and derivation of thyroid cancer: C cell story revisited. Eur.Thyroid J. 2016;5(2):79–93. doi: 10.1159/000447333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu L.S., Milan S.A. Management of microcarcinomas (papillary and medullary) of the thyroid. Curr. Opin. Oncol. 2013;25(1):27–32. doi: 10.1097/CCO.0b013e328359feea. [DOI] [PubMed] [Google Scholar]
- 17.Cooper D.S., Doherty G.M., Haugen B.R., Kloos R.T., Lee S.L., Mandel S.J., et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 18.Rossing M., Nygaard B., Nielsen F.C., Bennedbæk F.N. High prevalence of papillary thyroid microcarcinoma in Danish patients: a prospective study of 854 consecutive patients with a cold thyroid nodule undergoing fine-needle aspiration. Eur.Thyroid J. 2012;1(2):110–117. doi: 10.1159/000338921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayrak E., Serter R. Coexistence of papillary and medullary thyroid carcinoma: a rare entity. <sb:contribution><sb:title>Turk. J.</sb:title> </sb:contribution><sb:host><sb:issue><sb:series><sb:title>Endocrinol. Metab.</sb:title></sb:series></sb:issue></sb:host>. 2020;24(2) [Google Scholar]
- 20.So Y.K., Seo M.Y., Son Y.I. Prophylactic central lymph node dissection for clinically node-negative papillary thyroid microcarcinoma: influence on serum thyroglobulin level, recurrence rate, and postoperative complications. Surgery. 2012;151(2):192–198. doi: 10.1016/j.surg.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Biscolla R.P., Ugolini C., Sculli M., Bottici V., Castagna M.G., Romei C., et al. Medullary and papillary tumors are frequently associated in the same thyroid gland without evidence of reciprocal influence in their biologic behavior. Thyroid. 2004;14(11):946–952. doi: 10.1089/thy.2004.14.946. [DOI] [PubMed] [Google Scholar]
- 22.Ceolin L., da Silveira Duval M.A., Benini A.F., Ferreira C.V., Maia A.L. Medullary thyroid carcinoma beyond surgery: advances, challenges, and perspectives. Endocr. Relat. Cancer. 2019;26(9):R499–R518. doi: 10.1530/ERC-18-0574. [DOI] [PubMed] [Google Scholar]
- 23.Ito Y., Miyauchi A., Inoue H., Fukushima M., Kihara M., Higashiyama T., et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J. Surg. 2010;34(1):28–35. doi: 10.1007/s00268-009-0303-0. [DOI] [PubMed] [Google Scholar]
- 24.Wong R.L., Kazaure H.S., Roman S.A., Sosa J.A. Simultaneous medullary and differentiated thyroid cancer: a population-level analysis of an increasingly common entity. Ann. Surg. Oncol. 2012;19(8):2635–2642. doi: 10.1245/s10434-012-2357-8. [DOI] [PubMed] [Google Scholar]
- 25.Huang B.Y., Hseuh C., Chao T.C., Lin K.J., Lin J.D. Well-differentiated thyroid carcinoma with concomitant Hashimoto's thyroiditis present with less aggressive clinical stage and low recurrence. Endocr. Pathol. 2011;22(3):144–149. doi: 10.1007/s12022-011-9164-9. [DOI] [PubMed] [Google Scholar]