Abstract
Aims
To determine the impact of anesthesia encountered and to optimize the treatment of perioperative pulmonary arterial hypertension (PAH) in an effort to improve perioperative management and reduce complications.
Methods
We conducted a retrospective analysis of scoliosis patients with PAH who underwent scoliosis surgery.
Results
During this period, we identified a total of 22 patients. Their mean age was 22.18 ± 2.11 years. 16 PAH patients (72.72%) received PAH-specific treatment. Only Propofol-based TIVA was used intraoperatively. During the procedure, pulmonary artery catheters and PICCO catheters were placed in all patients to monitor intraoperative and postoperative mPAP, MAP, PRVI and SRVI. During tracheal intubation and intraoperative awake testing, mPAP generally tended to increase in all patients. 6 patients (27.27%) received intraoperative PAH-Specific therapy. All patients received oral sildenafil (75-100 mg/d orally), and 9 patients received postoperative oral sildenafil combined with nebulized iloprost (20 μg/d); intravenous treprostinil (2 ng/kg/min started and titrated to 10-17.5 ng/kg/min); or bosentan (250 mg/d) postoperatively. 7 patients (31.82%) reported postoperative complications, including 2 cases of respiratory failure requiring reintubation, 1 case of right heart failure, 2 cases of superficial surgical site infection, 1 case of fluid and electrolyte and acid-base imbalances, 2 cases of pneumonia and 1 case of pulmonary oedema with fluid overload. Two patients developed more than 1 postoperative complication. No in-hospital death occurred.
Conclusions
The anesthetic management of scoliosis patients with PAH is important task that, like its own surgery, relies on the input of the multidisciplinary team for its success. Close monitoring, optimization of systemic blood pressure, pain control, oxygenation and ventilation, avoidance of exacerbating factors, and the use of vasopressors and pulmonary vasodilators when necessary are essential elements of management.
Keywords: anesthetic management, pulmonary arterial hypertension, scoliosis surgery
Introduction
Severe scoliosis has numerous adverse effects on the cardiorespiratory system. Previous studies have shown that scoliosis compromises chest-wall movement and is a causative factor in restrictive ventilatory disorders including reduced forced vital capacity (FVC) as well as a decrease in the percentage of predicted FVC (%FVC). Several early studies indicated that untreated scoliosis increases mortality caused by respiratory failure and right heart failure.1,2 Pulmonary arterial hypertension (PAH) is thought contribute to the development of cardiac consequences. In scoliosis patients with PAH, anesthesia and scoliosis surgery are associated with a significant increase in morbidity and mortality, mainly due to right ventricular failure, arrhythmias, postoperative hypoxemia, and myocardial ischemia. 3 Preoperative risk assessment and successful management of scoliosis patients with PAH undergoing scoliosis surgery involve an understanding of the pathophysiology of the disease, screening of patients at-risk for PAH, analysis of preoperative and operative risk factors, comprehensive multidisciplinary planning, careful intraoperative management, and early recognition and treatment of postoperative complications.
Here, we report a cases series of scoliosis patients with PAH who successfully underwent scoliosis surgery according to the 2015 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines, in order to provide a reference for the perioperative management of such cases.
Methods
Patient Selection
This was a single-center retrospective study. The institutional review board of the XXX approved this study, and the ethics committee waived the requirements for written informed consent. The medical records of all scoliosis patients with PAH who underwent scoliosis surgery between 2013 and 2020 at the XXX were reviewed retrospectively. All cases were confirmed with pulmonary artery catheterization. Mild pulmonary hypertension was defined as a mean pulmonary artery pressure between 25 and 49 mmHg, and severe pulmonary hypertension was defined a as mean pulmonary artery pressure ≥50 mmHg or sPAP ≥70 mmHg. Pulmonary hypertensive crisis (PHC) was defined as an acute increase in pulmonary arterial pressure (PAP) with an sPAP/systolic arterial pressure ratio >.8 with acute right heart (RV) failure and hypoxemia.
Data Collection
Data extraction included patients’ demographics including age, race–ethnicity, comorbidities, New York Heart Association functional state before, during, and after scoliosis surgery, 6-minute walk test, breath-hold test and any other existing co-morbidity. Preoperative pulmonary arterial pressure (PAP)- pulmonary artery catheter (PAC), mean PAP (mPAP)-PAC, pulmonary vascular resistance index (PVRI)-PAC, systemic vascular resistance index (SVRI)- pulse contour cardiac output (PICCO), mean arterial pressure (MAP), cardiac index (CI)-PAC, intraoperative PAH-specific therapy, operative time and postoperative PAH therapy, Post-operative duration of mechanical ventilation (PPDMV), complications, and vasopressor dose were among the parameters investigated.
Statistical Analysis
Statistical analyses were performed using SPSS version 22.0 (IBM, Armonk, New York, USA). Data were presented as the mean ± standard deviations (SD) for normally distributed continuous variables and numbers for categorical variables.
Results
Basic Demographic Characteristics of Scoliosis Patients with Pulmonary Arterial Hypertension Underwent Scoliosis Surgery
From 2013 to 2020, a total of 724 scoliosis patients were treated at our hospital, with 137 cases of idiopathic scoliosis, 212 cases of congenital scoliosis, 114 cases of neuromuscular scoliosis, 129 cases of degenerative scoliosis, 47 cases of traumatic scoliosis, 3 cases of tumour scoliosis, and 82 cases of inflammatory or specific infectious scoliosis. For patients with moderate-to-severe PAH, PAH-specific therapy was initiated before surgery. Most of these patients underwent scoliosis surgery under general anesthesia. A pulmonary artery catheter was placed before the patient was transferred to the operating room.
Twenty-two scoliosis patients were identified with PAH, with an incident rate of 3.04%. Their mean ages were 22.18 ± 2.11 years. Of the 22 patients with combined PAH who underwent scoliosis surgery, 19 patients (86%) had neuromuscular and congenital scoliosis. Their baseline characteristics, and management before scoliosis surgery were listed in Table 1. 13 patients (59.09%) were newly diagnosed with PAH during their hospitalization. 9 patients (40.91%) were previously diagnosed with PAH, and only 2 (9.10%) had received PAH specific therapy. 16 patients (72.73%) had other cardiopulmonary abnormalities, including 14 cases (63.63%) extremely pulmonary dysfunction, 11 (50.00%) structural malformation of heart. 12 (54.55%) had a history of respiratory failure, defined as arterial oxygen tension (PaO2) lower than 60 mmHg with a normal or high arterial carbon dioxide tension (PaCO2). Of these 22 patients, 13 (59.09%) were wheel chair bound. All patients recovered in the surgical intensive care unit for 3 to 5 days and continued to receive treatment for PAH. Once a patient was hemodynamically stabilized, the patient was transferred to the general ward for additional care.
Table 1.
Demographic Characteristics and Clinical Features.
| Parameter | Value |
|---|---|
| Age (years) | 31.6 ± 3.2 |
| Weight (kg) | 35.1 ± 12.9 |
| Cobb’s angle (º) | 127.5 ± 26.8 |
| Height (cm) | 126.2 ± 13.8 |
| Arm length (cm) | 146.6±19.1 |
| HR (beats/min) | 89.2 ± 26.1 |
| BP (mmHg) | 123.5 ± 36.5 |
| SpO2 (%) | 89.4 ± 7.8 |
| Na+ (mmol/L) | 138.6 ± 2.4 |
| K+ (mmol/L) | 3.9 ± 0.4 |
| Blood urea nitrogen (BUN) (mmol/L) | 4.7 ± 1.3 |
| Creatinine (μmol/L) | 42.5 ± 6.4 |
| Hb (g/dl) | 126.3 ± 11.4 |
| WBC (×109/L) | 8.4 ± 1.9 |
| PLT (×109/L) | 218.2 ± 31.7 |
Values are expressed as mean±SD.
Preoperative Therapy and Management
16 PAH patients (72.72%) received PAH-specific therapy (oral sildenafil, inhaled iloprost, or treprostinil) after initial diagnosis, 3 to 6 months before scoliosis surgery. They received sildenafil alone or in combination with inhaled iloprost, treprostinil or bosentan (Table 2). All 22 scoliosis patients received traction with a cephalopelvic ring upon admission. 10 scoliosis patients with combined type 2 respiratory failure were on noninvasive-ventilator assisted breathing. All of these patients received oxygen therapy. Only 2 patients received preoperative anticoagulation to prevent thrombosis and digoxin for heart failure. All patients were monitored postoperatively in the intensive care unit and received carefully planned pain management strategies to reduce sympathetic activation and increased pulmonary vascular resistance (PVR), and immediate postoperative monitoring in the intensive care unit. In addition to the above medical measures, all patients were asked to blow up balloons and sing exercises, and for patients with limited mobility, they are also asked to stake the stairs to improve their lung function.
Table 2.
Baseline Characteristics and Management of Scoliosis Patients with Pulmonary Arterial Hypertension.
| Patients | Age (years) | Type of Scoliosis | WHO FC at Hospital Admission | PAH-specific or Other Therapy | SpO2 (%) at Hospital Admission | SPAP-ECHO (mmHg) at Hospital Admission | 6MWD at Hospital Admission(m) | NT-proBNP at PAH Diagnosis (ng/L) | PAH Severity | Type 2 Respiratory Failure |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | IS | II | None or none | 96 | 74 | 481 | 485 | Low risk | No |
| 2 | 24 | NS | III | B 125 mg/d, N 20 mg/d or NIV | 90 | 82 | 248 | 671 | High risk | Yes |
| 3 | 19 | CS | III | None or none | 92 | 76 | 501 | 1083 | High risk | No |
| 4 | 23 | NS | III | S 75 mg/d or NIV | 90 | 89 | 420 | 893 | High risk | Yes |
| 5 | 24 | IS | II | S 75 mg/d or none | 96 | 55 | 486 | 512 | Moderate risk | No |
| 6 | 26 | NS | IV | S 100 mg/d, Ilo 20 µg/d, F 20 mg/d, D 0.25 mg/d, LMWH or NIV | 84 | 98 | 120 | 2630 | High risk | Yes |
| 7 | 21 | IS | II | B 125 mg/d or none | 94 | 68 | 508 | 413 | No | |
| 8 | 24 | NS | IV | S 100 mg/d, B 125 mg/d, F 20 mg/d, D 0.25 mg/d, LMWH or NIV | 86 | 96 | 294 | 1937 | High risk | Yes |
| 9 | 25 | NS | IV | S 100 mg/d, N 20 mg/d or NIV | 80 | 113 | 286 | 2346 | High risk | Yes |
| 10 | 21 | IS | III | None or none | 89 | 63 | 483 | 964 | High risk | No |
| 11 | 22 | CS | III | S 100 mg/d or none | 91 | 82 | 462 | 861 | Moderate risk | No |
| 12 | 22 | IS | II | S 75 mg/d or none | 92 | 54 | 496 | 635 | Moderate risk | No |
| 13 | 26 | NS | III | Ilo 20 µg/d or NIV | 88 | 75 | 381 | 1359 | High risk | Yes |
| 14 | 21 | CS | II | S 75 mg/d or none | 94 | 72 | 452 | 394 | Moderate risk | No |
| 15 | 19 | IS | III | None or none | 91 | 94 | 284 | 619 | Moderate risk | No |
| 16 | 20 | IS | II | None or none | 93 | 48 | 452 | 268 | Moderate risk | No |
| 17 | 20 | CS | IV | None or NIV | 82 | 93 | 157 | 1649 | High risk | Yes |
| 18 | 23 | NS | III | S 100 mg/d, Ilo 20 µg/d or NIV | 89 | 64 | 424 | 866 | High risk | Yes |
| 19 | 24 | CS | III | None or NIV | 84 | 81 | 392 | 946 | High risk | Yes |
| 20 | 21 | CS | II | S 75 mg/d or none | 93 | 61 | 436 | 268 | Low risk | No |
| 21 | 20 | IS | I | None or none | 95 | 49 | 447 | 123 | Low risk | No |
| 22 | 22 | NS | III | S 100 mg/d or NIV | 88 | 68 | 299 | 967 | High risk | Yes |
NS: Neuromuscular Scoliosis; IS: Idiopathic Scoliosis; CS: Congenital Scoliosis; B: Bosentan; N: Nifedipine; NIV: Non-invasive Ventilator therapy; S: Sildenafifil; Ilo: Iloprost; F: Furosemide; D: Digoxin; LMWH: Low-molecular–weight heparin; PHS: Pulmonary Arterial Hypertension.
Intraoperative Details of Scoliosis Surgery With Pulmonary Arterial Hypertension
Preoperatively, patients were classified as class 4 according to American Society of Anesthesiologists classification. The majority of procedures were performed in the prone position (81.82%, n = 18), with 4 patients receiving the lateral position due to excessive kyphoscoliosis. After local oral mucosal anesthesia and cricothyroid membrane puncture anesthesia, tracheal intubation was performed. 18 (81.82%) patients were guided by fiberoptic bronchoscope and 4 (18.18%) by visible light wand. Intraoperatively, only propofol-based total intravenous anesthesia (TIVA) was used with various neuromuscular blocking agents (Rocuronium or Cisatracurium), analgesics (Remifentanil, Sufentanil and Oxycodone), and adjuvants (Dexmedetomidine). The median operative time for primary procedures were 386 minutes (IQR 276–469). The estimated total intraoperative blood loss was 1130 mL (IQR 860–2600). 4 patients (18.18%) underwent revisional scoliosis surgery; their median operative time and estimated intraoperative blood loss were 513 minutes (IQR 457-587) and 1684 mL (IQR 1350-2073), respectively (Table 3).
Table 3.
Perioperative Data of Scoliosis Patients with Pulmonary Arterial Hypertension.
| Patients | PAP-PAC Pre-operative (mmHg) | Mean PAP-PAC Pre-operative (mmHg) | PVRI-PAC Pre-operative (dynes·s/cm5·m2) | SVRI-Picco Pre-operative (dynes·s/cm5 m2) | MAP Pre-operative (mmHg) | CI-PAC Pre-operative (L/min·m2) | Operative Body Position | Intraoperative PAH-specific Therapy | Operative Duration (min) | Intraoperative Blood Loss (ml) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62/34 | 43 | 384 | 2238 | 86 | 3.1 | Prone position | None | 324 | 1050 |
| 2 | 96/71 | 79 | 592 | 1986 | 72 | 2.6 | Prone position | Ilo 20 µg | 486 | 1680 |
| 3 | 83/60 | 68 | 684 | 2461 | 80 | 2.4 | Prone position | Ilo 20 µg | 596 | 2608 |
| 4 | 96/45 | 62 | 692 | 1793 | 71 | 2.7 | Prone position | None | 686 | 1840 |
| 5 | 74/34 | 47 | 319 | 1933 | 92 | 3.4 | Prone position | None | 351 | 1294 |
| 6 | 112/68 | 83 | 1011 | 2468 | 106 | 2.0 | Lateral position | Tre 2 ng/kg/min | 468 | 1845 |
| 7 | 52/34 | 40 | 336 | 2018 | 81 | 3.1 | Prone position | None | 513 | 860 |
| 8 | 110/63 | 79 | 916 | 1930 | 96 | 1.8 | Lateral position | Tre 2 ng/kg/min | 512 | 2684 |
| 9 | 86/45 | 59 | 715 | 2096 | 84 | 2.2 | Prone position | Tre 2 ng/kg/min | 608 | 1682 |
| 10 | 76/40 | 52 | 496 | 2461 | 90 | 2.7 | Prone position | None | 691 | 2643 |
| 11 | 94/48 | 63 | 618 | 2715 | 106 | 2.4 | Prone position | None | 705 | 1620 |
| 12 | 61/48 | 52 | 351 | 2184 | 89 | 3.1 | Prone position | None | 439 | 2662 |
| 13 | 82/61 | 68 | 634 | 2291 | 96 | 2.6 | Prone position | None | 563 | 1686 |
| 14 | 69/54 | 59 | 412 | 2683 | 105 | 3.2 | Prone position | None | 324 | 1832 |
| 15 | 90/42 | 58 | 643 | 1925 | 76 | 2.6 | Prone position | None | 584 | 1680 |
| 16 | 61/54 | 56 | 346 | 1897 | 81 | 3.8 | Prone position | None | 431 | 2086 |
| 17 | 98/47 | 64 | 851 | 2843 | 103 | 1.9 | Lateral position | Tre 2 ng/kg/min | 572 | 1368 |
| 18 | 92/40 | 57 | 761 | 2034 | 96 | 2.7 | Prone position | None | 496 | 968 |
| 19 | 106/60 | 75 | 944 | 3012 | 112 | 2.4 | Lateral position | None | 536 | 1094 |
| 20 | 58/39 | 45 | 346 | 2433 | 102 | 3.6 | Prone position | None | 463 | 1389 |
| 21 | 55/38 | 44 | 358 | 2196 | 92 | 3.6 | Prone position | None | 582 | 2068 |
| 22 | 82/66 | 71 | 539 | 2681 | 107 | 2.8 | Prone position | None | 468 | 1968 |
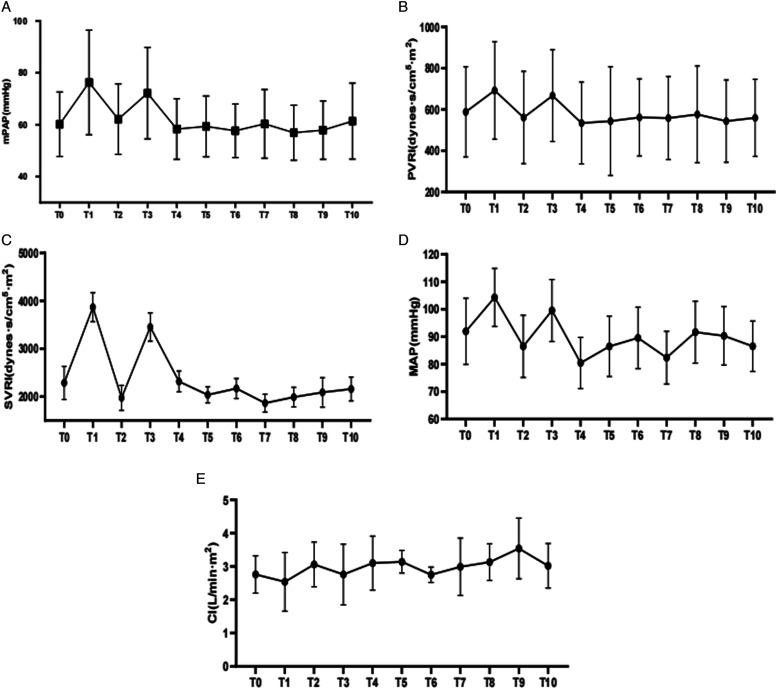
Pulmonary artery catheters were placed in all patients before they were transferred to the operating room. Intraoperative and postoperative monitoring of mPAP, PVR index, systemic vascular resistance (SVR) index, and cardiac output was performed. When the intraoperative mean PAP/mean systemic arterial pressure ratio increased, iloprost was inhaled or intravenous treprostinil was administered to control pulmonary hypertension. Intraoperatively, 2 patients received iloprost inhalation and 5 patients received treprostinil intravenously. Most pulmonary artery catheters were removed within 3 to 5 days after surgery when the patient’s condition became relatively stable. The bispectral index (BIS) monitor was used as a guide for anesthetic depth to reduce incidents of sudden hemodynamic disturbances. Somatosensory evoked potentials (SSEPs) and myogenic motor evoked potentials (MMEPs) were monitored in all procedures. During the operation, we also monitored invasive arterial blood pressure and pulse-indicated continuous cardiac output (PICCO). 16 patients experienced intraoperative hypotension (systemic blood pressure < 90/60 mmHg) and were treated with phenylephrine; noradrenaline; or epinephrine to maintain hemodynamic stability. During tracheal intubation and intraoperative awake testing, mPAP generally tended to increase in all patients (Figure.1).
Figure 1.
Perioperative hemodynamic parameters over 72 hours for mPAP (A), PVRI (B), SVTI (C), Map (D) and CI(E). mPAP: mean systemic pulmonary arterial pressure; PVRI: pulmonary vascular resistance index; SVRI: systemic vascular resistance index; MAP: mean systemic arterial pressure; CI: cardiac index. The horizontal axis of the coordinates indicatingT0, at induction; T1, at intubation; T2, at skin incision; T3, at intraoperative awake-test; T4, at the end of the procedure; T5, 12 hours postoperatively; T6, 24 hours postoperatively; T7, 36 hours postoperatively; T8, 48 hours postoperatively; T9, 60 hours postoperatively; T10, 72 hours postoperatively.
Postoperative Management and Clinic Outcomes
Echocardiography was performed daily after scoliosis surgery. LMWH was started 24 hours after surgery. The pulmonary artery catheter was removed on the third to fifth postoperative day without catheter-related complications. No patient died postoperatively due to pulmonary hypertension and related complications. The mean PAP, PVR index, mean systemic arterial pressure, SVR index, and cardiac index were almost equivalent preoperatively and 72 hours postoperatively (Figure.1). All patients were treated with oral sildenafil (75-100 mg/d orally), and 9 received oral sildenafil combined with nebulized iloprost (20 μg/d); intravenous treprostinil (starting at 2 ng/kg/min and titrated to 10-17.5 ng/kg/min); or bosentan (250 mg/d) based on PAP measurements by pulmonary artery catheter (Table 4).
Table 4.
Postoperative Management of Scoliosis Patients with Pulmonary Arterial Hypertension.
| Patients | Post-operative Pulmonary Arterial Hypertension Therapies | Other Therapies | PPDMV (h) | Post-operative Complications | Dosage of Vasopressor |
|---|---|---|---|---|---|
| 1 | S 75 mg/d, Ilo 20 µg/d, LMWH 5000IU/d | None | 3 | None | Norepinephrine .03 mg/kg/min |
| 2 | B 125 mg/d, N 20 mg/d, LMWH 5000IU/d | Non-invasive ventilator therapy | 11 | None | Norepinephrine .05 mg/kg/min |
| 3 | S 100 mg/d, Ilo 20 µg/d, LMWH 5000IU/d | CRRT | 16 | FEAI | Epinephrine .02 mg/kg/min |
| 4 | S 75 mg/d, LMWH 5000IU/d | Non-invasive ventilator therapy | 37 | Pulmonary edema | Norepinephrine .05 mg/kg/min |
| 5 | S 75 mg/d, Ilo 20 µg/d, LMWH 5000IU/d | None | 4 | None | None |
| 6 | S 100 mg/d, Ilo 20 µg/d, furosemide 20 mg/d,digoxin,0.25 mg/d, LMWH 5000 IU/d | Non-invasive ventilator therapy | 61 | Respiratory failure, pneumonia | Epinephrine .02 mg/kg/min |
| 7 | Bosentan 125 mg/d, LMWH 5000IU/d | None | 11 | None | Norepinephrine .01 mg/kg/min |
| 8 | S 100 mg/d, bosentan 125 mg/d, furosemide 20 mg/d, digoxin,0.25 mg/d, LMWH 5000 IU/d | Non-invasive ventilator therapy | 72 | Respiratory failure, pneumonia | Epinephrine .01 mg/kg/min, dobutamine 5 µg/kg/min |
| 9 | S 100 mg/d, Nifedipine 20 mg/d, LMWH 5000IU/d | Non-invasive ventilator therapy | 28 | None | Norepinephrine .05 mg/kg/min, dobutamine 5 µg/kg/min |
| 10 | S 100 mg/d, Nifedipine 20 mg/d, LMWH 5000IU/d | None | 16 | None | Norepinephrine .03 mg/kg/min |
| 11 | S 100 mg/d, Ilo 20 µg/d, LMWH 5000IU/d | None | 12 | Surgical site infection | Norepinephrine .04 mg/kg/min |
| 12 | S 75 mg/d, Ilo 20 µg/d, LMWH 5000IU/d | None | 9 | None | None |
| 13 | Ilo 20ug/d, LMWH 5000IU/d | Non-invasive ventilator therapy | 24 | None | Norepinephrine .04 mg/kg/min |
| 14 | S 75 mg/d, LMWH 5000IU/d | None | 16 | Surgical site infection | None |
| 15 | S 75 mg/d, LMWH 5000IU/d | None | 33 | None | Epinephrine .01 mg/kg/min |
| 16 | S 75 mg/d, LMWH 5000IU/d | None | 26 | None | None |
| 17 | S 100 mg/d, Nifedipine 20 mg/d, furosemide 20 mg/d, digoxin,0.25 mg/d, LMWH | Non-invasive ventilator therapy | 63 | Right heart failure | Epinephrine .03 mg/kg/min and dobutamine 5 µg/kg/min |
| 18 | S 100 mg/d, Ilo 20 ug/d, LMWH 5000IU/d | Non-invasive ventilator therapy | 37 | None | Norepinephrine .05 mg/kg/min |
| 19 | S 100 mg/d, LMWH 5000IU/d | Non-invasive ventilator therapy | 28 | None | Norepinephrine .05 mg/kg/min |
| 20 | S 75 mg/d, Ilo 20 ug/d, LMWH 5000IU/d | None | 19 | None | None |
| 21 | S 75 mg/d, LMWH 5000IU/d | None | 8 | None | None |
| 22 | S 100 mg/d, Ilo 20 µg/d, LMWH 5000IU/d | Non-invasive ventilator therapy | 42 | None | None |
PPDMV: Post-operative duration of mechanical ventilation; FEAI: Fluid and electrolyte and acid-base imbalances.
Early postoperative complications (≤30 d) were reported in 7 patients (31.82%). Major early complications included respiratory failure requiring reintubation (n = 2), right heart failure (n = 1). Minor early complications included superficial surgical site infection (n = 2), fluid and electrolyte and acid-base imbalances (n = 1), pneumonia (n = 2), pulmonary edema with fluid overload (n = 1). Two patients developed >1 early postoperative complications. Overall, 4 patients developed cardiopulmonary complications. All patients received post-operative analgesia, including oral NSAIDs and intravenous oxycodone. There were no postoperative deaths in this cohort.
Discussion
PAH is a known risk factor for perioperative complications. Most of the literature regarding the role of PAH in perioperative morbidity and mortality in patients undergoing noncardiac surgery is based on small series and remains poorly defined. 4 PAH is currently not listed in the American Heart Association/American College of Cardiology Foundation Practice Guideline for noncardiac surgery as an independent risk factor for postoperative complications. 5 Despite the paucity of data, patients with PAH are often counseled against having elective procedures because early and sudden postoperative deaths have been reported. 6
Potential complications, including hypotension, respiratory compromise, or RV failure, may occur intraoperatively or in the postoperative period. 7 Despite the limited literature, several factors have been identified as potential predictors of increased risk of perioperative complications in patients with PAH undergoing noncardiac surgery. Patients with PAH are unable to accommodate alterations in right ventricular (RV) preload or afterload induced by fluid shifts, medications, or changes in the autonomic nervous system precipitated by hypoxia or hypercapnia. 8 These factors become magnified in situations of added stress such as surgical intervention. Systemic hypotension and arrhythmias may precipitate RV ischemia, further worsening RV function.9,10 Patient and surgical characteristics and choice of anesthetic technique are crucial factors in perioperative management. The 2 main principles of perioperative management are the prevention of systemic hypotension (risk of RV ischemia) and the prevention of acute elevations in pulmonary arterial pressure (risk of RV failure).11-13 Close monitoring, optimization of systemic blood pressure (BP), pain control, oxygenation and ventilation, avoidance of exacerbating factors, and use of vasopressors and pulmonary vasodilators as necessary are essential elements of management.8,12,14 Understanding the pathophysiology, cause, and severity of PAH in the individual perioperative patient allows accurate risk assessment, optimization of PAH and RV function prior to surgery, and appropriate intraoperative and postoperative management.2,15,16
Appropriate preoperative evaluation and medical optimization, as well as close monitoring during the perioperative period to avoid noxious stimuli, are essential for success. Perioperative medical management of these complex patients often requires a multidisciplinary approach with input from anesthesiologists, PAH experts, pharmacists, and surgeons. In our clinical practice, perioperative management was based on the literature and the expertise of the authors in managing these patients, as there are no evidence-based guidelines.17,18 Based on the evidence, the patients with PAH undergoing scoliosis surgery are also at increased risk and be evaluated for the cause and severity of the PAH prior to surgery. In addition to the assessment of external scoliosis, we performed a thorough history and physical examination, ECG, chest radiograph and Doppler echocardiography to assess the structure and function of the RV and LV and valves. As an anesthesiologist, several key issues should be considered in the preoperative evaluation, including the risk-benefit ratio of the surgery, the anesthetic technique to be used, the potential for the surgery to disrupt fluid balance or increase PVR, and the availability of necessary tools to prevent and treat PAH and acute right heart failure should they occur. The principles of management include the avoidance of systemic hypotension (risk of RV ischemia), myocardial depression (risk of RV failure due to diminished contractility), acute elevations of PAP (risk of RV failure due to increased impedance), inadequate pain control (risk of increased sympathetic tone and PVR), and respiratory depression.19-21
Specific medications being used to treat PAH, such as oral sildenafil, inhaled iloprost, or treprostinil therapy must be continued, as discontinuation induces aa PAH crisis.8,11,22 Even though some of these medications can inhibit platelet aggregation, this is usually a minor effect and excessive surgical bleeding has not been reported. Throughout the scoliosis surgery procedure, we maintained oxygenation well and avoided systemic hypotension, which could have led right heart ischemia. The standard American Society of Anesthesiologists monitors was used in all scoliosis patients. In addition, we applied an indwelling arterial catheter to monitor the systemic BP during induction of general anesthesia. We also monitored central venous pressure to assess changes in RV filling pressure and to detect new or worsening of tricuspid insufficiency. Since PA catheter monitoring allows accurate assessment of PAP for proper treatment with pulmonary vasodilators, measurement of CO and mixed venous oxygen saturation, as well as accurate measurement of central venous pressure and pulmonary artery pressure. Because of the ability to accurately assess PAP for appropriate treatment with pulmonary vasodilators, measure CO, mixed venous oxygen saturation, calculate PVR, and accurately measure central venous pressure and pulmonary wedge pressure to determine the need for vasopressors and fluids, all of our patients with scoliosis combined with PAH were monitored with PA catheter. We did not perform intraoperative TEE monitoring due to the position restrictions of scoliosis surgery.
All scoliosis patients underwent general anesthesia. The choice of anesthetic technique and anesthetic management are crucial factors in maintaining cardiovascular stability in patients with PAH. However, regardless of the anesthetic technique used, an adequate level of anesthesia should be maintained to produce effective pain control. Good oral mucosal and tracheal surface anesthesia is particularly important because the patient’s neck is held in place by an external fixation brace (cephalopelvic ring), the patient’s neck is difficult to tilt posteriorly, and all patients were intubated while awake. All the intubation were performed in a rapid and smooth manner by an experienced person. Throughout conscious tracheal intubation, we preoxygenated with 100% oxygen to achieve an end-tidal oxygen concentration of more than 90%, which mitigates the increased risk of hypoxemia that often occurs after induction due to reduced functional residual capacity. Patients were also instructed to breathe deeply to avoid an increase in arterial CO 2 and to achieve adequate oxygenation. Anesthetics may depress myocardial contractility, decrease SVR and venous return, increase PVR, or exacerbate hypoxia or hypercapnia. Etomidate has the least effect on myocardial contractility and SVR, and in combination with opioids is usually our induction agent of choice. IV or nebulized milrinone or epoprostenol, IV nitroglycerin, inhaled nitric oxide, or nebulized epoprostenol or iloprost can all be used before induction to prevent pulmonary hypertensive responses, although their efficacy has not been studied adequately and selection depends largely on whether they are used preoperatively. When titrating anesthetics, it should be remembered that the metabolism of drugs may be altered in patients with chronic severe PAH due to prolonged venous congestion and visceral dysfunction. During maintenance, we avoid exacerbating risk factors that exacerbate impaired RV, such as hypothermia, hypoxia, hypercarbia, acidosis, and hyper- or hypovolemia. To achieve these goals, blood gases, temperature and ventilation need to be monitored and adequate depth of anesthesia needs to be maintained. A lung-preserving ventilator strategies using low tidal volumes may help avoid overinflation of the lungs and minimize PVR by keeping lung volume close to functional residual capacity. Scoliosis surgery carries greater risks for patients with PAH because of the long operative time, volume shifts and blood loss, and the impact on cardiovascular or pulmonary function. In particular, patients with combined thoracic deformities and intraoperative thoracoplasty may experience a temporary or permanent increase in PVR.
In addition, scoliosis correction may increase intra-abdominal pressure enough to impair pulmonary compliance or reduce venous return. Postoperatively, patients are at risk of worsening PAH values and RV ischemia as the effects of the anesthetics used during surgery, as well as opioids, wear off. Pain increases PVR, but opioids used systemically can have the same effect, as they tend to cause respiratory acidosis. In this case, regional blocks may be beneficial as they relieve pain without inhibiting respiratory drive. We also routinely use adjuvant therapy with NSAIDs intraoperatively to improve pain control.
A feature of the intraoperative management of scoliosis surgery is that in order to determine the function of the spinal cord, the surgeon will require the anesthesiologist to carry out a wake-up test after the main steps of the operation have been completed. In scoliosis patients with combined PAH, this is a huge risk. This is why we gave an adequate amount of analgesic medications, including oxycodone or NSIADs during intraoperative arousal in these patients. Arousal times are usually longer than in other patients with scoliosis without PAH. Despite this, during our PA catheter monitoring, we found that the patient’s pulmonary artery pressure was elevated during intraoperative arousal.
Recommendation
For all patients with scoliosis, we recommend TransThoracic Echocardiography (TTE) prior to admission, which provides a clearer picture of the structure of the heart chambers, changes in valve motion and blood flow spectrum, and indirectly infers pressure changes in the pulmonary circulation. When TTE shows a combination of PAH, patients should undergo right heart catheterization upon admission to accurately measure pulmonary artery pressure, which is the “gold standard” for evaluating the accuracy of various non-invasive manometric methods. In addition to accurately measuring pulmonary artery pressure, it can be used to determine pulmonary artery wedge pressure and to measure the oxygen content of the heart chambers, as well as to assess the degree of hemodynamic compromise and to test pulmonary vascular reactivity.
Conclusions
PAH is a high-risk factor for scoliosis surgeries. Careful preoperative evaluation should be performed in all patients with PAH, and those deemed at higher risk by virtue of significant reductions in functional capacity or abnormalities on echocardiogram should undergo a preoperative right-sided heart catheterization to better characterize their severity. Careful planning among PAH specialists, anesthesiologists, and surgeons can help avoid unnecessary complications. Multiple pharmacotherapies, including specific PAH drugs, vasopressors, and orthomimetics, have been used to treat PAH patients in the perioperative period, but have not been systematically studied. On the other hand, observation of fundamental management principles such as optimization of fluid volume, systemic BP, and acid base balance; avoidance of hypoxemia and hypercapnia; and good pain control are clearly important to achieve the desired surgical outcomes.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was financially supported by the project of Health and Family Planning Commission of Chengdu (2018002) and the project of Health and Family Planning Commission of Sichuan province (18PJ181).
ORCID iDs
Chuandong Zheng https://orcid.org/0000-0002-0300-0714
References
- 1.Koumbourlis AC. Scoliosis and the respiratory system. Paediatr Respir Rev. 2006;7(2):152-160. [DOI] [PubMed] [Google Scholar]
- 2.Lin Y, Shen J, Chen L, et al. Cardiopulmonary function in patients with congenital scoliosis: An observational study. J Bone Joint Surg Am. 2019;101(12):1109-1118. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y, Tan H, Rong T, et al. Impact of thoracic cage dimension and geometry on cardiopulmonary function in patients with congenital scoliosis: A prospective study. Spine. 2019;44(20):1441-1448. [DOI] [PubMed] [Google Scholar]
- 4.Aguirre MA, Lynch I, Hardman B. Perioperative management of pulmonary hypertension and right ventricular failure during noncardiac surgery. Adv Anesth. 2018;36(1):201-230. [DOI] [PubMed] [Google Scholar]
- 5.Halperin JL, Levine GN, Al-Khatib SM, et al. Further evolution of the ACC/AHA clinical practice guideline recommendation classification system: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2016;133(14):1426-1428. [DOI] [PubMed] [Google Scholar]
- 6.Schisler T, Marquez JM, Hilmi I, et al. Pulmonary Hypertensive Crisis on Induction of Anesthesia: Recommendations for Safe Practice; Proceedings of the seminars in cardiothoracic and vascular Anesthesia, F; Los Angeles, CA: SAGE Publications Sage CA; 2017. [DOI] [PubMed] [Google Scholar]
- 7.Soroceanu A, Burton DC, Oren JH, et al. Medical complications after adult spinal deformity surgery: Incidence, risk factors, and clinical impact. Spine. 2016;41(22):1718-1723. [DOI] [PubMed] [Google Scholar]
- 8.Gupta B, Kakkar K, Gupta L, et al. Anesthetic Considerations for a Parturient with Pulmonary Hypertension; Proceedings of the Indian Anaesthetists Forum, F. Mumbai, India: Medknow Publications; 2017. [Google Scholar]
- 9.Maguire K. Adolescent Idiopathic Scoliosis Patients are at Increased Risk for Pulmonary Hypertension Which Reverses after Corrective Scoliosis Surgery; Proceedings of the 2014 AAP National Conference and Exhibition, F. Itasca, IL: American Academy of Pediatrics; 2014. [Google Scholar]
- 10.Sarwahi V, Borlack RE, Dworkin A, et al. Adolescent idiopathic scoliosis patients are at increased risk for pulmonary hypertension which reverses after scoliosis surgery. Spine J. 2014;14(11):S153-S154. [Google Scholar]
- 11.Przybylski R, Hedequist DJ, Nasr VG, et al. Adverse perioperative events in children with complex congenital heart disease undergoing operative scoliosis repair in the contemporary era. Pediatr Cardiol. 2019;40(7):1468-1475. [DOI] [PubMed] [Google Scholar]
- 12.Helal SM, Abd Elaziz AA, Dawoud AG. Anesthetic considerations during intraoperative neurophysiological monitoring in spine surgery. Menoufia Med J. 2018;31(4):1187. [Google Scholar]
- 13.Tellermann J, Sablinskis M, Machado PRR, et al. Long-term response to vasoactive treatment in a case of kyphoscoliosis-associated pulmonary hypertension. Am J Case Rep. 2019;20:1505-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bersot CD, Guimarães JE, Mercante R, et al. Intraoperative Neurophysiologic Monitoring in Spinal Cord Surgery: A Brief Review. https://www.researchgate.net/publication/348154686_. January 2, 2021. [Google Scholar]
- 15.Smilowitz NR, Armanious A, Bangalore S, et al. Cardiovascular outcomes of patients with pulmonary hypertension undergoing noncardiac surgery. Am J Cardiol. 2019;123(9):1532-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steppan J, Diaz-Rodriguez N, Barodka VM, et al. Focused review of perioperative care of patients with pulmonary hypertension and proposal of a perioperative pathway. Cureus. 2018;10(1):e2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inao T, Amano M, Hashimoto S, et al. Rapid improvement of severe pulmonary hypertension due to scoliosis-related restrictive ventilatory disorder. Intern Med. 2021:60(20):3289-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo JS, So KY, Kim SH. Perioperative anesthetic considerations in patients with pulmonary hypertension undergoing non-cardiac and non-obstetric surgeries. Med Biol Sci Eng. 2019;2(2):31-39. [Google Scholar]
- 19.Gruenbaum BF, Gruenbaum SE. Neurophysiological monitoring during neurosurgery: Anesthetic considerations based on outcome evidence. Curr Opin Anaesthesiol. 2019;32(5):580-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De la Garza Ramos R, Goodwin CR, Abu-Bonsrah N, et al. Patient and operative factors associated with complications following adolescent idiopathic scoliosis surgery: An analysis of 36,335 patients from the nationwide inpatient sample. J Neurosurg Pediatr. 2016;18(6):730-736. [DOI] [PubMed] [Google Scholar]
- 21.Bronheim R, Khan S, Carter E, Sandhaus RA, Raggio C: Scoliosis and cardiopulmonary outcomes in osteogenesis imperfecta patients. Spine. 2019, 44(15):1057-1063. [DOI] [PubMed] [Google Scholar]
- 22.Latham GJ, Yung D. Current understanding and perioperative management of pediatric pulmonary hypertension. Paediatr Anaesth. 2019;29(5):441-456. [DOI] [PubMed] [Google Scholar]