Abstract
Exercise and consumption of plant-based foods rich in polyphenols are attractive therapeutic approaches for the prevention and treatment of Parkinson’s disease (PD). Few studies, however, have examined the neuroprotective efficacy of combining these treatment modalities against PD. Therefore we investigated whether combining voluntary running and consumption of blueberry juice (BBJ) was more efficacious against 6-hydroxydopamine (6-OHDA) toxicity than either treatment alone. Four weeks of running before and after intrastriatal 6-OHDA reduced amphetamine-induced rotational behavior and loss of substantia nigra dopamine (DA) neurons. BBJ consumption alone had no ameliorative effects, but when combined with exercise, behavioral deficits and nigrostriatal DA neurodegeneration were reduced to a greater extent than exercise alone. The neuroprotection observed with exercise alone was associated with an increase in striatal glial cell-lined derived neurotrophic factor (GDNF), whereas combining exercise and BBJ was associated with an increase in nigral GDNF. These results suggest that polyphenols may potentiate the protective effects of exercise and that differential regulation of GDNF expression underlies protection observed with exercise alone versus combined treatment with consumption of BBJ.
Keywords: Parkinson’s disease, Neuroprotection, Exercise, Polyphenols, Blueberry juice, GDNF
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that over time robs patients of the ability to move effectively in the absence of pharmacotherapy (Stocchi & Olanow, 2004). Its characteristic motor dysfunctions are caused by loss of midbrain dopamine (DA) neurons in the substantia nigra (SN) and depletion of striatal DA (Hornykiewicz, 2006). To date, the most effective pharmacotherapy for PD is DA replacement therapy using levodopa (Olanow & Stocchi, 2018); however, this treatment has no ameliorative effects upon the underlying pathology. Thus, additional treatments are needed that alter the disease course in addition to attenuating symptoms.
Over the last two decades, there has been increased interest in the impact of exercise and nutrition on neurodegenerative diseases. Exercise or diets incorporating certain plant-based foods, such as teas and berries or their extracted juices have been associated with a lower risk of PD (Tsai et al., 2002, Chen et al., 2005, Gao et al., 2007, Gao et al., 2012). Furthermore, exercise appears to attenuate the motor, cognitive, and affective deficits in patients with mild to moderate PD (Goodwin et al., 2008, da Silva et al., 2018, Schenkman et al., 2018). In accordance with the clinical literature, various forms of motor therapy are generally effective in reducing behavioral deficits in animal models of PD (Tillerson et al., 2001, Fisher et al., 2004, Moroz et al., 2004, O'Dell et al., 2007, Archer and Fredriksson, 2010, Dutra et al., 2012, Aguiar et al., 2013), and in some cases, also reduce damage to the nigrostriatal pathway induced by DA neurotoxins (Tillerson et al., 2001, Cohen et al., 2003, Tillerson et al., 2003, Yoon et al., 2007, Tajiri et al., 2010, Aguiar et al., 2016a).
Plant-based foods, as well as beverages rich in flavonoids, have been shown to reduce age-related cognitive and motor decline in humans and in laboratory animals (Shukitt-Hale et al., 2006, Krikorian et al., 2010a, Krikorian et al., 2010b, Rendeiro et al., 2012, Shukitt-Hale et al., 2015). Specifically, improvements in balance and coordination in aged animals were observed with diets containing polyphenol-rich grape juice, blueberries, strawberries, blackberries, and walnuts (Shukitt-Hale et al., 2006, Joseph et al., 2009, Shukitt-Hale et al., 2009, Willis et al., 2009). Flavonoids have potent antioxidant and anti-inflammatory properties (Kelsey et al., 2010, Cassidy et al., 2015), which could serve to protect DA neurons from diverse insults. Indeed, polyphenols or polyphenol-rich foods markedly protect DA-containing neurons against toxicity both in vitro (Jin et al., 2001, Levites et al., 2002, Nie et al., 2002, Pan et al., 2003, Guo et al., 2005, Mercer et al., 2005) and in vivo (Datla et al., 2001, Levites et al., 2001, Stromberg et al., 2005, Zbarsky et al., 2005, Chaturvedi et al., 2006, Guo et al., 2007).
The inclusion of plant-based foods in the diet in conjunction with exercise may promote a healthy lifestyle. Indeed, exercise combined with a diet rich in fruits and vegetables decreases the incidence of cardiovascular disease and diabetes (Buttar et al., 2005, Khera et al., 2016, Lambert et al., 2018). A similar strategy reduced age-related cognitive dysfunction greater than exercise or diet alone (Milgram et al., 2004), and may also be an effective neuroprotective treatment strategy against Alzheimer’s disease (Pop et al., 2010, Walker et al., 2015, Zhang et al., 2016). However, this treatment strategy has not been widely examined for PD. Further, what has been reported in the literature does not lend support for this strategy as a viable neurorestorative treatment approach for PD, i.e., capable of reversing the neurodegeneration once it develops (Eshraghi-Jazi et al., 2012).
We have now examined the neuroprotective effects of voluntary running, alone and in combination with the consumption of flavonoid-rich blueberry juice (BBJ) in a unilateral intrastriatal 6-OHDA rat model of PD (Sauer and Oertel, 1994, Cohen et al., 2011). Blueberries have one of the highest antioxidant capacities of fruits and vegetables (Pellegrini et al., 2004; Huang et al., 2012). Further, a blueberry extract has shown efficacy in a 6-OHDA model of PD (Stromberg et al., 2005). The effect of these treatment modalities on protein levels of glial cell-lined derived neurotrophic factor (GDNF) was also investigated as a potential mechanism of action.
Materials and methods
Reagents
6-OHDA and all other reagents were of the highest available purity and were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless indicated otherwise. Knudsen's Just Blueberry juice (J.M. Smucker Co, Orrville, OH), consisting of BBJ concentrate and filtered water, was purchased in bulk from Vitacost and stored at 4 °C for up to 2 weeks.
Animals
Three-month old male Fischer 344/Brown Norway hybrid rats (National Institute of Aging, Bethesda, MD) were weighed at the start of the experiment and weekly thereafter. All animals were housed individually in standard cages with or without attached running wheels (Lafayette Instruments, Lafayette, IN). The wheels were 14″ in diameter and consisted of 0.0625″ stainless steel rods mounted every 0.25″ with 4.3″ wide running surfaces and free wheel resistance of < 6 g. Rats were maintained on a 12 h light/dark cycle with food (Tekland global 18% rodent diet; Harlan Laboratories, Indianapolis, IN) and drinking solution available ad libitum. Doors to the running wheels remained closed until the commencement of the experiment. All procedures were performed in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the VA Pittsburgh Healthcare System.
Experimental procedure
Animals were given a one-week period of acclimation before being randomly assigned to the following groups: control solution + sedentary (CS + SED), control solution + exercise (CS + EX), BBJ + sedentary (BBJ + SED), and BBJ + exercise (BBJ + EX) (Fig. 1). Rats continued to be maintained on the Tekland global 18% rodent diet and either BBJ (diluted with water to produce a 20% solution) or a control solution consisting of water supplemented with 1.5% sugar, the amount of sugar contained in the 20% diluted BBJ. Drinking solutions were prepared fresh daily. Fluid and food intake were recorded daily.
Fig. 1.
Experimental design and timeline of the study depicted in a flow chart.
Rats in the exercise group had unlimited access to running wheels throughout the experiment and running was continuously monitored via computer. Rats were maintained on their respective drinking solution with or without access to running wheels for four weeks prior to and four weeks after infusion of 6-OHDA. A subset of animals from each treatment group was euthanized after the initial four weeks of therapeutic intervention to assess the effects of exercise and/or BBJ intake on GDNF protein levels in the SN and striatum at the time point that 6-OHDA would be introduced.
Administration of 6-OHDA
After four weeks of exercise and/or BBJ, animals were anesthetized with isoflurane (3–4% in 100% O2 for induction and 1–2% in 100% O2 for maintenance of anesthetic plane) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). Vehicle (0.7 mg/ml ascorbic acid in 0.9% sterile saline) or 6-OHDA (6 µg/0.5 µl) in a volume of 0.5 µl was delivered intrastriatally via a 10 µl Hamilton syringe mounted on a Quintessential motorized stereotaxic injector (Stoelting Co., Wood Dale, IL) at 0.5 µl/min at the following coordinates: AP +0.7, ML -2.9 and DV -5.5. After infusion, the syringe was kept in place for an additional 5 min to allow for diffusion. The wound was then secured with surgical staples. After recovery from surgery, animals were immediately returned to their pre-surgical experimental condition for an additional four weeks.
Amphetamine-induced rotations
Amphetamine-induced rotational behavior, a measure of the loss of DA after a unilateral lesion (Przedborski et al., 1995), was assessed four weeks after 6-OHDA infusion. Animals were jacketed and tethered to a sensor attached to the top of the stainless steel rotameter bowl (Rota Count 8; Columbus Instruments, Columbus, OH). Once securely in the bowls, animals received D-amphetamine-sulfate (3 mg/kg free base, i.p.) and turning was monitored for 1 h.
Immunohistochemical analysis
Five days after assessment of amphetamine-induced rotational behavior, animals were deeply anesthetized with sodium pentobarbital (50 mg/kg, ip) and sacrificed via transcardial perfusion with chilled solutions of 0.1 M phosphate buffer (PB) and 4% paraformaldehyde (ThermoFisher Scientific, Pittsburgh, PA), each containing sodium fluoride. Brains were removed, post-fixed for 30 min in 4% paraformaldehyde followed by 30% sucrose at 4 °C until submersed, and then sectioned at 35 µm or 60 µm for SN and striatum, respectively.
Tyrosine hydroxylase terminal density in the striatum
Free-floating striatal sections were washed in 10 mM phosphate buffered saline (PBS), pH 7.6, prior to and between antibody incubations. Sections were blocked for 1 h at room temperature (RT) in a solution containing 5% bovine serum albumin, 0.1% glycine, 5% goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA), and 0.2% Triton X-100 followed by incubation in rabbit anti-tyrosine hydroxylase (TH) antibody (1:1000, # P40101, Pel Freez Biologicals, Rogers, AK) overnight on a rotator at 4 °C. After a series of 3 washes in PBS, sections were incubated with goat anti-rabbit Alexa Fluor 750-conjugated secondary antibody (1:1000, A-21039, Invitrogen, Carlsbad, CA) for 2 h at RT, washed, and then mounted on gelatin-coated slides. After sections had dried at RT, they were coverslipped with Fluoromount mounting media (Southern Biotech, Birmingham, AL). Slides were scanned using an infrared Odyssey Imager (LI-COR Biosciences, Lincoln, NE) and dorsal striatal TH density was quantified using the Odyssey software version 3.0. An average of 6 sections/animal were stained and quantified.
Tyrosine hydroxylase cell counts in the SN
Free-floating SN sections were washed as described above before incubation in primary antibodies for microtubule-associated protein 2 (MAP2) (1:2000; MAB3418, EMD Millipore, Billerica, MA) and TH (1:2000; AB1542, EMD Millipore, Billerica, MA) for 72 h at 4 °C. Subsequently, sections were washed in PBS (3 ×10 min) followed by incubation with the following secondary antibodies: Cy3-conjugated anti-sheep antibody (1:500; 713–155–147 Jackson Immunoresearch, West Grove, PA) and Alexafluor-conjugated 647 anti-mouse antibody (1:500; A31571 Invitrogen, Carlsbad, CA) for 1 h at RT. Tissue sections were then washed and incubated with Hoechst 33342 (1:5000) for nuclear staining for 5 min and after a final wash, were mounted and coverslipped using gelvatol mounting media. An automated Nikon 90i upright fluorescence microscope equipped with Q-imaging Retiga CCD camera (Nikon, Melville, NY) was used to capture images from nigral sections. Quantitative analysis was performed when TH, MAP2, and Hoechst 33342 channels colocalized (Tapias et al., 2013, Tapias et al., 2014).
GDNF protein levels in SN and striatum
After a 4-week treatment period with exercise and/or BBJ, rats were euthanized by decapitation, brains were harvested, and SN and striatum were dissected and immediately frozen on dry ice. Tissue samples were stored at -80 °C until the assessment of GDNF by Elisa. Frozen tissue samples were homogenized in lysis buffer (20X volume/weight) containing 1X Halt protease and phosphatase inhibitor cocktail (ThermoFisher Scientific, Pittsburgh, PA). Elisa kits were used to assess nigral and striatal protein levels of GDNF (#BEK-2230, Biosensis, Temecula, CA) following the manufacturer’s instructions.
Statistical analysis
Data were analyzed by Two-way or Three-way ANOVA with post-hoc Tukey multiple comparison analysis using GraphPad Prism software (v. 7.0c; La Jolla, CA). Treatment-related changes over time were assessed with repeated factorial analysis also with post hoc Tukey multiple comparison analysis. A comparison of running data was analyzed by multiple t-tests. ANOVA testing, as well as the family-wise error rate of the Tukey multiple comparison tests, and t-tests were deemed significant at the 0.05 level.
Results
Weight
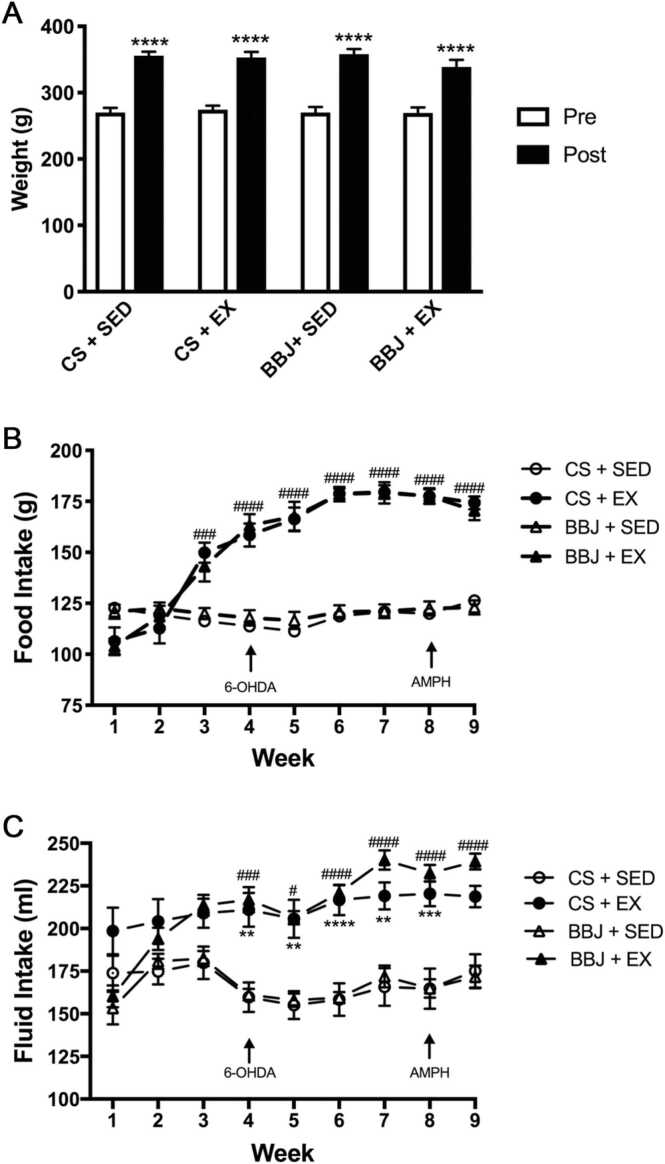
There was no significant difference in body weight among groups at any time during the experiment (Fig. 2A).
Fig. 2.
A. Exercise, BBJ consumption, or the combination did not affect body weight over the course of the experiment. Average weight of the animals measured at the beginning and the end of the experiment. Data are expressed as mean ± SEM (n = 20–24/group). **** p < 0.0001 vs. the beginning weight. B. Exercise increased weekly food intake. Average weekly food intake throughout the experimental time course. Data are expressed as mean ± SEM. ###, #### p < 0.001 and p < 0.0001, respectively CS + EX and BBJ + EX vs. sedentary counterparts; n = 20–24/group. C. Exercise increased weekly fluid intake. The weekly amount of the control solution (CS) or BBJ consumed over the course of the experiment. Data are expressed as mean ± SEM (n = 20–24/group). **, *** , **** p < 0.01, p < 0.001, and p < 0.0001, respectively CS + SED vs. CS + EX; #, ###, #### p < 0.05, p < 0.001, p < 0.0001, respectively BBJ + SED vs. BBJ + EX.
Food consumption
Food consumption in animals treated with vehicle or 6-OHDA did not differ, and as such, these animals were combined for subsequent statistical analysis. Sedentary animals consumed food at a stable rate throughout the experiment whereas the exercising animals steadily increased their food intake and surpassed the food intake of their sedentary counterparts by week 3 (Fig. 2B; n = 20 −24/group). At this time point, food consumption of exercising animals was 20–30% higher than the sedentary animals (p < 0.001). This difference in food intake further increased over time to reach a maximum of 50% at week 6 (p < 0.0001 weeks 5–9 exercise animals vs. sedentary animals). BBJ consumption did not affect food intake in either sedentary or exercising animals.
Fluid Intake
Fluid consumption did not differ between animals that received either intrastriatal infusion of vehicle or 6-OHDA; therefore within each beverage treatment group, these animals were combined for subsequent statistical analysis. The drinking solution did not influence the amount of fluid consumed as animals given BBJ consumed the same amount of fluid as their sedentary or exercising counterpart given the control solution (Fig. 2C; n = 20–24/group). In contrast, exercising animals drank approximately 35% more fluid than their sedentary counterparts and this increased consumption persisted from week 4 to the end of the experiment.
Running distance
Animals in both exercising groups steadily increased their daily running amounts over the first month of the experiment. The daily running distances of exercising animals were 7257 ± 1124 m (CS + EX) and 8280 ± 1220 m (BBJ ± EX) by day 28, the day before vehicle or 6-OHDA infusion (Fig. 3A; n = 20–24/group). The evening after surgery, the amount of running in both groups decreased by 30–40% compared to pre-surgical distances. However, within 3–4 days post-surgery, animals from each group had reached and were maintaining their pre-surgical running distances. There was no difference in running distance between the two groups at any time point.
Fig. 3.
A. BBJ consumption had no effect on running distance. The time course of distance ran in meters (m) by exercising animals given either CS or BBJ. Data are expressed as mean ± SEM (n = 20–24/group). B. BBJ potentiated the exercised-induced reduction in amphetamine rotations. The number of rotations in one hour after intraperitoneal amphetamine injection. Data are expressed as mean ± SEM. **** p < 0.0001 vs. vehicle treated counterpart. ##, #### p < 0.01 and 0.0001 respectively vs. sedentary counterpart. $ p < 0.05 vs. exercising animals given the CS; n = 10–12/group.
Amphetamine-induced rotational behavior
Animals that received vehicle infusion into the striatum did not rotate in response to amphetamine (Fig. 3B; 10–12/group). Four weeks after unilateral intrastriatal injection of 6-OHDA, the lesioned sedentary animals given either CS or BBJ rotated 520 ± 55 and 558 ± 75 times/hour in response to amphetamine, respectively, which is in line with previous studies using the striatal 6-OHDA infusion model (Ahmad et al., 2005, Yuan et al., 2005, Bagga et al., 2015). There was no significant difference in the number of rotations between these two groups. Exercise alone significantly decreased the number of amphetamine-induced rotations by 42% down to 302.3 ± 47.8 rotations/hour in animals given the CS compared to their sedentary counterpart (p < 0.01). The combined effect of exercise and BBJ significantly decreased the number of amphetamine-induced rotations by 80% down to 130 ± 29.4 rotations/hour compared to sedentary animals given BBJ. Notably, it decreased the number of rotations by 66% compared to exercising animals given the control solution (p < 0.05).
Striatal TH immunoreactive fiber density
TH striatal density, used as a measure of the integrity of the nigrostriatal pathway, was reduced to 27.5 ± 1.7% by 6-OHDA in sedentary animals that received the control solution compared to vehicle-treated control animals (Fig. 4A-B; n = 10–12/group). Quantitative analysis showed that neither BBJ consumption nor exercise alone attenuated the loss of striatal TH projections induced by 6-OHDA. Nevertheless, when both strategies were used in combination, striatal TH density was increased to 45.7 ± 3.8% of vehicle-treated control animals. This change reflected a 66% increase compared to 6-OHDA-treated sedentary animals maintained on the control solution (Fig. 4B; p < 0.05).
Fig. 4.
Combined treatment with exercise and BBJ partially preserved striatal TH. A. Representative immunofluorescent images displaying striatal TH loss in CS + SED, CS + EX, BBJ + SED, and BBJ + EX treated rats. B. The percentage of TH-immunoreactivity (TH-ir) remaining in the striatum after treatment with the CS or BBJ with or without exercise. Data are expressed as mean ± SEM (n = 10–12/group) and are represented as a percentage of vehicle control animals. (* p < 0.05 vs. CS + SED).
Tyrosine hydroxylase cell counts in the SN
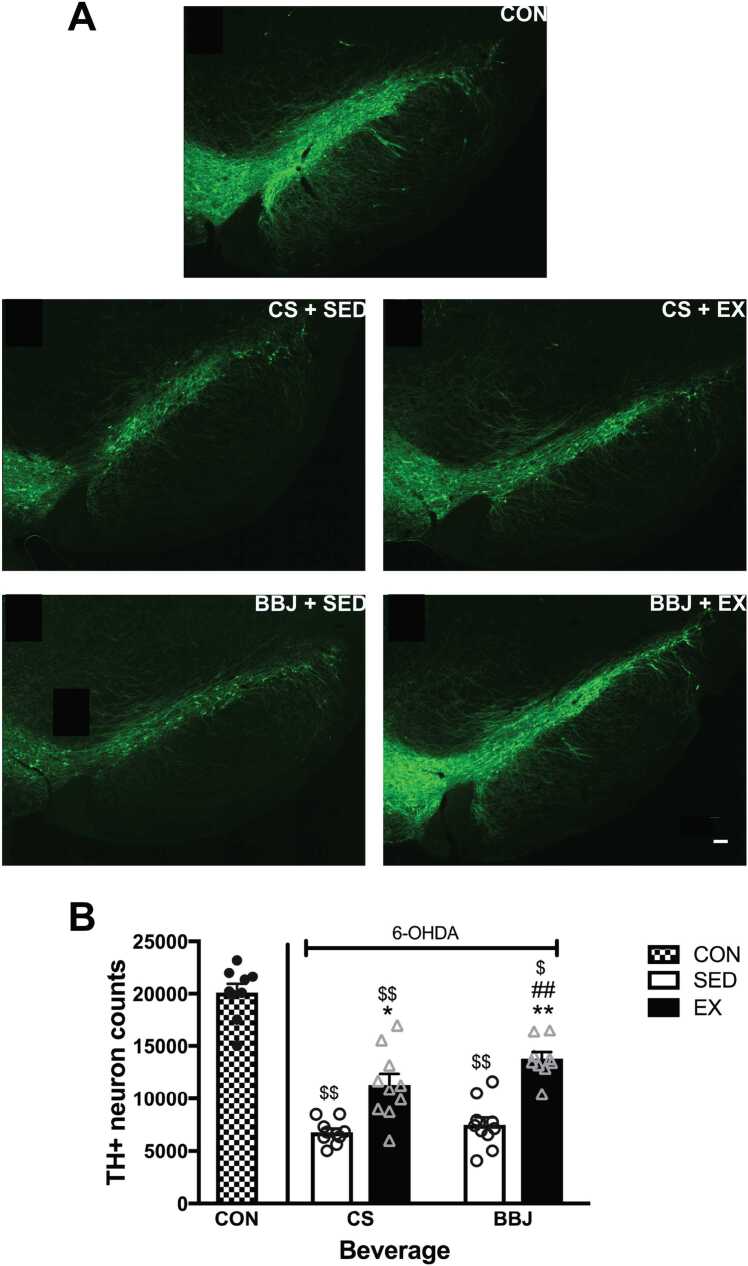
To further evaluate the level of neuroprotection, the number of nigral DA neurons was estimated in all treatment groups. Fluorescent images depicted a robust loss of DA neurons in the SN following 6-OHDA and significant protection mediated by exercise alone and exercise combined with BBJ (Fig. 5A-B; n = 10–12/group). The quantitative assessment of TH+ nigral neurons demonstrated that intrastriatal 6-OHDA reduced the number of nigral TH+ cells by 66% in sedentary animals given CS in comparison to vehicle-treated controls (20,098 ± 1804 vs. 6794 ± 472 neurons; p < 0.0001) (Fig. 5B). Importantly, exercise significantly increased the number of TH+ cells by 66% when compared to sedentary rats given the CS (6794 ± 472 vs. 11,290 ± 1327 neurons; p < 0.05). In contrast, BBJ intake did not attenuate 6-OHDA-induced cell death, as the number of TH+ neurons in this group (7424 ± 1221) was not significantly different from sedentary animals maintained on the CS. Combined treatment with exercise and BBJ significantly reduced the loss of TH+ neurons (13,486 ± 961) by 81% when compared to 6-OHDA-treated sedentary animals given the BBJ (p < 0.01) and by 99% when compared to 6-OHDA-treated sedentary animals that drank the CS (p < 0.01). However, combined treatment with BBJ and exercise was not significantly different from exercise alone.
Fig. 5.
Nigral TH+cells were partially spared by exercise with and without BBJ. A. Representative fluorescent images of SN stained for TH in vehicle treated control (CON), CS + SED, CS + EX, BBJ + SED, and BBJ + EX treated animals. B. TH+ nigral cell counts measured in animals given CS or BB juice with or without access to a running wheel. Vehicle-injected animals that served as control had 20,098 ± 1804 cells in the SN. Exercise alone and combined with BBJ attenuated the loss of SN TH+ cells to the same extent. BBJ alone did not affect 6-OHDA-induced TH+ cell loss in the SN. Data are expressed as mean ± SEM (n = 10–12/group). * and ** p < 0.05 and 0.01 vs. sedentary counterpart. ## p < 0.01 vs. CS + SED. $ and $$ p < 0.001 and 0.0001 vs. CON. Scale bar = 100 µm.
GDNF protein expression in SN and STR
We measured GDNF levels to ascertain if increases in this neurotrophic factor were associated with the protection elicited by voluntary running and if the greater protection observed with combined treatment of exercise and BBJ resulted in a more substantial increase in GDNF. GDNF protein levels in the SN were not significantly altered by either BBJ consumption or voluntary wheel running alone (Fig. 6A; n = 7–8/group). However, when combined with exercise, BBJ increased the levels of GDNF in the SN compared to sedentary animals maintained on the CS by 141.7% (28.7 ± 4.2 vs. 69.4 ± 6.6 pg/ml; p < 0.05). Striatal GDNF increased by 48% in exercising animals that drank the CS compared to their sedentary counterparts (Fig. 6B; p < 0.01; n = 7–8/group). In contrast, GDNF levels were decreased by 45% in the rat striatum in response to BBJ as compared to the CS-drinking sedentary animals ( p < 0.01). BBJ intake prevented the exercise-induced increase in striatal GDNF such that GDNF levels in exercising animals that received BBJ was significantly lower than their exercising CS counterpart ( p < 0.01).
Fig. 6.
A. Nigral GDNF was enhanced with combined treatment with exercise and BBJ. The effect of exercise and/or BBJ on GDNF protein levels in the SN. Data are expressed as mean ± SEM (n = 7–8/group). * p < 0.05 vs. CS + SED. B. Striatal GDNF was increased with exercise but decreased with BBJ consumption.. The effect of exercise and/or BBJ on GDNF protein levels in the striatum. Data are expressed as mean ± SEM (n = 7–8/group). ** p < 0.01 vs. CS counterpart. ## p < 0.01 vs. sedentary counterpart.
Discussion
We sought to examine whether voluntary running and BBJ, when used alone or in combination, could mitigate the damage to the nigrostriatal pathway induced by striatal 6-OHDA infusion. Our findings show that voluntary exercise alone improved 6-OHDA-induced behavioral deficits and attenuated the loss of DA neurons in the SN without affecting DA terminal loss in the striatum. BBJ consumption alone did not protect against 6-OHDA toxicity as assessed behaviorally or immunohistochemically. However, BBJ consumption together with exercise resulted in a greater reduction in amphetamine-induced rotational behavior when compared to exercise alone and significantly protected against the loss of striatal DA terminals. Combined treatment also attenuated 6-OHDA-induced DA cell death; however, this reduction was not significantly greater than exercise alone.
Studies have consistently reported that exercise is beneficial against behavioral deficits induced by 6-OHDA lesions (Cohen et al., 2003, Mabandla et al., 2004, Moroz et al., 2004, O'Dell et al., 2007, Aguiar et al., 2013, Landers et al., 2013). In agreement with these studies, we observed that voluntary wheel running for four weeks before and following 6-OHDA administration decreased substantially the rotational behavior induced by amphetamine. We also found that exercise attenuated the loss of DA neurons in the SN without affecting 6-OHDA-induced TH terminal loss in the striatum, similar to results observed with voluntary exercise-induced protection against MPTP (Gerecke et al., 2010), and acute toxicity induced by 6-OHDA infusion into the medial forebrain bundle (Mabandla et al., 2009, Tsai et al., 2019). Behavioral protection in the absence of preservation of TH immunoreactivity in the striatum may indicate that the phenotypic loss of TH in the striatum does not accurately depict the state of dopaminergic neurotransmission within the striatum. Indeed, we have previously shown with GDNF-induced protection against 6-OHDA toxicity that at a time when the striatum was showing a significant loss of TH immunoreactivity, striatal DA content was normal (Cohen et al., 2011), suggesting the possibility that remaining terminals compensated for this loss of TH and maintained DA levels, hence dopaminergic neurotransmission. Alternatively, behavioral improvement could be driven by protection of TH immunoreactive nigral neurons. Inhibition of TH activity specifically in the SN caused motor deficits in the absence of any effects on striatal DA levels (Salvatore et al., 2019), and caloric restriction-related protection has been shown to correlate with increased protein levels of nigral TH and DA content while decreasing striatal TH protein and DA levels (Salvatore et al., 2017). Thus, partial preservation of nigral TH-immunoreactive cells may have played a pivotal role in attenuating amphetamine-induced rotations observed with exercise in the current study.
Interestingly, despite the consistent observance of an amelioration of behavioral deficits with voluntary exercise (Mabandla et al., 2004, Moroz et al., 2004, Howells et al., 2005, O'Dell et al., 2007, Mabandla et al., 2009, Aguiar et al., 2013, Landers et al., 2013, Hsueh et al., 2018, Tsai et al., 2019), only a few studies have reported attenuation of damage to the dopaminergic nigrostriatal pathway (Mabandla et al., 2009, Tsai et al., 2019), as most of these studies either did not assess the integrity of the dopaminergic nigrostriatal pathway after 6-OHDA toxicity (Mabandla et al., 2004, Landers et al., 2013) or reported negative results (Moroz et al., 2004; Howells et al., 2005; O’Dell et al., 2007; Aguiar et al., 2013; Hsueh et al., 2018). The two studies that observed partial protection of nigral cell counts utilized an acute model of 6-OHDA toxicity in which the toxin was infused into the medial forebrain bundle. To our knowledge, the neuroprotective benefit of voluntary exercise on the nigrostriatal pathway in a progressive 6-OHDA rat model of PD (i.e. striatal infusion of 6-OHDA followed by protracted loss of DA cells in SN) has not been previously reported. Instead, improvements in behavioral performance in these other studies employing voluntary exercise as a therapeutic intervention in a progressive 6-OHDA model could be attributed to an enhancement of compensatory mechanisms known to ensue in this DA system upon the occurrence of degeneration less than 90–95% (Zigmond et al., 1984, O'Dell et al., 2007). In contrast, evidence suggests that different types of forced exercise, such as treadmill running and forced limb use, protect against progressive 6-OHDA-induced nigrostriatal damage (Yoon et al., 2007, Tajiri et al., 2010, Aguiar et al., 2016b). We surmised from a detailed examination of these published studies that vigorous exercise that occurs within seven days of a toxic insult consistently confers protection against degeneration of DA neurons and/or terminals. Indeed, the most potent “therapeutic window” for exercise appears to be within 72 h of toxin infusion (Tillerson et al., 2001). In studies that failed to observe protection of DA neurons and/or terminals with voluntary running, exercise commenced outside the 72 h critical window (Aguiar et al., 2013) or animals failed to maintain a high level of running (i.e., ran < 1500 m/day) in the initial days after 6-OHDA infusion (O'Dell et al., 2007). In our experiment, rats ran on average 5000 m/day during the initial period after 6-OHDA infusion. The underlying reason for differences in post-6-OHDA running between our study and O′Dell et al., (2007) could relate to the strain of rats used, the number of 6-OHDA injection sites, or the length of the pre-lesion running period. A relationship between the amount of running and protection of DA cells against MPTP toxicity has been previously described (Gerecke et al., 2010). Thus, the maintenance of this higher level of voluntary running within the therapeutic window of 72 h post-6-OHDA infusion may have played a pivotal role in the protection against the death of nigral DA neurons induced by 6-OHDA in the current study. Together, these studies suggest that a threshold level of running must be attained around the time of neurotoxic insult to reduce damage to the nigrostriatal pathway.
BBJ consumption alone had no ameliorative effects on amphetamine-induced rotational behavior, nor did it lessen the impact of 6-OHDA on DA neurons and terminals in our study. Numerous studies have reported that administration of either single or complex mixtures of polyphenols (i.e. extracts or juices) derived from plant-based foods attenuates damage incurred from 6-OHDA and other DA neurotoxins (Stromberg et al., 2005, Chaturvedi et al., 2006, Guo et al., 2007, Jin et al., 2008, Eshraghi-Jazi et al., 2012). In most of these studies, however, animals were given a higher percentage of juice (e.g., 50% juice) than that given in the current study (20%), high doses of single polyphenols were administered, or animals received concentrated mixtures of fruit or tea extract. Twenty percent BBJ may contain lower concentrations of protective polyphenols than extracts, and this could have ultimately resulted in smaller amounts of polyphenols reaching relevant areas of the brain (i.e., striatum and SN).
Despite the lack of protection observed with BBJ alone, when exercise and BBJ consumption were combined, the number of amphetamine-induced rotations was decreased greater than exercise alone, and there was partial preservation of the dopaminergic nigrostriatal pathway at the terminal and cell body level. Wheel running is known to increase intake of food and water (Afonso & Eikelboom, 2003), and 3–4 wks into the experiment, exercising animals were consuming significantly higher amounts of fluid than their sedentary counterparts. Thus, exercising animals maintained on the BBJ in effect received a higher “dose” of BBJ and its accompanying polyphenols than sedentary animals maintained on BBJ, as there was no difference in body weight across groups. Moreover, exercise may have altered the bioavailability of the pertinent phytochemical(s) in the BBJ through changes in absorption, metabolism, elimination (Piatkowski et al., 1993, Medina et al., 2012), and/or blood-brain barrier permeability (Sharma et al., 1991, Watson et al., 2005, Bailey et al., 2011), which may have resulted in higher concentrations of polyphenols reaching relevant areas in the exercising animals. Consumption of a 50% grape solution, which is higher than the 20% BBJ solution used in the present study, reduced amphetamine-induced rotations after nigral 6-OHDA infusion (Eshraghi-Jazi et al., 2012), therefore, the greater protection observed in exercising animals that consumed BBJ may have been partially mediated by increased consumption and/or enhanced bioavailability of polyphenols contained within the BBJ.
Although 50% grape juice alone attenuated 6-OHDA-induced behavioral deficits after nigral infusion of 6-OHDA (Eshraghi-Jazi et al., 2012), the combined effect of treadmill running and consumption of flavonoid-rich grape juice in that study did not result in greater attenuation of behavioral deficits versus individual treatment. In that study, the intervention was instituted two weeks after 6-OHDA infusion into the SN, which causes an acute and fast DA deficiency. The larger lesion, the temporal relation between the intervention and 6-OHDA treatment, as well as the site of infusion, may underlie the different behavioral outcomes observed between our study and Eshraghi-Jazi et al. (Eshraghi-Jazi et al., 2012). Moreover, whereas we used voluntary running in an exercise wheel, these authors used forced running on a treadmill and this may have introduced a stressful component. Indeed, stress was shown to negate running-induced protection against 6-OHDA-induced behavioral deficits (Howells et al., 2005) and to potentiate 6-OHDA related decreases in striatal GDNF and neurotrophin-3 (Ngema & Mabandla, 2017). Additional studies are needed to examine the efficacy of combined intervention when administered after degeneration of the nigrostriatal pathway has commenced.
The mechanism by which exercise protects in PD models has been postulated to involve regulation of neurotrophic factor signaling (Cohen et al., 2003, Tajiri et al., 2010, Lau et al., 2011, Churchill et al., 2017). Similar mechanisms are reported to underlie the beneficial effects of polyphenols (Leem et al., 2014, Patil et al., 2014). We previously showed that the protection against 6-OHDA afforded by forced exercise via unilateral forelimb casting correlated with an increase in striatal GDNF protein levels (Cohen et al., 2011). In the current study, we observed an increase in striatal GDNF in response to running, consistent with previous studies (Tajiri et al., 2010). However, combined treatment with BBJ and running did not elicit a greater GDNF increase in the striatum. In fact, GDNF with combined treatment was significantly lower than running alone and was no different than sedentary animals that drank the control solution. Lower striatal GDNF in the combined treatment group compared to running alone may have been driven by the unexpected decrease in striatal GDNF caused by BBJ intake alone, which was countered by concurrent treatment with exercise. The reason for this decrease is unknown but may be related to the potential pro-oxidant capacity of polyphenols contained within the BBJ. Indeed, Tapias et al. (Tapias et al., 2014) demonstrated that pomegranate juice, another polyphenol-rich beverage, exacerbated rotenone toxicity, resulting in increased oxidative stress, inflammatory responses, caspase activation, and nigrostriatal DA degeneration. Thus the pro-oxidant activity of polyphenols within BBJ could potentially underlie the lack of protection against 6-OHDA observed with BBJ alone in the current study.
Our observed decrease in GDNF with BBJ contrasts with findings in the hippocampus, where administration of polyphenols or polyphenol-rich foods increased neurotrophic factors, particularly BDNF (Li et al., 2009, Rahvar et al., 2011, Stringer et al., 2015). However, diminished striatal BDNF and NGF levels have been reported in response to polyphenols derived from olives (De Nicolo et al., 2013, Carito et al., 2014), suggesting that regulation of neurotrophic signaling in response to polyphenols may be brain region and polyphenol specific. Although, combined treatment with BBJ and exercise did not augment the running-associated increase in striatal GDNF, nigral levels were significantly enhanced only in those rats subjected to both BBJ and voluntary running. Both exercise and BBJ alone resulted in non-significant increases in nigral GDNF. Combined treatment may have therefore resulted in a cumulative significant increase in nigral GDNF resulting in more nigral neurons maintaining connections with, and thereby stabilizing, more striatal TH terminals than that observed with exercise alone. A similar phenomenon was observed with infusion of the gdnf gene via viral delivery into the SN compared to striatal gdnf gene infusion (Kozlowski et al., 2000), where more connections were preserved between the striatum and SN with nigral gdnf infusion after 6-OHDA infusion that correlated with enhanced neurotransmission in the striatum (Smith et al., 2005). Thus, the enhanced protection of the nigrostriatal pathway afforded by BBJ consumption combined with exercise may have involved increased signaling through the GDNF signaling pathway within the SN.
Few studies have examined the beneficial effects of combined treatment with a polyphenol-rich diet and exercise in a PD model, much less the mechanism by which such benefits may be exerted. Enhancement of neurotrophic signaling is an overlapping theme in the interaction between polyphenols and exercise (Fahnestock et al., 2012, Joseph et al., 2012, Zhang et al., 2016). This augmentation correlates with a decrease in oxidative stress (Opii et al., 2008) and pro-death signaling (Snigdha et al., 2011). Indeed, epicatechin combined with exercise in a mouse model of Alzheimer’s disease potentiated BDNF levels and downstream signaling targets in association with an improvement in cognitive deficits (Zhang et al., 2016). In a cerebral ischemia model, increased protection with concurrent treatment with exercise and resveratrol or quercetin, two polyphenolic constituents of BBJ, was associated with enhanced signaling via the BDNF/TrkB pathway (Shi et al., 2016), and augmentation of pro-survival signaling molecules (Chang et al., 2014). Our results suggest a similar potentiation of pro-survival signaling may be involved in the greater protection observed with exercise and BBJ intake in our PD model.
Epidemiological data suggests that exercise or diets rich in polyphenols reduce the risk of developing PD (Tsai et al., 2002, Chen et al., 2005, Gao et al., 2007, Gao et al., 2012) and may be an adjunct symptomatic treatment for PD patients. Our results raise the possibility that combined use of exercise and consumption of polyphenol-rich foods may impart neuroprotective benefits greater than either of these treatments alone. Rats in the current experiment exercised vigorously, and although it has been inferred that such intense exercise is needed for protection (Gerecke et al., 2010), whether such intense exercise is also needed if it is paired with intake of a polyphenol-rich diet is unknown. If lower levels of exercise paired with intake of polyphenol-rich foods can impart greater benefit, this would be of particular clinical significance as those individuals who are unable or unwilling to perform exercise at such a high level could still reap the benefits of this combined therapy. Additional studies examining different “doses” of exercise with polyphenol intake are therefore needed. As PD is an age-related neurological disorder that affects motor function, it will also be clinically relevant to access whether similar results can be achieved with exercise and a polyphenol-rich diet in older animals as they are known to run significantly less than their younger counterparts. It will also be important to assess if combined intervention is beneficial when instituted after damage has already occurred. Whereas some studies have shown that the motor and non-motor symptoms of PD can be attenuated with moderate to intense exercise in PD patients (Schenkman et al., 2018, Tollár et al., 2019, van der Kolk et al., 2019), the ability of patients to maintain these high levels of exercise as the disease progresses will wane. If concurrent maintenance on a polyphenol-rich diet can potentiate the effects of these lower levels of exercise on symptom relief, even in the absence of effects on the underlying pathology, the benefits of exercise may be extended into the latter stages of the disease.
In conclusion, we show that voluntary running ameliorated behavioral impairment and nigral DA neuronal death induced by 6-OHDA. BBJ consumption per se did not attenuate nigrostriatal damage or behavioral deficits in 6-OHDA-treated animals but potentiated the effects of exercise by improving behavioral performance and reducing the loss of striatal DA terminals concomitant with partial preservation of nigral DA neurons. Exercise or the consumption of polyphenol-rich foods have both separately been advanced as beneficial to PD patients (Tsai et al., 2002, Chen et al., 2005, Gao et al., 2007, Gao et al., 2012). The present data suggest that maintaining a polyphenol-rich diet concurrent with exercise may prove more beneficial than either treatment alone, and thus may be a viable neuroprotective strategy against PD.
CRediT authorship contribution statement
Sandra L. Castro: Investigation, Writing – original draft. Victor Tapias: Investigation, Writing – original draft, Visualization. Ronald Gathagan: Investigation. Alexander Emes: Investigation. Taylor E. Brandon: Investigation, , Writing – original draft, Writing – review & editing. Amanda D. Smith: Conceptualization, Methodology, Validation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors have no conflict of interests to disclose.
Acknowledgments
This work was supported by the National Institute of Health (ADS: NS45698) and the Department of Veterans Affairs (ADS: B6612R). The funding source was not involved in any aspect of this study.
References
- Afonso V.M., Eikelboom R. Relationship between wheel running, feeding, drinking, and body weight in male rats. Physiol. Behav. 2003;80:19–26. doi: 10.1016/s0031-9384(03)00216-6. [DOI] [PubMed] [Google Scholar]
- Aguiar A.S., Jr., Duzzioni M., Remor A.P., Tristao F.S., Matheus F.C., Raisman-Vozari R., Latini A., Prediger R.D. Moderate-intensity physical exercise protects against experimental 6-hydroxydopamine-induced hemiparkinsonism through Nrf2-antioxidant response element pathway. Neurochem. Res. 2016;41:64–72. doi: 10.1007/s11064-015-1709-8. [DOI] [PubMed] [Google Scholar]
- Aguiar A.S., Jr., Lopes S.C., Tristao F.S., Rial D., de Oliveira G., da Cunha C., Raisman-Vozari R., Prediger R.D. Exercise Improves Cognitive Impairment and Dopamine Metabolism in MPTP-Treated Mice. Neurotox. Res. 2016;29:118–125. doi: 10.1007/s12640-015-9566-4. [DOI] [PubMed] [Google Scholar]
- Aguiar A.S., Jr., Moreira E.L., Hoeller A.A., Oliveira P.A., Cordova F.M., Glaser V., Walz R., Cunha R.A., Leal R.B., Latini A., Prediger R.D. Exercise attenuates levodopa-induced dyskinesia in 6-hydroxydopamine-lesioned mice. Neuroscience. 2013;243:46–53. doi: 10.1016/j.neuroscience.2013.03.039. [DOI] [PubMed] [Google Scholar]
- Ahmad M., Saleem S., Ahmad A.S., Ansari M.A., Yousuf S., Hoda M.N., Islam F. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Hum. Exp. Toxicol. 2005;24:137–147. doi: 10.1191/0960327105ht509oa. [DOI] [PubMed] [Google Scholar]
- Archer T., Fredriksson A. Physical exercise attenuates MPTP-induced deficits in mice. Neurotox. Res. 2010;18:313–327. doi: 10.1007/s12640-010-9168-0. [DOI] [PubMed] [Google Scholar]
- Bagga V., Dunnett S.B., Fricker R.A. The 6-OHDA mouse model of Parkinson’s disease - Terminal striatal lesions provide a superior measure of neuronal loss and replacement than median forebrain bundle lesions. Behav. Brain Res. 2015;288:107–117. doi: 10.1016/j.bbr.2015.03.058. [DOI] [PubMed] [Google Scholar]
- Bailey D.M., Evans K.A., McEneny J., Young I.S., Hullin D.A., James P.E., Ogoh S., Ainslie P.N., Lucchesi C., Rockenbauer A., Culcasi M., Pietri S. Exercise-induced oxidative-nitrosative stress is associated with impaired dynamic cerebral autoregulation and blood-brain barrier leakage. Exp. Physiol. 2011;96:1196–1207. doi: 10.1113/expphysiol.2011.060178. [DOI] [PubMed] [Google Scholar]
- Buttar H.S., Li T., Ravi N. Prevention of cardiovascular diseases: role of exercise, dietary interventions, obesity and smoking cessation. Exp. Clin. Cardiol. 2005;10:229–249. [PMC free article] [PubMed] [Google Scholar]
- Carito V., Venditti A., Bianco A., Ceccanti M., Serrilli A.M., Chaldakov G., Tarani L., De Nicolo S., Fiore M. Effects of olive leaf polyphenols on male mouse brain NGF, BDNF and their receptors TrkA, TrkB and p75. Nat. Prod. Res. 2014;28:1970–1984. doi: 10.1080/14786419.2014.918977. [DOI] [PubMed] [Google Scholar]
- Cassidy A., Rogers G., Peterson J.J., Dwyer J.T., Lin H., Jacques P.F. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults. Am. J. Clin. Nutr. 2015;102:172–181. doi: 10.3945/ajcn.115.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.C., Yang Y.R., Wang P.S., Wang R.Y. Quercetin enhances exercise-mediated neuroprotective effects in brain ischemic rats. Med. Sci. Sports Exerc. 2014;46:1908–1916. doi: 10.1249/MSS.0000000000000310. [DOI] [PubMed] [Google Scholar]
- Chaturvedi R.K., Shukla S., Seth K., Chauhan S., Sinha C., Shukla Y., Agrawal A.K. Neuroprotective and neurorescue effect of black tea extract in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Neurobiol. Dis. 2006;22:421–434. doi: 10.1016/j.nbd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Chen H., Zhang S.M., Schwarzschild M.A., Hernan M.A., Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–669. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- Churchill M.J., Pflibsen L., Sconce M.D., Moore C., Kim K., Meshul C.K. Exercise in an animal model of Parkinson's disease: Motor recovery but not restoration of the nigrostriatal pathway. Neuroscience. 2017;359:224–247. doi: 10.1016/j.neuroscience.2017.07.031. [DOI] [PubMed] [Google Scholar]
- Cohen A.D., Tillerson J.L., Smith A.D., Schallert T., Zigmond M.J. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J. Neurochem. 2003;85:299–305. doi: 10.1046/j.1471-4159.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- Cohen A.D., Zigmond M.J., Smith A.D. Effects of intrastriatal GDNF on the response of dopamine neurons to 6-hydroxydopamine: time course of protection and neurorestoration. Brain Res. 2011;1370:80–88. doi: 10.1016/j.brainres.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva F.C., Iop R.D.R., de Oliveira L.C., Boll A.M., de Alvarenga J.G.S., Gutierres Filho P.J.B., de Melo L., Xavier A.J., da Silva R. Effects of physical exercise programs on cognitive function in Parkinson's disease patients: A systematic review of randomized controlled trials of the last 10 years. PloS one. 2018;13 doi: 10.1371/journal.pone.0193113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datla K.P., Christidou M., Widmer W.W., Rooprai H.K., Dexter D.T. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson's disease. Neuroreport. 2001;12:3871–3875. doi: 10.1097/00001756-200112040-00053. [DOI] [PubMed] [Google Scholar]
- De Nicolo S., Tarani L., Ceccanti M., Maldini M., Natella F., Vania A., Chaldakov G.N., Fiore M. Effects of olive polyphenols administration on nerve growth factor and brain-derived neurotrophic factor in the mouse brain. Nutrition. 2013;29:681–687. doi: 10.1016/j.nut.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Dutra M.F., Jaeger M., Ilha J., Kalil-Gaspar P.I., Marcuzzo S., Achaval M. Exercise improves motor deficits and alters striatal GFAP expression in a 6-OHDA-induced rat model of Parkinson’s disease. Neurol. Sci. 2012;33:1137–1144. doi: 10.1007/s10072-011-0925-5. [DOI] [PubMed] [Google Scholar]
- Eshraghi-Jazi F., Alaei H., Azizi-Malekabadi H., Gharavi-Naini M., Pilehvarian A., Ciahmard Z. The effect of red grape juice and exercise, and their combination on parkinson(’)s disease in rats. Avicenna J. Phytomed. 2012;2:90–96. [PMC free article] [PubMed] [Google Scholar]
- Fahnestock M., Marchese M., Head E., Pop V., Michalski B., Milgram W.N., Cotman C.W. BDNF increases with behavioral enrichment and an antioxidant diet in the aged dog. Neurobiol. Aging. 2012;33:546–554. doi: 10.1016/j.neurobiolaging.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B.E., Petzinger G.M., Nixon K., Hogg E., Bremmer S., Meshul C.K., Jakowec M.W. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci. Res. 2004;77:378–390. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- Gao X., Cassidy A., Schwarzschild M.A., Rimm E.B., Ascherio A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology. 2012;78:1138–1145. doi: 10.1212/WNL.0b013e31824f7fc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Chen H., Fung T.T., Logroscino G., Schwarzschild M.A., Hu F.B., Ascherio A. Prospective study of dietary pattern and risk of Parkinson disease. Am. J. Clin. Nutr. 2007;86:1486–1494. doi: 10.1093/ajcn/86.5.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerecke K.M., Jiao Y., Pani A., Pagala V., Smeyne R.J. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res. 2010;1341:72–83. doi: 10.1016/j.brainres.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin V.A., Richards S.H., Taylor R.S., Taylor A.H., Campbell J.L. The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 2008;23:631–640. doi: 10.1002/mds.21922. [DOI] [PubMed] [Google Scholar]
- Guo S., Bezard E., Zhao B. Protective effect of green tea polyphenols on the SH-SY5Y cells against 6-OHDA induced apoptosis through ROS-NO pathway. Free Radical Biol. Med. 2005;39:682–695. doi: 10.1016/j.freeradbiomed.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Guo S., Yan J., Yang T., Yang X., Bezard E., Zhao B. Protective effects of green tea polyphenols in the 6-OHDA rat model of Parkinson’s disease through inhibition of ROS-NO pathway. Biol. Psychiatr. 2007;62:1353–1362. doi: 10.1016/j.biopsych.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. The discovery of dopamine deficiency in the parkinsonian brain. J. Neural Transm. Suppl. 2006:9–15. doi: 10.1007/978-3-211-45295-0_3. [DOI] [PubMed] [Google Scholar]
- Howells F.M., Russell V.A., Mabandla M.V., Kellaway L.A. Stress reduces the neuroprotective effect of exercise in a rat model for Parkinson’s disease. Behav. Brain Res. 2005;165:210–220. doi: 10.1016/j.bbr.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Hsueh S.C., Chen K.Y., Lai J.H., Wu C.C., Yu Y.W., Luo Y., Hsieh T.H., Chiang Y.H. Voluntary Physical Exercise Improves Subsequent Motor and Cognitive Impairments in a Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2018:19. doi: 10.3390/ijms19020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.Y., Zhang H.C., Liu W.X., Li C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B. 2012;13:94–102. doi: 10.1631/jzus.B1100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C.F., Shen S.R., Sr., Zhao B.L. Different effects of five catechins on 6-hydroxydopamine-induced apoptosis in PC12 cells. J. Agric. Food Chem. 2001;49:6033–6038. doi: 10.1021/jf010903r. [DOI] [PubMed] [Google Scholar]
- Jin F., Wu Q., Lu Y.F., Gong Q.H., Shi J.S. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur. J. Pharmacol. 2008;600:78–82. doi: 10.1016/j.ejphar.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Joseph J.A., Shukitt-Hale B., Willis L.M. Grape juice, berries, and walnuts affect brain aging and behavior. J. Nutr. 2009;139:1813s–1817s. doi: 10.3945/jn.109.108266. [DOI] [PubMed] [Google Scholar]
- Joseph M.S., Ying Z., Zhuang Y., Zhong H., Wu A., Bhatia H.S., Cruz R., Tillakaratne N.J., Roy R.R., Edgerton V.R., Gomez-Pinilla F. Effects of diet and/or exercise in enhancing spinal cord sensorimotor learning. PloS one. 2012;7 doi: 10.1371/journal.pone.0041288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey N.A., Wilkins H.M., Linseman D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. 2010;15:7792–7814. doi: 10.3390/molecules15117792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera A.V., Emdin C.A., Drake I., Natarajan P., Bick A.G., Cook N.R., Chasman D.I., Baber U., Mehran R., Rader D.J., Fuster V., Boerwinkle E., Melander O., Orho-Melander M., Ridker P.M., Kathiresan S. Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. New Engl. J. Med. 2016;375:2349–2358. doi: 10.1056/NEJMoa1605086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski D.A., Connor B., Tillerson J.L., Schallert T., Bohn M.C. Delivery of a GDNF gene into the substantia nigra after a progressive 6-OHDA lesion maintains functional nigrostriatal connections. Exp. Neurol. 2000;166:1–15. doi: 10.1006/exnr.2000.7463. [DOI] [PubMed] [Google Scholar]
- Krikorian R., Nash T.A., Shidler M.D., Shukitt-Hale B., Joseph J.A. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Brit. J. Nutr. 2010;103:730–734. doi: 10.1017/S0007114509992364. [DOI] [PubMed] [Google Scholar]
- Krikorian R., Shidler M.D., Nash T.A., Kalt W., Vinqvist-Tymchuk M.R., Shukitt-Hale B., Joseph J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010;58:3996–4000. doi: 10.1021/jf9029332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert K., Hokayem M., Thomas C., Fabre O., Cassan C., Bourret A., Bernex F., Feuillet-Coudray C., Notarnicola C., Mercier J., Avignon A., Bisbal C. Combination of nutritional polyphenols supplementation with exercise training counteracts insulin resistance and improves endurance in high-fat diet-induced obese rats. Sci. Rep. 2018;8:2885. doi: 10.1038/s41598-018-21287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers M.R., Kinney J.W., Allen D.N., van Breukelen F. A comparison of voluntary and forced exercise in protecting against behavioral asymmetry in a juvenile hemiparkinsonian rat model. Behav. Brain Res. 2013;248:121–128. doi: 10.1016/j.bbr.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Lau Y.S., Patki G., Das-Panja K., Le W.D., Ahmad S.O. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. Eur. J. Neurosci. 2011;33:1264–1274. doi: 10.1111/j.1460-9568.2011.07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem E., Nam J.H., Jeon M.T., Shin W.H., Won S.Y., Park S.J., Choi M.S., Jin B.K., Jung U.J., Kim S.R. Naringin protects the nigrostriatal dopaminergic projection through induction of GDNF in a neurotoxin model of Parkinson’s disease. J. Nutr. Biochem. 2014;25:801–806. doi: 10.1016/j.jnutbio.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Levites Y., Weinreb O., Maor G., Youdim M.B., Mandel S. Green tea polyphenol (-)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J. Neurochem. 2001;78:1073–1082. doi: 10.1046/j.1471-4159.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- Levites Y., Youdim M.B., Maor G., Mandel S. Attenuation of 6-hydroxydopamine (6-OHDA)-induced nuclear factor-kappaB (NF-kappaB) activation and cell death by tea extracts in neuronal cultures. Biochem. Pharmacol. 2002;63:21–29. doi: 10.1016/s0006-2952(01)00813-9. [DOI] [PubMed] [Google Scholar]
- Li Q., Zhao H.F., Zhang Z.F., Liu Z.G., Pei X.R., Wang J.B., Cai M.Y., Li Y. Long-term administration of green tea catechins prevents age-related spatial learning and memory decline in C57BL/6 J mice by regulating hippocampal cyclic amp-response element binding protein signaling cascade. Neuroscience. 2009;159:1208–1215. doi: 10.1016/j.neuroscience.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Mabandla M., Kellaway L., St, Clair Gibson A., Russell V.A. Voluntary running provides neuroprotection in rats after 6-hydroxydopamine injection into the medial forebrain bundle. Metab. Brain Dis. 2004;19:43–50. doi: 10.1023/b:mebr.0000027416.13070.c3. [DOI] [PubMed] [Google Scholar]
- Mabandla M.V., Kellaway L.A., Daniels W.M., Russell V.A. Effect of exercise on dopamine neuron survival in prenatally stressed rats. Metab. Brain Dis. 2009;24:525–539. doi: 10.1007/s11011-009-9161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina S., Dominguez-Perles R., Garcia-Viguera C., Cejuela-Anta R., Martinez-Sanz J.M., Ferreres F., Gil-Izquierdo A. Physical activity increases the bioavailability of flavanones after dietary aronia-citrus juice intake in triathletes. Food Chem. 2012;135:2133–2137. doi: 10.1016/j.foodchem.2012.07.080. [DOI] [PubMed] [Google Scholar]
- Mercer L.D., Kelly B.L., Horne M.K., Beart P.M. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: investigations in primary rat mesencephalic cultures. Biochem. Pharmacol. 2005;69:339–345. doi: 10.1016/j.bcp.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Milgram N.W., Head E., Zicker S.C., Ikeda-Douglas C., Murphey H., Muggenberg B.A., Siwak C.T., Tapp P.D., Lowry S.R., Cotman C.W. Long-term treatment with antioxidants and a program of behavioral enrichment reduces age-dependent impairment in discrimination and reversal learning in beagle dogs. Exp. Gerontol. 2004;39:753–765. doi: 10.1016/j.exger.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Moroz I.A., Pecina S., Schallert T., Stewart J. Sparing of behavior and basal extracellular dopamine after 6-hydroxydopamine lesions of the nigrostriatal pathway in rats exposed to a prelesion sensitizing regimen of amphetamine. Exp. Neurol. 2004;189:78–93. doi: 10.1016/j.expneurol.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Ngema P.N., Mabandla M.V. Post 6-OHDA lesion exposure to stress affects neurotrophic factor expression and aggravates motor impairment. Metab. Brain Dis. 2017;32:1061–1067. doi: 10.1007/s11011-017-9988-1. [DOI] [PubMed] [Google Scholar]
- Nie G., Jin C., Cao Y., Shen S., Zhao B. Distinct effects of tea catechins on 6-hydroxydopamine-induced apoptosis in PC12 cells. Arch. Biochem. Biophys. 2002;397:84–90. doi: 10.1006/abbi.2001.2636. [DOI] [PubMed] [Google Scholar]
- O'Dell S.J., Gross N.B., Fricks A.N., Casiano B.D., Nguyen T.B., Marshall J.F. Running wheel exercise enhances recovery from nigrostriatal dopamine injury without inducing neuroprotection. Neuroscience. 2007;144:1141–1151. doi: 10.1016/j.neuroscience.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Olanow C.W., Stocchi F. Levodopa: a new look at an old friend. Mov. Disord. 2018;33:859–866. doi: 10.1002/mds.27216. [DOI] [PubMed] [Google Scholar]
- Opii W.O., Joshi G., Head E., Milgram N.W., Muggenburg B.A., Klein J.B., Pierce W.M., Cotman C.W., Butterfield D.A. Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer’s disease. Neurobiol. Aging. 2008;29:51–70. doi: 10.1016/j.neurobiolaging.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T., Jankovic J., Le W. Potential therapeutic properties of green tea polyphenols in Parkinson’s disease. Drugs Aging. 2003;20:711–721. doi: 10.2165/00002512-200320100-00001. [DOI] [PubMed] [Google Scholar]
- Patil S.P., Jain P.D., Sancheti J.S., Ghumatkar P.J., Tambe R., Sathaye S. Neuroprotective and neurotrophic effects of Apigenin and Luteolin in MPTP induced parkinsonism in mice. Neuropharmacology. 2014;86:192–202. doi: 10.1016/j.neuropharm.2021.108876. [DOI] [PubMed] [Google Scholar]
- Pellegrini N., Serafini M., Colombi B., Del Rio D., Salvatore S., Bianchi M., Brighenti F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2004;9:2812–2819. doi: 10.1093/jn/133.9.2812. [DOI] [PubMed] [Google Scholar]
- Piatkowski T.S., Day W.W., Weiner M. Increased renal drug metabolism in treadmill-exercised Fischer-344 male rats. Drug Metab. Dispos. 1993;21:474–479. [PubMed] [Google Scholar]
- Pop V., Head E., Hill M.A., Gillen D., Berchtold N.C., Muggenburg B.A., Milgram N.W., Murphy M.P., Cotman C.W. Synergistic effects of long-term antioxidant diet and behavioral enrichment on beta-amyloid load and non-amyloidogenic processing in aged canines. J. Neurosci. 2010;30:9831–9839. doi: 10.1523/JNEUROSCI.6194-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S., Levivier M., Jiang H., Ferreira M., Jackson-Lewis V., Donaldson D., Togasaki D.M. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience. 1995;67:631–647. doi: 10.1016/0306-4522(95)00066-r. [DOI] [PubMed] [Google Scholar]
- Rahvar M., Nikseresht M., Shafiee S.M., Naghibalhossaini F., Rasti M., Panjehshahin M.R., Owji A.A. Effect of oral resveratrol on the BDNF gene expression in the hippocampus of the rat brain. Neurochem. Res. 2011;36:761–765. doi: 10.1007/s11064-010-0396-8. [DOI] [PubMed] [Google Scholar]
- Rendeiro C., Vauzour D., Kean R.J., Butler L.T., Rattray M., Spencer J.P., Williams C.M. Blueberry supplementation induces spatial memory improvements and region-specific regulation of hippocampal BDNF mRNA expression in young rats. Psychopharmacology. 2012;223:319–330. doi: 10.1007/s00213-012-2719-8. [DOI] [PubMed] [Google Scholar]
- Salvatore M.F., McInnis T.R., Cantu M.A., Apple D.M., Pruett B.S. Tyrosine Hydroxylase Inhibition in Substantia Nigra Decreases Movement Frequency. Mol. Neurobiol. 2019;56:2728–2740. doi: 10.1007/s12035-018-1256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore M.F., Terrebonne J., Cantu M.A., McInnis T.R., Venable K., Kelley P., Kasanga E.A., Latimer B., Owens C.L., Pruett B.S., Yu Y., Luedtke R., Forster M.J., Sumien N., Ingram D.K. Dissociation of Striatal Dopamine and Tyrosine Hydroxylase Expression from Aging-Related Motor Decline: Evidence from Calorie Restriction Intervention. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017;73:11–20. doi: 10.1093/gerona/glx119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer H., Oertel W.H. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Schenkman M., Moore C.G., Kohrt W.M., Hall D.A., Delitto A., Comella C.L., Josbeno D.A., Christiansen C.L., Berman B.D., Kluger B.M., Melanson E.L., Jain S., Robichaud J.A., Poon C., Corcos D.M. Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients With De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 2018;75:219–226. doi: 10.1001/jamaneurol.2017.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H.S., Cervos-Navarro J., Dey P.K. Increased blood-brain barrier permeability following acute short-term swimming exercise in conscious normotensive young rats. Neurosci. Res. 1991;10:211–221. doi: 10.1016/0168-0102(91)90058-7. [DOI] [PubMed] [Google Scholar]
- Shi N., Zhu C., Li L. Rehabilitation training and resveratrol improve the recovery of neurological and motor function in rats after cerebral ischemic injury through the sirt1 signaling pathway. BioMed. Res. Int. 2016;2016:1732163. doi: 10.1155/2016/1732163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukitt-Hale B., Bielinski D.F., Lau F.C., Willis L.M., Carey A.N., Joseph J.A. The beneficial effects of berries on cognition, motor behaviour and neuronal function in ageing. Brit. J. Nutr. 2015;114:1542–1549. doi: 10.1017/S0007114515003451. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B., Carey A., Simon L., Mark D.A., Joseph J.A. Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition. 2006;22:295–302. doi: 10.1016/j.nut.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B., Kalt W., Carey A.N., Vinqvist-Tymchuk M., McDonald J., Joseph J.A. Plum juice, but not dried plum powder, is effective in mitigating cognitive deficits in aged rats. Nutrition. 2009;25:567–573. doi: 10.1016/j.nut.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Smith A.D., Kozlowski D.A., Bohn M.C., Zigmond M.J. Effect of AdGDNF on dopaminergic neurotransmission in the striatum of 6-OHDA-treated rats. Exp. Neurol. 2005;193:420–426. doi: 10.1016/j.expneurol.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Snigdha S., Berchtold N., Astarita G., Saing T., Piomelli D., Cotman C.W. Dietary and behavioral interventions protect against age related activation of caspase cascades in the canine brain. PloS One. 2011;6 doi: 10.1371/journal.pone.0024652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocchi F., Olanow C.W. Continuous dopaminergic stimulation in early and advanced Parkinson's disease. Neurology. 2004;62:S56–S63. doi: 10.1212/wnl.62.1_suppl_1.s56. [DOI] [PubMed] [Google Scholar]
- Stringer T.P., Guerrieri D., Vivar C., van Praag H. Plant-derived flavanol (-)epicatechin mitigates anxiety in association with elevated hippocampal monoamine and BDNF levels, but does not influence pattern separation in mice. Trans. Psychiatr. 2015;5 doi: 10.1038/tp.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg I., Gemma C., Vila J., Bickford P.C. Blueberry- and spirulina-enriched diets enhance striatal dopamine recovery and induce a rapid, transient microglia activation after injury of the rat nigrostriatal dopamine system. Exp. Neurol. 2005;196:298–307. doi: 10.1016/j.expneurol.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Tajiri N., Yasuhara T., Shingo T., Kondo A., Yuan W., Kadota T., Wang F., Baba T., Tayra J.T., Morimoto T., Jing M., Kikuchi Y., Kuramoto S., Agari T., Miyoshi Y., Fujino H., Obata F., Takeda I., Furuta T., Date I. Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Res. 2010;1310:200–207. doi: 10.1016/j.brainres.2009.10.075. [DOI] [PubMed] [Google Scholar]
- Tapias V., Cannon J.R., Greenamyre J.T. Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson’s disease. Neurobiol. Aging. 2014;35:1162–1176. doi: 10.1016/j.neurobiolaging.2013.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapias V., Greenamyre J.T., Watkins S.C. Automated imaging system for fast quantitation of neurons, cell morphology and neurite morphometry in vivo and in vitro. Neurobiol. Dis. 2013;54:158–168. doi: 10.1016/j.nbd.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillerson J.L., Caudle W.M., Reveron M.E., Miller G.W. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neuroscience. 2003;119:899–911. doi: 10.1016/s0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- Tillerson J.L., Cohen A.D., Philhower J., Miller G.W., Zigmond M.J., Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J. Neurosci. 2001;21:4427–4435. doi: 10.1523/JNEUROSCI.21-12-04427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollár J., Nagy F., Hortobágyi T. Vastly different exercise programs similarly improve parkinsonian symptoms: a randomized clinical trial. Gerontology. 2019;65:120–127. doi: 10.1159/000493127. [DOI] [PubMed] [Google Scholar]
- Tsai C.H., Lo S.K., See L.C., Chen H.Z., Chen R.S., Weng Y.H., Chang F.C., Lu C.S. Environmental risk factors of young onset Parkinson’s disease: a case-control study. Clinical Neurol. Neurosurg. 2002;104:328–333. doi: 10.1016/s0303-8467(02)00027-6. [DOI] [PubMed] [Google Scholar]
- Tsai W.L., Chen H.Y., Huang Y.Z., Chen Y.H., Kuo C.W., Chen K.Y., Hsieh T.H. Long-Term Voluntary Physical Exercise Exerts Neuroprotective Effects and Motor Disturbance Alleviation in a Rat Model of Parkinson’s Disease. Behav. Neurol. 2019;2019:4829572. doi: 10.1155/2019/4829572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kolk N.M., de Vries N.M., Kessels R.P.C., Joosten H., Zwinderman A.H., Post B., Bloem B.R. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: a double-blind, randomised controlled trial. Lancet. Neurol. 2019;18:998–1008. doi: 10.1016/S1474-4422(19)30285-6. [DOI] [PubMed] [Google Scholar]
- Walker J.M., Klakotskaia D., Ajit D., Weisman G.A., Wood W.G., Sun G.Y., Serfozo P., Simonyi A., Schachtman T.R. Beneficial effects of dietary EGCG and voluntary exercise on behavior in an Alzheimer’s disease mouse model. J. Alzheimer’s Dis. 2015;44:561–572. doi: 10.3233/JAD-140981. [DOI] [PubMed] [Google Scholar]
- Watson P., Shirreffs S.M., Maughan R.J. Blood-brain barrier integrity may be threatened by exercise in a warm environment. American J. Physiol. 2005;288:R1689–R1694. doi: 10.1152/ajpregu.00676.2004. [DOI] [PubMed] [Google Scholar]
- Willis L.M., Shukitt-Hale B., Cheng V., Joseph J.A. Dose-dependent effects of walnuts on motor and cognitive function in aged rats. Brit. J. Nutr. 2009;101:1140–1144. doi: 10.1017/S0007114508059369. [DOI] [PubMed] [Google Scholar]
- Yoon M.C., Shin M.S., Kim T.S., Kim B.K., Ko I.G., Sung Y.H., Kim S.E., Lee H.H., Kim Y.P., Kim C.J. Treadmill exercise suppresses nigrostriatal dopaminergic neuronal loss in 6-hydroxydopamine-induced Parkinson’s rats. Neurosci. Lett. 2007;423:12–17. doi: 10.1016/j.neulet.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Yuan H., Sarre S., Ebinger G., Michotte Y. Histological, behavioural and neurochemical evaluation of medial forebrain bundle and striatal 6-OHDA lesions as rat models of Parkinson’s disease. J. Neurosci. Methods. 2005;144:35–45. doi: 10.1016/j.jneumeth.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Zbarsky V., Datla K.P., Parkar S., Rai D.K., Aruoma O.I., Dexter D.T. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic. Res. 2005;39:1119–1125. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wu H., Huang H. Epicatechin Plus Treadmill Exercise are Neuroprotective Against Moderate-stage Amyloid Precursor Protein/Presenilin 1 Mice. Pharmacogn. Mag. 2016;12:S139–S146. doi: 10.4103/0973-1296.182174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond M.J., Acheson A.L., Stachowiak M.K., Stricker E.M. Neurochemical compensation after nigrostriatal bundle injury in an animal model of preclinical parkinsonism. Arch. Neurol. 1984;41:856–861. doi: 10.1001/archneur.1984.04050190062015. [DOI] [PubMed] [Google Scholar]