Abstract
Introduction:
In breast cancer patients treated with the anti-estrogen tamoxifen, low concentrations of the active metabolite endoxifen are associated with more disease recurrence. We hypothesized that we could increase endoxifen concentrations by induction of its formation and inhibition of its metabolism by co-administration of probenecid.
Methods:
We conducted a crossover study and measured endoxifen concentrations in patients on steady-state tamoxifen monotherapy and after 14 days of combination treatment with probenecid. Eleven evaluable patients were included.
Results:
Treatment with tamoxifen and probenecid resulted in a 26% increase of endoxifen area under the plasma concentration–time curve from 0 to 24 h (AUC0–24h) compared to tamoxifen monotherapy (95% confidence interval [CI]: 8–46%; p < 0.01), while the maximum observed endoxifen concentration increased with 24% (95% CI: 7–44%; p < 0.01). The metabolic ratio of endoxifen to tamoxifen increased with 110% (95% CI: 82–143%; p < 0.001) after the addition of probenecid.
Conclusion:
Probenecid resulted in a clinically relevant increase of endoxifen concentrations in breast cancer patients treated with adjuvant tamoxifen. This combination therapy could provide a solution for patients with a CYP2D6-poor metabolizer phenotype or endoxifen concentrations below the threshold despite earlier tamoxifen dose.
Keywords: breast cancer, endoxifen, metabolism, probenecid, tamoxifen
Introduction
Tamoxifen is a selective estrogen receptor modulator, frequently used in the adjuvant treatment of estrogen receptor–positive breast cancer. 1 It is a prodrug that undergoes metabolization to its most active metabolite endoxifen by cytochrome P450 (CYP) 2D6 and 3A4 enzymes. 2 Despite 5 years of adjuvant treatment with tamoxifen, one-third of patients develop disease recurrence within 15 years. 3 Importantly, systemic endoxifen concentrations are correlated with breast cancer relapse. Patients with endoxifen concentrations above the therapeutic threshold were found to have a 26% lower chance of disease recurrence. 4
The therapeutic threshold value for endoxifen has been defined at 14–16 nM, which is achieved by 75–80% of tamoxifen users.4,5 This large variance in endoxifen concentrations is mainly the result of interpatient variability in CYP2D6 activity, due to a high prevalence of functional polymorphisms in the CYP2D6 gene.6,7 Enzyme activity is based on the presence of functional alleles. Patients with an extensive metabolizer phenotype have normal CYP2D6 activity, whereas intermediate metabolizers (IMs) and poor metabolizers (PMs) have reduced and little or no enzyme function, respectively. Hence, the biotransformation of tamoxifen to endoxifen is compromised in patients with an IM and—to a greater extent—a PM CYP2D6 phenotype. It results in lower endoxifen plasma concentrations compared with CYP2D6-extensive metabolizers. 8 While patients with an IM CYP2D6 phenotype usually reach therapeutic endoxifen concentrations after tamoxifen dose escalation, this is rarely the case for patients with a PM CYP2D6 phenotype. This subgroup is consequently more prone to disease recurrence. 5
Therefore, we sought a solution to increase systemic endoxifen exposure in this population by interfering with tamoxifen metabolism. After endoxifen formation by CYP2D6 and 3A4 (phase-1 metabolism), endoxifen undergoes glucuronidation to the inactive endoxifen-glucuronide by UDP-glucuronosyltransferases (UGTs) in order to be excretable.9,10 Aside from the impact of CYP activity on endoxifen concentrations, it has been demonstrated that its concentration is also influenced by functional UGT variants. 9
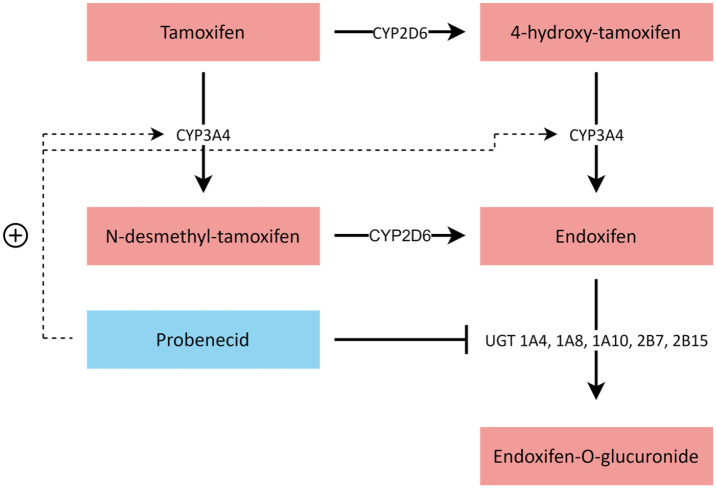
We hypothesized that administration of the CYP3A4 inducer and pan-UGT inhibitor probenecid would result in increased endoxifen concentrations by a mechanism of a two-fold nature, namely by means of induction of tamoxifen to endoxifen transformation and inhibition of endoxifen glucuronidation (Figure 1). Probenecid is a uricosuric agent, nowadays seldom used in the treatment of gout. 11 It has a mild and predictable toxicity profile (i.e. gastrointestinal complaints, headache, and rash), which does not overlap with that of tamoxifen. 12 Probenecid has already been demonstrated to alter drug exposure by both CYP3A4 induction and UGT inhibition in several in vitro and clinical studies.13–17 Here, we report the results of a prospective crossover study on the influence of probenecid on endoxifen concentrations in breast cancer patients treated with adjuvant tamoxifen with an impaired CYP2D6 phenotype.
Figure 1.
Tamoxifen metabolism and hypothesized mechanism of CYP3A4 induction and UGT inhibition by probenecid. After administration, tamoxifen is metabolized to N-desmethyl-tamoxifen and 4-hydroxy-tamoxifen, mainly by CYP3A4 and CYP2D6, respectively. Next, N-desmethyl-tamoxifen and 4-hydroxy-tamoxifen are metabolized to endoxifen, mainly by CYP2D6 and CYP3A4, respectively. Endoxifen gets glucuronidated by UGTs to the inactive endoxifen-glucuronide.
CYP, cytochrome P450; UGT, UDP-glucuronosyltransferase.
Materials and methods
Study design
The primary objective of this trial was to compare the area under the plasma concentration–time curve from 0 to 24 h (AUC0–24h) of endoxifen with and without concomitant use of probenecid. A relative difference in AUC0–24h of endoxifen of at least 25% was considered clinically relevant. 18 Assuming a standard deviation of the difference of 25%, a total of 11 evaluable patients were required to detect a difference, given 90% power and a two-sided alpha of 0.05. 19
Secondary objectives were to compare the AUC0–24h of tamoxifen, the maximum observed plasma concentration (Cmax) of endoxifen and tamoxifen and the AUC0–24h-based metabolic ratios endoxifen to tamoxifen, N-desmethyl-tamoxifen (NDM) to tamoxifen, endoxifen to 4-OH-tamoxifen (4-OH), 4-OH to tamoxifen, endoxifen to NDM, and 4beta-hydroxycholesterol (4β-OHC) to cholesterol with and without concomitant use of probenecid. Adverse events were graded using the Common Terminology Criteria for Adverse Events, version 5.0 (CTCAE, version 5, National Cancer Institute, Bethesda, MD).
We conducted a one-way crossover study consisting of two phases. All patients entered the study on tamoxifen monotherapy and crossed over to combination treatment with probenecid after 7 days, which lasted for 14 days. Probenecid (Biokanol Pharma GmbH, Rastatt, Germany) was administered at a dose of 1000 mg twice daily. Tamoxifen was administered once daily at a fixed dose of 20 mg according to standard of care or 40 mg because of prior dose escalation due to endoxifen concentrations below the threshold. In order to ensure steady-state concentrations, medication adherence was assessed from 3 months before start until end of study. At the end of each phase, patients were hospitalized for 24 h after drug administration to obtain 14 blood samples at predefined time points for pharmacokinetic analysis. Blood samples were processed to plasma and stored at −80°C until analysis.
Patients
Eligible patients had a confirmed diagnosis of breast cancer and were on adjuvant tamoxifen treatment for at least 3 months to guarantee steady-state concentrations. Patients had to have a PM or IM CYP2D6 phenotype based on CYP2D6 genotype screening. 6 For complete inclusion and exclusion criteria, see the Supplementary Materials and Methods.
The study protocol (MEC 20-0188) was approved by the institutional review board (METC Erasmus MC) and was registered on March 9, 2020, in the Netherlands Trial Register (NL8444). All patients provided written informed consent before study entry.
Pharmacogenetic and pharmacokinetic analysis
CYP2D6 genotype was assessed by the Infiniti test (Autogenomics, Carlsbad, CA) and the Quantstudio test (Thermo Fisher Scientific, Waltham, MA). Plasma samples were analyzed for tamoxifen, NDM, 4-OH and endoxifen concentrations by a validated liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) method in accordance with U.S. Food and Drug Administration (FDA) bioanalytical method validation guidelines.20,21 A non-compartmental pharmacokinetic analysis of concentrations was performed using Phoenix WinNonlin, version 8.1 (Certara, Princeton, NJ). 4β-OHC to cholesterol ratios were determined as described previously. 22
Statistical analysis
Analyses of AUC0–24h, metabolic ratios and Cmax observations were performed on log-transformed data because these are assumed to follow a log-normal distribution. Estimates for the mean differences were obtained using a paired t-test and are shown as ratios of the geometric means with corresponding 95% confidence intervals (CIs) by taking the exponent of the results from the paired t-test. The relation between the ratio 4β-OHC to cholesterol and the ratio NDM to tamoxifen was analyzed using the Pearson’s correlation coefficient. Analysis of treatment-related adverse events was of descriptive nature.
Results
Patients
A total of 11 evaluable patients taking tamoxifen on steady state, with a median age of 54 years (range: 34–77 years) were enrolled between May 2020 and October 2020. Four patients had a PM phenotype and seven patients an IM phenotype for CYP2D6. Six patients, including all patients with a PM phenotype, used tamoxifen at a dose of 40 mg daily at the time of inclusion. Patient characteristics at baseline are listed in Table 1.
Table 1.
Patient characteristics at baseline.
| Characteristic | n (%) or median [range] | |
|---|---|---|
| Female | 11 | (100) |
| Age, years | 54 | [34–77] |
| Body mass index, kg/m2 | 24.0 | [20.8–32.8] |
| WHO Performance status | ||
| 0 | 11 | (100) |
| Tamoxifen dose | ||
| 20 mg | 5 | (45) |
| 40 mg | 6 | (55) |
| Time on adjuvant tamoxifen, months | 6.7 | [3.7–17.7] |
| Time since dose escalation, months | 3.1 | [3.0–6.7] |
| CYP2D6 phenotype | ||
| Intermediate metabolizer | 7 | (64) |
| Poor metabolizer | 4 | (36) |
| Previous treatment | ||
| Surgery | 10 | (91) |
| Radiotherapy | 8 | (73) |
| Chemotherapy | 4 | (36) |
| Ethnic origin | ||
| Caucasian | 11 | (100) |
WHO, World Health Organization.
Endoxifen concentrations
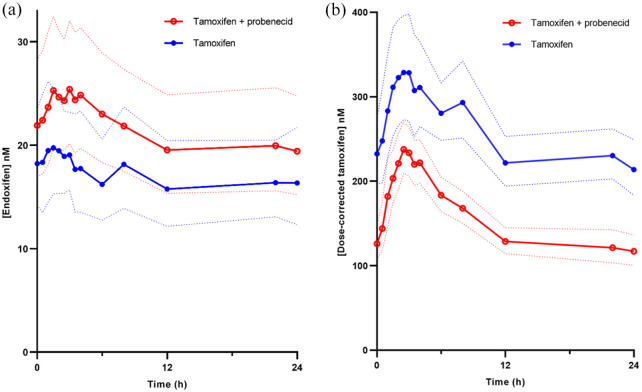
We measured endoxifen concentrations in all patients, treated with tamoxifen at steady state and compared these concentrations to endoxifen concentrations after 14 days of concomitant use of probenecid (1000 mg twice daily). Treatment with tamoxifen and probenecid resulted in a 26% increase of endoxifen AUC0–24h compared to tamoxifen monotherapy (95% CI: 8–46%; p < 0.01; geometric mean 505 vs 402 nmol·h/L; Figure 2(a)). The Cmax of endoxifen was 24% higher when patients used concomitant probenecid (95% CI: 7–44%; p < 0.01; geometric mean 27.4 vs 22.0 nM; Table 2).
Figure 2.
Plasma concentrations of endoxifen and tamoxifen with and without probenecid. Geometric mean plasma concentration vs time profiles of endoxifen (a) and dose-corrected tamoxifen (b) are shown for tamoxifen monotherapy (blue) and tamoxifen with probenecid combination therapy (red). Confidence bands indicate the 95% CI (n = 11).
Table 2.
Pharmacokinetic parameters of endoxifen and tamoxifen (n = 11).
| Pharmacokinetic parameter | Tamoxifen monotherapy (CV%) | Tamoxifen with probenecid (CV%) | Relative difference (%) (95% CI) | p |
|---|---|---|---|---|
| Endoxifen | ||||
| AUC0–24h (nmol·h/L) | 402 (43) | 505 (41) | 26 (8 to 46) | <0.01 |
| Cmax (nM) | 22.0 (46) | 27.4 (41) | 24 (7 to 44) | <0.01 |
| Tamoxifen | ||||
| AUC0–24h (nmol·h/L) | 8844 (45) | 5286 (46) | −40 (−47 to −33) | <0.001 |
| Cmax (nM) | 532 (48) | 357 (47) | −33 (−42 to −22) | <0.001 |
| Metabolic ratios | ||||
| Endoxifen/tamoxifen | 0.05 (72) | 0.10 (58) | 110 (82 to 143) | <0.001 |
| NDM/tamoxifen | 2.39 (16) | 3.26 (12) | 36 (23 to 50) | <0.001 |
| Endoxifen/4-OH | 3.97 (41) | 5.69 (36) | 43 (27 to 63) | <0.001 |
| 4-OH/tamoxifen | 0.01 (32) | 0.02 (32) | 47 (33 to 61) | <0.001 |
| Endoxifen/NDM | 0.02 (80) | 0.03 (64) | 55 (41 to 70) | <0.001 |
| 4β-OHC/cholesterol | 13.94 (60) | 13.66 (84) | −2 (−46 to 77) | 0.94 |
4β-OHC, 4β-hydroxy-cholesterol; 4-OH, 4-hydroxy-tamoxifen; AUC0–24h, area under the plasma concentration–time curve from 0 to 24 h; CI, confidence interval; Cmax, maximum observed plasma concentration; CV%, coefficient of variation; NDM, N-desmethyl-tamoxifen. AUC0–24h and Cmax are displayed as geometric mean. Metabolic ratios are ratios of the geometric mean.
In patients with a CYP2D6 PM phenotype, endoxifen AUC0–24h increased with 41% (95% CI: 2–95%; p = 0.04; geometric mean 404 vs 287 nmol·h/L) during combined treatment with probenecid. While in patients with a CYP2D6 IM phenotype, endoxifen AUC0–24h increased with 18% (95% CI: −4% to 44%; p = 0.09; geometric mean 573 vs 487 nmol·h/L).
Tamoxifen and other metabolite concentrations
Tamoxifen AUC0–24h during concomitant use of probenecid decreased with 40% (95% CI: −47 to −33%; p < 0.001; geometric mean 5286 vs 8844 nmol·h/L) compared to tamoxifen monotherapy (Figure 2b). Tamoxifen Cmax decreased with 33% (95% CI: −42 to −22%; p < 0.001; geometric mean 357 vs 532 nM) due to the addition of probenecid. The ratio endoxifen to tamoxifen during combination therapy increased with 110% (95% CI: 82–143%; p < 0.001; geometric mean 0.10 vs 0.05) compared to tamoxifen monotherapy (Table 2).
The ratio NDM to tamoxifen and the ratio endoxifen to 4-OH (as a measure for CYP3A4 activity; Figure 1) increased with 36% (95% CI: 23–50%; p < 0.001; geometric mean 3.26 vs 2.39) and 43% (95% CI: 27–63%; p < 0.001; geometric mean 5.69 vs 3.97), respectively (Table 2).
The ratio endoxifen to NDM and the ratio 4-OH to tamoxifen (as a measure for CYP2D6 activity; Figure 1) increased with 55% (95% CI: 41–70%; p < 0.001; geometric mean 0.03 vs 0.02) and 47% (95% CI: 33–61%; p < 0.001; geometric mean 0.02 vs 0.01), respectively (Table 2).
CYP3A4 activity
To determine CYP3A4 metabolic activity with and without concomitant probenecid, we determined the ratio 4β-OHC to cholesterol, an established endogenous marker of CYP3A4 activity. 23 The ratio 4β-OHC to cholesterol did not change (2%; 95% CI: −46 to 77%; p = 0.94; 13.66 vs 13.93). The fold change of the ratio 4β-OHC to cholesterol was correlated with the fold change of the ratio NDM to tamoxifen (Pearson’s correlation coefficient r = 0.67; p = 0.02; Supplemental Figure S1).
Treatment-related adverse events
Observed adverse events during combination treatment were relatively mild. Probenecid treatment–related adverse effects included hypokalemia, neutropenia, nausea, headache, dizziness, increased creatinine, and leukopenia, and were all grade 1 or 2 (Supplemental Table S1). Except for muscle cramps, which occurred two times more, tamoxifen-related adverse events did not increase during combination therapy, compared to monotherapy. There were no severe or serious adverse events (CTCAE grade ⩾ 3) observed.
Discussion
In this study, we demonstrated that probenecid causes a clinically relevant increase in endoxifen plasma concentrations in breast cancer patients treated with tamoxifen. This finding was accompanied by a decrease of tamoxifen concentrations during concomitant administration of probenecid. We determined concentrations of other tamoxifen metabolites in order to elucidate the mechanisms involved in these changes. Analysis of the tamoxifen metabolites NDM and 4-OH showed an increase of all CYP-mediated tamoxifen-to-metabolite or metabolite-to-endoxifen conversions occurring in the phase-1 metabolism of tamoxifen. These findings implicated at least an induction of CYP3A4 and/or CYP2D6, causing the reported shifts in endoxifen and tamoxifen plasma concentrations. Therefore, we subsequently determined 4β-OHC to cholesterol ratios with and without probenecid. The 4β-OHC to cholesterol ratio is an endogenous marker of CYP3A4/5 activity, which has previously proven utility in confirming CYP3A4 induction by rifampicin, administered in combination with tamoxifen. 24 However, in this study, no significant alterations could be detected in CYP3A4 functionality with or without probenecid administration. Yet, the fold change of the 4β-OHC-to-cholesterol ratio was correlated with the fold change of the NDM to tamoxifen ratio; both a CYP3A4-mediated conversion. This confirms the value of the 4β-OHC to cholesterol ratio as a genuine marker for CYP3A4 functional activity. Potential upregulation of CYP2D6 could not be investigated due to lack of an endogenous plasma marker for CYP2D6. The observed alterations in tamoxifen and endoxifen concentrations indicate a major effect of probenecid on the phase-1 metabolism of tamoxifen but cannot assess an effect on endoxifen glucuronidation.
To our knowledge, this is the first study to show the feasibility of increasing endoxifen concentrations by a pharmacological intervention, which could be especially important for patients with a PM phenotype for CYP2D6. A meta-analysis of 29 studies, including 13,000 tamoxifen users, demonstrated that PM patients on average had endoxifen concentrations of 8.8 ± 7.2 nM. 25 Furthermore, patients with a PM phenotype on average benefit the least of tamoxifen dose escalation, due to a lower increase of endoxifen concentrations per fixed increase of tamoxifen dose. It was found that on average for each 10 mg increase in tamoxifen dosage, patients with a PM phenotype only had a 1.2 nM increase of endoxifen, compared to a population average increase of 7.8 nM. 26 In a population of 145 tamoxifen users, 100% of PM patients and 34% of IM patients had endoxifen concentrations below the threshold of 16 nM. Despite tamoxifen dose escalation, only 36% and 79% of these patients reached the threshold, respectively. 27 Moreover, another study in 353 tamoxifen users demonstrated that dose escalation is not feasible for patients with a PM phenotype. 28 These observations stress the need for a solution, other than a dose escalation, to increase endoxifen concentrations in these patients to therapeutic concentrations.
Here, we demonstrated that in patients with a PM phenotype for CYP2D6, endoxifen concentrations increased to a greater extent compared to patients with an IM phenotype. Therefore, the currently proposed intervention is of greatest interest for this subgroup of patients. Although patients in this study were not selected on sub-therapeutic endoxifen concentrations, all patients with a PM phenotype had endoxifen trough concentrations below the therapeutic threshold at baseline. These concentrations increased to borderline therapeutic concentrations after co-treatment with probenecid, demonstrating the effectiveness of this intervention. In addition, as no serious side-effects occurred after 14 days of combination treatment, the feasibility of the intervention was also shown. In clinical practice, probenecid is administered as a uricosuric drug up to 1000 mg twice daily for several years. 29 Absolute contraindications for probenecid are scarce, and despite long-term administration, toxicity is generally mild. 12 This reflects our own observations on low drug toxicity and warrants further investigation of this likely tolerable combination in long-term treatment.
Our study is limited by the short duration of the treatment intervention. However, the main goal of this study was assessment of pharmacokinetics for proof of concept, for which the parameters used were sufficient. Validation of our findings in a larger group of patients is required prior to implementation of this intervention in clinical practice. A second limitation is the quantification of relevant metabolites. Although we determined several metabolites of tamoxifen and performed a phenotypical analysis of drug metabolism, we could not analyze all concentrations of relevant metabolites and activity of all conversions involved in the complex metabolism of tamoxifen. A third limitation is the purely systemic measurement of endoxifen concentrations performed in this study, contrarily to measurements in the target cancer cell. However, the study was performed according to current guidelines in the pharmacological field because such targeted measurements are practically impossible in this population, which is being treated in the adjuvant setting. Nonetheless, differences between systemic and intra-tumoral drug exposure is a relevant topic.
This study shows that probenecid can be used to increase endoxifen concentrations in breast cancer patients treated with tamoxifen. This combination therapy could provide a solution for patients with endoxifen concentrations below the threshold despite earlier tamoxifen dose escalation or in case of tamoxifen-related toxicity at lower doses.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221081075 for Influence of probenecid on endoxifen systemic exposure in breast cancer patients on adjuvant tamoxifen treatment by Stefan A. J. Buck, C. Louwrens Braal, Maaike M. Hofman, Esther Oomen-de Hoop, Peter de Bruijn, Inge M. Ghobadi Moghaddam-Helmantel, Koen G. A. M. Hussaarts, Mijntje B. Vastbinder, Quirine C. van Rossum-Schornagel, Ron H. N. van Schaik, Agnes Jager, Stijn L. W. Koolen and Ron H. J. Mathijssen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359221081075 for Influence of probenecid on endoxifen systemic exposure in breast cancer patients on adjuvant tamoxifen treatment by Stefan A. J. Buck, C. Louwrens Braal, Maaike M. Hofman, Esther Oomen-de Hoop, Peter de Bruijn, Inge M. Ghobadi Moghaddam-Helmantel, Koen G. A. M. Hussaarts, Mijntje B. Vastbinder, Quirine C. van Rossum-Schornagel, Ron H. N. van Schaik, Agnes Jager, Stijn L. W. Koolen and Ron H. J. Mathijssen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-3-tam-10.1177_17588359221081075 for Influence of probenecid on endoxifen systemic exposure in breast cancer patients on adjuvant tamoxifen treatment by Stefan A. J. Buck, C. Louwrens Braal, Maaike M. Hofman, Esther Oomen-de Hoop, Peter de Bruijn, Inge M. Ghobadi Moghaddam-Helmantel, Koen G. A. M. Hussaarts, Mijntje B. Vastbinder, Quirine C. van Rossum-Schornagel, Ron H. N. van Schaik, Agnes Jager, Stijn L. W. Koolen and Ron H. J. Mathijssen in Therapeutic Advances in Medical Oncology
Footnotes
Author contributions: Stefan A. J. Buck: Conceptualization; Formal analysis; Investigation; Project administration; Writing – original draft; Writing – review & editing.
C. Louwrens Braal: Conceptualization; Writing – review & editing.
Maaike M. Hofman: Investigation; Project administration; Writing – review & editing.
Esther Oomen-de Hoop: Formal analysis; Methodology.
Peter de Bruijn: Investigation; Writing – review & editing.
Inge M. Ghobadi Moghaddam-Helmantel: Investigation; Writing – review & editing.
Koen G. A. M. Hussaarts: Conceptualization; Writing – review & editing.
Mijntje B. Vastbinder: Investigation; Writing – review & editing.
Quirine C. van Rossum-Schornagel: Investigation; Writing – review & editing.
Ron H. N. van Schaik: Methodology; Writing – review & editing.
Agnes Jager: Conceptualization; Writing – original draft; Writing – review & editing.
Stijn L. W. Koolen: Conceptualization; Writing – original draft; Writing – review & editing.
Ron H. J. Mathijssen: Conceptualization; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Stefan A. J. Buck  https://orcid.org/0000-0003-1367-8403
https://orcid.org/0000-0003-1367-8403
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Stefan A. J. Buck, Department of Medical Oncology, Erasmus MC Cancer Institute, Doctor Molewaterplein 40, P.O. Box 2040, 3000CA Rotterdam, The Netherlands.
C. Louwrens Braal, Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, The Netherlands.
Maaike M. Hofman, Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, The Netherlands
Esther Oomen-de Hoop, Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, The Netherlands.
Peter de Bruijn, Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, The Netherlands.
Inge M. Ghobadi Moghaddam-Helmantel, Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, The Netherlands
Koen G. A. M. Hussaarts, Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, The Netherlands
Mijntje B. Vastbinder, Department of Internal Medicine, IJsselland Hospital, Capelle aan den IJssel, The Netherlands
Quirine C. van Rossum-Schornagel, Department of Internal Medicine, Franciscus Gasthuis & Vlietland, Schiedam, The Netherlands
Ron H. N. van Schaik, Department of Clinical Chemistry, Erasmus University Medical Center, Rotterdam, The Netherlands
Agnes Jager, Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, The Netherlands.
Stijn L. W. Koolen, Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, The NetherlandsDepartment of Hospital Pharmacy, Erasmus University Medical Center, Rotterdam, The Netherlands
Ron H. J. Mathijssen, Department of Medical Oncology, Erasmus MC Cancer Institute, Rotterdam, The Netherlands
References
- 1. Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med 1998; 339: 1609–1618. [DOI] [PubMed] [Google Scholar]
- 2. Sanchez-Spitman AB, Swen JJ, Dezentje VO, et al. Clinical pharmacokinetics and pharmacogenetics of tamoxifen and endoxifen. Expert Rev Clin Pharmacol 2019; 12: 523–536. [DOI] [PubMed] [Google Scholar]
- 3. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011; 378: 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Madlensky L, Natarajan L, Tchu S, et al. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther 2011; 89: 718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saladores P, Murdter T, Eccles D, et al. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenomics J 2015; 15: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sachse C, Brockmoller J, Bauer S, et al. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet 1997; 60: 284–295. [PMC free article] [PubMed] [Google Scholar]
- 7. van der Lee M, Allard WG, Vossen R, et al. Toward predicting CYP2D6-mediated variable drug response from CYP2D6 gene sequencing data. Sci Transl Med 2021; 13: eabf3637. [DOI] [PubMed] [Google Scholar]
- 8. Borges S, Desta Z, Li L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther 2006; 80: 61–74. [DOI] [PubMed] [Google Scholar]
- 9. Blevins-Primeau AS, Sun D, Chen G, et al. Functional significance of UDP-glucuronosyltransferase variants in the metabolism of active tamoxifen metabolites. Cancer Res 2009; 69: 1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. King CD, Rios GR, Green MD, et al. UDP-glucuronosyltransferases. Curr Drug Metab 2000; 1: 143–161. [DOI] [PubMed] [Google Scholar]
- 11. Robbins N, Koch SE, Tranter M, et al. The history and future of probenecid. Cardiovasc Toxicol 2012; 12: 1–9. [DOI] [PubMed] [Google Scholar]
- 12. Strilchuk L, Fogacci F, Cicero AF. Safety and tolerability of available urate-lowering drugs: a critical review. Expert Opin Drug Saf 2019; 18: 261–271. [DOI] [PubMed] [Google Scholar]
- 13. Uchaipichat V, Mackenzie PI, Guo XH, et al. Human udp-glucuronosyltransferases: isoform selectivity and kinetics of 4-methylumbelliferone and 1-naphthol glucuronidation, effects of organic solvents, and inhibition by diclofenac and probenecid. Drug Metab Dispos 2004; 32: 413–423. [DOI] [PubMed] [Google Scholar]
- 14. Qian Y, Sherbini A, Matin B, et al. Inhibition of 2-methoxyestradiol glucuronidation by probenecid. J Pharm Pharmacol 2015; 67: 1585–1592. [DOI] [PubMed] [Google Scholar]
- 15. Markowitz JS, Devane CL, Liston HL, et al. The effects of probenecid on the disposition of risperidone and olanzapine in healthy volunteers. Clin Pharmacol Ther 2002; 71: 30–38. [DOI] [PubMed] [Google Scholar]
- 16. Kim KA, Oh SO, Park PW, et al. Effect of probenecid on the pharmacokinetics of carbamazepine in healthy subjects. Eur J Clin Pharmacol 2005; 61: 275–280. [DOI] [PubMed] [Google Scholar]
- 17. Smith DA. Induction and drug development. Eur J Pharm Sci 2000; 11: 185–189. [DOI] [PubMed] [Google Scholar]
- 18. EMA. Guideline on the investigation of drug interactions, 2012, https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-drug-interactions-revision-1_en.pdf
- 19. Jager NG, Rosing H, Schellens JH, et al. Tamoxifen dose and serum concentrations of tamoxifen and six of its metabolites in routine clinical outpatient care. Breast Cancer Res Treat 2014; 143: 477–483. [DOI] [PubMed] [Google Scholar]
- 20. FDA. Bioanalytical method validation guidance for industry, 2018, https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf
- 21. Binkhorst L, Mathijssen RH, Ghobadi Moghaddam-Helmantel IM, et al. Quantification of tamoxifen and three of its phase-I metabolites in human plasma by liquid chromatography/triple-quadrupole mass spectrometry. J Pharm Biomed Anal 2011; 56: 1016–1023. [DOI] [PubMed] [Google Scholar]
- 22. de Graan AJ, Sparreboom A, de Bruijn P, et al. 4beta-hydroxycholesterol as an endogenous CYP3A marker in cancer patients treated with taxanes. Br J Clin Pharmacol 2015; 80: 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diczfalusy U, Nylen H, Elander P, et al. 4beta-Hydroxycholesterol, an endogenous marker of CYP3A4/5 activity in humans. Br J Clin Pharmacol 2011; 71: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Binkhorst L, van Gelder T, Loos WJ, et al. Effects of CYP induction by rifampicin on tamoxifen exposure. Clin Pharmacol Ther 2012; 92: 62–67. [DOI] [PubMed] [Google Scholar]
- 25. Hwang GS, Bhat R, Crutchley RD, et al. Impact of CYP2D6 polymorphisms on endoxifen concentrations and breast cancer outcomes. Pharmacogenomics J 2018; 18: 201–208. [DOI] [PubMed] [Google Scholar]
- 26. Fox P, Balleine RL, Lee C, et al. Dose escalation of tamoxifen in patients with low endoxifen level: evidence for therapeutic drug monitoring-the TADE study. Clin Cancer Res 2016; 22: 3164–3171. [DOI] [PubMed] [Google Scholar]
- 27. Braal CL, Jager A, Hoop EO, et al. Therapeutic drug monitoring of endoxifen for tamoxifen precision dosing: feasible in patients with hormone-sensitive breast cancer. Clin Pharmacokinet. Epub ahead of print 17 November 2021. DOI: 10.1007/s40262-021-01077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hertz DL, Deal A, Ibrahim JG, et al. Tamoxifen dose escalation in patients with diminished CYP2D6 activity normalizes endoxifen concentrations without increasing toxicity. Oncologist 2016; 21: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Probenecid oral, https://reference.medscape.com/drug/probenecid-342832
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221081075 for Influence of probenecid on endoxifen systemic exposure in breast cancer patients on adjuvant tamoxifen treatment by Stefan A. J. Buck, C. Louwrens Braal, Maaike M. Hofman, Esther Oomen-de Hoop, Peter de Bruijn, Inge M. Ghobadi Moghaddam-Helmantel, Koen G. A. M. Hussaarts, Mijntje B. Vastbinder, Quirine C. van Rossum-Schornagel, Ron H. N. van Schaik, Agnes Jager, Stijn L. W. Koolen and Ron H. J. Mathijssen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359221081075 for Influence of probenecid on endoxifen systemic exposure in breast cancer patients on adjuvant tamoxifen treatment by Stefan A. J. Buck, C. Louwrens Braal, Maaike M. Hofman, Esther Oomen-de Hoop, Peter de Bruijn, Inge M. Ghobadi Moghaddam-Helmantel, Koen G. A. M. Hussaarts, Mijntje B. Vastbinder, Quirine C. van Rossum-Schornagel, Ron H. N. van Schaik, Agnes Jager, Stijn L. W. Koolen and Ron H. J. Mathijssen in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-3-tam-10.1177_17588359221081075 for Influence of probenecid on endoxifen systemic exposure in breast cancer patients on adjuvant tamoxifen treatment by Stefan A. J. Buck, C. Louwrens Braal, Maaike M. Hofman, Esther Oomen-de Hoop, Peter de Bruijn, Inge M. Ghobadi Moghaddam-Helmantel, Koen G. A. M. Hussaarts, Mijntje B. Vastbinder, Quirine C. van Rossum-Schornagel, Ron H. N. van Schaik, Agnes Jager, Stijn L. W. Koolen and Ron H. J. Mathijssen in Therapeutic Advances in Medical Oncology