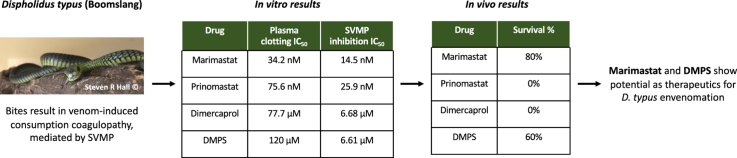
Abstract
Snakebite envenoming affects more than 250,000 people annually in sub-Saharan Africa. Envenoming by Dispholidus typus (boomslang) results in venom-induced consumption coagulopathy (VICC), whereby highly abundant prothrombin-activating snake venom metalloproteinases (SVMPs) consume clotting factors and deplete fibrinogen. The only available treatment for D. typus envenoming is the monovalent SAIMR Boomslang antivenom. Treatment options are urgently required because this antivenom is often difficult to source and, at US$6000/vial, typically unaffordable for most snakebite patients. We therefore investigated the in vitro and in vivo preclinical efficacy of four SVMP inhibitors to neutralise the effects of D. typus venom; the matrix metalloproteinase inhibitors marimastat and prinomastat, and the metal chelators dimercaprol and DMPS. The venom of D. typus exhibited an SVMP-driven procoagulant phenotype in vitro. Marimastat and prinomastat demonstrated equipotent inhibition of the SVMP-mediated procoagulant activity of the venom in vitro, whereas dimercaprol and DMPS showed considerably lower potency. However, when tested in preclinical murine models of envenoming using mixed sex CD1 mice, DMPS and marimastat demonstrated partial protection against venom lethality, demonstrated by prolonged survival times of experimental animals, whereas dimercaprol and prinomastat failed to confer any protection at the doses tested. The preclinical results presented here demonstrate that DMPS and marimastat show potential as novel small molecule-based therapeutics for D. typus snakebite envenoming. These two drugs have been previously shown to be effective against Echis ocellatus VICC in preclinical models, and thus we conclude that marimastat and DMPS should be further explored as potentially valuable early intervention therapeutics to broadly treat VICC following snakebite envenoming in sub-Saharan Africa.
Keywords: Small molecules, Drugs, Boomslang, Snakebite, SVMP
Abbreviations: SVMP, snake venom metalloproteinase; VICC, venom-induced consumption coagulopathy
Graphical abstract
Highlights
-
•
Snake venom metalloproteinases mediate pathology of Dispholidus typus envenoming.
-
•
Small molecule drugs were tested for inhibition of D. typus venom in vitro and in vivo.
-
•
Performance in in vitro experiments did not predict in vivo efficacy.
-
•
Marimastat and DMPS show potential as novel therapeutics for D. typus envenomation.
1. Introduction
More than 250,000 cases of snakebite envenoming are estimated to occur annually in sub-Saharan Africa (sSA) (Halilu et al., 2019), disproportionately affecting those in rural, impoverished communities without adequate access to healthcare (Longbottom et al., 2018; Harrison et al., 2019). Venom-induced consumption coagulopathy (VICC) is a common manifestation of snakebite envenoming, during which procoagulant venom toxins consume clotting factors resulting in the ensuing depletion of fibrinogen and, ultimately, coagulopathy (Berling and Isbister, 2015). Several clotting factors are the target for procoagulant snake venom toxins, and these include Factor X, Factor V, fibrinogen and prothrombin (Berling and Isbister, 2015). While infrequent, envenomings by the rear fanged African colubrid Dispholidus typus (boomslang) are characterised by causing VICC (Lakier and Fritz, 1969; Matell et al., 1973; Gomperts and Demetriou, 1977). The venom of this species is known to potently activate prothrombin (Debono et al., 2017), resulting in the liberation of thrombin, and the subsequent downstream consumption of fibrinogen and fibrin, causing dysregulation of coagulation (Debono et al., 2017, 2020; Ainsworth et al., 2018). The activation of prothrombin is likely the result of snake venom metalloproteinases (SVMPs) (Ainsworth et al., 2018; Debono et al., 2020), which are the dominant toxin type present in the venom and account for almost 75% of the proteinaceous toxins (Pla et al., 2017; Kamiguti et al., 2000). Other minor toxin families identified in the D. typus venom proteome (each constituting <10% of the venom proteome) include three-finger toxins, phospholipases A2 (PLA2s), cysteine-rich secretory proteins (CRISPs), snake venom serine proteases (SVSPs) and C-type lectin-like toxins (Pla et al., 2017), though their contribution to envenoming pathology remains unclear.
Dispholidus typus is broadly distributed throughout much of sub-Saharan Africa and whilst incidences of envenoming are rare, the rapid and severe VICC consequences pose considerable clinical challenges. This is because the only specific treatment for D. typus envenoming is the monospecific F(ab’)2 antivenom “SAIMR Boomslang” (South African Vaccine Producers Pty Ltd), which has limited availability outside of the Southern Africa Economic Community, and costs as much as US$6050 per vial (Krüger and Lemke, 2019). Given that D. typus exhibits a broad geographical distribution throughout much of sub-Saharan Africa, the only specific treatment for envenomings caused by this species is largely unobtainable for snakebite victims who either cannot afford, or do not have access to the antivenom (Ainsworth et al., 2018), and thus investigating novel treatments is a research priority.
More generally, it is well recognised that conventional polyclonal antibody-based antivenoms have several shortcomings, despite being life-saving therapeutics. In addition to often being unaffordable to many snakebite victims, they are associated with high rates of adverse reactions (de Silva et al., 2016; Potet et al., 2019), and have poor dose efficacy, with only ∼10–20% of the active immunoglobulins recognising and binding to venom toxins (Casewell et al., 2010). Logistically, antivenoms are poorly suited for the rural locations in which they are typically required; for example many antivenoms rely on cold chain transport and storage and must be administered intravenously by trained staff in healthcare facilities (World Health Organization, 2010). Indeed, up to 75% of deaths from snakebite are estimated to occur before patients are able to reach healthcare facilities, thus there is a compelling need to identify novel snakebite treatments that could be administered in the community soon after a bite (Bulfone et al., 2018).
To this end, small molecule-based drugs (i.e. ‘toxin inhibitors’) have received considerable interest as novel snakebite therapeutics, both as individual treatments or in combination with existing antivenoms (Bulfone et al., 2018; Williams et al., 2019; Clare et al., 2021). Small molecule drugs have a number of potentially advantageous characteristics over antivenoms, including improved affordability and stability, oral delivery format, higher tolerability (Clare et al., 2021), and improved tissue penetration (Rucavado et al., 2000; Layfield et al., 2020). Previously, Ainsworth et al. demonstrated the in vitro inhibitory effect of the metal chelator EDTA against SVMP-mediated prothrombin activation caused by D. typus venom, suggesting that small molecule inhibitors may be effective therapeutics for D. typus envenoming (Ainsworth et al., 2018). In the same study, Ainsworth et al. demonstrated in a murine preclinical model that EDTA was protective against the lethal effects of Echis ocellatus venom, an African viper which, similar to D. typus venom, contains a high abundance of SVMP toxins (Ainsworth et al., 2018), including prothrombin activators, and causes VICC in envenomed victims (Warrell et al., 1977; Arias et al., 2017). Other small molecule drugs with SVMP-inhibiting potential include other metal chelators, such as dimercaprol and DMPS (2,3-dimercapto-1-propanesulfonic acid) (Xie et al., 2020a, 2020b; Albulescu et al., 2020a), and the mimetic matrix metalloproteinase inhibitors marimastat, batimastat and prinomastat (Layfield et al., 2020; Arias et al., 2017; Howes et al., 2007). Marimastat and batimastat were found to effectively inhibit SVMP activity and reduce haemorrhagic pathologies in murine models of E. ocellatus envenoming (Arias et al., 2017), and inhibit the procoagulant effects of several viper venoms in vitro24,25. Similarly, prinomastat (AG-3340) showed inhibitory activity against the haemorrhagic effects of both purified SVMPs and the crude venom of E. ocellatus28. The metal chelators dimercaprol and DMPS have also been shown to inhibit SVMP activity of E. ocellatus venom in vitro, with DMPS also demonstrating in vivo preclinical neutralisation of venom lethality and haemorrhage (Albulescu et al., 2020a). While D. typus venom is abundant with SVMPs, PLA2 toxins are also thought to contribute to the coagulopathy induced by this venom (Slagboom et al., 2020). The small molecule drug varespladib has been extensively investigated as an inhibitor of venom PLA2 toxins found in a range of snake species, with such studies showing potent neutralisation of PLA2 activity (Xie et al., 2020a, 2020b, 2020c) and associated anticoagulant, haemorrhagic, myotoxic and neurotoxic pathologies (Wang et al., 2018; Bryan-Quirós et al., 2019; Bittenbinder et al., 2018; Lewin et al., 2018a, 2018b). Thus, small molecule treatments for snakebite have the potential to overcome the species-specific and geographically-restrictive limitations associated with current antivenom.
Despite these promising recent research outcomes, further investigation is required to explore the inhibitory breadth and potency of small molecule toxin inhibitors due to the ubiquitous variability in snake venom composition and therefore, also, the variant toxin specificities of these inhibitory small molecule drugs. In particular, despite overarching similarities in venom composition and ensuing snakebite pathology between Echis spp. and D. typus9, the SVMPs of D. typus have evolved their prothrombin activating ability independently of those found in the venom of Echis spp. (Debono et al., 2020). Consequently, building on previous principles demonstrated for Echis spp. (Arias et al., 2017; Xie et al., 2020a; Albulescu et al., 2020a, 2020b), in this study four small molecule drugs were investigated in vitro and in vivo to assess their inhibitory potential against the venom of D. typus. To do so, we applied in vitro metalloproteinase and coagulation bioassays on crude and nanofractionated venom, and in vivo murine models of envenoming to assess neutralisation of venom lethality.
2. Methods
2.1. Venoms
Lyophilised D. typus venom (Product code L1403, origin South Africa, purity >99%) was sourced from Latoxan (Portes les Valence, France) and stored at 4 °C to ensure long-term stability. Prior to use, venom was resuspended in PBS (pH 7.4, Gibco) at 1 mg/mL for in vitro experiments and 5 mg/mL for in vivo experiments.
2.2. Drug preparations for in vitro studies against crude venom
The small molecule SVMP inhibitors tested were; dimercaprol (2,3-dimercapto-1-propanol, ≥98% iodometric, Cat no: 64,046, Sigma), DMPS (2,3-dimercapto-1-propane-sulfonic acid sodium salt monohydrate, 98%, Cat no: H56578, Alfa Aesar), marimastat ((2S,3R)-N4-[(1S)-2,2-Dimethyl-1-[(methylamino)carbonyl]propyl]-N1,2-dihydroxy-3-(2-methylpropyl)butanediamide, >98%, Cat no: 2631, Tocris Bioscience), prinomastat hydrochloride (Cat no: HY-12170A, >98%, MedChemExpress). Varespladib (2-[[3-(2-Amino-2-oxoacetyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl]oxy]-acetic acid, Cat no: SML1100, >98% HPLC, Sigma) was used as a small molecule drug control. All drugs were reconstituted in dimethyl sulfoxide (DMSO) (Sigma) to 10 mM stocks and stored at −20 °C. Daughter plates were created at 1 mM concentrations in 384-well format to allow the creation of assay-ready plates using a VIAFLO 384 electronic pipette (Integra). Daughter plates and assay-ready plates were stored at −20 °C, with the latter used within a month of creation. For the SVMP assay 0.91 μL of each drug was plated (final reaction volume of 91 μL), while 0.5 μL was plated for the coagulation assay (final reaction volume of 50 μL). For marimastat, prinomastat and varespladib, dose response curves were created at a final concentration range of 10 μM to 4.8 pM using a two-fold dilution (50 μL drug into 50 μL of DMSO), with each concentration tested in duplicate. For DMPS and dimercarpol, dose response curves were created at a final concentration range of 160 μM to 76.3 pM using a two-fold dilution (50 μL drug into 50 μL of DMSO), with each concentration tested in duplicate.
2.3. In vitro neutralisation of coagulopathic crude venom activity
To assess the inhibitory potency of the selected drugs against coagulopathic venom activity we used a previously described absorbance-based plasma clotting assay (Still et al., 2017). Citrated bovine plasma (VWR) was defrosted and centrifuged at 858×g for 5 minutes to remove precipitates before use. Thereafter, 100 ng of venom in 10 μL PBS was added to each well in the 384-well assay-ready plate (containing 0.5 μL of 1 mM of inhibitor) using a VIAFLO 384, the plate was then briefly spun down in a Platefuge (Benchmark Scientific) and incubated at 37 °C for 25 min, followed by a further 5 min acclimatisation at room temperate. Next, 20 μL of 20 mM CaCl2 was added using a MultiDrop 384 Reagent Dispenser (ThermoFisher Scientific), followed by the immediate addition of 20 μL citrated bovine plasma. The plate was then immediately read for kinetic absorbance at 595 nm for 116 min using a FLUOstar Omega platereader (BMG Labtech).
Assays were performed in triplicate and each assay contained technical duplicates at each dose. Positive control values were generated using DMSO + venom, and negative control values were generated using DMSO in the absence of venom. All compounds were analysed for their ability to return clotting to normal at the timepoint at which the positive and negative absorbance values were furthest apart. For this, the raw values were normalised to show percentage of normal clotting, e.g. a value of 100% meant the compound returned clotting to that of the negative control. These percentage values were plotted and fitted with a nonlinear regression curve for the normalised response (variable slope) to calculate the IC50 data and 95% confidence intervals for each compound using GraphPad Prism 9.0 (GraphPad Software, San Diego, USA). Multiple comparisons one-way ANOVA test was used to compare IC50 values generated for each replicate plate, using GraphPad Prism 9.0.
2.4. Venom nanofractionation
To further explore the inhibitory specificity of the selected drugs, we fractionated D. typus into toxin constituents and repeated the plasma bioassay. Venom nanofractionation (Slagboom et al., 2020; Zietek et al., 2018) was performed on a UPLC system ('s Hertogenbosch, The Netherlands) controlled by Shimadzu Lab Solutions software. Venom solution was prepared by dissolving lyophilised D. typus venom into water (purified by Milli-Q Plus system, Millipore) to a concentration of 5.0 mg/mL and stored at −80 °C until use. For each analysis, 50 μL venom solution (1.0 mg/mL) was injected by a Shimadzu SIL-30AC autosampler after diluting the stock venom solutions (5.0 ± 0.1 mg/mL) in Milli-Q water. A Waters XBridge reversed-phase C18 column (4.6 × 100 mm column with a 5 μm particle size and a 300 Å pore size) was used for gradient separation at 30 °C. Mobile phase A was composed of 98% water, 2% acetonitrile (ACN) (Biosolve) and 0.1% formic acid (FA) (Biosolve), while mobile phase B was composed of 98% ACN, 2% water and 0.1% FA. The total solvent flow rate was maintained at 0.5 mL/min and the gradients were run as follows: linear increase of eluent B from 0 to 50% in 20 min followed by a linear increase to 90% B in 4 min, then isocratic elution at 90% for 5 min, subsequently the eluent B was decreased from 90% to 0% in 1 min followed by an equilibration of 10 min at 0% B. The column effluent was split as two parts (9:1), with the smaller fraction (10%) sent to a Shimadzu SPD-M20A prominence diode array detector. The larger fraction (90%) was directed to a FractioMate nanofractionator (SPARK-Holland & VU) and fractions collected onto transparent 384-well plates (F-bottom, rounded square well, polystyrene, without lid, clear, non-sterile; Greiner Bio One). The nanofractionator was controlled by FractioMator software (Spark-Holland) to collect fractions continuously at a resolution of 6 s/well. After collection, the well plates with venom fractions were dried overnight in a Christ Rotational Vacuum Concentrator (RVC 2–33 CD plus, Zalm en Kipp, Breukelen, The Netherlands), to remove any solvent remaining in the wells. The vacuum concentrator was equipped with a cooling trap maintained at −80 °C during operation. The dried plates were then stored at −20 °C until bioassaying. Note that during reversed-phase separation, some toxins might denature during separation conditions (i.e. due to FA and/or ACN). This unfortunately cannot be circumvented as the FA is required to achieve sufficient separation resolution and ionisation efficiency in mass spectrometry. This study limitation has been discussed thoroughly elsewhere (Slagboom et al., 2020).
2.5. In vitro neutralisation of coagulopathic venom toxin fractions
The small molecule inhibitors marimastat ((2S,3R)-N4-[(1S)-2,2-Dimethyl-1-[(methylamino)carbonyl] propyl]-N1,2-dihydroxy-3-(2-methylpropyl) butanediamide), prinomastat hydrochloride (AG-3340 hydrochloride), dimercaprol (2,3-Dimercapto-1-propanol), DMPS (2,3-dimercapto-1-propane-sulfonic acid sodium salt monohydrate) and varespladib (A-001) were purchased from Sigma-Aldrich. Bovine plasma (Sodium Citrated, Sterile Filtered, Product Code: S0260) was purchased from Biowest. For assay preparation, the CaCl2 (Biosolve), which was used to de-citrate plasma to initiate coagulation in the coagulation assay, was dissolved in Milli-Q water to 20 mM. The inhibitors were dissolved in DMSO (≥99.9%, Sigma-Aldrich) to a concentration of 10 mM and stored at −20 °C. The plasma was defrosted and then centrifuged at 805×g for 4 min in a 5810 R centrifuge (Eppendorf) to remove possible particulate matter. The inhibitor stock solutions were diluted in PBS buffer to the described concentrations, then 10 μL of each diluted inhibitor solution was pipetted to all wells of plate containing freeze-dried nanofractionated venom fractions by a VWR Multichannel Electronic Pipet, followed by centrifuging the plate for 1 min at 805×g. Next, a pre-incubation step for 30 min at room temperature was performed. Final concentrations of inhibitor solutions used for the coagulation bioassay were 20, 4, 0.8, 0.16 and/or 0.032 μM (with corresponding DMSO final concentrations of 0.02%, 0.004%, 0.0008%, 0.00016% and 0.000032%, respectively). After this incubation step, the HTS coagulation assay was performed as described by Still et al. (2017). A Multidrop 384 Reagent Dispenser (Thermo Fisher Scientific) was used to dispense 20 μL of CaCl2 solution onto all wells of the 384-well plates, followed by 20 μL plasma after rinsing of the Multidrop with deionized water between dispensing. Kinetic absorbance measurements were conducted immediately for 100 min at 595 nm at 25 °C using a Varioskan Flash Multimode Reader (Thermo Fisher Scientific). Venom-only analyses were performed as control experiments, for which 10 μL PBS instead of inhibitor solution was added to all wells of the vacuum centrifuge-dried nanofractionated plates. Each nanofractionation analysis was performed in at least duplicate.
The resulting coagulation chromatograms were plotted as described by Slagboom et al. (2020), with each chromatogram reconstructed to display ‘very fast coagulation’, ‘slightly/medium increased coagulation’ and ‘anticoagulation’. To plot the very fast coagulation chromatogram, the average slope of the first 5 min in the assay was plotted, and for the slightly/medium coagulation chromatogram the average slope of the first 20 min was plotted. For anticoagulant chromatogram the final (end-point) read at 100 min was plotted. Clotting velocities were all plotted against the venom nanofractionation time, producing positive peaks for procoagulant compounds and negative peaks for anticoagulant compounds.
2.6. In vitro neutralisation of venom SVMP activity
The SVMP activity of crude D. typus venom in the presence of inhibitors or vehicle control (DMSO), was measured using a quenched fluorogenic substrate (ES010 Mca-KPLGL-Dpa-AR-NH2, R&D Biosystems; substrate for matrix metalloprotease (MMP) −1, −2, −7, −8, −9, −12, −13, −14, −15, −16, a disintegrin and metalloproteinase (ADAM)10, ADAM17/TACE, Cathepsin D and Cathepsin E), in line with principles previously described (Albulescu et al., 2020a). The substrate was suspended in reaction buffer (150 mM NaCl, 50 mM Tris-HCl pH 7.5) and used at a final concentration of 10 μM (supplied as a 6.2 mM stock). Reactions consisted of 1 μg of venom (1 μg in 15 μL PBS) co-incubated with 0.91 μL of 1 mM of inhibitor. The 384 well plate (Greiner) was briefly spun down in a Platefuge (Benchmark Scientific) and incubated at 37 °C for 25 min, with an additional 5 min acclimatisation at room temperate, before the final addition of the freshly diluted fluorogenic substrate (75 μL of 12.1 μM). The plate was immediately run on a CLARIOstar platereader (BMG Labtech) at an excitation wavelength of 320–10 nm and emission wavelength of 420–10 nm with 10 flashes per well at 25 °C for 100 cycles (each cycle time 79 s). The assay was performed independently in duplicate. The end-reads were calculated for each sample at the time where all fluorescence curves had typically reached a plateau (maximum fluorescence). SVMP activity was calculated for each test condition as a percentage of the mean of the DMSO only wells (100% activity), with a baseline of the marimastat 10 μM controls representing 0% activity. IC50 values were calculated from the percentage inhibition values by fitting a nonlinear regression curve for the normalised response (variable slope) for each compound using GraphPad Prism 9.0 (GraphPad Software, San Diego, USA). The best-fit IC50 values for each replicate were compared to identify significant differences between the IC50 values for each drug using one-way multiple comparisons ANOVA analysis in GraphPad Prism 9.0.
2.7. In vivo neutralisation of venom lethality
2.7.1. Animal ethics
All animal experiments were performed using protocols approved by the Animal Welfare and Ethical Review Boards of the Liverpool School of Tropical Medicine and the University of Liverpool, under project licence (P58464F90) approved by the UK Home Office in accordance with the UK Animal (Scientific Procedures) Act 1986.
2.7.2. Animal maintenance
CD1 mice (18–20 g) were sourced from Charles River (UK) and acclimatised for a minimum of one week before experimentation. Male mice were used for all experiments except for the prinomastat drug only control group. Mice were grouped in cages of five, with room conditions of approximately 22 °C at 40–50% humidity, with 12/12 hour light cycles, and given ad lib access to CRM irradiated food (Special Diet Services, UK) and reverse osmosis water in an automatic water system. Mice were housed in specific-pathogen free facilities in Techniplast GM500 cages containing Lignocell bedding (JRS, Germany), Sizzlenest zigzag fibres as nesting material (RAJA), and supplied with environmental enrichment materials.
2.7.3. Co-incubation model of preclinical efficacy
The median murine lethal dose (LD50) for D. typus venom administered by intravenous injection was previously determined as 22.29 μg per mouse (Ainsworth et al., 2018). To determine the efficacy of small molecule inhibitors against D. typus venom, a refined version of the WHO recommended antivenom ED50 neutralisation experiments was used, in which ∼4 x LD50 doses of venom (90 μg) were pre-incubated with each small molecule inhibitor. Drug stocks were freshly prepared to allow for a ratio of 1:1.33 venom to inhibitor as previously defined by marimastat in vivo testing against other snake venoms (Albulescu et al., 2020b). Drugs tested in vivo were dimercaprol (2,3-dimercapto-1-propanol ≥98% iodometric, Cat no: 64,046, Sigma-Aldrich), marimastat (>98% HPLC, Cat no: M2699, Sigma-Aldrich), and prinomastat hydrochloride (≥95% HPLC, Cat no: PZ0198, Merck), all resuspended at 1 mg/mL in water, and DMPS (2,3-dimercapto-1-propanesulfonic acid sodium salt monohydrate, 95%, Cat no: H56578, Alfa Aesar) resuspended at 1 mg/mL in PBS. Groups of five mice received experimental doses that consisted of either: (a) venom only (4 x LD50 dose) or (b) venom (4 x LD50 dose) with drug (118 μg) or (c) drug only (118 μg) to assess drug safety. The control group was the venom only group, against which all drug treatments were compared. Each experimental group comprised five animals as this was previously determined to be the minimum number of animals required to produce statistically significant results (World Health Organization, 2018), although the prinomastat only (no venom) control group contained only four due to the misdosing of one animal. No randomisation was used to allocate experimental groups – mice were randomly allocated into cages of five prior to the experiment, and each cage formed one treatment group. No criteria for including or excluding animals was applied, and all data points were included in analyses. A total of 45 mice were used. All experimental doses were prepared to a volume of 200 μL in PBS and incubated at 37 °C for 30 minutes prior to intravenous injection via the tail vein. Animals were monitored for humane endpoints (loss of righting reflex, seizure, external haemorrhage) for 6 hours, and any animals showing such signs were immediately euthanised by rising concentrations of carbon dioxide. All observations were performed by mixed gender experimenters who were blinded to the drug group allocation. Time of death, number of deaths and number of survivors were recorded, where deaths and times of death represent implementation of humane endpoint-dictated euthanasia. Kaplan-Meier survival plots were generated using GraphPad Prism 9.0 (GraphPad Software, San Diego, USA) and log-rank (Mantel-Cox) tests were used to statistically compare the survival times between groups treated with and without drug.
3. Results
3.1. Small molecule drugs have varying effects on the procoagulant activity of crude D. typus venom
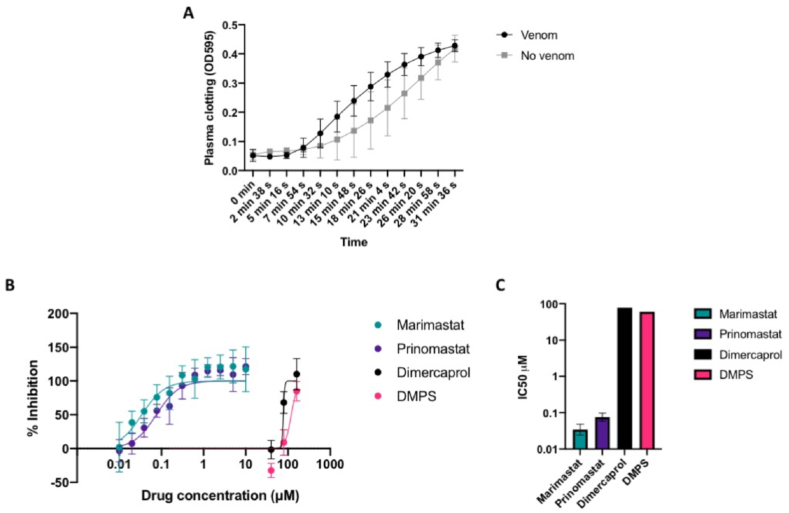
The addition of D. typus venom to bovine plasma in the coagulation assay resulted in earlier stimulation of clotting compared to the no venom control (natural clotting), highlighting the procoagulant nature of this venom (Fig. 1A). The effects of the small molecules against the procoagulant activity of crude D. typus venom are shown in Fig. 1. Weak venom-inhibitory effects were observed for the metal chelators dimercaprol and DMPS at micromolar concentrations. As shown in Fig. 1B, concentrations of 160 μM showed strong inhibitory activity for both drugs, but this inhibitory effect rapidly decreased at lower concentrations, with no effect observed at concentrations of 10 μM and lower. IC50 values were determined to be 77.7 μM for dimercaprol and 120 μM for DMPS (Fig. 1C), although due to the small number of data points between 0 and 100% inhibition these values must be interpreted with caution and 95% confidence intervals were unable to be calculated. Contrastingly, the peptidomimetic matrix metalloproteinase inhibitors marimastat and prinomastat potently neutralised the procoagulant effects of D. typus venom (Fig. 1B). The IC50 values for marimastat and prinomastat were determined to be 34.2 nM (95% CI 24.2–48.5 nM) and 75.6 nM (95% CI 58.6–97.7 nM) respectively (Fig. 1C), demonstrating that marimastat was significantly more potent in this assay than prinomastat (p = 0.01). The PLA2 inhibitor varespladib (control non-SVMP inhibiting drug used throughout) had no neutralising effect on venom-induced coagulation at any of the tested drug concentrations (Supplemental File S1), a result in line with our expectations of procoagulant venom activity being mediated by SVMP toxins.
Fig. 1.
Effects of D. typus venom, and inhibition by small molecule drugs, on in vitro plasma clotting measured by absorbance at 595 nm (OD595).A) Clotting as indicated by the increase in OD595 in the presence of crude D. typus venom (black circles) compared to normal clotting in the absence of venom (grey squares). Data points represent the mean of twelve individual values recorded over three independent technical replicates, and error bars represent standard deviation. B) Inhibitory activity of marimastat (teal circles), prinomastat (dark purple circles), dimercaprol (black circles) and DMPS (pink circles) over a two-fold serial dilution curve, from which IC50 values were calculated. Inhibitory activity is expressed as a percentage of normal clotting, where 100% inhibition represents return of clotting to normal plasma clotting levels. Data points represent the mean of six individual values recorded over three independent technical replicates, and error bars represent standard deviation. C) Calculated IC50 values for marimastat, prinomastat, dimercaprol and DMPS. Data points represent the best fit IC50 value and error bars represent 95% confidence intervals (not calculated for dimercaprol and DMPS).
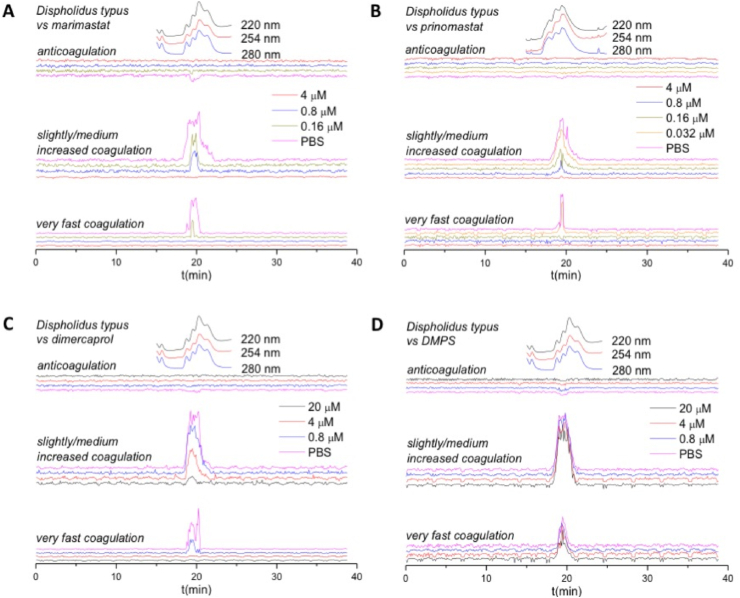
3.2. Inhibitory effects of small molecule drugs on nanofractionated D. typus venom coagulotoxins
To further characterise the coagulopathic activity of D. typus venom, and to better explore the specificity of the various small molecule inhibitors against specific toxins, we repeated the coagulation assay experiments using nanofractionated venom. As previously described, this method uses fractionated venom as the basis for measurements of the velocity of clotting in different wells in comparison to control wells, with procoagulant toxins producing positive peaks in the resulting bioassay chromatogram and anticoagulant toxins producing negative peaks (Still et al., 2017). In the venom-only analysis, broad positive peaks (18.4–22.0 min) were observed for both the ‘very fast coagulation’ chromatograms and the ‘slightly/medium increased coagulation’ chromatograms, indicative of an overall procoagulant effect of the venom. Detected bioactivities of D. typus venom were correlated with previously generated LC-MS and proteomics data (Slagboom et al., 2020). From this data, a candidate toxin mass of 23 kDa was identified for the procoagulant activity, which is within the range of SVMPs. The inhibitory effects of marimastat and prinomastat on nanofractionated D. typus venom toxins are depicted in Fig. 2A and B respectively. The peaks in the very fast and slight/medium increased coagulation chromatograms decreased with increasing concentrations of both marimastat and prinomastat, indicative of a dose-dependent restoration of normal clotting velocity. All coagulopathic activities were inhibited at 0.8 μM marimastat and 0.16 μM prinomastat for very fast coagulation chromatograms, and at 4 μM for both inhibitory molecules for slightly/medium increased coagulation activity. The inhibitory effects of dimercaprol and DMPS on nanofractionated D. typus venom toxins are depicted in Fig. 2C and D, respectively. By increasing the concentration range of dimercaprol, the procoagulant activity of D. typus venom was inhibited, with very fast coagulation activity fully inhibited at 4 μM, and slightly/medium increased coagulation activity at 20 μM. However, no substantial inhibition of procoagulant venom activity was observed with DMPS at any tested concentration up to 20 μM. These results reflect the considerable potency differences observed between the peptidomimetic inhibitors and the metal chelators in the crude venom plasma bioassays.
Fig. 2.
Reconstructed coagulation chromatograms for nanofractionated D. typus venom toxins in the presence of different concentrations of A) marimastat, B) prinomastat, C) dimercaprol, and D) DMPS. The negative peaks indicate anticoagulant activity where velocity is lower than the assay solution in control wells without venom toxins, and the positive peaks indicate procoagulant activity where velocity is higher than that in control wells without venom toxins. The top superimposed chromatograms are characteristic profiles of the UV trace at 220, 254 and 280 nm. PBS indicates venom only samples where PBS was used as a control for the inhibitors. Traces with different colours indicate different concentrations (final) of inhibitors in the assay.
A very weak signal (peak centre at 19.6 min) was also detected in terms of anticoagulant venom activity. A previous study observed a much clearer negative anticoagulant peak with D. typus venom, though the venom was applied at a five-fold higher concentration (5.0 mg/mL venom) than that used in this study (1.0 mg/mL venom) (Slagboom et al., 2020). While varespladib has previously been demonstrated to be a potent inhibitor of anticoagulant venom activities induced by PLA2 toxins (Bittenbinder et al., 2018; Kazandjian et al., 2021; Youngman et al., 2020; Liu et al., 2021), in this study it produced no inhibitory effects on venom-induced coagulation, whether procoagulant or anticoagulant, at the maximal drug dose tested (20 μM) (Supplemental File S2). However, due to the weak anticoagulant venom activity observed in these experiments, the assay window for measuring such inhibition is limited.
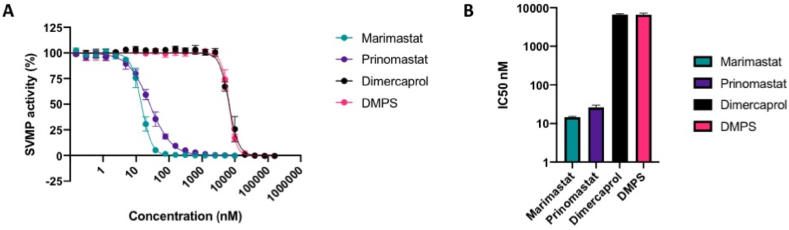
3.3. Small molecule drugs inhibit crude D. typus venom SVMP activity
To determine the specific inhibitory effects of the selected small molecule drugs on SVMP toxin activity, we performed IC50 screens of the five different toxin inhibitors in a previously defined kinetic enzymatic SVMP assay using crude D. typus venom. As previously observed (Alomran et al., 2021), D. typus venom demonstrated strong SVMP-specific activity in this assay. The inhibitory effects of the matrix metalloproteinase inhibitors marimastat and prinomastat and the metal chelators DMPS and dimercaprol against the venom SVMP activity of D. typus are displayed in Fig. 3. Marimastat and prinomastat demonstrated nanomolar IC50 values of 14.5 (95% CI 13.9–15.2 nM) and 25.9 nM (95% CI 22.6–29.7 nM) respectively, and complete inhibition of venom activity at 156 nM for marimastat and 2.5 μM for prinomastat (Fig. 3A). Inhibition of SVMP activity by dimercaprol and DMPS as measured by IC50 values was significantly lower than that observed for marimastat and prinomastat (p < 0.02 for all comparisons). Dimercaprol and DMPS demonstrated highly comparable inhibition of D. typus SVMP activity with complete inhibition obtained at 40 μM and IC50 values of 6.68 (95% CI 6.36–7.02 μM) and 6.61 μM (95% CI 6.38–6.87 μM), respectively (Fig. 3B), with no significant differences between the two IC50 values. As anticipated, the control drug used in this study, the PLA2 inhibitor varespladib, showed no inhibitory activity at any of the concentrations tested (maximum concentration 10 μM) (Supplemental File S3).
Fig. 3.
In vitro inhibition of D. typus crude venom SVMP activity by small molecule inhibitors. A) SVMP activity of D. typus crude venom in the presence of marimastat (teal circles), prinomastat (dark purple circles), dimercaprol (black circles) and DMPS (pink circles) over a two-fold serial dilution curve, from which IC50 values were calculated. Data points represent the percentage of crude venom SVMP activity generated from the mean of four individual values recorded over two independent technical replicates, and error bars represent standard deviation. B) IC50 values of SVMP inhibition for marimastat, prinomastat, dimercaprol and DMPS.
3.4. Marimastat and DMPS provide some protection against D. typus venom-induced lethality in vivo
Given that inhibition of SVMP and coagulotoxic activities in vitro have previously been demonstrated to translate into varying degrees of in vivo protection against systemic envenoming (Albulescu et al., 2020a, 2020b) we next tested the capability of the four SVMP-inhibiting small molecule drugs to protect against D. typus venom-induced lethality in vivo. To do so, we used a modified version of the WHO-recommended protocol of murine venom neutralisation (ED50 assay). All five experimental animals treated intravenously with 4 x LD50 doses of D. typus venom (90 μg) succumbed to the lethal venom effects within the first hour of the experiment (mean 17 minutes, range 1–40 minutes), as shown in Fig. 4. The intravenous co-delivery of the small molecule drugs preincubated with D. typus venom revealed that both prinomastat and dimercaprol failed to protect against venom-induced lethality at the single therapeutic dose tested (118 μg), with all experimental animals in these groups succumbing to venom lethality within the first 30 minutes (mean 7.8 minutes for both groups; prinomastat range 4–21 minutes; dimercaprol range 1–18 minutes), in a highly comparable manner to the venom only control. Contrastingly, DMPS and marimastat both showed a significant degree of protection against D. typus venom-induced lethality. Three of the five experimental animals dosed with DMPS were protected for the duration of the experiment (6 hours), with two deaths occurring within the first hour, resulting in a mean survival time of 224 minutes compared to 17.2 minutes in the venom only control group (log-rank test, p = 0.047). Of the animals dosed with marimastat, four of the five animals were protected for the duration of the experiment, with mean survival times of 316.2 minutes compared to 17.2 minutes in the venom only control group (log-rank test, p = 0.002). The single non-surviving experimental animal in this group was euthanised at 141 minutes. No adverse effects were observed in experimental drug -only control animals and, consequently, all survived the duration of the experiment (Fig. 4).
Fig. 4.
The small molecule drugs marimastat and DMPS significantly increase the survival times of mice receiving lethal doses of D. typus venom. Data shown as Kaplan-Meier survival graphs for experimental animals (n = 5 per group, except prinomastat only group where n = 4) treated with either: 90 μg (4 x LD50) of D. typus venom only (magenta), 118 μg of drug only (cyan) or 90 μg venom and 118 μg drug (black). Treatments were pre-incubated at 37 °C for 30 minutes prior to intravenous injection via the tail vein and animals were monitored for 6 hours. Data is shown for: A) DMPS, B) dimercaprol, C) marimastat, and D) prinomastat.
4. Discussion
Conventional animal-derived antivenom, although a life-saving treatment, has numerous deficiencies that impair its utility in the treatment of snakebite. Although a seemingly effective antivenom for treating D. typus envenoming is available in South Africa, it is often difficult to source in other regions of the continent and can be catastrophically unaffordable for patients (Krüger and Lemke, 2019), and this scenario encapsulates the challenges faced by snakebite victims the world over. There is therefore an urgent need to develop alternative/supplementary therapeutics that are stable, effective, affordable and available in remote rural areas where medical access is limited. Small molecule inhibitors that can broadly neutralise a class of key toxins in snake venom following oral administration are possible solutions in this regard (Clare et al., 2021) and Phase II clinical trials for small molecule inhibitors of snakebite are underway (ClinicalTrials.gov, 2021). Rapid-onset pathologies such as VICC, together with tissue damage induced by SVMPs, are only partially neutralised by antibody based antivenoms (Gutiérrez et al., 2006), which suffer from poor tissue distribution due to the inherent large size of antibodies (Gutiérrez et al., 2017). By contrast, the drastically smaller size of the inhibitors tested in this study enables favourable properties of rapid and effective tissue penetration and potential for oral delivery, due to their pharmacokinetic and physicochemical properties (Lewin et al., 2018a, 2018b; Kini et al., 2018). Moreover, repurposing these small molecule inhibitors that are either licensed drugs or phase I-approved drug candidates could significantly shorten drug development times as safety profiles, pharmacokinetics, bioavailability and tolerance data on these molecules have already been attained (Kini et al., 2018; Lewin et al., 2016; Knudsen et al., 2019). Current evidence of the utility of small molecule inhibitors against snakebite indicates that they may be particularly effective as first line, early intervention therapeutics and/or bridging therapies for initial and adjunct treatment in community settings, before patients are able to access antivenom in healthcare centres (Bulfone et al., 2018; Howes et al., 2007; Lewin et al., 2016).
In this study we assessed the ability of four small molecule inhibitors to neutralise D. typus venom toxin activities in vitro and in vivo. In line with previous findings (Debono et al., 2017, 2020; Ainsworth et al., 2018; Pla et al., 2017), our study demonstrates that D. typus venom toxicity is largely conferred by SVMP toxins, which are likely responsible for causing coagulopathy in vivo. Our data from in vitro assays of D. typus venom activity demonstrate that the matrix metalloproteinase inhibitors marimastat and prinomastat are potent inhibitors of the SVMP-mediated procoagulant effects of this venom, with both compounds demonstrating similar inhibitory activity in in vitro assays of plasma coagulation and SVMP activity. Indeed, marimastat and prinomastat showed nanomolar IC50 values in the crude venom plasma coagulation assay and crude venom SVMP assay, and both drugs showed inhibitory effects at low micromolar concentrations in the plasma coagulation assay with nanofractionated venom. However, and in contrast with in vitro SVMP-inhibiting prowess, in the in vivo murine models of envenoming assays, all animals in the prinomastat group succumbed to lethality from D. typus venom, whilst an equivalent amount of marimastat conferred 80% protection. This was unexpected and hints at different levels of drug exposure and metabolism in this single dose intravenous-delivered model, as both matrix metalloproteinase inhibiting drugs showed potent inhibitory activity in our in vitro assays and have been shown in other studies to neutralise the in vivo lethal effects of E. ocellatus venom in murine models (Arias et al., 2017; Albulescu et al., 2020a). Studies by other groups have similarly shown the impressive in vitro inhibitory activities of marimastat and prinomastat against SVMP activity in rattlesnakes and a wide range of palearctic vipers (Chowdhury et al., 2021a; Seneci et al., 2021) and strong inhibitory effects of prinomastat against the anticoagulant activity of spitting cobra venoms (Chowdhury et al., 2021b).
The metal chelators dimercaprol and DMPS demonstrated lower potency than marimastat and prinomastat in the in vitro studies of SVMP activity and plasma coagulation, which is in agreement with previous studies (Albulescu et al., 2020a, 2020b; Chowdhury et al., 2021a), whilst the PLA2 inhibitor varespladib, used as a non-SVMP inhibiting control, produced no inhibitory effects on the venom. Of the two metal chelators, DMPS showed the weakest inhibitory activity in in vitro assays of venom bioactivity. Despite this reduced in vitro potency in comparison with the peptidomimetic matrix metalloproteinase inhibitors, DMPS conferred a degree of protection against venom lethality in murine models of envenoming, with significant increases in mean survival times and 60% of mice surviving until the end of the experimental time window. None of the experimental animals dosed with the other metal chelator, dimercaprol, survived the experiment, despite the comparable mechanism of action and in vitro inhibitory potency in the coagulation and SVMP assays. These results contrast with our previous preclinical study investigating E. ocellatus envenoming, which found that both dimercaprol and DMPS provided protection against lethal effects in this same intravenous murine model, though are consistent with DMPS exhibiting superior preclinical efficacy (Albulescu et al., 2020a).
The notable discrepancies between the in vitro and in vivo experiments described herein exemplifies the complexity associated with relying on in vitro potency-based screens as a means to predict the efficacy of small molecule drugs in in vivo experiments. While efficacy data gained from in vitro experiments is undoubtedly an essential prerequisite prior to preclinical efficacy testing, substantial differences in inhibitor potency at this step does not preclude preclinical efficacy, which ultimately is dictated by drug exposure. Equally, the preclinical model utilised here, consisting of the pre-incubation of drug with venom followed by intravenous co-delivery, is largely detached from the clinical scenario of a snakebite. While this is the WHO-recommended method for preclinical assessment of antivenom efficacy, and thus is a logical starting point for assessing preclinical efficacy, this method does not reflect the biodistribution of venom during early envenoming and uses a non-clinically relevant route of venom injection. The pharmacokinetics of snake envenoming demonstrate that venoms undergo an initial absorption phase when administered through the intramuscular or subcutaneous routes, which are more representative of a snakebite, whereas the intravenous route bypasses the absorption phase to the distribution phase and finally the elimination phase (Sanhajariya et al., 2018). Furthermore, the intravenous pre-incubation assay does not take into account the pharmacokinetics/pharmacodynamics of the unbound test inhibitor (Gutiérrez et al., 2021). Thus, the lack of efficacy observed with prinomastat (compared with marimastat) here, for example, may be the result of a lack of in vivo dose optimisation and thus sub-optimal exposure. Further work is required to better define the pharmacokinetic and pharmacodynamic profiles of small molecule drugs in preclinical models of snakebite envenoming to inform the design of optimised preclinical dosing regimens applicable for use in more biologically-realistic models of envenoming (e.g. “challenge then treat models”) (Albulescu et al., 2020a, 2020b; Lewin et al., 2018a; Gutiérrez et al., 2017; Knudsen et al., 2020), and taking into account plasma stability, drug metabolism, and appropriate compartmental models of venom and small molecule inhibitors.
In sub-Saharan Africa, VICC is only known to be commonly caused by Echis spp. and D. typus, and the venoms of these snakes have been shown to converge on similar SVMP-rich venom composition profiles (Ainsworth et al., 2018), suggesting that D. typus venom may be amenable to neutralisation by previously identified inhibitors of Echis venoms. This study investigated the ability of repurposed small molecule inhibitors to effectively neutralise D. typus venom activity in vitro and in vivo, and identified the SVMP inhibiting drugs DMPS and marimastat as two lead compounds that provide a significant degree of preclinical protection against the lethal effects of D. typus venom. Previous studies have demonstrated that both DMPS and marimastat also provide preclinical action against E. ocellatus venom (Albulescu et al., 2020a, 2020b), and the present study may expand the range of snake species and, consequentially, the number of snakebite victims of that could potentially benefit from receiving an early intervention with small molecule therapeutics. While considerable work remains to be done before such toxin inhibitors can progress into snakebite clinical trials, our findings here provide a strong rationale to continue such work, and ultimately progress towards the long term future goal of clinically evaluating the efficacy of such small molecule drugs in all cases of diagnostically indicated VICC following snakebite envenoming in sub-Saharan Africa.
Credit author statement
Stefanie K Menzies: Methodology, Investigation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Visualization. Rachel H Clare: Methodology, Investigation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Visualization, Funding acquisition. Chunfang Xie: Methodology, Investigation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Visualization, Funding acquisition. Adam Westhorpe: Investigation, Formal analysis, Data curation, Writing – review & editing. Steven R Hall: Investigation, Writing – review & editing. Rebecca J Edge: Investigation, Writing – review & editing. Jaffer Alsolaiss: Investigation, Writing – review & editing. Edouard Crittenden: Investigation, Writing – review & editing. Amy E Marriott: Investigation, Writing – review & editing. Robert A Harrison: Investigation, Writing – review & editing. Jeroen Kool: Conceptualization, Writing – review & editing, Supervision, Funding acquisition. Nicholas R Casewell: Conceptualization, Investigation, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition.
Funding
This research was funded in whole, or in part, by the Wellcome Trust, grant numbers as detailed below. For the purpose of open access, the authors have applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. R.H.C. acknowledges funding support from the Director's Catalyst Fund at LSTM [supported by Wellcome Institutional Strategic Support Fund 3 (204806/Z/16/Z) and LSTM Internal Funding]. N.R.C. acknowledges a UK Medical Research Council research grant (MR/S00016X/1) and a Sir Henry Dale Fellowship (200517/Z/16/Z) jointly funded by Wellcome and the Royal Society. N.R.C and J.K. acknowledge funding provided by a Wellcome project grant (221712/Z/20/Z). C.X. acknowledges funding support from the China Scholarship Council (CSC) fellowship (201706250035).
Ethics statement
All animal experiments were performed using protocols approved by the Animal Welfare and Ethical Review Boards of the Liverpool School of Tropical Medicine and the University of Liverpool, under project licence (P58464F90) approved by the UK Home Office in accordance with the UK Animal (Scientific Procedures) Act 1986.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Rachel H Clare reports financial support was provided by Wellcome Trust. Jeroen Kool reports financial support was provided by Wellcome Trust. Nicholas R Casewell reports financial support was provided by Wellcome Trust. Nicholas R Casewell reports financial support was provided by Medical Research Council. Nicholas R Casewell reports financial support was provided by The Royal Society. Chunfang Xie reports financial support was provided by China Scholarship Council.
Acknowledgements
We thank the staff in the Biomedical Service Unit of the University of Liverpool for their support in the maintenance and care of the study mice. We are grateful to Laura-Oana Albulescu for discussions regarding this work and comments on the manuscript.
Handling Editor: Ray Norton
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.toxcx.2022.100118.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ainsworth S., Slagboom J., Alomran N., et al. The paraspecific neutralisation of snake venom induced coagulopathy by antivenoms. Commun. Biol. 2018;1(1):1–14. doi: 10.1038/s42003-018-0039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albulescu L.O., Hale M.S., Ainsworth S., et al. Preclinical validation of a repurposed metal chelator as an early-intervention therapeutic for hemotoxic snakebite. Sci. Transl. Med. 2020;12(542) doi: 10.1126/scitranslmed.aay8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albulescu L.O., Xie C., Ainsworth S., et al. A therapeutic combination of two small molecule toxin inhibitors provides broad preclinical efficacy against viper snakebite. Nat. Commun. 2020;11(1):6094. doi: 10.1038/s41467-020-19981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alomran N., Alsolaiss J., Albulescu L.O., et al. Pathology-specific experimental antivenoms for haemotoxic snakebite: the impact of immunogen diversity on the in vitro cross-reactivity and in vivo neutralisation of geographically diverse snake venoms. PLoS Negl. Trop. Dis. 2021;15(8) doi: 10.1371/journal.pntd.0009659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias A.S., Rucavado A., Gutiérrez J.M. Peptidomimetic hydroxamate metalloproteinase inhibitors abrogate local and systemic toxicity induced by Echis ocellatus (saw-scaled) snake venom. Toxicon Off. J. Int. Soc. Toxinol. 2017;132:40–49. doi: 10.1016/j.toxicon.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Berling I., Isbister G.K. Hematologic effects and complications of snake envenoming. Transfus. Med. Rev. 2015;29(2):82–89. doi: 10.1016/j.tmrv.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Bittenbinder M.A., Zdenek C.N., Op den Brouw B., et al. Coagulotoxic cobras: clinical implications of strong anticoagulant actions of African spitting Naja venoms that are not neutralised by antivenom but are by LY315920 (varespladib) Toxins. 2018;10(12):516. doi: 10.3390/toxins10120516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan-Quirós W., Fernández J., Gutiérrez J.M., Lewin M.R., Lomonte B. Neutralizing properties of LY315920 toward snake venom group I and II myotoxic phospholipases A2. Toxicon. 2019;157:1–7. doi: 10.1016/j.toxicon.2018.11.292. [DOI] [PubMed] [Google Scholar]
- Bulfone T.C., Samuel S.P., Bickler P.E., Lewin M.R. Developing small molecule therapeutics for the initial and adjunctive treatment of snakebite. J. Trop. Med. 2018;2018:1–10. doi: 10.1155/2018/4320175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casewell N.R., Cook D.A.N., Wagstaff S.C., et al. Pre-clinical assays predict Pan-African Echis viper efficacy for a species-specific antivenom. PLoS Negl. Trop. Dis. 2010;4(10) doi: 10.1371/journal.pntd.0000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury A., Zdenek C.N., Lewin M.R., et al. Venom-induced blood disturbances by palearctic viperid snakes, and their relative neutralization by antivenoms and enzyme-inhibitors. Front. Immunol. 2021;12:688802. doi: 10.3389/fimmu.2021.688802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury A., Lewin M.R., Zdenek C.N., Carter R., Fry B.G. The relative efficacy of chemically diverse small-molecule enzyme-inhibitors against anticoagulant activities of African spitting cobra (Naja species) venoms. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.752442. https://www.frontiersin.org/article/10.3389/fimmu.2021.752442 Accessed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare R.H., Hall S.R., Patel R.N., Casewell N.R. Small molecule drug discovery for neglected tropical snakebite. Trends Pharmacol. Sci. 2021;42(5):340–353. doi: 10.1016/j.tips.2021.02.005. [DOI] [PubMed] [Google Scholar]
- de Silva H.A., Ryan N.M., de Silva H.J. Adverse reactions to snake antivenom, and their prevention and treatment. Br. J. Clin. Pharmacol. 2016;81(3):446–452. doi: 10.1111/bcp.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debono J., Dobson J., Casewell N.R., et al. Coagulating colubrids: evolutionary, pathophysiological and biodiscovery implications of venom variations between boomslang (Dispholidus typus) and twig snake (Thelotornis mossambicanus) Toxins. 2017;9(5):171. doi: 10.3390/toxins9050171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debono J., Dashevsky D., Nouwens A., Fry B.G. The sweet side of venom: glycosylated prothrombin activating metalloproteases from Dispholidus typus (boomslang) and Thelotornis mossambicanus (twig snake) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020;227:108625. doi: 10.1016/j.cbpc.2019.108625. [DOI] [PubMed] [Google Scholar]
- Gomperts E.D., Demetriou D. Laboratory studies and clinical features in a case of boomslang envenomation. South Afr. Med. J. Suid-Afr Tydskr Vir Geneeskd. 1977;51(6):173–175. [PubMed] [Google Scholar]
- Gutiérrez J.M., Theakston R.D.G., Warrell D.A. Confronting the neglected problem of snake bite envenoming: the need for a global partnership. PLoS Med. 2006;3(6) doi: 10.1371/journal.pmed.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J.M., Solano G., Pla D., et al. Preclinical evaluation of the efficacy of antivenoms for snakebite envenoming: state-of-the-art and challenges ahead. Toxins. 2017;9(5):163. doi: 10.3390/toxins9050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J.M., Albulescu L.O., Clare R.H., et al. The search for natural and synthetic inhibitors that would complement antivenoms as therapeutics for snakebite envenoming. Toxins. 2021;13(7):451. doi: 10.3390/toxins13070451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halilu S., Iliyasu G., Hamza M., Chippaux J.P., Kuznik A., Habib A.G. Snakebite burden in Sub-Saharan Africa: estimates from 41 countries. Toxicon. 2019;159:1–4. doi: 10.1016/j.toxicon.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Harrison R.A., Casewell N.R., Ainsworth S.A., Lalloo D.G. The time is now: a call for action to translate recent momentum on tackling tropical snakebite into sustained benefit for victims. Trans. R. Soc. Trop. Med. Hyg. 2019;113(12):835–838. doi: 10.1093/trstmh/try134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes J.M., Theakston R.D.G., Laing G.D. Neutralization of the haemorrhagic activities of viperine snake venoms and venom metalloproteinases using synthetic peptide inhibitors and chelators. Toxicon Off. J. Int. Soc. Toxinol. 2007;49(5):734–739. doi: 10.1016/j.toxicon.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Kamiguti A.S., Theakston R.D.G., Sherman N., Fox J.W. Mass spectrophotometric evidence for P-III/P-IV metalloproteinases in the venom of the Boomslang (Dispholidus typus) Toxicon. 2000;38(11):1613–1620. doi: 10.1016/S0041-0101(00)00089-1. [DOI] [PubMed] [Google Scholar]
- Kazandjian T.D., Arrahman A., Still K.B.M., et al. Anticoagulant activity of Naja nigricollis venom is mediated by phospholipase A2 toxins and inhibited by varespladib. Toxins. 2021;13(5):302. doi: 10.3390/toxins13050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini R.M., Sidhu S.S., Laustsen A.H. Biosynthetic Oligoclonal antivenom (BOA) for snakebite and next-generation treatments for snakebite victims. Toxins. 2018;10(12):534. doi: 10.3390/toxins10120534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen C., Ledsgaard L., Dehli R.I., Ahmadi S., Sørensen C.V., Laustsen A.H. Engineering and design considerations for next-generation snakebite antivenoms. Toxicon Off. J. Int. Soc. Toxinol. 2019;167:67–75. doi: 10.1016/j.toxicon.2019.06.005. [DOI] [PubMed] [Google Scholar]
- Knudsen C., Casewell N.R., Lomonte B., Gutiérrez J.M., Vaiyapuri S., Laustsen A.H. Novel snakebite therapeutics must Be tested in appropriate rescue models to robustly assess their preclinical efficacy. Toxins. 2020;12(9):528. doi: 10.3390/toxins12090528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger H.J., Lemke F.G. Fatal boomslang bite in the northern cape. Afr. J. Emerg. Med. 2019;9(1):53–55. doi: 10.1016/j.afjem.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakier J.B., Fritz V.U. Consumptive coagulopathy caused by a boomslang bite. South Afr. Med. J. Suid-Afr Tydskr Vir Geneeskd. 1969;43(34):1052–1055. [PubMed] [Google Scholar]
- Layfield H.J., Williams H.F., Ravishankar D., et al. Repurposing cancer drugs batimastat and marimastat to inhibit the activity of a group I metalloprotease from the venom of the western diamondback rattlesnake. Crotalus atrox. Toxins. 2020;12(5):309. doi: 10.3390/toxins12050309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin M., Samuel S., Merkel J., Bickler P. Varespladib (LY315920) appears to Be a potent, broad-spectrum, inhibitor of snake venom phospholipase A2 and a possible pre-referral treatment for envenomation. Toxins. 2016;8(9):248. doi: 10.3390/toxins8090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin M.R., Gutiérrez J.M., Samuel S.P., et al. Delayed oral LY333013 rescues mice from highly neurotoxic, lethal doses of papuan Taipan (Oxyuranus scutellatus) venom. Toxins. 2018;10(10):380. doi: 10.3390/toxins10100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin M.R., Gilliam L.L., Gilliam J., et al. Delayed LY333013 (oral) and LY315920 (intravenous) reverse severe neurotoxicity and rescue Juvenile pigs from lethal doses of Micrurus fulvius (Eastern coral snake) venom. Toxins. 2018;10(11) doi: 10.3390/toxins10110479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.C., Wu C.J., Hsiao Y.C., et al. Snake venom proteome of Protobothrops mucrosquamatus in Taiwan: delaying venom-induced lethality in a rodent model by inhibition of phospholipase A2 activity with varespladib. J. Proteonomics. 2021;234:104084. doi: 10.1016/j.jprot.2020.104084. [DOI] [PubMed] [Google Scholar]
- Longbottom J., Shearer F.M., Devine M., et al. Vulnerability to snakebite envenoming: a global mapping of hotspots. Lancet. 2018;392(10148):673–684. doi: 10.1016/S0140-6736(18)31224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matell G., Nyman D., Werner B., Wilhelmsson S. Consumption coagulopathy caused by a boomslang bite: a case report. Thromb. Res. 1973;3(2):173–182. doi: 10.1016/0049-3848(73)90067-4. [DOI] [Google Scholar]
- Pla D., Sanz L., Whiteley G., et al. What killed Karl Patterson Schmidt? Combined venom gland transcriptomic, venomic and antivenomic analysis of the South African green tree snake (the boomslang), Dispholidus typus. Biochim. Biophys. Acta Gen. Subj. 2017;1861(4):814–823. doi: 10.1016/j.bbagen.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potet J., Smith J., McIver L. Reviewing evidence of the clinical effectiveness of commercially available antivenoms in sub-Saharan Africa identifies the need for a multi-centre, multi-antivenom clinical trial. PLoS Negl. Trop. Dis. 2019;13(6) doi: 10.1371/journal.pntd.0007551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randomized, double-blinded, placebo-controlled Study to Evaluate the safety, tolerability, and Efficacy of a multi-dose Regimen of oral varespladib-Methyl in subjects Bitten by venomous snakes. clinicaltrials.gov. 2021. https://clinicaltrials.gov/ct2/show/NCT04996264 Accessed. [Google Scholar]
- Rucavado A, Escalante T, Franceschi A, et al. Inhibition of local hemorrhage and dermonecrosis induced by Bothrops asper snake venom: effectiveness of early in situ administration of the peptidomimetic metalloproteinase inhibitor batimastat and the chelating agent CaNa EDTA. Am. J. Trop. Med. Hyg. 63. Published online 2000. [PubMed]
- Sanhajariya S., Duffull S.B., Isbister G.K. Pharmacokinetics of snake venom. Toxins. 2018;10(2):73. doi: 10.3390/toxins10020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneci L., Zdenek C.N., Chowdhury A., et al. A clot twist: extreme variation in coagulotoxicity mechanisms in Mexican neotropical rattlesnake venoms. Front. Immunol. 2021;12:612846. doi: 10.3389/fimmu.2021.612846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagboom J., Mladić M., Xie C., et al. High throughput screening and identification of coagulopathic snake venom proteins and peptides using nanofractionation and proteomics approaches. PLoS Negl. Trop. Dis. 2020;14(4) doi: 10.1371/journal.pntd.0007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Still K.B.M., Nandlal R.S.S., Slagboom J., Somsen G.W., Casewell N.R., Kool J. Multipurpose HTS coagulation analysis: assay development and assessment of coagulopathic snake venoms. Toxins. 2017;9(12) doi: 10.3390/toxins9120382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang J., Zhang D., Xiao H., Xiong S., Huang C. Exploration of the inhibitory potential of varespladib for snakebite envenomation. Molecules. 2018;23(2):391. doi: 10.3390/molecules23020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell D.A., Davidson N., Greenwood B., et al. Poisoning by bites of the saw-scaled or carpet viper (Echis carinatus) in Nigeria. Q. J. Med. 1977;46:33–62. [PubMed] [Google Scholar]
- Williams H.F., Layfield H.J., Vallance T., et al. The urgent need to develop novel strategies for the diagnosis and treatment of snakebites. Toxins. 2019;11(6) doi: 10.3390/toxins11060363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2010. Guidelines for the Prevention and Clinical Management of Snakebite in Africa.https://www.who.int/publications-detail-redirect/9789290231684 Accessed. [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2018. WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins.https://www.who.int/snakebites/resources/Snake_antivenom_immunoglobulins_WHO_TRS1004_Annex5.pdf?ua=1 [Google Scholar]
- Xie C., Albulescu L.O., Bittenbinder M.A., et al. Neutralizing effects of small molecule inhibitors and metal chelators on coagulopathic Viperinae snake venom toxins. Biomedicines. 2020;8(9):297. doi: 10.3390/biomedicines8090297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Slagboom J., Albulescu L.O., et al. Neutralising effects of small molecule toxin inhibitors on nanofractionated coagulopathic Crotalinae snake venoms. Acta Pharm. Sin. B. 2020;10(10):1835–1845. doi: 10.1016/j.apsb.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Albulescu L.O., Still K.B.M., et al. Varespladib inhibits the phospholipase A2 and coagulopathic activities of venom components from hemotoxic snakes. Biomedicines. 2020;8(6):165. doi: 10.3390/biomedicines8060165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman N.J., Walker A., Naude A., Coster K., Sundman E., Fry B.G. Varespladib (LY315920) neutralises phospholipase A2 mediated prothrombinase-inhibition induced by Bitis snake venoms. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020;236:108818. doi: 10.1016/j.cbpc.2020.108818. [DOI] [PubMed] [Google Scholar]
- Zietek B.M., Mayar M., Slagboom J., et al. Liquid chromatographic nanofractionation with parallel mass spectrometric detection for the screening of plasmin inhibitors and (metallo)proteinases in snake venoms. Anal. Bioanal. Chem. 2018;410(23):5751–5763. doi: 10.1007/s00216-018-1253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.