Abstract
Background:
Iron overload is one of the main factors that increase morbidity and mortality in patients with non-transfusion dependent thalassemia (NTDT).
Aim:
This study aimed at investigating the prevalence and severity of iron overload in Chinese NTDT patients.
Methods:
we analyzed serum ferritin (SF), liver iron concentration (LIC) and cardiac T2* in 178 Chinese NTDT in this cross-sectional study.
Results:
The median SF level was 996.00(27.15–19704.00) ng/ml and the median LIC value was 8.90(0.60–43.00) mg Fe/g dry weight (dw). The youngest patient with liver iron overload was 5 years old with 5.6 mg Fe/g dw in LIC. The median cardiac T2* was 33.06(7.46–75.08) ms. 6 patients had cardiac T2*⩽20ms. The patients with β thalassemia intermedia and HbE/β thalassemia showed a statistically significant lower Hb and higher values of SF and LIC than those of hemoglobin H disease patients. On multivariate logistic regression analysis, patients in ⩾ age 30-year old had a significant higher risk for iron overload (OR: 77.75, 95% CI: 8.76–690.49) in the age group. The detailed analysis of proportions of different LIC indicate in > 30-year old group, 76.8% patients suffered from moderate and severe LIC.
Conclusion:
Our study provides a strong support for the novel findings that Chinese NTDT patients have a high prevalence of iron overload. The first assessment of MRI LIC should be performed as early as 5 years old. Then, NTDT patients > 30 years old may suffer with a high burden of iron overload.
Keywords: Chinese thalassemic patients, iron overload, non-transfusion-dependent thalassemia
Introduction
Thalassemia syndromes are hereditary hemolytic diseases resulting from unbalance synthesis of globin chains of the hemoglobin molecule, leading to abnormal red blood cells. It can be divided into two groups, transfusion-dependent thalassemia (TDT) and non-transfusion-dependent thalassemia (NTDT). NTDT is a spectrum of thalassemia, relative to those with TDT, NTDT just needs occasional transfusion in special circumstances like as infection, growth retardation, or pregnancy. 1 The NTDT group largely includes β thalassemia intermedia (β-TI), HbE/β thalassemia, and hemoglobin H disease (HbH disease). 2 High value of iron overload was associated with a significantly increase risk of complications in NTDT, like cardiac dysfunction, chronic liver disease, hepatic cirrhosis, diabetes mellitus. 3 Chronic hepatocellular iron deposition is a key factor in the process of liver fibrosis and cirrhosis, pulmonary hypertension, thrombosis and osteoporosis in NTDT patients.4–6 Many studies already indicated without treatment, iron overload in NTDT patients keeps on accumulating and iron–related morbidity often appears beyond 10 years of age (or later at 15 years in HbH disease).7,8 A database of 584 Thalassemia intermedia (TI) patients demonstrated that the key role of advancing age in many complications in TI, specifically iron overload. 9 A positive correlation has been confirmed between advancing age and iron overload in liver. 10 Theoretically, the aging NTDT patients should display higher LIC values due to increasing iron deposition in liver. However, the age-related changes of liver iron overload in NTDT patients have been rarely reported.
Thalassemia International Federation (TIF) recommends that assessment of iron overload by either SF or MRI for NTDT patients starts from 10 years of age. The observational studies identified a significant positive correlation between SF and LIC in NTDT patients.8,11,12 SF and LIC are the useful markers to monitor the iron chelation therapy. The thresholds of SF and LIC in chelation treatment: SF levels ⩾ 800 ng/ml can highly predict LIC ⩾ 5 mg Fe/g dry weight(dw) for initiating chelation therapy; SF thresholds above 2000 ng/ml were highly predictive of LIC ⩾ 7 mg Fe/g dw, so the chelation treatment should be escalated to further reduce iron overload.13,14 Currently, there are three kinds of iron chelating agents used in clinic: deferoxamine (DFO), deferiprone (DFP) and deferasirox (DFX), which were introduced to China in 1998, 2003 and 2010, respectively.
For most NTDT patients in developing countries including China. Previous literature indicates a high frequency of thalassemia in the population of southern China, mainly south of the Yangtze River, particularly in the provinces of Guangxi, Guangdong, Yunnan, Fujian and Sichuan. The previous meta-analysis revealed a higher prevalence of prevalence of α-thalassemia was 7.88% in China. 15 The most common α-thalassemia mutation in mainland China is --SEA which induces the health burden resulting from Hb H diseases and Hb Bart’s hydrops fetalis. Non-deletion types of Hb H disease (--/αTα) usually display more severe than the deletion types (--/-α) with early anemic symptoms, jaundice, splenomegaly and a higher proportion of required blood transfusion. 16 The prevalence of β-thalassemia was 2.21%, the most common β-globin gene mutation was CD41/42 in southern China. In addition, the prevalence of α + β-thalassemia in mainland China was found to be 0.48%. 15
There is limited data of iron overload in patients with NTDT in Chinese real world when the lack of special care occurs. To answer the questions about the iron overload, we designed this study to describe the prevalence of iron overload in mainland Chinese NTDT patients and evaluate age-related elasticity changes of liver iron overload in NTDT patients.
Subjects and methods
Patients
This cross-sectional study included 178 NTDT patients, who attended the Department of Hematology at First Affiliated Hospital of Guangxi Medical University, China from January 2017 to May 2019. NTDT was defined as thalassemia disease that received not more than 3 times red blood cell transfusion per year in the past 5 years. Patients who had contraindications for MRI and patients with fever or active infections that may interfere with the blood test results were excluded. The study protocol was approved by the Medical Ethics Committee of First Affiliated Hospital of Guangxi Medical University [approval number: NO. 2017(KY-E-094), and assured that all methods were performed in accordance with the relevant guidelines and regulations. All of patients provided their written informed consent to participate in this study. Adults(⩾18) finished the informed consent by themselves. And for minors (<18), the informed consents were obtained from the guardians on the behalf of the minors’ participants involved in this study. The medical records were reviewed for thalassemia diagnosis and genotypes, transfusion history, history of splenectomy, comorbidities, and the maximum ferritin level. All patients were evaluated for complete blood counts, liver function tests, SF levels, and MRI examinations for cardiac T2* and LIC by the local institute. The reporting of this study conforms to the STROBE statement, and the checklist is available as Supplementary Material.
Serum ferritin
Laboratory tests of procoagulant blood specimen were performed 2 weeks after the last blood transfusion. Measurements were carried out by an Electrochemiluminescence immunoassay (COBASE E 601, Roch, USA).
MRI protocols
LIC measurement was performed by MRI analysis (Ferriscan®-Resonance Health, Australia); myocardial iron deposition was assessed as previously described by our group. 17 MRI evaluations was performed in all patients. LIC < 3 mg Fe/g dw and cardiac T2* > 20ms was considered normal. Abnormal LIC can be divided into mild: 3–<7 mg Fe/g dw, moderate: 7–<15mg Fe/g dw, severe: ⩾ 15 mg Fe/g dw. 13 To NTDT patients who cannot be screened for MRI, SF thresholds for chelation therapy is recommended into initial chelation >800 ng/ml, interruption of chelation <300 ng/ml and escalating chelation dose >2000 ng/ml.
Statistical analysis
Data were expressed as mean ± one standard deviation, or median (range) as appropriate. Mann Whitney U test or t test were used for comparison of continuous variables between groups. The significance of the correlation between parameters was assessed using Spearman rank as the data was not normally distributed. Categorical data were compared using the chi-square test. Binary logistic regression analysis was performed for the risk factors of iron overload. Statistical analyses were carried out using SPSS Statistics 16.0 (SPSS Inc., USA). A p value less than 0.05 was considered statistically significant, and all p values were two sided.
Results
Patients’ characteristics
A total of 178 patients were recruited in this study, including 106(59.55%) men. A total of 99 (55.6%) had HbH disease, whereas 79 (44.4%) with β-TI and HbE/β thalassemia. The median age was 23 years old (range, 4–63). Some 63 (35.39%) patients were splenectomized. The mean hemoglobin level was 84.5(40.90–117.00) g/L, whereas the median numbers of transfusion were 4.25(0–360.00) Unit. 142 patients (79.78%) were non-chelated. The median SF level of 175 NTDT patients was 996(27.15–19,704.00) ng/ml. LIC was detected in 176 patients (99%) and the median LIC value was 8.90(0.60–43.00) mg Fe/g dw. Cardiac T2*was assessed in 106 patients (59.55 %) since 72 patients refused to or cannot tolerate the test, and the median cardiac T2* was 33.06(7.46–75.08) ms. The 6 patients (5.66%) (3 with HbH disease and 3 with β-TI) had cardiac T2*<20 ms (Table 1), 83.3% patients of them with splenectomy, they rare transfused, but the Hemoglobin levels was not low and with severe serum and liver iron deposition. Patients with β-TI and HbE/β thalassemia showed a statistically significant lower Hb and higher values of SF and LIC than those of HbH disease patients (Table 2). In this study, 73 patients had complications, including 12 patients undergo anemic heart disease is defined according the echocardiogram results, 14 had gallstone, 10 had extramedullary hematopoiesis, according to their fasting and 2 h postprandial blood glucose levels, 27 patients had hypoglycemic, 17 had impaired glucose tolerance, and only 1 had diabetes. Only 1 patient had hypothyroidism, 4 had subclinical hypothyroidism, and none had hyperthyroidism, 18 had elevated parathyroid hormone. Iron overload was positively correlated with the occurrence of complications (r = 0.264, p < 0.001).
Table 1.
The main characteristics of NTDT patients along with the main results of the study (n = 178).
| Characteristics | No.of patients n (%) |
|---|---|
| Male | 106(59.55) |
| Age(years), median(range) | 23(4–63) |
| Age group | |
| <10 | 16(8.99) |
| 10–<20 | 55(30.90) |
| 20–<30 | 55(30.90) |
| ⩾30 | 52(29.21) |
| Splenectomy | |
| Yes | 63(35.39) |
| No | 115(64.61) |
| Hb(g/L), median(range) | 84.55(40.90–117.00) |
| Blood transfusion number (IU), median (range) |
4.25(0–360.00) |
| Previous chelation therapy | |
| DFO | 12(6.74) |
| DFP | 2(1.12) |
| DFX | 11(6.18) |
| DFO + DFX | 5(2.81) |
| DFO + DFP | 5(2.81) |
| DFX + DFP | 1(0.56) |
| None | 142(79.78) |
| SF(ng/mL), median(range) | 996.00(27.15–19704.00) |
| SF category | |
| <300 | 23(13.14) |
| 300–<800 | 54(30.86) |
| 800–<2500 | 64(36.57) |
| ⩾2500 | 34(19.43) |
| LIC(mg Fe/g dw), median(range) | 8.90(0.60–43.00) |
| LIC* category | |
| <3.0 | 36(20.45) |
| 3.0–<7 | 41(23.30) |
| 7–<15 | 42(23.86) |
| ⩾15 | 57(32.39) |
| Cardiac T2*(ms), median(range) | 33.06(7.46–75.08) |
| <10 | 1(0.94) |
| 10–<20 | 5(4.72) |
| ⩾20 | 100(94.34) |
DFO, deferoxamine; DFP, deferiprone; DFX, deferasirox; Hb, hemoglobin; LIC*, liver iron concentration; NTDT, non-transfusion-dependent thalassemia; SF, serum ferritin.
Cardiac T2* refers to cardiac magnetic resonance relaxometry T2*.
Table 2.
The comparison of indices of Hb and iron overload of NTDT patients.
| Indexes | Genotype | p | |
|---|---|---|---|
| HbH disease (n = 99) | β TI/HbE/β (n = 79) | ||
| Hb (g/L), median(range) | 91.00(40.90–117.00) | 79.00(42.80–116.80) | <0.001 |
| SF (ng/ml), median(range) | 695.90(27.15–19704.00) | 1519.00(159.00–9951.94) | <0.001 |
| LIC (mg Fe/g dw), median(range) | 6.50(0.60–43.00) | 14.55(0.60–43.00) | <0.001 |
Hb, hemoglobin; LIC, liver iron concentration; NTDT, non-transfusion-dependent thalassemia; SF, serum ferritin.
Correlation between LIC, SF, age and Hb in NTDT patients
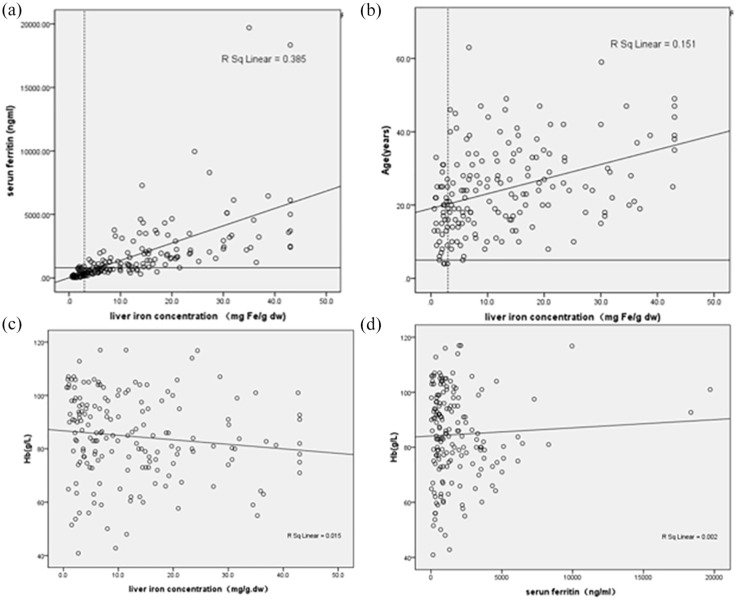
There was a strong correlation between SF and LIC (r = 0.83, p < 0.001) (Figure 1(a)). There was a statistically significant positive correlation between age and LIC (r = 0.41, p < 0.001) (Figure 1(b)). There were 7 patients (3.6%) (5 with HbH disease, 1 with β-TI and 1 with HbE/β thalassemia) < 10 years old found liver iron overload. The youngest patient with liver iron overload was 5 years old with 5.6 mg Fe/g dw in LIC (Figure 1(b)). There was a statistically significant negative correlation between Hb and LIC (r = -0.19, p = 0.01) (Figure 1(c)). No correlation was found between Hb and SF (r = -0.07, p = 0.38). (Figure 1(d)). No correlation between LIC and Hb, cardiac T2* values can be verified.
Figure 1.
Correlation among age, Hb, serum ferritin and LIC in NTDT patients. (a) Correlation between SF and liver iron concentration (r = 0.83, p < 0.001). The dotted line represents reference range for LIC of 3.0 mg Fe/g dw, and the solid line represents SF of 800 ng/ml, (b) LIC was positively associated with age correlation (r = 0.41, p < 0.001). The dotted line represents reference range for LIC of 3.0 mg Fe/g dw, and the solid line represents age of 5 year, (c) correlation between Hb and liver iron concentration (r = -0.19, p = 0.01) and (d) correlation between Hb and serum ferritin (r = -0.07, p = 0.38).
Age-related elasticity changes of liver iron overload in NTDT patients
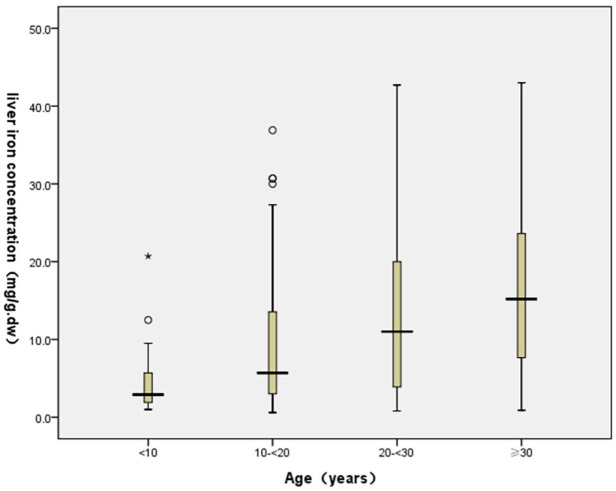
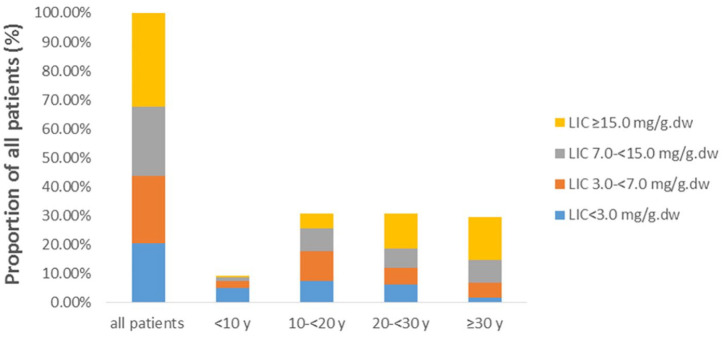
When the NTDT patients were stratified into the various age groups (<10, 10–20 <, 20–30 <, and ⩾ 30), there were 16 (8.99%) patients aged < 10, 55 (30.90%) aged between 10 and 20 years, 55 (30.90%) aged between 20 and 30 years, and 52 (29.21%) aged ⩾ 30 years. The age-related dynamic changes of liver iron overload in patients with NTDT were determined using SF and LIC and are illustrated in Tables 3 and 4. The correlation analysis between age and SF, LIC revealed that liver iron deposition in patients with <20-year old group was mainly normal or mild level (SF < 800 ng/ml: 56.36–80.0%; LIC < 3 mg Fe/g.dw: 24.07–56.25%). In ⩾ 20 years of age group, NTDT patients showed moderate to severe level of liver iron overload (SF ⩾ 800 ng/ml: 66.35%; LIC ⩾ 7 mg Fe/g.dw: 67.59%), particularly in ⩾ 30 years of age group, SF ⩾ 800 ng/ml: 74.00%; LIC ⩾ 7 mg Fe/g.dw: 76.92%. In addition, the level of LIC in patients ⩾ 30-year old group was significantly higher than those in other groups. The detailed analysis of proportions of different LIC indicated in ⩾ 30 -year old group, 76.92% patients suffered from moderate and severe LIC, of whom 50.00% had severe LIC (Tables 3 and 4, Figures 2 and 3). We compared the distribution of SF and LIC between patients with and without splenectomy, and the results showed that the distribution of SF in splenectomy groups was significantly different, and with splenectomy patients suffered from moderate and severe SF, 42.62% of the patients underwent splenectomy with severe LIC overload, however, the distribution were not different. (Tables 3 and 4).
Table 3.
Comparison of distribution of serum ferritin levels in different age and splenectomy groups (n,%).
| Indexs | SF (ng/ml) | X2 | p | ||
|---|---|---|---|---|---|
| <800 | 800–< 2500 | ⩾2500 | |||
| Age (years) | 19.67 | 0.003 | |||
| <10 | 12(80.00) | 1(6.67) | 2(13.33) | ||
| 10–<20 | 31(56.36) | 17(30.91) | 7(12.73) | ||
| 20–<30 | 21(38.18) | 23(41.82) | 11(20.00) | ||
| ⩾30 | 13(26.00) | 23(46.00) | 14(28.00) | ||
| Splenectomy | 23.83 | <0.001 | |||
| No | 63(56.25) | 37(33.03) | 12(10.71) | ||
| Yes | 14(22.22) | 27(42.86) | 22(34.92) | ||
SF, serum ferritin.
Table 4.
Comparison of distribution of LIC levels in different age and splenectomy groups (n,%).
| Indexes | LIC (mg Fe/g dw) | X2 | p | |||
|---|---|---|---|---|---|---|
| <3 | 3–< 7 | 7–<15 | ⩾15 | |||
| Age (years) | 33.94 | <0.001 | ||||
| <10 | 9(56.25) | 4(25.00) | 2(12.50) | 1(6.25) | ||
| 10–<20 | 13(24.07) | 18(33.33) | 14(25.93) | 9(16.67) | ||
| 20–<30 | 11(20.37) | 10(18.52) | 12(22.22) | 21(38.89) | ||
| ⩾30 | 3(5.77) | 9(17.31) | 14(26.92) | 26(50.00) | ||
| Splenectomy | 5.67 | 0.13 | ||||
| No | 28(24.35) | 28(24.35) | 28(24.35) | 31(26.96) | ||
| Yes | 8(13.11) | 13(21.31) | 14(22.95) | 26(42.62) | ||
LIC, liver iron concentration.
Figure 2.
Blox-plot demonstrating the distribution trend between LIC and age groups.
Figure 3.
Shift in proportion of patients with severe, moderate, mild and normal LIC values. The proportion of severe LIC in ⩾ 30-year old NTDT group is significantly higher than that in other groups (p = 0.001).
Multivariate analyses of risk factors for iron overload in NTDT patients
Multivariate analyses of the effects of variables including age, thalassemia genotype, gender, splenectomy status and hemoglobin level on iron overload risk are shown in Table 5. The independent significant risk factors for having iron overload were age (for 10–<20 years old, OR:6.01, 95% CI: 1.07–33.90; for 20–<30 years old, OR:13.62, 95% CI: 2.34–79.35; and for ⩾ 30 years old, OR 77.75, 95% CI: 8.76–690.49). In age group, the distribution in different age groups provided a significant trend in iron overload response to age group As expected, β-TI (OR:4.67, 95% CI: 1.30–16.82) and splenectomy (OR:3.37, 95% CI: 1.10–10.30) were found as independent risk factors for iron overload. In hemoglobin level group, a nearly 5-fold independent increase in confirmed iron overload risk in 60–<90 g/L group compare with ⩾ 90 g/L group (OR:4.61, 95% CI,1.27–16.65).
Table 5.
Multivariate analyses of risk factors for iron overload in NTDT patients.
| Characteristics | OR | 95% CI of OR | p-Value |
|---|---|---|---|
| Age group (years) | |||
| <10 | 1.00 | – | – |
| 10–<20 | 6.01 | 1.07–33.90 | 0.04 |
| 20–<30 | 13.62 | 2.34–79.35 | 0.004 |
| ⩾30 | 77.75 | 8.76–690.49 | < 0.001 |
| Genotype group | |||
| α-Thalassemia | 1.00 | – | – |
| β- thalassemia Intermedia | 4.67 | 1.30–16.82 | 0.02 |
| β-Thalassemia/Hb E | 7.11 | 0.68–74.36 | 0.10 |
| Female gender | 1.16 | 0.39–3.44 | 0.79 |
| Splenectomy | 3.37 | 1.10–10.30 | 0.03 |
| Hb group(g/L) | |||
| <60 | 4.30 | 0.44–41.93 | 0.21 |
| 60–<90 | 4.61 | 1.27–16.65 | 0.02 |
| ⩾90 | 1.00 | – | – |
95% CI, 95% confidence interval; NTDT, non-transfusion-dependent thalassemia; OR, odds ratio.
Discussion
NTDT is characterized by limited or no requirement for regular blood transfusion. Recently, there is increasing evidence that NTDT may be associated with a variety of clinical complications later in life. Iron overload is one of the major complications in NTDT and is strongly correlated with various morbidities in NTDT patients therefore early detection and prompt treatment is needed. Our study provides insight into the prevalence of iron overload in mainland Chinese NTDT patients which have not previously been reported. Based on the data from 178 NTDT patients, compared with reported research in non-Chinese NTDT patients, we have identified a high prevalence of iron overload in the mainland Chinese NTDT patients, especially LIC. The iron overload in patients with β-TI and HbE/β thalassemia are more serious than those in HbH disease patients. The youngest patient with liver iron overload was 5 years old. NTDT patients ⩾ 30 years old with irregular treatment may suffer with a high burden of iron overload. These findings are rarely reported and provide more clinical features of NTDT patients in China, especially iron overload. In this manuscript we report that the minimum age for liver iron overload is 5 years, which is earlier than previously reported in the literature. This probably due to the following factors : most patients suffering from thalassemia do not live in first world countries and have limited access to modern medicine technology. In China, due to ethnicity, and underlying genotypes, the low income, inadequate health insurance, shortage of blood, limited availability of chelators and lack of specific knowledge of the disease, the NTDT patients were poorly-chelated and modestly transfused in our study, the hemoglobin levels is lower than international’s, which induce severe ineffective erythropoiesis, resulting in severe iron deposition.
In China, our previous published paper has already proved that TDT patients in China were poorly-chelated and had a high burden of iron overload, 6 years old TDT patient was tested cardiac iron overload. 17 To NTDT patients, as early as 2015, few studies about Chinese NTDT patients were reported. A retrospective review from 6 registered centers (the OPTIMAL CARE study) reported that 584 NTDT patients’ mean age was 25.44 ± 13.86 (2–76 years) and SF level was 967.5 ± 853.9 (17–10,793) ng/mL. 9 In another study of 91 patients with NTDT, 59% had hepatic iron overload (LIC levels: 8.5 ± 6.7 mg Fe/g dw and SF levels: 767 ± 692 ng/ml). 18 In our study, the median age of our NTDT patients was 23 (4–63) years old, the median SF level was 996(27.15–19,704.00) ng/ml and the median LIC value was 8.90(0.60–43.00) mg Fe/g dw. Our data described the clinical characteristics of mainland Chinese NTDT patients and displayed more severity of iron overload in Chinese NTDT population compared with other reported data. To our knowledge, this was the largest study on iron overload of Chinese NTDT population. Thinking about its reason, in China, while progress has been made for the control and treatment for thalassemia trait over the last decade, the lack of disease specific knowledge, lack of specialist physicians and the limited resources within the health insurance system pose challenges to optimum care are included in common factors. A considerable proportion of NTDT patients are insufficiently transfused and poorly-chelated. Therefore, health care for patients suffering with NTDT in China continues to pose a great challenge to the public health services.
As we all know, many patients who undergo splenectomy appear to restore Hb levels in the short term by about 10–20 g/l, splenectomy is an important factor for thalassemia, Chinprateep et al. 19 use cardiac MRI to measure the left ventricular diastolic function in thalassemia major patients with normal left ventricular systolic function and showed that Homozygous beta-thalassemia and splenectomy were strong predictors of the left ventricular diastolic dysfunction (LVDD). A multi-center study in Thailand of review of disease-related complications and management in adult patients with thalassemia showed that severe iron overload started earlier in patients with TDT than NTDT and was associated with diabetes mellitus, and splenectomy and advanced age were important risk factors for developing major complications in both groups, 20 also in a prospective analysis for prevalence of complications in Thai non-transfusion-dependent Hb E/β-thalassemia and Hb H disease, splenectomy, degree of anemia, and iron overload seem to be determining risks of developing these complications. 21 Similar to what is reported in the literature, in our study patients undergoing splenectomy had moderate to severe serum iron overload, and in a multifactorial analysis, splenectomy was a risk factor for iron overload complications. It has been suggested that the intact spleen may be a reservoir of excess iron and may have a possible scavenging effect on iron free fractions including non-transferrin-bound iron (NTBI), which explains the higher serum level of NTBI in splenectomised TI patients. 22
As well known, the association between iron overload and morbidity in NTDT patients has been strongly confirmed and the need for chelation therapy is quite apparent. 23 Without treatment, iron overload in NTDT patients continues to accumulate, and most of patients eventually touch LIC thresholds of clinical significance. Serum ferritin levels ⩾ 800 ng/dl and LIC levels > 5 mg Fe/g dw in NTDT patients have been associated with a significantly greater risk of long-term morbidities. 1 Previous studies already strongly demonstrated that the main independent risk factors for complications in NTDT were naivety to RBC transfusion therapy, naivety to iron chelation therapy, splenectomy.23,24 In the ORIENT study, the mean SF level for 52 β-thalassemia intermedia patients was 826.9 ± 69.5 ng/mL (72–2065.6 ng/mL). Among them, 48.1% patients were osteoporosis, 19.2% had extramedullary hematopoiesis, and 17.3% had liver disease, 7.7% patients suffered diabetes mellitus. 25 Among the OPTIMAL CARE study, 22.8% had experienced osteoporosis, 17.3% had evidence of hypogonadism and 14% experienced a thrombotic event. 9 Our current study about liver fibrosis on Chinese NTDT patients with iron overload showed that the serum levels of three well-established markers of liver fibrosis (blood collagen type IV, precollagen type III and hyaluronic acid) were significantly increased in 105 NTDT patients and these were related to liver iron overload even in the absence of hepatitis. 26 In this study, among 178 Chinese NTDT patients, 98 (56.0%) patients had SF > 800 ng/ml and 99 (56.25%) patients had LIC > 7 mg Fe/g dw. The prevalence of diverse complications in NTDT patients with iron overload was 47.86%, for endocrine-related complications was 36.43%. It means that Chinese NTDT patients suffer with serious iron overload and the risk of multiple complications in Chinese NTDT is quite high.
Since iron overload in NTDT patients is associated with the development of serious morbidities, it is important to management of iron overload. Among the many risk factors for iron overload, patient age is reported to be a very critical factor. 7 Our data showed a trend toward a significant increasing prevalence of iron overload in the older age categories. It also indicated a significant correlation between LIC and age (r = 0.41, p < 0.001). Our analysis has illustrated that the levels of LIC in patients ⩾ 30-year old group are significantly higher than those in other groups. The detailed analysis of proportions of different LIC indicated in ⩾ 30 -year old group, 76.92% patients suffered from moderate and severe LIC, in which 50.0% was in severe LIC. Moreover, in our study, the multivariate analyses of risk factors for iron overload indicated that age ⩾ 30 -year old is the main risk factor in NTDT patients. The OPTIMAL CARE study already revealed that the prevalence of complications in NTDT patients increases with age and the high prevalence of morbidities has been observed as they advance in age. 9 The previous studies also demonstrated that age > 20 years, hemoglobin < 7 g/dl, cumulative RBC transfusion > 10 units are the clinical risk factors for hepatic iron overload. 18 Therefore, it is very important and necessary to evaluate iron overload in patients with NTDT and to optimize chelation therapy base on the assessment by LIC and serum ferritin at the optimal age. Our study can provide an important clinical implication. For NTDT patients who are lack blood transfused and poorly-chelated, especially in developing countries, patients ⩾ 30 years of age with NTDT may have a high burden of iron overload and may have a prevalence of moderate and severe LIC. These NTDT patients need to be offered close clinical follow up as they age to avoid disease-related morbidity. Our study may help develop guidelines to the clinical assessment and management of iron overload in NTDT patients in developing countries.
In conclusion, we present the findings of a large cohort of Chinese NTDT patients. Because of the lack of disease knowledge, lack of blood transfusion and poorly chelation, the vast majority of Chinese NTDT patients show a high prevalence of iron overload. Our results show the effects of poor chelation of young patients and that the first assessment of LIC should take place as early as 5 years of age. The age of patient is a risk factor of iron overload in NTDT patient. For patients ⩾ 30 years of age, particularly those with insufficient transfusion and poor chelation, this group of patients should be evaluated for LIC to avoid disease-related morbidity.
Limitations of the study
The main limitations of present work are: (1) it was a non-randomized observational investigation in a single-center study with a relatively small sample size. (2) Cardiac MRI scans could not be performed in all cases. (3) Wide gene spectrum in China, and mix genotype of thalassemia were enrolled in this study. Although the evaluation and radiographic of these patients was a snapshot at a defined time point in their disease, we suggest that the data obtained justifies the implementation of a larger multicenter trial in the future.
Supplemental Material
Supplemental material, sj-doc-1-tah-10.1177_20406207221084639 for Iron overload status in patients with non-transfusion-dependent thalassemia in China by Yumei Huang, Gaohui Yang, Man Wang, Xiaoyun Wei, Lingyuan Pan, Jiaodi Liu, Yu Lei, Peng Peng, Liling Long, Yongrong Lai and Rongrong Liu in Therapeutic Advances in Hematology
Supplemental material, sj-docx-1-tah-10.1177_20406207221084639 for Iron overload status in patients with non-transfusion-dependent thalassemia in China by Yumei Huang, Gaohui Yang, Man Wang, Xiaoyun Wei, Lingyuan Pan, Jiaodi Liu, Yu Lei, Peng Peng, Liling Long, Yongrong Lai and Rongrong Liu in Therapeutic Advances in Hematology
Acknowledgments
We would like to thank all participants involved in this study.
Footnotes
Author contributions: Yumei Huang: Data curation; Writing – original draft; Writing – review & editing.
Gaohui Yang: Data curation; Writing – original draft.
Man Wang: Formal analysis; Methodology; Writing – original draft.
Xiaoyun Wei: Methodology.
Lingyuan Pan: Methodology.
Jiaodi Liu: Methodology.
Yu Lei: Methodology.
Peng: Methodology; Software.
Liling Long: Methodology; Software.
Yongrong Lai: Investigation; Methodology; Project administration; Writing – original draft; Writing – review & editing.
Rongrong Liu: Investigation; Methodology; Project administration; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by research grants from the National Natural Science Foundation of China (grant no.Y121 and 1598011-1), and the Guangxi key laboratory of thalassemia research (grant no.16-380-34), and Youth Science Foundation of Guangxi Medical University(GXMUYSF201917).
ORCID iD: Yongrong Lai  https://orcid.org/0000-0002-0371-1719
https://orcid.org/0000-0002-0371-1719
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yumei Huang, Department of Hematology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China.
Gaohui Yang, Department of Hematology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China.
Man Wang, Department of Hematology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China.
Xiaoyun Wei, Department of Hematology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China.
Lingyuan Pan, Department of Hematology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China.
Jiaodi Liu, Department of Hematology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China.
Yu Lei, Department of Hematology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China.
Peng, Department of Radiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China.
Liling Long, Department of Radiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China.
Yongrong Lai, Department of Hematology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi, China.
Rongrong Liu, Department of Hematology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi, China.
References
- 1. Musallam KM, Rivella S, Vichinsky E, et al. Non-transfusion-dependent thalassemias. Haematologica 2013; 98: 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weatherall DJ. The definition and epidemiology of non-transfusion-dependent thalassemia. Blood Rev 2012; 26(Suppl. 1): S3–S6. [DOI] [PubMed] [Google Scholar]
- 3. Musallam KM, Cappellini MD, Wood JC, et al. Iron overload in non-transfusion-dependent thalassemia: a clinical perspective. Blood Rev 2012; 26(Suppl. 1): S16–S19. [DOI] [PubMed] [Google Scholar]
- 4. Musallam KM, Cappellini MD, Wood JC, et al. Elevated liver iron concentration is a marker of increased morbidity in patients with beta thalassemia intermedia. Haematologica 2011; 96: 1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Musallam KM, Motta I, Salvatori M, et al. Longitudinal changes in serum ferritin levels correlate with measures of hepatic stiffness in transfusion-independent patients with beta-thalassemia intermedia. Blood Cells Mol Dis 2012; 49: 136–139. [DOI] [PubMed] [Google Scholar]
- 6. Maakaron JE, Cappellini MD, Graziadei G, et al. Hepatocellular carcinoma in hepatitis-negative patients with thalassemia intermedia: a closer look at the role of siderosis. Ann Hepatol 2013; 12: 142–146. [PubMed] [Google Scholar]
- 7. Taher AT, Musallam KM, El-Beshlawy A, et al. Age-related complications in treatment-naive patients with thalassaemia intermedia. Br J Haematol 2010; 150: 486–489. [DOI] [PubMed] [Google Scholar]
- 8. Lal A, Goldrich M, Haines D, et al. Heterogeneity of hemoglobin H disease in childhood. N Engl J Med 2011; 364: 710–718. [DOI] [PubMed] [Google Scholar]
- 9. Taher AT, Musallam KM, Karimi M, et al. Overview on practices in thalassemia intermedia management aiming for lowering complication rates across a region of endemicity: the OPTIMAL CARE study. Blood 2010; 115: 1886–1892. [DOI] [PubMed] [Google Scholar]
- 10. Taher AT, Musallam KM, Wood JC, et al. Magnetic resonance evaluation of hepatic and myocardial iron deposition in transfusion-independent thalassemia intermedia compared to regularly transfused thalassemia major patients. Am J Hematol 2010; 85: 288–290. [DOI] [PubMed] [Google Scholar]
- 11. Taher AT, Cappellini MD. Management of non-transfusion-dependent thalassemia: a practical guide. Drugs 2014; 74: 1719–1729. [DOI] [PubMed] [Google Scholar]
- 12. Cheong JW, Kim HJ, Lee KH, et al. Deferasirox improves hematologic and hepatic function with effective reduction of serum ferritin and liver iron concentration in transfusional iron overload patients with myelodysplastic syndrome or aplastic anemia. Transfusion 2014; 54: 1542–1551. [DOI] [PubMed] [Google Scholar]
- 13. Taher AT, Porter JB, Viprakasit V, et al. Defining serum ferritin thresholds to predict clinically relevant liver iron concentrations for guiding deferasirox therapy when MRI is unavailable in patients with non-transfusion-dependent thalassaemia. Br J Haematol 2015; 168: 284–290. [DOI] [PubMed] [Google Scholar]
- 14. Taher AT, Cappellini MD, Aydinok Y, et al. Optimising iron chelation therapy with deferasirox for non-transfusion-dependent thalassaemia patients: 1-year results from the THETIS study. Blood Cells Mol Dis 2016; 57: 23–29. [DOI] [PubMed] [Google Scholar]
- 15. Lai K, Huang G, Su L, et al. The prevalence of thalassemia in mainland China: evidence from epidemiological surveys. Sci Rep 2017; 7: 920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laosombat V, Viprakasit V, Chotsampancharoen T, et al. Clinical features and molecular analysis in Thai patients with HbH disease. Ann Hematol 2009; 88: 1185–1192. [DOI] [PubMed] [Google Scholar]
- 17. Yang G, Liu R, Peng P, et al. How early can myocardial iron overload occur in beta thalassemia major? PLoS ONE 2014; 9: e85379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tantiworawit A, Charoenkwan P, Hantrakool S, et al. Iron overload in non-transfusion-dependent thalassemia: association with genotype and clinical risk factors. Int J Hematol 2016; 103: 643–648. [DOI] [PubMed] [Google Scholar]
- 19. Chinprateep B, Ratanasit N, Kaolawanich Y, et al. Prevalence of left ventricular diastolic dysfunction by cardiac magnetic resonance imaging in thalassemia major patients with normal left ventricular systolic function. BMC Cardiovasc Disor 2019; 19: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chuncharunee S, Teawtrakul N, Siritanaratkul N, et al. Review of disease-related complications and management in adult patients with thalassemia: a multi-center study in Thailand. PLoS ONE 2019; 14: e0214148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ekwattanakit S, Siritanaratkul N, Viprakasit V. A prospective analysis for prevalence of complications in Thai nontransfusion-dependent Hb E/β-thalassemia and α-thalassemia (Hb H disease). Am J Hematol 2018; 93: 623–629. [DOI] [PubMed] [Google Scholar]
- 22. Taher AT, Musallam KM, Cappellini MD, et al. Optimal management of beta thalassaemia intermedia. Br J Haematol 2011; 152: 512–523. [DOI] [PubMed] [Google Scholar]
- 23. Saliba AN, Taher AT. Morbidities in non-transfusion-dependent thalassemia. Ann N Y Acad Sci 2016; 1368: 82–94. [DOI] [PubMed] [Google Scholar]
- 24. Taher AT, Musallam KM, Saliba AN, et al. Hemoglobin level and morbidity in non-transfusion-dependent thalassemia. Blood Cells Mol Dis 2015; 55: 108–109. [DOI] [PubMed] [Google Scholar]
- 25. Musallam KM, Cappellini MD, Daar S, et al. Serum ferritin level and morbidity risk in transfusion-independent patients with beta-thalassemia intermedia: the ORIENT study. Haematologica 2014; 99: e218–e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang M, Liu R, Liang Y, et al. Iron overload correlates with serum liver fibrotic markers and liver dysfunction: potential new methods to predict iron overload-related liver fibrosis in thalassemia patients. United European Gastroenterol J 2017; 5: 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-tah-10.1177_20406207221084639 for Iron overload status in patients with non-transfusion-dependent thalassemia in China by Yumei Huang, Gaohui Yang, Man Wang, Xiaoyun Wei, Lingyuan Pan, Jiaodi Liu, Yu Lei, Peng Peng, Liling Long, Yongrong Lai and Rongrong Liu in Therapeutic Advances in Hematology
Supplemental material, sj-docx-1-tah-10.1177_20406207221084639 for Iron overload status in patients with non-transfusion-dependent thalassemia in China by Yumei Huang, Gaohui Yang, Man Wang, Xiaoyun Wei, Lingyuan Pan, Jiaodi Liu, Yu Lei, Peng Peng, Liling Long, Yongrong Lai and Rongrong Liu in Therapeutic Advances in Hematology