Abstract
Background
Magnesium sulphate remains the drug of choice for both prevention and treatment of women with eclampsia. Regimens for administration of this drug have evolved over the years, but have not yet been formally evaluated.
Objectives
To assess the comparative effects of alternative regimens for the administration of magnesium sulphate when used for the care of women with pre‐eclampsia or eclampsia, or both.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (June 2010).
Selection criteria
Randomised trials comparing different regimens for administration of magnesium sulphate used for the care of women with pre‐eclampsia or eclampsia, or both.
Data collection and analysis
All four review authors assessed trial quality and extracted data independently.
Main results
We identified 17 studies of which six (866 women) met the inclusion criteria: two trials (451 women) compared regimens for women with eclampsia and four (415 women) for women with pre‐eclampsia.
Treatment of eclampsia: one trial compared loading dose alone with loading dose plus maintenance therapy for 24 hours (401 women). There was no clear difference between the groups in the risk ratio (RR) of recurrence of convulsions (RR 1.13, 95% confidence interval (CI) 0.42 to 3.05) or stillbirth (RR 1.13, 95% CI 0.66 to 1.92), and the CIs are wide. One trial compared a low dose regimen with a standard dose regimen over 24 hours (50 women). This study was too small for any reliable conclusions about the comparative effects.
Prevention of eclampsia: one trial compared intravenous with intramuscular maintenance regimen for 24 hours (17 women). This trial was too small for any reliable conclusions. Three trials compared short maintenance regimens postpartum with continuing for 24 hours after the birth (398 women), even taken together these trials were too small for any reliable conclusions.
Authors' conclusions
Although strong evidence supports the use of magnesium sulphate for prevention and treatment of eclampsia, trials comparing alternative treatment regimens are too small for reliable conclusions.
Plain language summary
Alternative magnesium sulphate regimens for women with pre‐eclampsia and eclampsia
This review found that not enough research has been carried out to show what is the best dose for magnesium sulphate for women with pre‐eclampsia or eclampsia, and how best to give it.
Pre‐eclampsia (or toxaemia) is a disorder that is usually associated with raised blood pressure (hypertension) and protein in the urine. It can occur at any time during the second half of pregnancy or in the first few weeks after delivery. Magnesium sulphate is effective in preventing eclampsia (a fit or seizure) in women who have pre‐eclampsia, and for treating women who experience an eclamptic convulsion. The review authors included six trials (866 women). Two of the trials (451 women) recruited women with eclampsia and four trials (415 women) recruited women with pre‐eclampsia. These randomised trials are too small to give reliable guidance on the advantages or disadvantages of the different regimens used. The included studies were carried out in both high‐income and low‐income countries.
Background
Pre‐eclampsia is a multisystem disorder that is usually associated with raised blood pressure (hypertension) and proteinuria. When severe, it can also involve the woman's liver, kidneys, clotting system or brain. The placenta can be affected too, leading to an increased risk of placental abruption, poor growth and early delivery for the baby. Around one in 10 women will have raised blood pressure at some time during pregnancy, and pre‐eclampsia is estimated to complicate between 2% to 8% of pregnancies (WHO 1988). It is the most common medical complication of pregnancy, and can occur at any time during the second half of pregnancy or the first few weeks after delivery. Only about 5% of women present for the first time in the days after delivery, however (Matthys 2004). For many women who have mild pre‐eclampsia the outcome is good, but severe disease can lead to death or serious problems for the woman or her baby, or both. The aetiology and diagnosis of pre‐eclampsia is discussed in more detail in the generic protocol of interventions for the prevention of pre‐eclampsia (Meher 2005).
Eclampsia, the occurrence of a convulsion (fit) in association with the syndrome of pre‐eclampsia, is a rare but serious complication of pregnancy. About one‐third of women will have their first fit after delivery of the baby (Knight 2007). Estimated to complicate around one in 2000 deliveries in Europe and other high‐income countries (Douglas 1994), and from one in 100 to 1700 deliveries in low‐ and middle‐income countries (Crowther 1985), eclampsia is associated with around 10% of maternal deaths and an estimated 50,000 women die each year having had an eclamptic convulsion (Duley 1992; Khan 2006). Anticonvulsants have long been used for women with eclampsia, in the belief that controlling the fits would improve outcome. More recently, they have also been advocated for preventing the onset of eclampsia in women with pre‐eclampsia (Magpie Trial 2002).
Magnesium sulphate for women with eclampsia
Magnesium sulphate was one of the earliest drugs suggested to have a specific anticonvulsant action in treatment of eclampsia. The first published account of this suggestion appeared in 1906 (Horn, from Chesley 1978). By the late 1920s, magnesium sulphate was being used for the treatment of women with eclampsia in both Europe (Chesley 1978) and the US (Dorsett 1926; Lazard 1925). Over the subsequent years, a range of alternative drugs have also been advocated for eclampsia. Diazepam (Lean 1968), for example, being cheap and readily available, rapidly became popular in both developed and developing countries. In the 1980s, phenytoin was proposed (Slater 1987) as having the theoretical advantage of controlling convulsions whilst avoiding sedation.
For decades, controversy raged as to which anticonvulsant was preferable for women with eclampsia (Dinsdale 1988; Donaldson 1992; Kaplan 1988; Sibai 1990). This controversy was concluded when a large randomised trial demonstrated that magnesium sulphate reduced the risk of further fits, and probably reduced the risk of maternal death, compared to either diazepam or phenytoin (Collab Trial 1995). Cochrane reviews confirm that magnesium sulphate is better than diazepam, phenytoin or lytic cocktail (usually a mixture of chlorpromazine, promethazine and pethidine) for treatment of women with eclampsia (Duley 2000; Duley 1995; Duley 2000a).
Magnesium sulphate for women with pre‐eclampsia
Anticonvulsants have also been advocated for women with pre‐eclampsia in the belief that they would prevent the onset of eclampsia, and so improve outcome. Initially, however, it was unclear whether a policy of using an anticonvulsant for women with pre‐eclampsia did more good than harm, to both her and her baby, than a policy of not using an anticonvulsant (Duley 1994). In 2002, a large randomised trial demonstrated that magnesium sulphate halved the risk of eclampsia compared to placebo (Magpie Trial 2002); this is confirmed by the systematic review (Duley 2003).
Alternative regimens for magnesium sulphate
When first introduced for women with eclampsia, magnesium sulphate was administered by the intravenous, intramuscular and subcutaneous routes, and at relatively low dose. For example, in one early report the total dose administered varied from 2 grams (g) to 6 g (Lazard 1925). Due to concern about toxicity, over the subsequent 20 years the dose was usually 10 g to 12 g in 24 hours (Eastman 1945; Stroganoff 1937). Later it was argued that larger doses might not only be safe but also have a greater therapeutic effect, and it was suggested that women be given 10 g as an intramuscular injection followed by 5 g every six hours (Eastman 1945). Having observed that plasma concentrations rise slowly after intramuscular injection, Pritchard 1955 suggested changing the loading dose to 4 g by intravenous injection, and increasing the maintenance dose to every four hours. This regimen is still widely used, particularly in the developing world where resources to support intravenous administration are often not available.
Potential disadvantages of the intramuscular route are pain and infection at the injection site. An alternative is to give the maintenance regimen by intravenous infusion. With this regimen the usual loading dose is also 4 g, followed by an infusion of 1 g per hour (Zuspan 1978). This is the standard intravenous regimen, widely used in many countries. Increasing the loading dose to 6 g (Sibai 1990) and the infusion rate to 2 g per hour (Sibai 1984) has also been suggested.
The regimens suggested by Pritchard and Zuspan are the two that have been evaluated in the randomised trials of anticonvulsants for women with eclampsia and pre‐eclampsia (Duley 1996; Duley 2003). In most of these trials, clinical monitoring was used: hourly measurement of urine output, with regular checking of the respiration rate and tendon reflexes. Serum monitoring of magnesium levels, which is expensive and not available in many settings, is not necessary. The effectiveness and safety of magnesium sulphate for women with eclampsia and pre‐eclampsia has been demonstrated with clinical monitoring alone (Duley 2000; Duley 2000a; Duley 2003; Duley 1995).
In low‐income countries, where the purchase price of magnesium sulphate is a substantial proportion of the overall cost of treatment (Simon 2006) and availability of staff trained in its administration may be limited, there is growing interest in the use of short regimens. In settings where women may require transfer over large distances, it is also important to know whether administration of a single dose of magnesium sulphate at community or primary care level, before transfer, would be beneficial, and if so what is the best regimen when they arrive in hospital
Side effects and adverse effects of magnesium sulphate
The most reliable data to date on the side effects and potential hazards of magnesium sulphate, compared to placebo, come from the Magpie Trial which recruited over 10,000 women (Magpie Trial 2002). The most common side effect is flushing (24% magnesium sulphate versus 3% placebo). Others are far less common and include nausea, vomiting, muscle weakness, thirst, headache, drowsiness and confusion. Although magnesium sulphate can lead to respiratory depression and respiratory arrest, these hazards appear to be rare. These data apply to women who receive similar regimens to those used in the trial. Higher dose regimens may be associated with a great risk of side effects and adverse effects.
If magnesium sulphate toxicity does occur, intravenous calcium gluconate is an effective antidote.
Mode of action for magnesium sulphate
Exactly how magnesium sulphate might control eclamptic convulsions is unclear. Magnesium may have a localised cerebral effect. For example, it may cause vasodilatation with subsequent reduction of cerebral ischaemia (Belfort 1992), or block some of the neuronal damage associated with ischaemia (Goldman 1988; Sadeh 1989), or both. A possible mechanism for vasodilatation is relaxation of smooth muscle, and it has been suggested that magnesium may have a generalised effect on all smooth muscle, including the peripheral vasculature and uterus. Alternatively, any effects of magnesium sulphate on control of eclamptic convulsions may be, wholly or partially, through its role as a blocker of N‐methyl‐D‐aspartate (NMDA) receptors in the brain. These NMDA receptors are activated in response to asphyxia, leading to calcium influx into the neurones, which causes cell injury. It is suggested that magnesium may block these receptors, so reducing calcium influx and protecting the neurones from damage.
Rationale for the review
Magnesium sulphate remains the drug of choice for both prophylaxis and treatment of women with eclampsia. Implementation of magnesium sulphate would be strengthened if guidelines and recommendations for practice could be based on reliable evidence about the comparative effects of alternative regimens. Regimens for administration of magnesium sulphate have evolved over the years, but have not been formally evaluated. It is therefore relevant to assess the pros and cons of alternative strategies for administration. As administration of magnesium sulphate requires regular supervision by trained staff, which is costly (Simon 2006), and higher doses may be associated with a greater risk of side effects and adverse events, it is particularly important to assess the minimum effect dose and duration of treatment. This review aims to assess the comparative effects of alternative regimens for magnesium sulphate.
Objectives
The aim of this review is to assess the comparative effects of alternative regimens for the administration of magnesium sulphate when used for the care of women with pre‐eclampsia or eclampsia, or both.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials that compare different regimens for administration of magnesium sulphate used for the care of women with pre‐eclampsia or eclampsia, or both. We excluded quasi‐random designs, such as those where allocation is by date of birth or hospital number, as well as those with a crossover design. We included cluster‐randomised trials.
Types of participants
Any women with a diagnosis of pre‐eclampsia or eclampsia, irrespective of whether this is during pregnancy, in labour or after delivery, and regardless of whether the pregnancy was single or multiple. We included women regardless of whether they had previously received magnesium sulphate therapy.
Types of interventions
All randomised comparisons of one method or regimen for giving magnesium sulphate with another. Comparisons could include different dose regimens, whether the intramuscular or intravenous route was used for maintenance therapy, and different durations of therapy.
Types of outcome measures
Outcomes include measures of clinical outcome and use of health service resources for the mother and, for women randomised before delivery, for the baby.
Primary outcomes
For all women
Eclampsia (for women with pre‐eclampsia), or recurrence of convulsions (for women with eclampsia).
Severe morbidity: women who have at least one of the following: liver failure, renal failure, HELLP syndrome (haemolysis, elevated liver enzymes and low platelets), disseminated intravascular coagulation, stroke and pulmonary oedema.
Side effects: such as respiratory depression, need for calcium gluconate, flushing, nausea or vomiting, problems at the injection site.
Treatment discontinued because of side effects or adverse effects.
For the baby
Death of the baby before discharge from hospital: including stillbirth, perinatal death and neonatal death.
Secondary outcomes
For all women
Maternal death.
Admission and length of stay in high‐dependency unit.
Poor blood pressure control: including hypertension, antihypertensive drugs, hypotension.
Need for additional anticonvulsant.
Severe morbidity: each of the outcomes included in 'severe morbidity' above (primary outcome 2) will be evaluated separately.
For women randomised before delivery
Mode of delivery: caesarean section or vaginal.
Induction or augmentation of labour.
Prolonged labour.
Placental abruption (separation of the placenta from the uterus).
Postpartum haemorrhage: 500 ml or more; 1000 ml or more.
Retained placenta.
For the baby
Admission to special care nursery or neonatal intensive care unit.
Respiratory morbidity: including need for ventilation, number of days ventilated, respiratory distress syndrome (immaturity of the lungs).
Other neonatal morbidity: including necrotising enterocolitis (bleeding in the bowel), any intraventricular haemorrhage (brain haemorrhage) and severe intraventricular haemorrhage.
Side effects: such as hypotension.
Development in childhood: including cerebral palsy and major neurodevelopmental delay.
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (June 2010).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We also searched the metaRegister of controlled trials (28 June 2010) using the terms 'magnesium pre‐eclampsia', 'magnesium preeclampsia', and 'magnesium eclampsia'.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
All four review authors carried out independent assessment of each citation for inclusion in the review. We resolved any differences in opinion by discussion.
Data extraction and management
All four review authors extracted data, and we resolved discrepancies through discussion. If we could not reach agreement, we excluded that item until further clarification was available from the authors. We entered data onto the Review Manager software (RevMan 2008), where a second review author checked them for accuracy.
Assessment of risk of bias in included studies
All four review authors independently assessed the quality of each included trial using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We described the methods used for generation of the randomisation sequence for each trial. We assessed each study for (i) quality of the concealment of allocation, (ii) completeness of follow up and (iii) blinding of the assessment of outcome as follows.
Selection bias (randomisation and allocation concealment)
We assessed concealment of allocation for each trial, using the following criteria:
Yes: adequate concealment of allocation, such as telephone randomisation, consecutively numbered, sealed opaque envelopes;
Unclear: unclear whether concealment of allocation was adequate;
No: inadequate concealment of allocation such as open random‐number tables, sealed envelopes that are not numbered or opaque.
Where the method of allocation concealment was unclear, we attempted to contact authors to provide further details.
We excluded studies with a quasi‐random design, such as allocation by alternate days or date of birth, as well as those with a crossover design.
Attrition bias (loss of participants, eg withdrawals, dropouts, protocol deviations)
We assessed completeness of follow up, and assigned a quality score, using the following criteria: (A) less than 5% of participants excluded from analysis; (B) 5% to 10% of participants excluded from analysis; (C) more than 10% and up to and including 20% of participants excluded from analysis.
Performance bias (blinding of participants, researchers and outcome assessment)
We describe blinding using the following criteria: (1) blinding of participants (yes/no/unclear or unspecified); (2) blinding of caregiver (yes/no/unclear or unspecified); (3) blinding of outcome assessment (yes/no/unclear or unspecified).
Measures of treatment effect
We carried out statistical analyses using the Review Manager software (RevMan 2008). We presented results as a summary risk ratio with 95% confidence intervals and, where relevant, as risk difference and number needed to treat. We used fixed‐effect meta‐analysis for combining data in the absence of significant heterogeneity if trials were sufficiently similar. If we found heterogeneity, we explored this by prespecified sensitivity and subgroup analysis, followed by random‐effects meta‐analysis if required.
Unit of analysis issues
We would have included cluster randomised trials in the analyses along with individually randomised trials. If cluster randomised trials are identified in a future update, we will adjust their standard errors using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population (Higgins 2009). If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We would consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We would also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
We will exclude crossover trials, as they are not an appropriate study design for assessment of the effects of an intervention during pregnancy.
Dealing with missing data
We analysed data for all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. If in the original reports participants were not analysed in the group to which they were randomised, and there was sufficient information in the trial report, we attempted to restore them to the correct group. If data were missing, whenever possible we sought clarification from the authors.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T2, I² and Chi² statistics. We regarded heterogeneity as substantial if T2 was greater than zero and either I2 was greater than 30% or there as a low P‐value (< 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there had been 10 or more studies in the meta‐analysis we planned to investigate reporting biases (such as publication bias) using funnel plots. We would assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes we would use the test proposed by Egger 1997, and for dichotomous outcomes we would use the test proposed by Harbord 2006. If we detect asymmetry in any of these tests or if it is suggested by a visual assessment, we plan to perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2008). We use fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and we judged the trials' populations and methods sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if we detected substantial statistical heterogeneity, we planned to use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. We would treat the random‐effects summary as the average range of possible treatment effects and we would discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful we would not combine trials.
If we used random‐effects analyses, we planned to present the results as the average treatment effect with its 95% confidence interval, and the estimates of T2 and I2.
Subgroup analysis and investigation of heterogeneity
We planned separate comparisons for women with eclampsia and those with pre‐eclampsia, and for different types of regimens.
If we had identified substantial heterogeneity, we would have investigated it using subgroup analyses and sensitivity analyses. In future updates, if we identify heterogeneity we would consider whether an overall summary was meaningful, and if it was, use random‐effects analysis to produce it.
We planned the following subgroup analyses, if sufficient data were available, based on:
for women with pre‐eclampsia, severity at trial entry: severe pre‐eclampsia, not severe pre‐eclampsia, unclear or mixed.
whether women were randomised before or after delivery: randomised before delivery, randomised after delivery, unclear or mixed (this analysis will be based on outcomes for the mother only);
route of administration of maintenance regimen: intravenous, intramuscular, unclear or mixed;
other aspects of the magnesium sulphate regimen: such as whether or not there was a loading dose; duration of treatment; whether combined with another type of anticonvulsant; setting such as primary or secondary care; type of monitoring;
previous magnesium sulphate therapy: magnesium sulphate therapy before trial entry, no magnesium sulphate before trial entry; unclear whether magnesium sulphate therapy.
Sensitivity analysis
We planned to carry out sensitivity analysis to explore the effect of trial quality. We would have excluded studies of poor quality (those with 'no' or 'unclear' for concealment of allocation or blinding) in order to assess for any substantive difference to the overall result.
We used only the main outcomes, listed above, for the subgroup and sensitivity analyses. There were insufficient data in any of the comparisons for sensitivity analysis.
Results
Description of studies
Included studies
We have included six trials (866 women) in this review:
Types of participants
Two trials (Bangladesh 2002; India 2007) (451 women) recruited women with eclampsia: in the largest study (Bangladesh 2002, 401 women) women were recruited regardless of whether or not they had delivered; in the small study (India 2007) all the women were recruited before delivery.
Four trials (South Africa 1994; USA 2005; USA 2006; India 2008) (415 women) recruited women with pre‐eclampsia. One small study (17 women) (South Africa 1994) recruited women with severe pre‐eclampsia or imminent eclampsia. Three studies (USA 2005; USA 2006; India 2008) recruited women postpartum: one recruited 98 women who had a diagnosis of severe pre‐eclampsia before delivery (USA 2005); another recruited 200 women who had mild pre‐eclampsia either before delivery, or up to two hours postpartum (USA 2006) and the third recruited 100 women with severe pre‐eclampsia who were postpartum (India 2008).
Types of intervention
For women with eclampsia
One trial (Bangladesh 2002) compared a loading dose of magnesium sulphate alone with a loading dose plus maintenance regimen. The loading dose was 4 g IV plus 6 g intramuscular (IM), and maintenance was 2.5 g IM every four hours. These doses are standard within Bangladesh. They are lower than doses used in most other countries, but have been agreed in Bangladesh as appropriate because Bangladeshi women are smaller than women from many other countries.
The other trial (India 2007) compared the Dhaka regimen from Bangladesh with the magnesium sulphate regimen recommended by Bhalla. Although the regimens are not described in the paper, the references cited are for the Dhaka regimen, as used in Bangladesh 2002 (see above) and for the Bhalla regimen (Bhalla 1994) which was 4 g intravenous (IV) plus 8 g IM as a loading dose, then 4 g IM every four hours for maintenance therapy.
For women with pre‐eclampsia
A small trial of women with severe pre‐eclampsia or imminent eclampsia (South Africa 1994) compared a high dose intravenous regimen of 6 g IV loading dose followed by 2 g per hour by intravenous infusion with the standard intramuscular regimen of 4 g IV plus 10 g IM loading dose, and 5 g every four hours for maintenance therapy.
Three trials recruited women postpartum (USA 2005; USA 2006; India 2008) and compared alternative maintenance regimens. One compared stopping maintenance therapy after the onset of diuresis with continuing for 24 hours after delivery (USA 2005); another compared individualised therapy based on clinical criteria with continuing for 24 hours (India 2008), and the third compared 12 hours postpartum maintenance therapy with 24 hours (USA 2006).
Excluded studies
We excluded 11 studies from the review. Nine studies did not report clinical outcomes (Brazil 2010; Thailand 1992; Thailand 1996; Thailand 1999; USA 1981; USA 1989; Bangladesh 2009; India 2009a; Nigeria 2009; India 2009); of these six are only published as abstracts (Brazil 2010; Thailand 1999; USA 1989; Bangladesh 2009; India 2009a; Nigeria 2009), a seventh was published in Thai and the English abstract suggested no clinical outcomes were available (Thailand 1992). One study was not a randomised trial (India 2009, and the last study compared magnesium sulphate with magnesium chloride (Iran 2005).
Risk of bias in included studies
Overall, there was little information on methods provided by study authors.
Sequence generation and concealment of allocation
Of the included trials, three reported adequate sequence generation (India 2007; USA 2005; USA 2006) and two adequate concealment of allocation (USA 2005; USA 2006. In USA 2005 sequence generation was by random number tables with block size of 10, and in USA 2006 by computer. For both these studies allocation concealment was by opaque sealed numbered envelopes.
Four trials (Bangladesh 2002; India 2007; South Africa 1994; India 2008) provided little or no information about sequence generation and allocation concealment. In Bangladesh 2002 allocation was described as “by lottery” which involved selecting a piece of paper from a box. There is no mention of how the information on the paper was concealed, to prevent the person selecting from knowing in advance what the allocation would be, nor is there any mention of a system to ensure pieces of paper could not be returned to the box and another selected for the same woman. Although India 2007 reported using random number tables for sequence generation, there is no information about concealment of allocation. In South Africa 1994 there is no description of either sequence generation or concealment of allocation. India 2008 is described as 'randomised', but with no further information.
Blinding
Blinding of the intervention was not mentioned for any of the included trials. However, blinding of clinicians and participants was unlikely to have been possible.
Follow up and exclusions
Post‐randomisation exclusions were not reported in any of these trials. There were no losses to follow up in South Africa 1994, Bangladesh 2002, USA 2005; India 2008 or India 2007. In USA 2006, four women (2%) were lost to follow up in the 24‐hour group.
Effects of interventions
Comparison 1: women with eclampsia: loading dose alone versus loading dose plus maintenance regimen for 24 hours
This comparison includes one trial, with 401 women. The trial evaluated the intramuscular maintenance regimen, and hence there are no data for the intravenous regimen.
Outcome for the women
There were insufficient data for any reliable conclusions about the differential effects on the two reported primary outcomes: recurrence of convulsion (risk ratio (RR) 1.13, 95% confidence interval (CI) 0.42 to 3.05), and maternal death (RR 0.89, 95% CI 0.37 to 2.14). There was no clear difference between the groups in the risk ratio of caesarean section (RR 1.02, 95% CI 0.88 to 1.18).
There were no data on any other measures of maternal morbidity or adverse effects.
Outcome for the babies
Stillbirth was the only outcome reported for the babies; there was no clear difference between the two treatment groups (RR 1.13, 95% CI 0.66 to 1.92).
Comparison 2: women with eclampsia: lower dose regimens versus standard dose regimens over 24 hours
This comparison includes one trial, with 50 women. The trial compared the low‐dose intramuscular regimen recommended in national guidelines for treatment of women with eclampsia in Bangladesh, with a standard intramuscular regimen used in India. Hence there are no data for intravenous regimens.
Outcome for the women
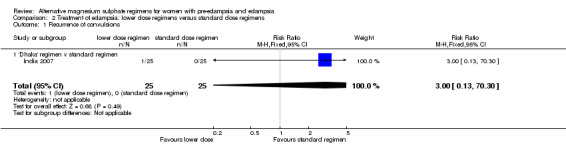
There were insufficient data for any reliable conclusions about the differential effects on the one reported primary outcome: recurrence of convulsions (RR 3.00, 95% CI 0.13 to 70.30).
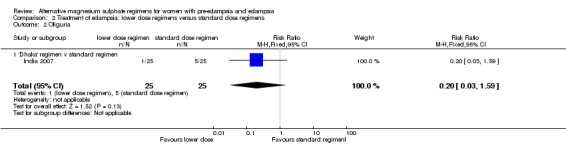
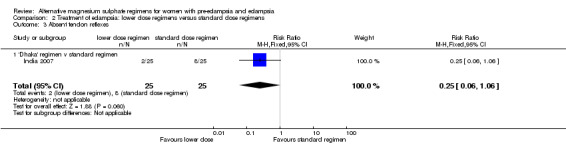
There were no clear differences between the two treatment groups in the risk ratio of oliguria (reduced urine output) (RR 0.20, 95% CI 0.03 to 1.59), and absent tendon reflexes (RR 0.25, 95% CI 0.06 to 1.06).
Outcome for the babies
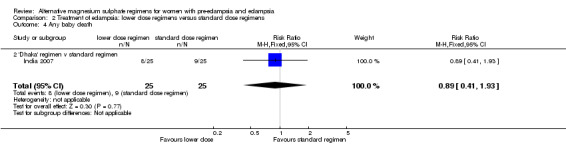
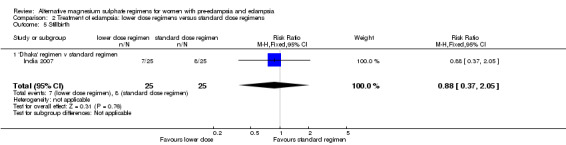
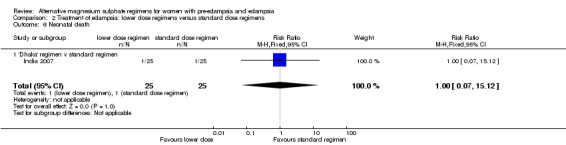
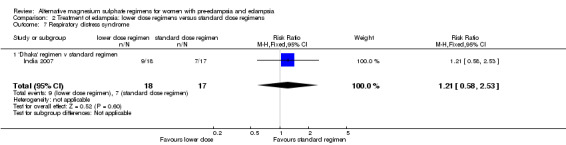
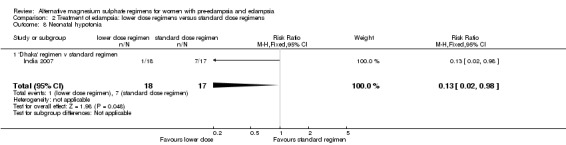
There was no clear difference between the treatment groups in the risk ratio of baby death (RR 0.89, 95% CI 0.41 to 1.93). Women allocated the lower dose, rather than standard, regimen were less likely to have babies who developed neonatal hypotonia, although the confidence intervals were wide (RR 0.13, 95% CI 0.02 to 0.98). There were insufficient data for reliable conclusions about the differential effects on other reported measures of neonatal morbidity: admission to special care baby unit (RR 2.36, 95% CI 0.53 to 10.58), respiratory distress syndrome (RR 1.21, 95% CI 0.58 to 2.53) or neonatal respiratory depression (RR 0.31, 95% CI 0.04 to 2.74).
Comparison 3: prevention of eclampsia: intravenous versus standard intramuscular maintenance regimen for 24 hours
This comparison includes one trial, with 17 women. The trial compared a high‐dose intravenous regimen for magnesium sulphate with a standard intramuscular regimen.
Outcome for the women
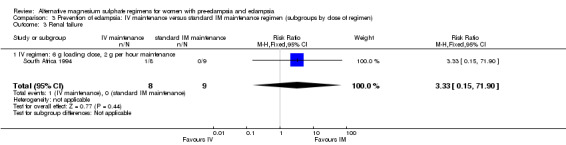
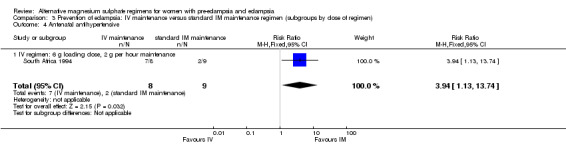
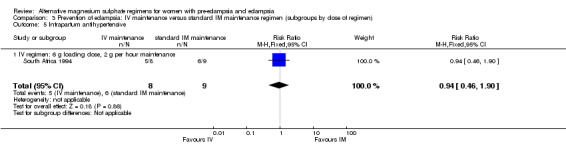
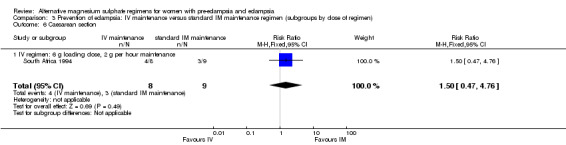
No women in this study developed eclampsia. Women allocated the standard intramuscular regimen were less likely to need antenatal antihypertensive therapy than those allocated the high‐dose intravenous regimen, although the confidence intervals were wide (RR 3.94, 95% CI 1.13 to 13.74). The trial was too small for any reliable conclusions about other reported measures of maternal morbidity: magnesium sulphate toxicity (RR 3.33, 95% CI 0.15 to 71.90); renal failure (RR 3.33, 95% CI 0.15 to 71.90); intrapartum antihypertensive therapy (RR 0.94, 95% CI 0.46 to 1.90); and caesarean section (RR 1.50, 95% CI 0.47 to 4.76).
Outcome for the babies
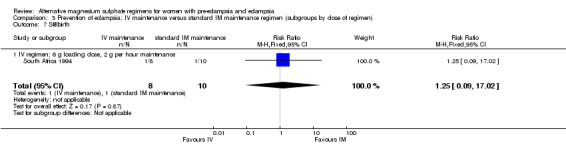
Stillbirth was the only outcome reported for the babies, and the study was too small for any reliable conclusions about the differential effect (RR 1.25, 95% CI 0.09 to 17.02).
Comparison 4: duration of postpartum maintenance regimen: short versus standard (24 hours) (subgroups by severity of pre‐eclampsia)
This comparison included three trials, involving 398 women. One trial recruited women with severe pre‐eclampsia and used onset of diuresis for the short regimen, another recruited women with women with severe pre‐eclampsia and used clinical criteria to terminate treatment in the experimental arm, and the third recruited women with mild pre‐eclampsia and used 12 hours as the short regimen.
Primary outcome
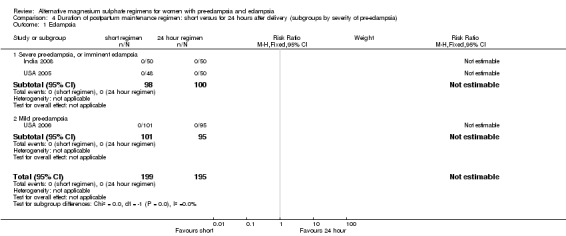
No women in these three trials developed eclampsia.
Secondary outcomes
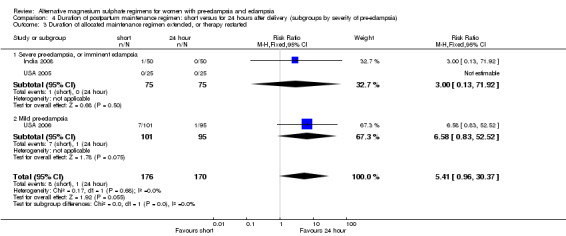
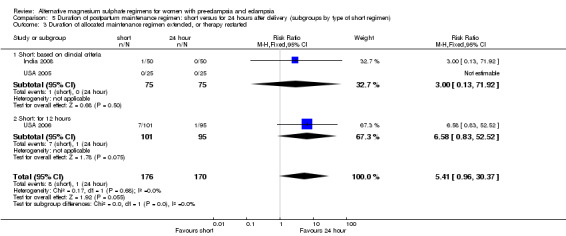
Unsurprisingly, women allocated a short maintenance regimen seemed more likely to have the allocated regimen extended or treatment restarted, although this difference did not achieve statistical significance (three trials, 346 women: RR 5.41, 95% CI 0.0.96 to 30.37).
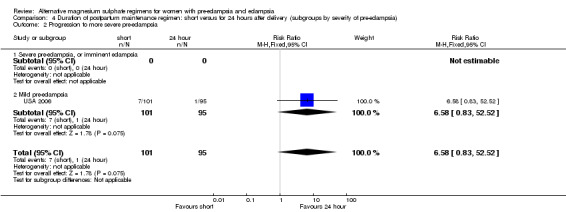
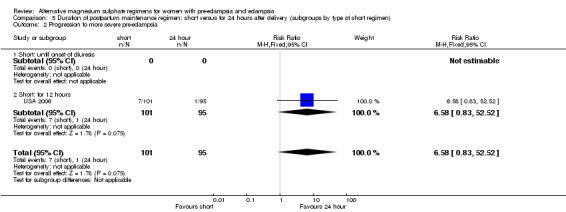
There were insufficient data for reliable conclusions about the differential effects on progression to more severe pre‐eclampsia (one trial, 196 women; RR 6.58, 95% CI 0.83 to 52.52) or antihypertensive drug at discharge (two trials, 198 women; RR 1.32, 95% CI 0.95 to 1.84).
Due to heterogeneity, data for length of postpartum hospital stay were not totaled across the subgroups.
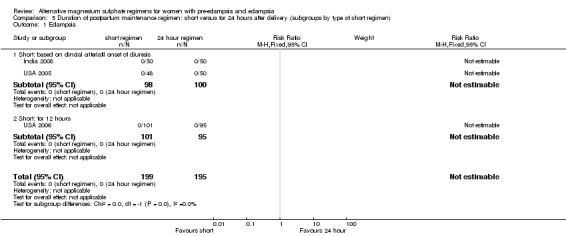
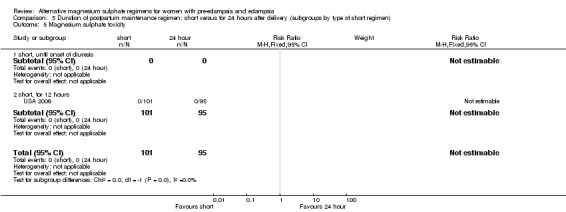
Comparison 5: duration of postpartum maintenance regimen: short versus standard (24 hours) (subgroups by type of short regimen)
The trials included in this comparison are the same as in comparison 4, and so it is not possible to distinguish between them.
Discussion
There is strong evidence from systematic reviews of randomised trials to support the use of magnesium sulphate for the prevention and treatment of women with eclampsia (Duley 1995; Duley 2000; Duley 2000a; Duley 2003). Nevertheless, there is little reliable evidence from randomised trials assessing the minimum effective dose, the comparative effects of alternative routes of administration (intravenous or intramuscular), or the ideal duration of therapy.
Summary of main results
We found six randomised trials with 866 women recruited; 451 with eclampsia and 415 with pre‐eclampsia. Even taken together, these trials are too small to provide reliable evidence about the comparative effects of alternative magnesium sulphate regimens for women with eclampsia or pre‐eclampsia.
Overall completeness and applicability of evidence
This review is based on a comprehensive search strategy, without language restriction. It is therefore, likely to be complete. The included studies were conducted in both high‐income and low‐income countries, and are therefore, widely applicable to the care of women with eclampsia and pre‐eclampsia.
All studies were conducted in a hospital setting. No trials have assessed the benefits and adverse effects of starting therapy before transfer to hospital, compared to waiting until the woman is admitted to hospital.
The main limitation of this review is the lack of data; the small number of studies with relatively small sample size, and missing data for several important outcomes.
Quality of the evidence
For two trials, sequence generation and allocation concealment were adequate. For the other four trials, it was unclear whether these were adequate. None of the trials reported blinding, and whilst it would have been difficult to blind the intervention, blinding of assessment of outcome could have been possible for some outcomes.
Potential biases in the review process
We attempted to minimise bias in a number of ways. We used a comprehensive search strategy. Although the trials register of the Cochrane Pregnancy and Childbirth Group is extensive, it is possible that some studies conducted in low‐ and middle‐income countries may not have been identified, if they were either not published or published in journals not indexed in widely accessible bibliographic databases. If such studies are identified we will include them in future updates of this review.
The review authors all independently assessed eligibility for inclusion, carried out data extraction and assessed risk of bias. This process aimed to minimise bias in conduct of the review.
Agreements and disagreements with other studies or reviews
We are not aware of any other reviews comparing different regimens for magnesium sulphate in women with pre‐eclampsia and eclampsia.
Other Cochrane Reviews compare magnesium sulphate with alternative drugs for women with eclampsia (Duley 1995; Duley 2000; Duley 2000a); evaluate magnesium sulphate for women with pre‐eclampsia (Duley 2003); and evaluate magnesium sulphate for neuroprotection following preterm birth (Doyle 2009).
Authors' conclusions
Implications for practice.
In the absence of reliable evidence from randomised trials to guide the choice of regimen for magnesium sulphate when used for women with pre‐eclampsia or eclampsia, clinicians are likely to choose either a regimen they are familiar with, or one that is recommended in local guidelines.
It seems sensible to choose regimens that have been used, and shown to be effective, in the randomised trials demonstrating effectiveness. For women with eclampsia, the largest trial is the Collaborative Eclampsia Trial (Collab Trial 1995), and for women with pre‐eclampsia the largest study is the Magpie Trial (Magpie Trial 2002). These studies used the same regimens for intramuscular (IM) and intravenous (IV) magnesium sulphate (IV: 4 g loading dose over 10 to 15 minutes followed by infusion of 1 g/hour over 24 hours. IM: 4 g IV and 10 g IM as loading dose followed by 5 g IM every 4 hours for 24 hours).
Implications for research.
There are several important questions about how best to use magnesium sulphate for women with eclampsia and pre‐eclampsia. These include: what is the minimum effective dose (loading and maintenance); what are the advantages and disadvantages of intramuscular and intravenous administration; can the initial dose be safely given at community or primary health care level before transfer to hospital, and when is best to stop treatment.
To address these questions will require large randomised trials of women with pre‐eclampsia or eclampsia which compare alternative regimens and assess the comparative effects on mortality, serious morbidity, adverse effects, and use of hospital resources for both the women and their babies. As eclampsia is most common in low‐ and middle‐income countries, the priority is to conduct trials relevant to maternal health in these settings.
What's new
| Date | Event | Description |
|---|---|---|
| 15 March 2011 | Amended | Contact details amended. |
Acknowledgements
Our thanks to Amita Suneja for access to unpublished data.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Treatment of eclampsia: loading dose alone versus loading dose + maintenance regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence of convulsions | 1 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.42, 3.05] |
| 1.1 Intravenous maintenance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Intramuscular maintenance | 1 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.42, 3.05] |
| 2 Maternal death | 1 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.37, 2.14] |
| 2.1 Intravenous maintenance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Intramuscular maintenance | 1 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.37, 2.14] |
| 3 Caesarean section | 1 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.88, 1.18] |
| 3.1 Intravenous maintenance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Intramuscular maintenance | 1 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.88, 1.18] |
| 4 Stillbirth | 1 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.66, 1.92] |
| 4.1 Intravenous maintenance | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Intramuscular maintenance | 1 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.66, 1.92] |
1.1. Analysis.
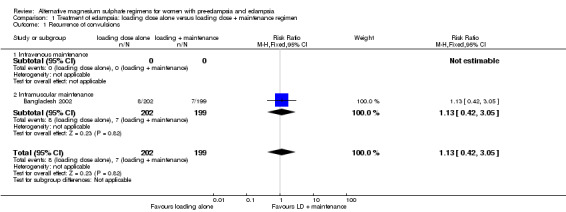
Comparison 1 Treatment of eclampsia: loading dose alone versus loading dose + maintenance regimen, Outcome 1 Recurrence of convulsions.
1.2. Analysis.
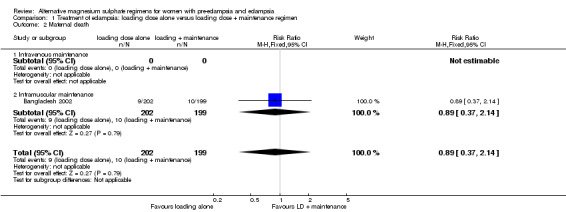
Comparison 1 Treatment of eclampsia: loading dose alone versus loading dose + maintenance regimen, Outcome 2 Maternal death.
1.3. Analysis.
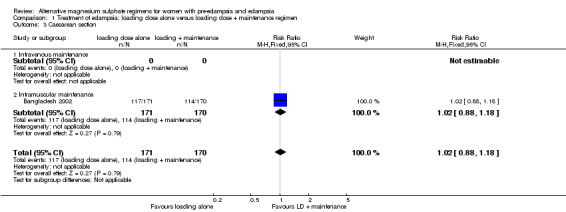
Comparison 1 Treatment of eclampsia: loading dose alone versus loading dose + maintenance regimen, Outcome 3 Caesarean section.
1.4. Analysis.
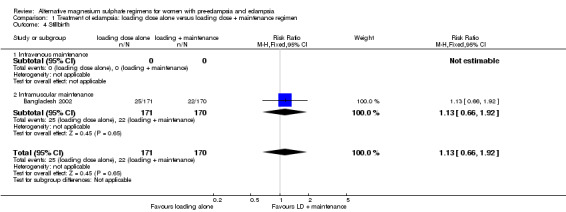
Comparison 1 Treatment of eclampsia: loading dose alone versus loading dose + maintenance regimen, Outcome 4 Stillbirth.
Comparison 2. Treatment of eclampsia: lower dose regimens versus standard dose regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence of convulsions | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.30] |
| 1.1 'Dhaka' regimen v standard regimen | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.30] |
| 2 Oliguria | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.59] |
| 2.1 'Dhaka' regimen v standard regimen | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.59] |
| 3 Absent tendon reflexes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 1.06] |
| 3.1 'Dhaka' regimen v standard regimen | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 1.06] |
| 4 Any baby death | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.41, 1.93] |
| 4.2 'Dhaka' regimen v standard regimen | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.41, 1.93] |
| 5 Stillbirth | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.37, 2.05] |
| 5.1 'Dhaka' regimen v standard regimen | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.37, 2.05] |
| 6 Neonatal death | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.12] |
| 6.1 'Dhaka' regimen v standard regimen | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.12] |
| 7 Respiratory distress syndrome | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.58, 2.53] |
| 7.1 'Dhaka' regimen v standard regimen | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.58, 2.53] |
| 8 Neonatal hypotonia | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.98] |
| 8.1 'Dhaka' regimen v standard regimen | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.98] |
| 9 Neonatal respiratory depression | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.04, 2.74] |
| 9.1 'Dhaka' regimen v standard regimen | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.04, 2.74] |
| 10 Admission to special care baby unit | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.53, 10.58] |
| 10.1 'Dhaka' regimen v standard regimen | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.53, 10.58] |
2.1. Analysis.
Comparison 2 Treatment of eclampsia: lower dose regimens versus standard dose regimens, Outcome 1 Recurrence of convulsions.
2.2. Analysis.
Comparison 2 Treatment of eclampsia: lower dose regimens versus standard dose regimens, Outcome 2 Oliguria.
2.3. Analysis.
Comparison 2 Treatment of eclampsia: lower dose regimens versus standard dose regimens, Outcome 3 Absent tendon reflexes.
2.4. Analysis.
Comparison 2 Treatment of eclampsia: lower dose regimens versus standard dose regimens, Outcome 4 Any baby death.
2.5. Analysis.
Comparison 2 Treatment of eclampsia: lower dose regimens versus standard dose regimens, Outcome 5 Stillbirth.
2.6. Analysis.
Comparison 2 Treatment of eclampsia: lower dose regimens versus standard dose regimens, Outcome 6 Neonatal death.
2.7. Analysis.
Comparison 2 Treatment of eclampsia: lower dose regimens versus standard dose regimens, Outcome 7 Respiratory distress syndrome.
2.8. Analysis.
Comparison 2 Treatment of eclampsia: lower dose regimens versus standard dose regimens, Outcome 8 Neonatal hypotonia.
2.9. Analysis.
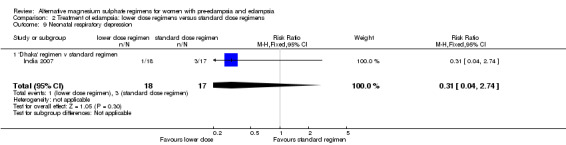
Comparison 2 Treatment of eclampsia: lower dose regimens versus standard dose regimens, Outcome 9 Neonatal respiratory depression.
2.10. Analysis.
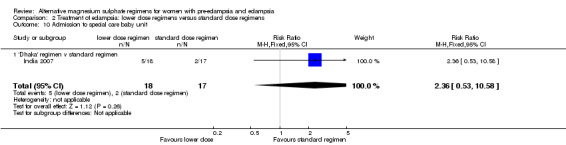
Comparison 2 Treatment of eclampsia: lower dose regimens versus standard dose regimens, Outcome 10 Admission to special care baby unit.
Comparison 3. Prevention of eclampsia: IV maintenance versus standard IM maintenance regimen (subgroups by dose of regimen).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eclampsia | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 IV regimen: 6 g loading dose, 2 g per hour maintenance | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Magnesium sulphate toxicity | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.15, 71.90] |
| 2.1 IV regimen: 6 g loading dose, 2 g per hour maintenance | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.15, 71.90] |
| 3 Renal failure | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.15, 71.90] |
| 3.1 IV regimen: 6 g loading dose, 2 g per hour maintenance | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.15, 71.90] |
| 4 Antenatal antihypertensive | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.94 [1.13, 13.74] |
| 4.1 IV regimen: 6 g loading dose, 2 g per hour maintenance | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.94 [1.13, 13.74] |
| 5 Intrapartum antihypertensive | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.46, 1.90] |
| 5.1 IV regimen: 6 g loading dose, 2 g per hour maintenance | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.46, 1.90] |
| 6 Caesarean section | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.47, 4.76] |
| 6.1 IV regimen: 6 g loading dose, 2 g per hour maintenance | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.47, 4.76] |
| 7 Stillbirth | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.09, 17.02] |
| 7.1 IV regimen: 6 g loading dose, 2 g per hour maintenance | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.09, 17.02] |
3.1. Analysis.
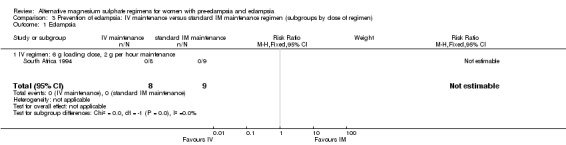
Comparison 3 Prevention of eclampsia: IV maintenance versus standard IM maintenance regimen (subgroups by dose of regimen), Outcome 1 Eclampsia.
3.2. Analysis.
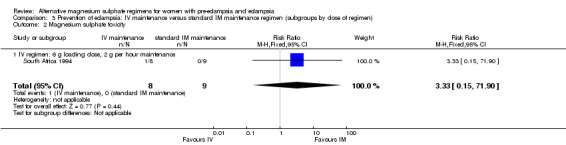
Comparison 3 Prevention of eclampsia: IV maintenance versus standard IM maintenance regimen (subgroups by dose of regimen), Outcome 2 Magnesium sulphate toxicity.
3.3. Analysis.
Comparison 3 Prevention of eclampsia: IV maintenance versus standard IM maintenance regimen (subgroups by dose of regimen), Outcome 3 Renal failure.
3.4. Analysis.
Comparison 3 Prevention of eclampsia: IV maintenance versus standard IM maintenance regimen (subgroups by dose of regimen), Outcome 4 Antenatal antihypertensive.
3.5. Analysis.
Comparison 3 Prevention of eclampsia: IV maintenance versus standard IM maintenance regimen (subgroups by dose of regimen), Outcome 5 Intrapartum antihypertensive.
3.6. Analysis.
Comparison 3 Prevention of eclampsia: IV maintenance versus standard IM maintenance regimen (subgroups by dose of regimen), Outcome 6 Caesarean section.
3.7. Analysis.
Comparison 3 Prevention of eclampsia: IV maintenance versus standard IM maintenance regimen (subgroups by dose of regimen), Outcome 7 Stillbirth.
Comparison 4. Duration of postpartum maintenance regimen: short versus for 24 hours after delivery (subgroups by severity of pre‐eclampsia).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eclampsia | 3 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Severe pre‐eclampsia, or imminent eclampsia | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Mild pre‐eclampsia | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Progression to more severe pre‐eclampsia | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.58 [0.83, 52.52] |
| 2.1 Severe pre‐eclampsia, or imminent eclampsia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Mild pre‐eclampsia | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.58 [0.83, 52.52] |
| 3 Duration of allocated maintenance regimen extended, or therapy restarted | 3 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.41 [0.96, 30.37] |
| 3.1 Severe pre‐eclampsia, or imminent eclampsia | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 3.2 Mild pre‐eclampsia | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.58 [0.83, 52.52] |
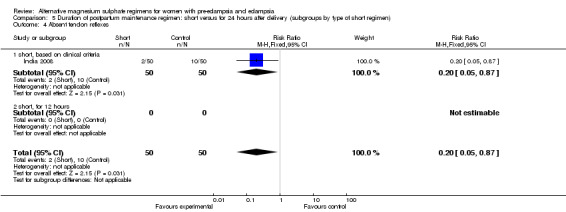
| 4 Absent tendon reflexes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.87] |
| 4.1 severe pre‐eclampsia, or imminent eclampsia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.87] |
| 4.2 mild pre‐eclampsia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Magnesium sulphate toxicity | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 severe pre‐eclampsia, or imminent eclampsia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 mild pre‐eclampsia | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Length of postpartum hospital stay (days) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Severe pre‐eclampsia, or imminent eclampsia | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.04, 0.84] |
| 6.2 Mild pre‐eclampsia | 1 | 196 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.47, 0.07] |
| 7 Antihypertensive drug at discharge | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.95, 1.84] |
| 7.1 severe pre‐eclampsia, or imminent eclampsia | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.95, 1.84] |
| 7.2 mild pre‐eclampsia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
Comparison 4 Duration of postpartum maintenance regimen: short versus for 24 hours after delivery (subgroups by severity of pre‐eclampsia), Outcome 1 Eclampsia.
4.2. Analysis.
Comparison 4 Duration of postpartum maintenance regimen: short versus for 24 hours after delivery (subgroups by severity of pre‐eclampsia), Outcome 2 Progression to more severe pre‐eclampsia.
4.3. Analysis.
Comparison 4 Duration of postpartum maintenance regimen: short versus for 24 hours after delivery (subgroups by severity of pre‐eclampsia), Outcome 3 Duration of allocated maintenance regimen extended, or therapy restarted.
4.4. Analysis.
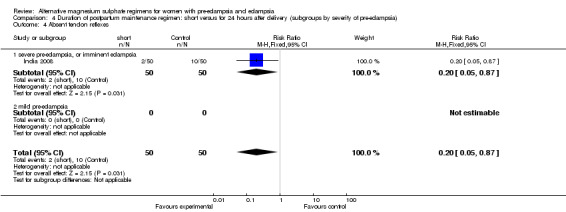
Comparison 4 Duration of postpartum maintenance regimen: short versus for 24 hours after delivery (subgroups by severity of pre‐eclampsia), Outcome 4 Absent tendon reflexes.
4.5. Analysis.
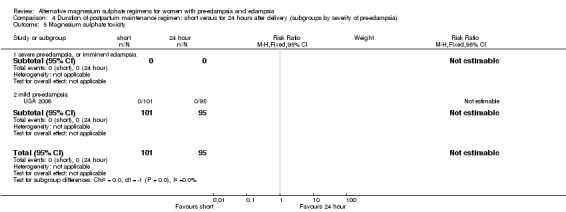
Comparison 4 Duration of postpartum maintenance regimen: short versus for 24 hours after delivery (subgroups by severity of pre‐eclampsia), Outcome 5 Magnesium sulphate toxicity.
4.6. Analysis.
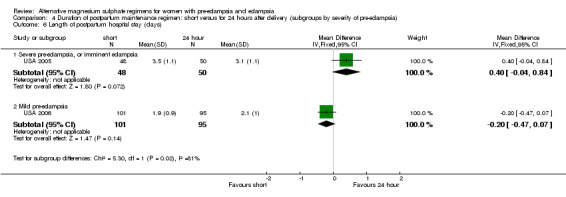
Comparison 4 Duration of postpartum maintenance regimen: short versus for 24 hours after delivery (subgroups by severity of pre‐eclampsia), Outcome 6 Length of postpartum hospital stay (days).
4.7. Analysis.
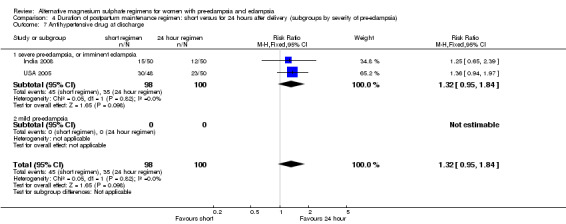
Comparison 4 Duration of postpartum maintenance regimen: short versus for 24 hours after delivery (subgroups by severity of pre‐eclampsia), Outcome 7 Antihypertensive drug at discharge.
Comparison 5. Duration of postpartum maintenance regimen: short versus for 24 hours after delivery (subgroups by type of short regimen).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eclampsia | 3 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Short: based on clincial criteriatil onset of diuresis | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Short: for 12 hours | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Progression to more severe pre‐eclampsia | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.58 [0.83, 52.52] |
| 2.1 Short: until onset of diuresis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Short: for 12 hours | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.58 [0.83, 52.52] |
| 3 Duration of allocated maintenance regimen extended, or therapy restarted | 3 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.41 [0.96, 30.37] |
| 3.1 Short: based on clincial criteria | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 3.2 Short: for 12 hours | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.58 [0.83, 52.52] |
| 4 Absent tendon reflexes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.87] |
| 4.1 short, based on clinical criteria | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.87] |
| 4.2 short, for 12 hours | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Magnesium sulphate toxicity | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 short, until onset of diuresis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 short, for 12 hours | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
Comparison 5 Duration of postpartum maintenance regimen: short versus for 24 hours after delivery (subgroups by type of short regimen), Outcome 1 Eclampsia.
5.2. Analysis.
Comparison 5 Duration of postpartum maintenance regimen: short versus for 24 hours after delivery (subgroups by type of short regimen), Outcome 2 Progression to more severe pre‐eclampsia.
5.3. Analysis.
Comparison 5 Duration of postpartum maintenance regimen: short versus for 24 hours after delivery (subgroups by type of short regimen), Outcome 3 Duration of allocated maintenance regimen extended, or therapy restarted.
5.4. Analysis.
Comparison 5 Duration of postpartum maintenance regimen: short versus for 24 hours after delivery (subgroups by type of short regimen), Outcome 4 Absent tendon reflexes.
5.5. Analysis.
Comparison 5 Duration of postpartum maintenance regimen: short versus for 24 hours after delivery (subgroups by type of short regimen), Outcome 5 Magnesium sulphate toxicity.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bangladesh 2002.
| Methods | Sequence generation: women were 'randomly allocated by lottery'. Allocation concealment: to recruit, someone 'selected a piece of paper from a box to determine' the treatment group'. Follow up: complete. Blinding: not stated, but unlikely to have been any blinding. | |
| Participants | 401 women (mean age ˜23 yrs, mean gestational age ˜36 wks) with eclampsia (85% antepartum, 15% postpartum). Excluded: if contraindication to MgSO4 (oliguria, renal failure, absent tendon reflexes); comatose, already on MgSO4; or decision to continue the pregnancy. | |
| Interventions | Exp: loading dose only. MgSO4 4 g IV over 15 to 20 minutes, then 6.g IM (3 g into each buttock). Control: loading dose plus maintenance regimen. Loading dose of MgSO4 as above, then 2.5 g IM every 4 hours for 24 hours. If recurrent convulsion, a further 2.5 g IV, and maintenance therapy in both groups. If DBP > 110 mmHg, 20 g hydralazine IV. |
|
| Outcomes | All women: further fits, maternal death. Women randomised before delivery: caesarean section. Babies: stillbirth. |
|
| Notes | Not mentioned whether any pregnancies were multiple. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | 'Randomly assigned by lottery'. They 'randomly selected a piece of paper from a box to determine [treatment group]'. |
| Allocation concealment? | Unclear risk | No mention of allocation concealment. |
| Blinding? All outcomes | Unclear risk | Not mentioned, but in view of the intervention it is unlikely there was blinding of the clinician or participant. Blinding of assessment may have been possible for some outcomes. |
India 2007.
| Methods | Sequence generation: using a 'Tippet table', which is a random number table. Allocation concealment:: not mentioned. Follow up: no losses. Blinding: not mentioned, but unlikely to have been any blinding. | |
| Participants | 50 women with antepartum eclampsia. Excluded: women with renal failure, pulmonary oedema, or if they received MgSO4 before hospital admission. | |
| Interventions | Exp: Dhaka regimen. Not described in the paper, but the reference provided states 4 g IV + 6 g IM loading dose MgSO4, then 2.5 g IM every 4 hours for 24 hours. Control: Bhalla regimen: not described in the paper, but the reference provided states 4 g IV + 8 g IM MgSO4 loading dose, then 4 g IM every 4 hours for 24 hours. |
|
| Outcomes | Women: recurrence of convulsions, oliguria, absent knee jerk reflex. Babies: stillbirth, neonatal death, admission to NICU, RDS, hypotonia, respiratory depression, jaundice, requirement for calcium gluconate. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Randomised using a Tippet table', which is tables of random numbers. No other information. |
| Allocation concealment? | Unclear risk | No information about concealment of allocation. |
| Blinding? All outcomes | Unclear risk | Not mentioned, but in view of the intervention it is unlikely there was blinding of the clinician. Blinding of participant would have been possible, as would blinding of assessment for some outcomes. |
India 2008.
| Methods | See risk of bias table below. Of 105 potentially eligible women 2 did not meet inclusion criteria and 3 refused to participate. | |
| Participants | 100 women with severe pre‐eclampsia postpartum, who had been given MgSO4 from induction until birth. Criteria for severe pre‐eclampsia included systolic BP ≥160 mmHg, diastolic BP ≥100 mmHg, proteinuria ≥3+ or ≥5g/24 hours, and signs or symptoms such as headache, visual disturbance, epigastric pain and thrombocytopenia. Excluded if history of seizures, derranged renal function, oliguria, myasthenia gravis. |
|
| Interventions | Exp: MgSO4 continued until clinical criteria met: no headache, visual symptoms, or epigastric pain; spontaneous diuresis ≥100 ml/hour for 2 hours; >50% of hourly BP <150/100 mmHg and none >160/110 mmHg. Control: MgSO4 continued for 24 hous after birth. |
|
| Outcomes | Duration of MgSO4 therapy, eclampsia, therapy restarted, side effects, loss of patellar reflex | |
| Notes | Abstract only published, additional information from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random numbers |
| Allocation concealment? | Unclear risk | No information |
| Blinding? All outcomes | High risk | Blinding not mentioned, but unlikely in view of the interventions. |
South Africa 1994.
| Methods | Sequence generation: 'randomly allocated', no further information. Allocation concealment: not mentioned. Follow up: complete. Blinding: not mentioned. | |
| Participants | 17 women with severe pre‐eclampsia or imminent eclampsia: proteinuria ≥ 1+ on dipstick, DBP ≥ 110 mmHg and not settled after 4 hours. Imminent eclampsia if also signs and symptoms (such as headache, epigastric pain or hyperreflexia). 4 women in the IM group and 2 in the IV group had imminent eclampsia. | |
| Interventions | Exp: IV bolus maintenance regimen: 6 g IV MgSO4 loading dose in 200 ml saline over 15 min, then 2 g hourly for 24 hours. Control: standard IM maintenance regimen: 4 g IV MgSO4 in 200 ml saline over 15 min plus 10 g IM (5 g in each buttock) loading dose, then 5 g IM every 4 hours for 24 hours. |
|
| Outcomes | Women: eclampsia, magnesium toxicity, intrapartum antihypertensive, antenatal antihypertensive, caesarean section. Baby: fresh stillbirth. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomly allocated', no further information. |
| Allocation concealment? | Unclear risk | No information about concealment of allocation. |
| Blinding? All outcomes | Unclear risk | Not mentioned, but in view of the intervention it is unlikely there was blinding of the clinician or participant. Blinding of assessment may have been possible for some outcomes. |
USA 2005.
| Methods | Sequence generation: using random number tables, with block size of 10. Allocation concealment: individual opaque sealed envelopes. Women assigned 'in numeric order'. Follow up: no losses. Blinding: not mentioned. | |
| Participants | 98 women (mean age ˜24 yrs, 53% primigravida) with severe pre‐eclampsia, needing MgSO4 postpartum. Criteria included: sustained SBP ≥ 160 mmHg; sustained DBP ≥ 110 mmHg; proteinuria ≥ +3 or ≥ 5 g in 24 hour urine collection; oliguria (< 500 ml 24 hours or < 30 ml/hr for 2 hours unresponsive to IV fluid challenge); presence of persistent headache, visual disturbances, or epigastric of right upper quadrant pain; thrombocytopenia; impaired liver function; pulmonary oedema or cyanosis; fetal growth restriction; chronic hypertension with superimposed pre‐eclampsia. | |
| Interventions | Exp: maintenance infusion of MgSO4 2 g/hour until onset of diuresis (2 consecutive hours of urine output > 100 ml/hour). Control: maintenance infusion of MgSO4 2 g/hour until 24 hours after delivery. All women had MgSO4,4 g IV loading dose over 20 min, then 2 g/hour infusion before delivery. |
|
| Outcomes | Women: eclampsia, need to restart therapy, antihypertensive at discharge, postpartum hospital stay. Baby: not relevant as postpartum randomisation. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Using random number tables, with block size of 10. |
| Allocation concealment? | Low risk | Individual opaque sealed envelopes. Women assigned 'in numeric order'. |
| Blinding? All outcomes | Unclear risk | Not mentioned, but in view of the intervention it is unlikely there was blinding of the clinician or participant. Blinding of assessment may have been possible for some outcomes. |
USA 2006.
| Methods | Sequence generation: using computer‐generated random number tables in blocks of 10. Allocation concealment: consecutively numbered, sealed opaque envelopes. Follow up: 4 women (2%) lost to follow up as data could not be found, all from the 24‐hour regimen group. Blinding: not mentioned. | |
| Participants | 200 women (mean age ˜25 yrs, 48% primiparous) with mild pre‐eclampsia diagnosed antepartum, intrapartum, or within 2 hours postpartum, who delivered at ≥ 34 weeks' gestation. Mild pre‐eclampsia was diagnosed if hypertension (SBP ≥140 mmHg or DBP ≥ 90 mmHg) and proteinuria (random catheterised sample ≥ +1). Excluded: severe pre‐eclampsia (based on symptoms, blood pressures, or laboratory evidence) at delivery or before randomisation. | |
| Interventions | Exp: 12 maintenance regimen: 2 g/hour for 12 hours after delivery.
Control: 24‐hour regimen: 2 g/hour for 24 hours after delivery. All women in both groups were already on IV MgSO4, having received a 4 g IV loading dose followed by 2 g/hour IV maintenance. |
|
| Outcomes | Women: eclampsia, progression to severe pre‐eclampsia, postpartum hospital stay, maintenance regimen extended, toxicity. Baby: not relevant as postpartum randomisation. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Using computer‐generated random number tables in blocks of 10. |
| Allocation concealment? | Low risk | Consecutively numbered, sealed opaque envelopes. |
| Blinding? All outcomes | Unclear risk | Not mentioned. |
DBP: diastolic blood pressure Exp: experimental IM: intramuscular IVH: intraventricular haemorrhage IV: intravenous MgSO4: magnesium sulphate NEC: necrotising enterocolitis NICU: neonatal intensive care unit RDS: respiratory distress syndrome SBP: systolic blood pressure wks: weeks yrs: years
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bangladesh 2009 | Limited information available, published as abstract only. Described as 'randomised by lottery' and no outcome data available. Participants: 100 women with eclampsia before birth Interventions: loading dose of 8 g IV vs loading dose of 10 g given as 4 g IV and 6 g IM. |
| Brazil 2010 | No clinical outcomes reported ‐ serum magnesium levels only. Methods: computerized random numbers for sequence generation, concealment of allocation with sequentially numbered opaque envelopes Participants: 29 women with imminent eclampsia or eclampsia. Interventions: maintenance regimen of 2 g/hour vs 1 g/hour. |
| India 2009 | Not a randomised trial. Allocation was to 'units within the department'. Study conducted over 5 years. Participants: 630 women with eclampsia ‐ 150 in IV group, 480 in IM group. Interventions: low dose intravenous regimen vs 'Pritchard' intramuscular regimen. |
| India 2009a | No clinical outcomes reported. Published as abstract only. Participants: 300 women with blood pressure ≥140/100 mmHg and proteinuria ≥1+ (30 mg/dl) Interventions: infusion using Springfusor pump vs manual IV loading dose and IM maintenance |
| Iran 2005 | Comparison on magnesium chloride with magnesium sulphate. Methods: 'randomised', no further information. 2 post randomisation exclusions. Participants: 68 women with mild pre‐eclampsia. Interventions: IV magnesium sulphate vs oral magnesium chloride. |
| Nigeria 2009 | No clinical outcomes reported. Published as abstract only. Methods: 'randomised' Participants: 72 women with eclampsia Interventions: low dose regimen (loading 4 g IV + 5 g IM, maintenance 2 g every 4 hours) vs standard therapy (loading 14 g, maintenance 5 g every 4 hours) |
| Thailand 1992 | No clinical outcomes. Information from English abstract of Thai paper. Methods: allocation by simple randomisation. No other information. Participants: 49 women with severe pre‐eclampsia or eclampsia. Interventions: maintenance regimen of 2 g/hour vs 1 g/hour. |
| Thailand 1996 | No clinical outcomes. Methods: 'double blind randomisation', blocked randomisation. Participants: 50 women with severe pre‐eclampsia and a singleton pregnancy. Interventions: 4 g IV loading dose with 1g/hour IV maintenance vs 4 g IV plus 10 g IM loading dose with 5 g IM every 4 hours maintenance. |
| Thailand 1999 | No clinical outcomes reported. Study published as abstract only. Methods: 'block randomisation'. No further information. Participants: 34 women with severe pre‐eclampsia. Interventions: maintenance regimen of 2 g/hour vs 1 g/hour. |
| USA 1981 | No clinical outcomes. Women used as their own controls in the analysis. Methods: Women observed for 30 min, then these data used as control for analysis. Randomised to IM of IV maintenance regimen. Participants: 19 women with mild pre‐eclampsia. |
| USA 1989 | No clinical outcomes. Published as abstract only. Methods: 'consecutively randomised', no further information. Participants: 40 women with pre‐eclampsia, mild or severe. Interventions: IM or IV maintenance regimen. |
vs: versus IM: intramuscular IV: intravenous
Differences between protocol and review
We have updated methods in accordance with the update Cochrane HandbookHiggins 2009.
Contributions of authors
The protocol was drafted and agreed with input from all review authors. Hosam E Matar (HEM) and Muhammad Qutayba Almerie (MQA) assessed the quality of trials, extracted and analysed data. Lelia Duley (LD) and David Hall (DH) also extracted data to check quality assessment, data extraction and data entry. LD, HEM and MQA wrote the first draft of the review, with input from DH. All review authors agreed the final version of the review.
Sources of support
Internal sources
-
University of Leeds, UK.
Employer, Lelia Duley
External sources
No sources of support supplied
Declarations of interest
Lelia Duley was the Principal Investigator for the Collaborative Eclampsia Trial and the Magpie Trial. Jim Neilson (Editor for this review) was a co‐investigator on the Magpie Trial.
Edited (no change to conclusions)
References
References to studies included in this review
Bangladesh 2002 {published data only}
- Begum A. Loading dose vs standard regime of magnesium sulphate in the management of eclampsia ‐ a randomized trial [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology (Book 2); 2000 Sept 3‐8; Washington DC, USA. 2000:47.
- Begum MR, Begum A, Quadir E. Loading dose versus standard regime of magnesium sulfate in the management of eclampsia: a randomized trial. Journal of Obstetrics and Gynaecology Research 2002;28:154‐9. [DOI] [PubMed] [Google Scholar]
India 2007 {published data only}
- Shilva, Saha SC, Kalra J, Prasad R. Safety and efficacy of low‐dose MgS04 in the treatment of eclampsia. International Journal of Gynecology & Obstetrics 2007;97(2):150‐1. [DOI] [PubMed] [Google Scholar]
India 2008 {published data only}
- Suneja A, Sinha S, Vaid N, Ahuja S. A prospective randomized controlled trial to individualize the duration of post partum magnesium sulfate therapy. Hypertension in Pregnancy 2008;27(4):504. [Google Scholar]
South Africa 1994 {published data only}
- Chissell S, Botha JH, Moodley J, McFadyen L. Intravenous and intramuscular magnesium sulphate regimens in severe pre‐eclampsia. South African Medical Journal 1994;84:607‐10. [PubMed] [Google Scholar]
USA 2005 {published data only}
- Fontenot MT, Lewis DF, Frederick JB, Wang Y, DeFranco EA, Groome LJ, et al. A prospective randomized trial of magnesium sulfate in severe preeclampsia: use of diuresis as a clinical parameter to determine the duration of postpartum therapy. American Journal of Obstetrics and Gynecology 2005;192:1788‐94. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang Y, Canzoneri BJ, Gu Y, Philibert L, Lewis DF. Prostacyclin and thromboxane levels in women with severe preeclampsia undergoing magnesium sulfate therapy during antepartum and postpartum periods. Hypertension in Pregnancy 2008;27(1):17‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
USA 2006 {published data only}
- Ehrenberg H, Mercer B. Abbreviated post‐partum magnesium sulfate therapy for women with mild preeclampsia [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S73. [DOI] [PubMed] [Google Scholar]
- Ehrenberg HM, Mercer BM. Abbreviated postpartum magnesium sulfate therapy for women with mild preeclampsia: a randomized controlled trial. Obstetrics & Gynecology 2006;108(4):833‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bangladesh 2009 {published data only}
- Begum K. A lower dose of magnesium sulphate for control of convulsion in eclamptic women of Bangladesh. International Journal of Gynecology & Obstetrics 2009; Vol. 107, issue Suppl 2:S124.
Brazil 2010 {published data only}
- Abbade JF, Costa RA, Martins AM, Borges VT, Rudge MV, Peraçoli JC. Zuspan's scheme versus an alternative magnesium sulfate scheme: Randomized clinical trial of magnesium serum concentrations. Hypertension in Pregnancy 2010;29(1):82–92. [DOI] [PubMed] [Google Scholar]
- Abbade JF, Costa RAA, Martins AMVC, Rudge MVC, Peracoli JC. Zuspan's scheme versus alternative scheme of magnesium sulphate to prevent or to treat eclampsia: comparison of magnesium serum concentrations [abstract]. Hypertension in Pregnancy 2006;25(Suppl 1):152. [Google Scholar]
India 2009 {published data only}
- Chowdhury JR, Chaudhuri S, Bhattacharyya N, Biswas PK, Panpalia M. Comparison of intramuscular magnesium sulfate with low dose intravenous magnesium sulfate regimen for treatment of eclampsia. Journal of Obstetrics and Gynaecology Research 2009;35(1):119‐25. [DOI] [PubMed] [Google Scholar]
India 2009a {published data only}
- Mundle S, Regi A, Biswas B, Bracken H, Easterling T, Winikoff B. Preeclampsia in low‐resource settings: a randomized trial of IV MgSO4 via flow controlled pump. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S278. [Google Scholar]
Iran 2005 {published data only}
- Ghahiri A, Berjis K. A comparison between intravenous magnesium sulfate and oral magnesium chloride in mild preeclampsia. Journal of Research in Medical Sciences 2005;10(1):6‐9. [Google Scholar]
Nigeria 2009 {published data only}
- Muhammad A, Ibrahim U, Nighat K, Muhammad Y, Abdullahi A. Low dose magnesium sulfate in the control of eclamptic fits: a randomized control trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S277‐8. [Google Scholar]
Thailand 1992 {published data only}
- Tongsong T, Dejkijwikrom W, Tansuwannont W, Jantachai U, Siangpura U. A comparison of intravenous magnesium sulfate regimens in preeclampsia‐eclampsia between rate 1 gram and 2 grams per hour. Siriraj Hospital Gazette 1992;44(7):509‐16. [Google Scholar]
Thailand 1996 {published data only}
- Manorot M, Tongsong T, Khettglang T. A comparison of serum magnesium sulfate levels in pregnant women with severe preeclampsia between intravenous and intramuscular magnesium sulfate regimens: a randomized controlled trial. Journal of the Medical Association of Thailand 1996;79(2):76‐82. [PubMed] [Google Scholar]
Thailand 1999 {published data only}
- Laiteerapong U, Leelahakorn S. Comparative study of serum magnesium levels attained from magnesium sulfate therapy for severe pre‐eclamptic patients between 1 gm/hr and 2 gm/hr regimen. Thai Journal of Obstetrics and Gynaecology 1999;11(4):281. [Google Scholar]
USA 1981 {published data only}
- Stallworth JC, Yeh SY, Petrie RH. The effect of magnesium sulfate on fetal heart rate variability and uterine activity. American Journal of Obstetrics and Gynecology 1981;140:702‐6. [DOI] [PubMed] [Google Scholar]
USA 1989 {published data only}
- Gloeb DJ, O'Sullivan MJ. Comparison of effects of intramuscular and intravenous magnesium sulfate regimens in pre‐eclamptic patients on intrapartum events and maternal/neonatal outcome. Proceedings of 9th Annual Meeting of the Society of Perinatal Obstetricians; 1989 Feb 1‐4; New Orleans, Louisiana, USA. 1989:494.
Additional references
Belfort 1992
- Belfort MA, Moise KJJ. Effect of magnesium sulfate on maternal brain blood flow in preeclampsia: a randomized, placebo controlled study. American Journal of Obstetrics and Gynecology 1992;167:661‐6. [DOI] [PubMed] [Google Scholar]
Bhalla 1994
- Bhalla AK, Dhall GI, Dhall K. A safer and more effective treatment regimen for eclampsia. Australian and New Zealand Journal of Obstetrics and Gynaecology 1994;34:144‐8. [DOI] [PubMed] [Google Scholar]
Chesley 1978
- Chesley L. Survey of management and case mortality. Hypertensive disorders in pregnancy. New York: Appleton‐Century‐Crofts, 1978:309‐40. [Google Scholar]
Collab Trial 1995
- Anonymous. Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet 1995;345:1455‐63. [PubMed] [Google Scholar]
Crowther 1985
- Crowther C. Eclampsia at Harare Maternity Hospital. An epidemiological study. South African Medical Journal 1985;68:927‐9. [PubMed] [Google Scholar]
Dinsdale 1988
- Dinsdale HB. Does magnesium sulfate treat eclamptic seizures? Yes. Archives of Neurology 1988;45:1360‐1. [DOI] [PubMed] [Google Scholar]
Donaldson 1992
- Donaldson JO. The case against magnesium sulfate for eclamptic convulsions. International Journal of Obstetric Anesthesia 1992;1:159‐66. [DOI] [PubMed] [Google Scholar]
Dorsett 1926
- Dorsett L. The intramuscular injection of magnesium sulfate for the control of convulsions in eclampsia. American Journal of Obstetrics and Gynecology 1926;11:227‐31. [Google Scholar]
Douglas 1994
- Douglas KA, Redman CWG. Eclampsia in the United Kingdom. BMJ 1994;309:1395‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2009
- Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004661.pub3] [DOI] [PubMed] [Google Scholar]
Duley 1992
- Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. British Journal of Obstetrics and Gynaecology 1992;99:547‐53. [DOI] [PubMed] [Google Scholar]
Duley 1994
- Duley L, Johanson R. Magnesium sulphate for pre‐eclampsia and eclampsia: the evidence so far. British Journal of Obstetrics and Gynaecology 1994;101:565‐7. [DOI] [PubMed] [Google Scholar]
Duley 1995
- Duley L, Henderson‐Smart DJ. Magnesium sulphate versus phenytoin for eclampsia. Cochrane Database of Systematic Reviews 1995, Issue 4. [DOI: 10.1002/14651858.CD000128] [DOI] [Google Scholar]
Duley 1996
- Duley L. Magnesium sulphate regimens for women with eclampsia: messages from the Collaborative Eclampsia Trial. British Journal of Obstetrics and Gynaecology 1996;103:103‐5. [DOI] [PubMed] [Google Scholar]
Duley 2000
- Duley L, Gulmezoglu AM. Magnesium sulphate versus lytic cocktail for eclampsia. Cochrane Database of Systematic Reviews 2000, Issue 3. [Art. No.: CD002960. DOI: 10.1002/14651858.CD002960] [DOI] [PubMed] [Google Scholar]
Duley 2000a
- Duley L, Henderson‐Smart DJ. Magnesium sulphate versus diazepam for eclampsia. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD000127] [DOI] [PubMed] [Google Scholar]
Duley 2003
- Duley L, Gulmezoglu AM, Henderson‐Smart DJ. Magnesium sulphate and other anticonvulsants for women with pre‐eclampsia. Cochrane Database of Systematic Reviews 2003, Issue 2. [Art. No.: CD000025. DOI: 10.1002/14651858.CD000025] [DOI] [PubMed] [Google Scholar]
Eastman 1945
- Eastman NJ, Steptoe PP. The management of pre‐eclampsia. Canadian Medical Association Journal 1945;52:562‐8. [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldman 1988
- Goldman R, Finkbeiner SM. Therapeutic use of magnesium sulfate in selected cases of cerebral ischemia and seizure. New England Journal of Medicine 1988;319:1224‐5. [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Kaplan 1988
- Kaplan PW, Lesser RP, Fisher RS, Repke JT, Hanley DF. No, magnesium sulfate should not be used in treating eclamptic seizures. Archives of Neurology 1988;45:1361‐4. [DOI] [PubMed] [Google Scholar]
Khan 2006
- Khan K, Wojdyla D, Say L, Gülmezoglu A, Look P. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066‐74. [DOI] [PubMed] [Google Scholar]
Knight 2007
- Knight M, on behalf of UKOSS. Eclampsia in the United Kingdom 2005. BJOG: an international journal of obstetrics and gynaecology 2007;114(9):1072‐8. [DOI] [PubMed] [Google Scholar]
Lazard 1925
- Lazard EM. A preliminary report on the intravenous use of magnesium sulphate in puerperal eclampsia. American Journal of Obstetrics and Gynecology 1925;9:178‐88. [DOI] [PubMed] [Google Scholar]
Lean 1968
- Lean TH, Ratnam SS, Sivasamboo R. The use of chlordiazepoxide in patients with severe pregnancy toxaemia. (A preliminary study of effects on the newborn infants). Journal of Obstetrics and Gynaecology of the British Commonwealth 1968;75:853‐5. [DOI] [PubMed] [Google Scholar]
Magpie Trial 2002
- Magpie Trial Collaboration Group. Do women with pre‐eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo‐controlled trial. Lancet 2002;359:1877‐90. [DOI] [PubMed] [Google Scholar]
Matthys 2004
- Matthys LA, Coppage KH, Lambers DS, Barton JR, Sibai BM. Delayed postpartum preeclampsia: an experience of 151 cases. American Journal of Obstetrics and Gynecology 2004;90:1464‐6. [DOI] [PubMed] [Google Scholar]
Meher 2005
- Meher S, Duley L, Prevention of Pre‐eclampsia Cochrane Review authors. Interventions for preventing pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2005, Issue 2. [Art. No.: CD005301. DOI: 10.1002/14651858.CD005301] [Google Scholar]
Pritchard 1955
- Pritchard JA. The use of magnesium ion in the management of eclamptogenic toxemias. Surgery, Gynecology and Obstetrics 1955;100:131‐40. [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Sadeh 1989
- Sadeh M. Action of magnesium sulfate in the treatment of preeclampsia‐eclampsia. Stroke 1989;20:1273‐5. [DOI] [PubMed] [Google Scholar]
Sibai 1984
- Sibai BM, Spinnato JA, Watson DL, Lewis JA, Anderson GD. Effect of magnesium sulfate on electroencephalographic findings in preeclampsia‐eclampsia. Obstetrics & Gynecology 1984;64:261‐6. [PubMed] [Google Scholar]
Sibai 1990
- Sibai BM. Magnesium sulfate is the ideal anticonvulsant in preeclampsia‐eclampsia. American Journal of Obstetrics and Gynecology 1990;162:1141‐5. [DOI] [PubMed] [Google Scholar]
Simon 2006
- Simon J, Gray A, Duley L on behalf of the Magpie Trial Collaborative Group. Cost‐effectiveness of prophylactic magnesium sulphate for 9996 women with pre‐eclampsia from 33 countries: economic evaluation of the Magpie Trial. BJOG: an international journal of obstetrics and gynaecology 2006;113:144–51. [DOI] [PubMed] [Google Scholar]
Slater 1987
- Slater RM, Smith WD, Patrick J, Mawer G. Phenytoin infusion in severe pre‐eclampsia. Lancet 1987;i:1417‐21. [DOI] [PubMed] [Google Scholar]
Stroganoff 1937
- Stroganoff W, Davidovitch O. Two hundred cases of eclampsia treated with magnesium sulphate. A preliminary report. Journal of Obstetrics and Gynaecology 1937;44:289‐99. [Google Scholar]
WHO 1988
- World Health Organization International Collaborative Study. Geographic variation in the incidence of hypertension in pregnancy. American Journal of Obstetrics and Gynecology 1988;158:80‐3. [PubMed] [Google Scholar]
Zuspan 1978
- Zuspan FP. Problems encountered in the treatment of pregnancy‐induced hypertension. American Journal of Obstetrics and Gynecology 1978;131:591‐7. [DOI] [PubMed] [Google Scholar]


