Abstract
Introduction:
Partial nephrectomy (PN) is associated with a non-negligible risk of postoperative cardiovascular morbidity and mortality. Identification of high-risk patients may enable optimization of perioperative management and consideration of alternative approaches. The authors aim to develop a procedure-specific cardiovascular risk index for PN patients and compare its performance to the widely used revised cardiac risk index (RCRI) and AUB-HAS2 cardiovascular risk index.
Methods:
The cohort was derived from the American College of Surgeons – National Surgical Quality Improvement Program (ACS-NSQIP) database. The primary outcome was the incidence of major adverse cardiovascular events (MACE), defined as 30-day postoperative incidence of myocardial infarction, stroke, or mortality. A multivariate logistic regression model was constructed; performance and calibration were evaluated using an ROC analysis and the Hosmer–Lemeshow test and compared to the RCRI and the AUB-HAS2 index.
Results:
In a cohort of 4795 patients, MACE occurred in 52 (1.1%) patients. A univariate analysis yielded 13 eligible variables for entry into the multivariate model. The final PN-A4CH model utilized six variables: Age ⩾75 years, ASA class >2, Anemia, surgical Approach, Creatinine >1.5, and history of Heart disease. Index ROC analysis provided a C-statistic of 0.81, calibration R2 was 0.99, and sensitivity was 85%. In comparison, the RCRI and AUB-HAS2 C-statistics were 0.59 and 0.68, respectively.
Conclusion:
This study proposes a novel procedure-specific cardiovascular risk index. The PN-A4CH index demonstrated good predictive ability and excellent calibration using a large national database and may enable further individualization of patient care and optimization of patient selection.
Keywords: cardiovascular diseases, kidney neoplasm, logistic models, nephrectomy, nephron sparing surgery, postoperative complications
Introduction
Renal cell carcinoma (RCC) is the most common primary renal malignancy, accounting for over 80% of all primary renal neoplasms, and occurs most frequently in adults aged between 50 and 70 years. 1 Incidence rates are increasing due to an increasing number of patients being diagnosed with early-stage tumors, and in 2021, it is estimated that over 70,000 cases of RCC will be diagnosed in the United States alone. 2 Partial nephrectomy (PN) is the gold standard for the management of localized kidney tumors smaller than 7 cm (cT1); as long as excision radicality can be achieved during surgical removal, it is also a viable option for select cT2 patients.3–5 Adoption of PN has also seen a parallel increase in the utilization of minimally invasive surgery (MIS), laparoscopic, and robotic-assisted techniques. 6
PN is a procedure which minimizes the adverse effects of radical nephrectomy (RN) while maintaining optimal oncologic management. In comparison to RN, PN offers improved survival outcomes in the early-stage kidney cancer with tumor sizes smaller than 4 cm. 7 In patients with tumor sizes between 4 and 7 cm, PN was found to have similar overall and cancer-specific survival rates to RN. 8 Moreover, postoperative renal function is better preserved in PN patients, leading to a lower risk of new-onset chronic kidney disease (CKD).9,10
Cardiovascular complications are uncommon post-PN; however, their incidence carries significant morbidity and is often associated with mortality.11,12 Preoperative estimation of patient cardiac risk is possible through the utilization of established universal surgical risk models, such as the revised cardiac risk index (RCRI), NSQIP-MICA index, and the recently published AUB-HAS2 cardiovascular risk index.13–16 However, the development of a procedure-specific risk index for PN, a procedure with relatively low morbidity and a select patient population, carries substantial merit as it enables better optimization of preoperative patient status and improved patient selection for PN versus alternative treatment modalities, such as surveillance or ablative therapy.
Methodology
Patient population
The study population consisted of 4795 patients who underwent PN and were registered in the American College of Surgeons – National Surgical Quality Improvement Program (ACS-NSQIP) between 2005 and 2012. 17 Datasets after 2012 were not included in our study as cardiac history variables were not captured beyond that point. 18 The ACS-NSQIP is a multicenter database which captures data on patients undergoing major surgical procedures; the data encompass over 150 variables, including demographics, preoperative and intraoperative factors, and 30-day postoperative morbidity and mortality outcomes. Data are de-identified and does not constitute human subject research; therefore, no institutional review board approval was required from the participating centers. Data are collected by trained and certified surgical clinical reviewers and entered to the ACS-NSQIP website. Data quality is ensured via an intra-rater reliability (IRR) audit of participating sites. Surgical procedures are categorized using common procedural terminology (CPT) codes. PN cases were selected using the CPT codes 50240 and 50543, coding for open surgery and MIS, respectively.
Ethical approval
The de-identified database (ACS-NSQIP) does not constitute human subject research; therefore, no institutional review board approval was required or attained from the participating centers. Moreover, this was a retrospective study using a de-identified national database; hence, informed consent was neither required nor attainable.
Clinical variables
All available preoperative factors pertaining to demographics, lifestyle, preoperative laboratory results, comorbidities, and surgery type were used in the analysis. Demographic and lifestyle factors included patient age, gender, body mass index (BMI), race, ethnicity, and smoking status within 1 year of surgery. Comorbidities history included history of heart disease (myocardial infarction, percutaneous intervention, cardiac surgery, or congestive heart failure), symptoms of cardiac disease (angina or dyspnea at rest or exertion), cerebrovascular events (history of transient ischemic attacks or stroke with or without residual neurological deficit), peripheral vascular disease (revascularization/amputation for peripheral vascular disease and rest pain in lower extremity), history of chronic obstructive pulmonary disease (COPD), hepatic disease (ascites or esophageal varices), renal disease (acute renal failure or dialysis), preoperative sepsis, diabetes mellitus, insulin dependence, hypertension, chronic corticosteroid use, unintentional weight loss (>10% 6 months before surgery), bleeding disorders, American Society of Anesthesiologists’ (ASA) classification, and transfusion of packed red blood cells (pRBC), anemia (preoperative hematocrit < 36% for females and < 41% for males), thrombocytopenia (platelet count < 150 × 103), abnormal creatinine (serum creatinine ⩾ 1.5 mg/dl). Laboratory values included hematocrit, platelet count, white blood cell count (WBC), sodium, creatinine, and blood urea nitrogen (BUN). Surgical approach (open or MIS) was also evaluated as a potential risk factor.
Primary outcome
The primary outcome measure was the occurrence of death, myocardial infarction, or stroke (MACE) within 30 days post surgery. Myocardial infarction was identified by electrocardiogram (ECG) changes indicative of an acute MI (one of: an ST-elevation > 1 mm in two or more contiguous leads, new left bundle branch, new Q-wave in two or more contiguous leads) or new elevation in troponin > 3 times the upper level of the reference range in the setting of suspected myocardial ischemia. Stroke was defined as developing an embolic, thrombotic, or hemorrhagic vascular accident or stroke with motor, sensory, or cognitive dysfunction that persists for ⩾ 24 h. Death was defined as mortality occurring intraoperatively or within 30 days postoperatively.
Statistical analysis
Model and index construction, and validation
A descriptive analysis was performed on all preoperative variables; associations were determined using the χ 2 test for categorical variables and the independent t-test for continuous variables. For model construction, an exploratory univariate logistic regression analysis was performed on all preoperative variables, and odds ratios (ORs) with 95% confidence intervals (CIs) and p-values were determined. Variables that had a two-sided p-value less than 0.1 at the univariate level were eligible for consideration in the multivariable analysis. For construction of the multivariable logistic regression model, all eligible variables were entered simultaneously at the first step, and a backward stepwise analysis was performed to generate a parsimonious model. Variables with loss of significance at the adjusted level were removed individually, and model comparisons were made. Clinical importance of variables was considered in preferential removal of variables. A total of six variables with statistical and clinical significance were included in the final model, and all demonstrated two-sided p-values less than 0.05. After construction of the final model, discrimination ability was determined using a receiver operating characteristic (ROC) curve and deriving the concordance statistic (C-statistic). Model calibration was assessed using the Hosmer–Lemeshow test for goodness of fit and contingency table. The novel index was named ‘PN-A4CH’, an abbreviation for Partial Nephrectomy – Age, Anemia, ASA class, (surgical) Approach; Creatinine; Heart disease. To create and assess the novel PN-A4CH index, all six variables were given equal weights, and index performance was assessed using an ROC curve and calibration determined by the Hosmer–Lemeshow test for goodness of fit and contingency tables. Percent risk for each respective index score ranging from 0 to 6 was calculated using the logistic regression probability formula: All statistical analysis was performed with IBM SPSS Statistics, v.26 (IBM Corp., Armonk, NY, USA). Statistical significance was set at an alpha level of 0.05.
Index comparisons
The novel PN-A4CH index performance was compared to the RCRI and AUB-HAS2 cardiovascular risk index. ROC curves and C-statistics were derived and compared. Index calibrations were determined using the Hosmer–Lemeshow test for goodness of fit, and the contingency table was used to derive the coefficients of determination (R2) for the expected versus observed proportions of MACE incidence. Sensitivity was calculated for each index, using a score of 2 points or higher to define the increased risk of postoperative cardiovascular morbidity.
Results
A cohort of 4795 patients met the eligibility criteria, with a median age (IQR) of 60 (51–68) years and 2779 (57.9%) were males. OPN was performed on 2103 (43.9%) of patients, while an MIS approach was undertaken 2629 (56.1%) times. Overall, the MACE outcome was present in 52 (1.1%) patients. Table 1 provides a summary of patient demographics, pre- and intraoperative factors, and postoperative cardiovascular outcomes.
Table 1.
Patient demographics, preoperative laboratory findings and incidents, medical history, and outcomes stratified by MACE incidence and total population.
| MACE (No) | MACE (Yes) | Total | p-value | ||
|---|---|---|---|---|---|
| N (% of 4743) | N (% of 52) | N (% of 4795) | |||
| Demographics | |||||
| Age ⩾ 75 years | 455 (9.6) | 16 (30.8) | 471 (9.8) | <0.001 | |
| Gender | Male | 2742 (57.8) | 36 (69.2) | 2778 (57.9) | 0.097 |
| Female | 2001 (42.2) | 16 (30.8) | 2017 (42.1) | ||
| Race | White | 3722 (78.5) | 44 (84.6) | 3766 (78.5) | 0.169 |
| Black | 397 (8.4) | 2 (3.9) | 399 (8.3) | ||
| Other | 115 (2.4) | 3 (5.8) | 118 (2.5) | ||
| Hispanic ethnicity | 235 (5) | 3 (5.8) | 238 (5) | 0.357 | |
| Smoker | 918 (19.4) | 11 (21.2) | 929 (19.4) | 0.744 | |
| Obese | 2485 (52.4) | 44 (84.6) | 2529 (52.8) | 0.853 | |
| ASA class > 2 | 2159 (45.5) | 23 (44.2) | 2182 (45.5) | <0.001 | |
| Surgical approach | MIS | 2677 (56.4) | 15 (28.9) | 2692 (56.1) | <0.001 |
| Open | 2066 (43.6) | 37 (71.2) | 2103 (43.9) | ||
| Preoperative laboratory | |||||
| Anemia | 1172 (24.7) | 27 (51.9) | 1199 (25.0) | <0.001 | |
| Thrombocytopenia | 310 (6.5) | 10 (19.2) | 320 (6.7) | 0.002 | |
| Abnormal creatinine | 300 (6.3) | 17 (32.7) | 317 (6.6) | <0.001 | |
| Medical history | |||||
| Hypertensive | 2814 (59.3) | 43 (82.7) | 2857 (59.6) | <0.001 | |
| Diabetic | 863 (18.2) | 11 (21.2) | 874 (18.2) | 0.583 | |
| Symptoms of heart disease | 360 (7.6) | 8 (15.4) | 368 (7.7) | 0.058 | |
| Insulin dependent | 253 (5.3) | 5 (9.6) | 258 (5.4) | 0.201 | |
| History of heart disease | 222 (4.7) | 10 (19.2) | 232 (4.8) | <0.001 | |
| History of COPD | 213 (4.5) | 8 (15.4) | 221 (4.6) | 0.002 | |
| History of stroke/TIA | 97 (2.1) | 4 (7.7) | 101 (2.1) | 0.023 | |
| History of bleeding disorder | 98 (2.1) | 3 (5.8) | 101 (2.1) | 0.096 | |
| History of PVD | 20 (0.4) | 0 (0) | 20 (0.4) | 1.000 | |
| Preoperative incidents | |||||
| Acute renal failure | 10 (0.2) | 0 (0) | 10 (0.2) | 1.000 | |
| Preoperative dialysis | 16 (0.3) | 2 (3.9) | 18 (0.4) | 0.016 | |
| Preoperative pRBC transfusion | 8 (0.2) | 0 (0) | 8 (0.2) | 1.000 | |
| Cardiovascular outcomes | |||||
| MACE (MI or stroke or death) | 0 (0) | 52 (100) | 52 (1.1) | – | |
| Myocardial infarction | 0 (0) | 25 (48.1) | 25 (0.5) | – | |
| Stroke | 0 (0) | 10 (19.2) | 10 (0.2) | – | |
| Death | 0 (0) | 22 (42.3) | 22 (0.5) | – | |
ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; MACE, major adverse cardiovascular events; MI, myocardial infarction; MIS, minimally invasive surgery; pRBC, packed red blood cells; PVD, peripheral vascular disease as a combination of history of revascularization or amputation or rest pain or gangrene due to vascular disease.
Obese indicates BMI ⩾ 30 kg/m2; anemia indicates hematocrit < 36% for females and < 41% for males; thrombocytopenia indicates platelet count < 150 × 103; abnormal creatinine indicates serum creatinine ⩾ 1.5 mg/dl; symptoms of heart disease include angina and dyspnea; history of heart disease include myocardial infarction or congestive heart failure or percutaneous intervention or cardiac surgery.
A univariate analysis was conducted (S1 Table), and 13 variables were considered for entry into the multivariate logistic model: age ⩾ 75 years (OR = 4.19, p < 0.001), anemia (OR = 3.3, p < 0.001), abnormal creatinine (OR = 7.2, p < 0.001), thrombocytopenia (OR = 3.41, p < 0.001), hypertension (OR = 3.3, p < 0.001), history of cardiac disease (OR = 4.9, p < 0.001), symptoms of cardiac disease (OR = 2.2, p = 0.04), History of Cerebrovascular Accident (CVA) (OR = 4.0, p = 0.009), COPD (OR = 3.9, p < 0.001), dialysis (OR = 11.8, p < 0.001), bleeding disorders (OR = 2.9, p = 0.08), ASA class > 2 (OR = 5.0, p < 0.001), and surgical approach (OR = 3.2, p < 0.001).
The final multivariate logistic regression model contained six clinically and statistically significant factors, with their ORs and 95% CIs as shown in Table 2. Model ROC analysis provided a C-statistic of 0.82 (95% CI: 0.77–0.87; p < 0.001). Calibration testing provided an R2 of 0.99. A 1000-sample Bootstrap analysis provided valid CIs for all included variables (S2 Table).
Table 2.
Estimates, standard errors, p-values, adjusted ORs, and 95% CIs for variables associated with MACE in the full PN-A4CH logistic regression model.
| Factor | Estimate | SE | p-value | Adjusted OR | 95% CI |
|---|---|---|---|---|---|
| Age (⩾ 75 years) | 0.779 | 0.322 | 0.016 | 2.18 | (1.16–4.09) |
| Anemia | 0.668 | 0.294 | 0.023 | 1.95 | (1.10–3.47) |
| ASA class (>2) | 1.124 | 0.399 | 0.005 | 3.08 | (1.41–6.73) |
| Approach (open surgery) | 1.032 | 0.312 | <0.001 | 2.81 | (1.52–5.17) |
| Creatinine (⩾1.5 mg/dl) | 1.230 | 0.324 | <0.001 | 3.42 | (1.81–6.45) |
| Heart disease | 1.006 | 0.371 | 0.007 | 2.74 | (1.32–5.66) |
Adjusted OR, adjusted odds ratio; ASA class, American Society of Anesthesiologists classification; 95% CI, 95% confidence interval; MACE, major adverse cardiovascular events; SE, standard error.
Approach is surgical approach; creatinine is preoperative serum creatinine in mg/dl; heart disease is history of myocardial infarction, percutaneous intervention, cardiac surgery, or congestive heart failure.
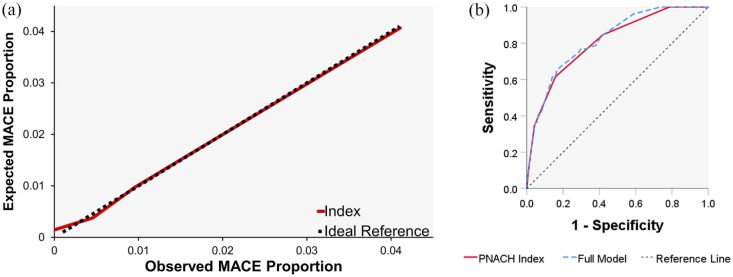
The final PN-A4CH index was derived by providing equal weights to all six variables. Index performance and calibration testing provided a C-statistic of 0.81 (95% CI: 0.75–0.87; p < 0.001) and R2 = 0.99, respectively (Figure 1).
Figure 1.
Receiver operated curve (ROC) for the full regression model and (b) the final PN-A4CH index versus the reference line, and (a) the observed versus expected proportions of postoperative major adverse cardiovascular events (MACE) versus to the ideal 45° line; indicating calibration of the final simplified PN-A4CH index.
The RCRI and AUB-HAS2 index provided C-statistics of 0.59 and 0.68, respectively, shown in (Figure 2) and Table 3. Sensitivity calculation resulted in 85% for the PN-A4CH index, 35% for the RCRI, and 21% for the AUB-HAS2 index.
Figure 2.
ROC curve comparison of the PN-A4CH, AUB-HAS2, and RCRI indices versus the reference line.
Table 3.
Comparison of PN-A4CH cardiac risk index with RCRI and AUB-HAS2 risk indices.
| Index | Score | MACE (No) | MACE (Yes) | Total | p-value | C-statistic (95% CI) |
|---|---|---|---|---|---|---|
| N (% of 4743) | N (% of 52) | N (%) | ||||
| RCRI | 0 | 0 (0) | 0 (0) | 0 (0) | <0.001 | 0.59 (0.51–0.68) |
| 1 | 3921 (82.7) | 34 (65.4) | 3955 (82.5) | |||
| 2 | 718 (15.1) | 11 (21.2) | 729 (15.2) | |||
| 3 | 91 (1.9) | 5 (9.6) | 96 (2) | |||
| 4 | 13 (0.3) | 2 (3.9) | 15 (0.3) | |||
| 5 | 0 (0) | 0 (0) | 0 (0) | |||
| 6 | 0 (0) | 0 (0) | 0 (0) | |||
| AUB-HAS2 | 0 | 3419 (72.1) | 21 (40.4) | 3440 (71.7) | <0.001 | 0.68 (0.59–0.76) |
| 1 | 1090 (23) | 20 (38.5) | 1110 (23.2) | |||
| 2 | 203 (4.3) | 6 (11.5) | 209 (4.4) | |||
| 3 | 30 (0.6) | 4 (7.7) | 34 (0.7) | |||
| 4 | 1 (0) | 1 (1.9) | 2 (0) | |||
| 5 | 0 (0) | 0 (0) | 0 (0) | |||
| 6 | 0 (0) | 0 (0) | 0 (0) | |||
| PN-A4CH | 0 | 1013 (21.4) | 0 (0) | 1013 (21.1) | <0.001 | 0.81 (0.75–0.87) |
| 1 | 1742 (36.8) | 8 (15.4) | 1750 (36.5) | |||
| 2 | 1237 (26.1) | 12 (23.1) | 1249 (26.1) | |||
| 3 | 548 (11.6) | 14 (26.9) | 562 (11.7) | |||
| 4 | 158 (3.3) | 13 (25) | 171 (3.6) | |||
| 5 | 40 (0.8) | 5 (9.6) | 45 (0.9) | |||
| 6 | 1 (0) | 0 (0) | 1 (0) |
AUB-HAS2, American University of Beirut HAS2 cardiovascular risk index; 95% CI, 95% confidence interval; MACE, major adverse cardiovascular events; PN-A4CH, partial nephrectomy cardiovascular risk index; RCRI, revised cardiac risk index.
Utilization of the PN-A4CH index may be simplified using the charted percentage risk estimations shown in Table 4. Such can be easy-to-use in the clinical setting and only requires addition of the risk index score with the respective estimated risk provided.
Table 4.
The PN-A4CH index scoring table with the percentage risk of 30-day MACE provided for respective scores.
| PN-A4CH score points total | % risk |
|---|---|
| 0 | 0.2 |
| 1 | 0.4 |
| 2 | 1.0 |
| 3 | 2.5 |
| 4 | 6.3 |
| 5 | 14.8 |
| 6 | 31.0 |
| Variable | Points |
| Age (⩾ 75 years) | 1 |
| Anemia | 1 |
| ASA class (>2) | 1 |
| Approach (open surgery) | 1 |
| Creatinine (⩾ 1.5 mg/dl) | 1 |
| Heart disease | 1 |
Age (years); anemia, yes: preoperative hematocrit < 36% for females and < 41% for males; Approach, open or minimally invasive surgery; ASA class, defined by the American Society of Anesthesiologists classification; creatinine, preoperative serum creatinine in mg/dl; heart disease, history of myocardial infarction, percutaneous intervention, cardiac surgery, or congestive heart failure; MACE, major adverse cardiovascular events; PN-A4CH, partial nephrectomy cardiovascular risk index.
Discussion
PN is the gold standard for cT1 kidney masses whenever feasible. 3 PN not only offers comparable oncologic outcomes to RN 19 but also offers better preservation of immediate and long-term kidney function.9,20 Due to less disruption in postoperative renal function, cardiovascular outcomes are improved with reduced incidence of MACE.21,22 As MACE incidence results in mortality or significant morbidity, estimating the risk of postoperative MACE is imperative; particularly as surgeons opt for PN to limit the functional morbidities of nephrectomy. We found that age ⩾ 75 years, anemia, abnormal creatinine (⩾ 1.5 mg/dl), history of heart disease, ASA class > 2, and open surgical approach all significantly increase the incidence of MACE within 30 days of PN.
First, age is known to be an independent risk factor for MACE, as demonstrated in a large Danish population cohort analysis which found that advanced age nearly doubled the risk of MACE. 23 Moreover, age has been utilized in other validated cardiovascular risk models, such as the AUB-HAS2 and Gupta scores.14,16 Similarly, ASA class has been incorporated in the Gupta score as it has been shown to be a reliable predictor of postoperative complications and mortality. 24 Our results highlight the importance of accounting for anemia as a risk factor for MACE after PN, and anemia is also an established risk factor for cardiovascular disease in the general population. 25 This is likely due to decreased tissue oxygenation and subsequent organ dysfunction, 26 and Wu et al. 27 demonstrated that even mild anemia was associated with an increased postoperative morbidity and mortality in a cohort of over 300,000 patients. Surgical approach is not included in widely used cardiovascular risk scores. Although strong evidence regarding postoperative cardiovascular morbidities is lacking in the field of PN, current evidence points toward a reduction of morbidity with MIS.28,29 In our study, we found that the traditional open approach for PN almost triples (OR = 2.81) the odds of MACE incidence, highlighting the importance of this risk factor and the need to explore its effect in other surgical procedures.
PN has been shown to decrease the incidence of cardiovascular adverse events by preserving more renal functionality than RN. 22 As such, preoperative creatinine values are strongly linked to cardiovascular morbidity and thus have been incorporated into the RCRI and the Gupta scores.13,16 A procedure-specific risk model, such as the PN-A4CH score, highlights the importance of preoperative renal function, as serum creatinine was attributed the highest odds ratio (OR = 3.42) of all six factors, hence reiterating the importance of this factor particularly in the PN population. History of heart disease was also attributed a high odds ratio (OR = 2.74), as in line with previous findings in major non-cardiac surgeries. 30 Other cardiovascular risk factors, such as histories of hypertension and stroke, were also found to be significant predictors for MACE, but only at the univariate level. Although history of stroke is one of the six RCRI factors, it may have overlapping contributions with ASA classification or history of heart disease. 13
The study results highlight the importance of developing procedure-specific tools to predict postoperative MACE. The universal scores available at our disposal, such as the AUB-HAS2 score and the RCRI score, are imperative in surgical practice. However, they lack procedure-specific intricacies as their construction used all major non-cardiac surgeries, without correcting for procedure- or population-specific characteristics. The authors believe that procedure-focused indices would help chaperon preoperative risk-prediction into the era of individualized medicine.
An important finding of this study is the added advantage of minimally invasive procedures in perioperative complications. It has been established that minimally invasive approaches offer the advantage of lower blood loss and reduced length of stay, when compared to the traditional open approach.31,32 However, we have shown from a large nation-wide database that traditional open PN confers a twofold increase in MACE, when compared to minimally invasive approaches.
Alternatively, it is possible to utilize the PN-A4CH index to stratify high-risk patients into alternative treatment modalities for small renal masses instead of pursuing the classical treatment of PN. For instance, in morbid patients with a high PN-A4CH score, active surveillance of small renal masses may be advocated, as active surveillance of small renal masses has been found to be of significant oncologic value in select patients.33,34 Alternatively, this score may assist in assigning well selected morbid patients to undergo tumor ablative therapies, which would otherwise be treated by PN, with decent oncologic outcomes. 35
Limitations
In this study, we constructed a novel procedure-specific cardiovascular risk-prediction index, described its statistical performance, and compared it to commonly used universal indices. Although the patient population utilized for this study is obtained from a large multicenter database, it is primarily based in North America. Moreover, the incidence of MACE events is a rare occurrence. Therefore, these results will require external validation using an independent cohort, preferably representing other countries or geographical areas. Moreover, the cohort data lack variables that are tumor- or procedure-specific, such as tumor stage, tumor complexity, ischemia time, or the use of cold or warm ischemia. Tumor-specific variables might have implications on the operative approach during PN and implications on postoperative morbidity. For instance, high complexity tumors with high RENAL score or high PADUA score may bleed more or require longer ischemia time and as a result confer a higher detriment to the cardiovascular system when compared to lower complexity tumors.
Conclusion
Our study proposes a novel procedure-specific cardiovascular risk index for patients undergoing PN. The PN-A4CH index includes six preoperative variables: Age ⩾ 75 years, ASA Class > 2, Anemia, surgical Approach, Creatinine > 1.5 mg/dl, and history of Heart disease. The PN-A4CH model demonstrated good predictive ability and excellent calibration using a large national database. The development and implementation of procedure-focused risk-prediction models may enable more individualization of patient care and further optimization of patient selection.
Supplemental Material
Supplemental material, sj-docx-1-tau-10.1177_17562872221084847 for Major adverse cardiovascular events following partial nephrectomy: a procedure-specific risk index by Ali A. Nasrallah, Habib A. Dakik, Nassib F. Abou Heidar, Jad A. Najdi, Oussama G. Nasrallah, Mazen Mansour, Hani Tamim and Albert El Hajj in Therapeutic Advances in Urology
Supplemental material, sj-docx-2-tau-10.1177_17562872221084847 for Major adverse cardiovascular events following partial nephrectomy: a procedure-specific risk index by Ali A. Nasrallah, Habib A. Dakik, Nassib F. Abou Heidar, Jad A. Najdi, Oussama G. Nasrallah, Mazen Mansour, Hani Tamim and Albert El Hajj in Therapeutic Advances in Urology
Acknowledgments
The authors thank Dr Viviane Chalhoub for her assistance in editing and revising the manuscript.
Footnotes
Author contributions: Ali A. Nasrallah: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Habib A. Dakik: Conceptualization; Investigation; Supervision; Writing – review & editing.
Nassib F. Abou Heidar: Conceptualization; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Jad A. Najdi: Writing – original draft; Writing – review & editing.
Oussama G. Nasrallah: Writing – original draft; Writing – review & editing.
Mazen Mansour: Writing – original draft; Writing – review & editing.
Hani Tamim: Data curation; Methodology; Writing – original draft; Writing – review & editing.
Albert El Hajj: Conceptualization; Investigation; Project administration; Supervision; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The de-identified database – the American College of Surgeons – National Surgical Quality Improvement Program (ACS-NSQIP) – does not constitute human subject research; therefore, no institutional review board approval was required or attained from the participating centers.
ORCID iD: Ali A. Nasrallah  https://orcid.org/0000-0001-7086-1410
https://orcid.org/0000-0001-7086-1410
Availability of data and material: Data are available at the American College of Surgeons – National Surgical Quality Improvement Program, and coding is fully available and can be provided by the authors.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ali A. Nasrallah, Division of Urology, Department of Surgery, American University of Beirut Medical Center, Beirut, Lebanon
Habib A. Dakik, Department of Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon
Nassib F. Abou Heidar, Division of Urology, Department of Surgery, American University of Beirut Medical Center, Beirut, Lebanon
Jad A. Najdi, Division of Urology, Department of Surgery, American University of Beirut Medical Center, Beirut, Lebanon
Oussama G. Nasrallah, Division of Urology, Department of Surgery, American University of Beirut Medical Center, Beirut, Lebanon
Mazen Mansour, Division of Urology, Department of Surgery, American University of Beirut Medical Center, Beirut, Lebanon.
Hani Tamim, Clinical Research Institute, American University of Beirut, Beirut, Lebanon.
Albert El Hajj, Division of Urology, Department of Surgery, American University of Beirut Medical Center, P.O. Box 11-0236, Riad El-Solh, 1107 2020 Beirut, Lebanon.
References
- 1. Garfield K, LaGrange CA. Renal cell cancer. Treasure Island, FL: StatPearls, 2020. [PubMed] [Google Scholar]
- 2. Du Z, Chen W, Xia Q, et al. Trends and projections of kidney cancer incidence at the global and national levels, 1990-2030: a Bayesian age-period modeling study. Biomark Res 2020; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campbell S, Uzzo RG, Allaf ME, et al. Renal mass and localized renal cancer: AUA guideline. J Urol 2017; 198: 520–529. [DOI] [PubMed] [Google Scholar]
- 4. Carbonara U, Simone G, Capitanio U, et al. Robot-assisted partial nephrectomy: 7-year outcomes. Minerva Urol Nephrol 2021; 73: 540–543. [DOI] [PubMed] [Google Scholar]
- 5. Minervini A, Grosso AA, Di Maida F. How to deal with renal cell carcinoma tumors > 7 cm: the role of nephron-sparing surgery. Eur Urol Open Sci 2021; 33: 42–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banegas MP, Harlan LC, Mann B, et al. Toward greater adoption of minimally invasive and nephron-sparing surgical techniques for renal cell cancer in the United States. Urol Oncol 2016; 34: 433.e9–433.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan H-J, Norton EC, Ye Z, et al. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA 2012; 307: 1629–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson RH, Siddiqui S, Lohse CM, et al. Partial versus radical nephrectomy for 4 to 7 cm renal cortical tumors. J Urol 2009; 182: 2601–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mir MC, Derweesh I, Porpiglia F, et al. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: a systematic review and meta-analysis of comparative studies. Eur Urol 2017; 71: 606–617. [DOI] [PubMed] [Google Scholar]
- 10. Anceschi U, Brassetti A, Tuderti G, et al. Risk factors for progression of chronic kidney disease after robotic partial nephrectomy in elderly patients: results from a multi-institutional collaborative series. Minerva Urol Nephrol. Epub ahead of print 22 June 2021. DOI: 10.23736/S2724-6051.21.04469-4. [DOI] [PubMed] [Google Scholar]
- 11. Puelacher C, Lurati Buse G, Seeberger D, et al. Perioperative myocardial injury after noncardiac surgery: incidence, mortality, and characterization. Circulation 2018; 137: 1221–1232. [DOI] [PubMed] [Google Scholar]
- 12. Mangano DT, Browner WS, Hollenberg M, et al. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med 1990; 323: 1781–1788. [DOI] [PubMed] [Google Scholar]
- 13. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100: 1043–1049. [DOI] [PubMed] [Google Scholar]
- 14. Dakik HA, Chehab O, Eldirani M, et al. A new index for pre-operative cardiovascular evaluation. J Am Coll Cardiol 2019; 73: 3067–3078. [DOI] [PubMed] [Google Scholar]
- 15. Dakik HA, Sbaity E, Msheik A, et al. AUB-HAS2 cardiovascular risk index: performance in surgical subpopulations and comparison to the revised cardiac risk index. J Am Heart Assoc 2020; 9: e016228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124: 381–387. [DOI] [PubMed] [Google Scholar]
- 17. American College of Surgeons. User guide for the 2012 ACS NSQIP procedure targeted participant use data file (PUF), https://www.facs.org/-/media/files/quality-programs/nsqip/ug12.ashx (2013, accessed 5 December 2020).
- 18. User guide for the 2014. ACS NSQIP procedure targeted participant use data file (PUF), https://www.facs.org/-/media/files/quality-programs/nsqip/nsqip_puf_userguide_2014.ashx (2014, accessed 5 December 2020).
- 19. Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011; 59: 543–552. [DOI] [PubMed] [Google Scholar]
- 20. Scosyrev E, Messing EM, Sylvester R, et al. Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol 2014; 65: 372–377. [DOI] [PubMed] [Google Scholar]
- 21. Weight CJ, Larson BT, Fergany AF, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol 2010; 183: 1317–1323. [DOI] [PubMed] [Google Scholar]
- 22. Capitanio U, Terrone C, Antonelli A, et al. Nephron-sparing techniques independently decrease the risk of cardiovascular events relative to radical nephrectomy in patients with a T1a–T1b renal mass and normal preoperative renal function. Eur Urol 2015; 67: 683–689. [DOI] [PubMed] [Google Scholar]
- 23. Hansen PW, Gislason GH, Jørgensen ME, et al. Influence of age on perioperative major adverse cardiovascular events and mortality risks in elective non-cardiac surgery. Eur J Intern Med 2016; 35: 55–59. [DOI] [PubMed] [Google Scholar]
- 24. Hackett NJ, De Oliveira GS, Jain UK, et al. ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int J Surg 2015; 18: 184–190. [DOI] [PubMed] [Google Scholar]
- 25. Miceli A, Romeo F, Glauber M, et al. Preoperative anemia increases mortality and postoperative morbidity after cardiac surgery. J Cardiothorac Surg 2014; 9: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation 2014; 130: e278–e333. [DOI] [PubMed] [Google Scholar]
- 27. Wu W-C, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA 2007; 297: 2481–2488. [DOI] [PubMed] [Google Scholar]
- 28. Porpiglia F, Mari A, Bertolo R, et al. Partial nephrectomy in clinical T1b renal tumors: multicenter comparative study of open, laparoscopic and robot-assisted approach (the RECORd project). Urology 2016; 89: 45–51. [DOI] [PubMed] [Google Scholar]
- 29. Wu Z, Li M, Liu B, et al. Robotic versus open partial nephrectomy: a systematic review and meta-analysis. PLoS ONE 2014; 9: e94878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hawn MT, Graham LA, Richman JS, et al. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013; 310: 1462–1472. [DOI] [PubMed] [Google Scholar]
- 31. Garisto J, Bertolo R, Dagenais J, et al. Robotic versus open partial nephrectomy for highly complex renal masses: comparison of perioperative, functional, and oncological outcomes. Urol Oncol 2018; 36: 471.e1–471.e9. [DOI] [PubMed] [Google Scholar]
- 32. Yerram NK, Dagenais J, Bryk DJ, et al. Trifecta outcomes in multifocal tumors: a comparison between robotic and open partial nephrectomy. J Endourol 2018; 32: 615–620. [DOI] [PubMed] [Google Scholar]
- 33. Pierorazio PM, Johnson MH, Ball MW, et al. Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. Eur Urol 2015; 68: 408–415. [DOI] [PubMed] [Google Scholar]
- 34. Jewett MAS, Mattar K, Basiuk J, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol 2011; 60: 39–44. [DOI] [PubMed] [Google Scholar]
- 35. Uhlig J, Strauss A, Rücker G, et al. Partial nephrectomy versus ablative techniques for small renal masses: a systematic review and network meta-analysis. Eur Radiol 2019; 29: 1293–1307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tau-10.1177_17562872221084847 for Major adverse cardiovascular events following partial nephrectomy: a procedure-specific risk index by Ali A. Nasrallah, Habib A. Dakik, Nassib F. Abou Heidar, Jad A. Najdi, Oussama G. Nasrallah, Mazen Mansour, Hani Tamim and Albert El Hajj in Therapeutic Advances in Urology
Supplemental material, sj-docx-2-tau-10.1177_17562872221084847 for Major adverse cardiovascular events following partial nephrectomy: a procedure-specific risk index by Ali A. Nasrallah, Habib A. Dakik, Nassib F. Abou Heidar, Jad A. Najdi, Oussama G. Nasrallah, Mazen Mansour, Hani Tamim and Albert El Hajj in Therapeutic Advances in Urology