Abstract
Aims
The aim of this study was to simultaneously analyze estrogen quinone-derived adducts, including 17β-estradiol-2,3-quinone (E2-2,3-Q) and 17β-estradiol-3,4-quinone (E2-3,4-Q), in human albumin (Alb) and hemoglobin (Hb) derived from breast cancer patients with five-year postoperative treatment without recurrence in Taiwan and to evaluate the treatment-related effects on the production of these adducts.
Settings and Design
Cohort
Methods and Material: Blood samples derived from breast cancer 5-year survivors without recurrence were collected. Albumin and hemoglobin adducts of E2-3,4-Q and E2-2,3-Q were analyzed to evaluate the degree of disposition of estrogen to quinones and to compare these adduct levels with those in patients before treatment.
Statistical Analysis
All data are expressed as mean ± standard deviation of three determinations. We used Student’s t-test to examine subgroups. Data were transformed to the natural logarithm and tested for normal distribution for parametric analyses. Linear correlations were investigated between individual adduct levels by simple regression. Statistical analysis was performed using the SPSS Statistics 20.0.
Results
Result confirmed that logged levels of E2-2,3-Q-derived adducts correlated significantly with those of E2-3,4-Q-derived adducts (correlation coefficient r=.336-.624). Mean levels of E2-2,3-Q-4-S-Alb and E2-3,4-Q-2-S-Alb in 5-year survivors were reduced by 60-70% when compared to those in the breast cancer patients with less than one year of diagnosis/preoperative treatment (P<.001).
Conclusions
Our findings add support to the theme that hormonal therapy including aromatase inhibitors and Tamoxifen may dramatically reduce burden of estrogen quinones. We hypothesize that combination of treatment-related effects and environmental factors may modulate estrogen homeostasis and diminish the production of estrogen quinones in breast cancer patients.
Keywords: biomarker, estrogen quinone, protein adducts, hormonal therapy
Introduction
Breast cancer is one of the common cancers found in Taiwanese women. The onset of breast cancer tends to occur at a younger age than in Western countries. 1 Development and application of biological markers to recognize high-risk individuals is needed. Conversion of 17β-estradiol (E2) to estrogen catechols, including 2-hydroxyestradiol (2-OH-E2) and 4-hydroxyestradiol (4-OH-E2), is mediated by CYP1A2/1A1 and CYP1B1.2-4 Both estrogen catechols undergo oxidation to form quinones, including estrogen-2,3-quinone (E2-2,3-Q) and estrogen-3,4-quinone (E2-3,4-Q).5,6 In combination with estrogen-derived mitogenesis, accumulation of estrogen quinones along with the subsequent generation of abasic sites and other types of DNA damage, contribute to estrogen-induced carcinogenesis.7-9
Many new tools in the field of molecular profiling have been developed for breast cancer to accurately predict outcomes and to estimate the benefit of adjuvant treatment. 10 Tumor tissue markers such as Gene expression assays-oncotypeDX are used to predict outcomes. 11 Our previous investigation indicated that elevation of background levels of estrogen quinone-derived blood protein adducts and abasic sites in leukocytes are biomarkers of developing breast cancer.12-14 Blood protein adducts have been used as surrogate markers for DNA adducts of several different classes of carcinogens.15-17 Protein adducts, including serum albumin (Alb) and hemoglobin (Hb) adducts, seem to be reliable biomarkers of early prediction of disease. We used the Alb and Hb adducts of quinonoid metabolites of estrogen as biomarkers to reflect the cumulative burden of reactive estrogen quinones in breast cancer patients before treatment. 18 We concluded that body burden of E2-2,3-Q and E2-3,4-Q is an important indicator of breast cancer risk. In this study, we refine our analytical method and measure the concentration of estrogen quinones in blood proteins derived from Taiwanese breast cancer patients with greater than five years of postoperative treatment without recurrence (5-year survivors) and compare these estrogen quinone adducts with those of breast cancer patients with less than one year of diagnosis/preoperative treatment (BCP) and healthy controls.12-14 We obtained evidence of significant reduction in the cumulative tissue doses of E2-2,3-Q and E2-3,4-Q adducts in breast cancer 5-year survivors when compared to those of BCP. We designate the products of reactions between estrogen quinones and blood proteins as E2-2,3-Q-1-S-Alb, E2-2,3-Q-4-S-Alb, and E2-3,4-Q-2-S-Alb for serum albumin, and E2-2,3-Q-1-S-Hb, E2-2,3-Q-4-S-Hb, and E2-3,4-Q-2-S-Hb for hemoglobin.
Subjects and Methods
Chemicals
E2-2, 4, 16, 16, 17-d5 ([2H5]-E2) was from C/D/N Isotope (Canada H9R 1H1). Acetone, acetonitrile, ethyl acetate, and methyl alcohol were obtained from TEDIA (Charlotte, NC288224, USA). E2, chloroform, N-Acetyl-L-Cysteine (NAC), L-Ascorbic acid (99%), trifluoroacetic acid anhydride (TFAA), methanesulfonic acid (MSA), human serum Alb, human Hb, and potassium nitrosodisulfonate were purchased from Sigma-Aldrich Inc. (St Louis, MO63178, USA).
Synthesis of Adducts of Estrogen Quinones With N-Acetyl-L-Cysteine
The products of reactions between estrogen quinones and NAC are designated as E2-2,3-Q-1-S-NAC, E2-2,3-Q-4-S-NAC, and E2-3,4-Q-2-S-NAC, and those with Hb as E2-2,3-Q-1-S-Hb, E2-2,3-Q-4-S-Hb, and E2-3,4-Q-2-S-Hb. Authentic standards of E2-2,3-Q-1-S-NAC, E2-2,3-Q-4-S-NAC, and E2-3,4-Q-2-S-NAC were synthesized following the procedure as described previously by Chen et al (2011). 18
Synthesis of Adducts of Estrogen Quinones With Human Globin and Albumin
To 30 mg (.11 mmole) of 17β-estradiol, 10 mL of acetone and then 16 mL of 10% acetic acid in water (v/v) were added. After the addition of 50 mg of potassium nitrosodisulfonate, the mixture was shaken for 15 min at room temperature. A second portion of potassium nitrosodisulfonate (50 mg) was added and the mixture was shaken for another 15 min. The estrogen quinones were extracted from the solution three times with chloroform (2mLx3). Chloroform was removed under a gentle stream of N2. 20 μL of acetonitrile was added to the residue and reactions were performed by adding estrogen quinones to a solution containing human globin and Alb (in 1X PBS; purchased from Sigma-Aldrich) for 30 min at 37°C. The reactions were terminated by adding 10 mM of ascorbic acid (final concentration) and by chilling in an ice bath. The modified protein was to serve as a positive control. 18
Synthesis of Isotopically Labeled Protein-Bound Internal Standards
Isotopically labeled protein bound internal standards were synthesized for the analysis of estrogen quinone-derived adducts according to the procedure previously described by Chen et al (2011). 18
Subjects
Inclusion criteria: Participants were female adults aged 20 years or over. The study size was arrived at a website calculation of Type I error rate .05, Power .7, ratio to control and different P0/P1 estimation. Exclusion criteria: those who were smoking or heavy drinkers as alcoholism victims or female cancers such as uterine endometrium cancers or ovary cancers. Healthy control participants, breast cancer patients with less than one year of diagnosis/preoperative treatment (BCP) and breast cancer patients with greater than five years of postoperative treatment without recurrence (5-year survivors) were recruited in a medical center and hospitals in central Taiwan between April 2014 and September 2017 (n=47). The participants provided venous blood for protein adduct analyses and completed questionnaires regarding age, occupation, disease history, cigarette smoking, and alcohol consumption, etc. Eleven of Alb and fifteen of Hb samples cannot be recovered during protein extraction. Overall, 36 of Alb and 32 of Hb samples were analyzed for the background levels of estrogen quinone-derived adducts. Participants diagnosed with ER (+) and/or progesterone receptor (+) breast cancer and received hormonal therapy including aromatase inhibitor (AI) and Tamoxifen. Additionally, healthy controls and BCP were recruited between May 2009 and May 2012 as previously described. 13 None of the subjects had history of alcohol-drinking nor cigarette-smoking. The mean ages for healthy controls, BCP, and 5-year survivors were 41.7, 49.5, and 55.3, respectively. The protocols were approved by the Institutional Review Board Committee of Changhua Christian Hospital (CCH IRB No. 131216). Each participant was provided informed consents after receiving detailed explanation of this study. The informed consent was obtained from the patients before blood collection. All blood samples were maintained at −80°C before protein isolation.
Isolation of Human Serum Albumin
After bringing the serum to room temperature, Alb was isolated as follows. A saturated solution of (NH4)2SO4 was added dropwise to the plasma until the final concentration of ammonium sulfate was 2.5 M. The solution was mixed with a vortex mixer, and the immunoglobulins were removed by centrifuging for 30 min at 3000g. The protein was purified by dialysis against 4×4 L of 1 mM ascorbic acid at 4°C using Spectra-Por 2 dialysis tubing (MWCO 12000-14000). The dialyzed proteins were lyophilized, weighed, and stored at −80°C prior to use.
Isolation of Globin
After removal of red blood cell membranes by centrifuging at 30,000g at 5°C for 20 min, the supernatant containing hemoglobin was purified by exhaustive dialysis (Spectra-Pore 1, 6000-8000 MWCO) against 4×3.5 L of deionized water at 4°C for over 24 h. Globin was isolated by adding the Hb solution dropwise to 10 volumes of ice-cold acetone containing .1% HCl, washed twice with ice-cold acetone, lyophilized, and stored under −80°C.
Measurement of Adducts
All cysteinyl adducts arising from estrogen quinones were assayed by the procedure as described by Chen et al, 2011. 18 Briefly, to an 8-ml vial containing 10 mg of protein, isotopically labeled protein-bound internal standards for estrogen quinones were added. After bringing samples to complete dryness in a vacuum oven (70°C), per-acetylation was achieved with the addition of 750 μL of TFAA and the reaction was allowed to proceed at 110°C for 30 min. After cooling to room temperature, adducts were cleaved by adding 20 μL of MSA and the mixture was heated at 110°C for an additional 30 min. The un-reacted anhydride was removed under a gentle stream of N2. One and a half mL of hexane was added to the residue and the hexane layer was washed twice with 2 mL of .1 M Tris buffer (pH 7.4) and once with 1 mL of deionized water. After concentrating the samples to 50 μL, a 2-μl aliquot was analyzed by gas chromatograph (GC) and mass spectrometer (MS). All analyses were conducted using an Agilent 6890 series GC coupled to an Agilent 5973N MS. A HP-5MS fused silica capillary column (30 m, .25-mm i.d., .25-μm film thickness) was used with He (99.999%) as the carrier gas at a flow rate of 1 mL/min. The MS transfer-line temperature was 250°C and the chemical ionization reagent gas (methane) pressure was 2.3×10−4 torr.
For characterization of adducts in the assay, our GC-MS was set to scan from m/Z 50 to m/Z 750 in electron impact and negative ion chemical ionization (NICI) modes. The GC oven temperature was held at 75°C for 2 min and increased at 6°C/min to 145°C, where it was held for 10 min. Late-eluting compounds were removed by increasing the oven temperature at 50°C/min to 260°C, where it was held for 5 min. The ion source temperature was set at 150°C and the injection-port temperature was 250°C in all cases.
For quantitative analysis of adducts, all conditions were similar to those described above for GC–NICI–MS except that the mass spectrometer was set to selected ion monitoring (SIM) mode. Fragment ions of the TFA-derivatives of estrogen quinone-derived adducts were monitored in NICI mode as follows: E2-2,3-Q-1-S-TFA, E2-2,3-Q-4-S-TFA, and E2-3,4-Q-2-S-TFA (m/Z 607); [2H3] E2-2,3-Q-4-S-TFA and E2-3,4-Q-2-S-TFA (m/Z 610); [2H4] E2-2,3-Q-1-S-TFA (m/Z 611). For E2-2,3-Q-1-S-TFA, E2-2,3-Q-4-S-TFA, and E2-2,3-Q-2-S-TFA, the quantitation was based on calibration against [2H3] E2-2,3-Q-4-S-TFA, [2H3] E2-3,4-Q-2-S-TFA, and [2H4] E2-2,3-Q-1-S-TFA, respectively. Standard curves were prepared over a range of .1–500 pmole by deriving authentic standards equivalent to those of samples.
Precision and Limit of Detection of the Assay
Based on a signal-to-noise ratio of 3, the limit of detection of the assay corresponds to 10 pmole/g for all adducts, assuming 10 mg of proteins were used for the assay. The precision, as indicated by estimated coefficients of variation, was estimated to be less than 15% (n=5).
Statistical Analysis
All data are expressed as mean ± standard deviation (SD) of three determinations. We used Student’s t-test to examine subgroups. Some Data were transformed to the natural logarithm and tested for normal distribution for parametric analyses. Linear correlations were investigated between individual adduct levels by simple regression. Two-tailed statistical significance was indicated by a P value less than .05. Statistical analysis was performed using the SPSS Statistics 20.0.
Results
Measurements of Estrogen Quinone-Derived Adducts in Human Serum Alb Derived From Breast Cancer 5-year Survivors
To examine the background levels of estrogen quinone-derived adducts in serum Alb derived from breast cancer 5-year survivors, we refined our previous protocol to allow measurements of all estrogen quinone-derived adducts in serum Alb. Figure 1 depicts the typical GC-NICI-MS chromatogram obtained in SIM mode after derivatization of 10 mg of Alb derived from a breast cancer 5-year survivors. This figure clearly demonstrates the presence of cysteinyl adducts of estrogen quinone on Alb after adducts cleavage. Levels of E2-2,3-Q-1-S-Alb, E2-2,3-Q-4-S-Alb, and E2-3,4-Q-2-S-Alb were summarized in Table 1. Adducts of E2-2,3-Q-4-S-Alb and E2-3,4-Q-2-S-Alb were detected in serum Alb of the study population. Levels of E2-2,3-Q-4-S-Alb and E2-3,4-Q-2-S-Alb were estimated to be between 43-933 and 10-177 pmole/g in these subjects, respectively.
Figure 1.
GC-NCI-MS chromatogram obtained in selected ion monitoring mode following the analysis of Hb derived from a 5-year survivor. (A) Ions m/z 607 corresponds to TFA derivatives of E2-2,3-Q and E2-3,4-Q. (B) The corresponding deuterated internal standard ions were m/z 610 for [2H3]E2-2,3-Q-4-S-TFA and [2H3]E2-3,4-EQ-2-S-TFA and (C) m/z611 for [2H4]E2-2,3-EQ-1-S-TFA.
Table 1.
Measurements of the background levels of estrogen quinone-derived Alb adducts in healthy controls, BCP* and 5-year survivors†.
| Adducts | Healthy Controls (n=75) | BCP (n=152) | 5-Year Survivors (n=36) |
|---|---|---|---|
| E2-2,3-Q-1-S-Alb (pmole/g-Alb) | |||
| Mean (SD‡) | ND§ | ND§ | ND§ |
| Median | ND§ | ND§ | ND§ |
| Range | ND§ | ND§ | ND§ |
| E2-2,3-Q-4-S-Alb (pmole/g-Alb) | |||
| Mean (SD‡) | 295 (116) | 403 (252) | 193 (192) |
| Median | 282 | 330 | 112 |
| Range | 66.6∼785 | 61.7∼1330 | 43∼933 |
| E2-3,4-Q-2-S-Alb (pmole/g-Alb) | |||
| Mean (SD‡) | 138 (57.9) | 688 (333) | 51 (36) |
| Median | 119 | 664 | 41 |
| Range | 66.6∼378 | 188∼1590 | 10∼177 |
| Ratio of mean levels of E2-3,4-Q-2-S-Alb to E2-2,3-Q-4-S-Alb | .468 | 1.71 | .264 |
BCP*: breast cancer patients with less than one year of diagnosis/preoperative treatment; 5-year survivors †: breast cancer patients with five year postoperative treatment without recurrence; SD‡: standard deviation; ND§: not detected. The limit of detection is defined as 10 pmole/g when 10 mg of proteins are assayed.
Measurements of Estrogen Quinone-Derived Adducts in Human Hb Derived From Breast Cancer 5-year Survivors
Adducts of E2-2,3-Q-4-S-Hb and E2-3,4-Q-2-S-Hb were detected in Hb of the participants. Levels of E2-2,3-Q-1-S-Hb, E2-2,3-Q-4-S-Hb, and E2-3,4-Q-2-S-Hb were summarized in Table 2. We observed that the concentrations of E2-2,3-Q-4-S-Alb and E2-3,4-Q-2-S-Alb were estimated to be between 143-345 and 195-706 pmole/g in these subjects, respectively.
Table 2.
Measurements of the background levels of estrogen quinone-derived Hb adducts in healthy controls, BCP* and 5-year survivors†.
| Adducts | Healthy Controls (n=147) | BCP (n=143) | 5-Year Survivors (n=32) |
|---|---|---|---|
| E2-2,3-Q-1-S-Hb (pmole/g-Hb) | |||
| Mean (SD‡) | ND§ | ND§ | ND§ |
| Median | ND§ | ND§ | ND§ |
| Range | ND§ | ND§ | ND§ |
| E2-2,3-Q-4-S-Hb (pmole/g-Hb) | |||
| Mean (SD‡) | 83 (39) | 478 (204) | 217 (41) |
| Median | 72 | 436 | 211 |
| Range | 36∼293 | 146∼1473 | 143∼345 |
| E2-3,4-Q-2-S-Hb (pmole/g-Hb) | |||
| Mean (SD‡) | 151 (72) | 956 (321) | 368 (125) |
| Median | 139 | 913 | 341 |
| Range | 69∼453 | 153∼2392 | 195∼706 |
| Ratio of mean levels of E2-3,4-Q-2-S-Alb to E2-2,3-Q-4-S-Alb | 1.82 | 2.00 | 1.70 |
BCP*: breast cancer patients with less than one year of diagnosis/preoperative treatment; 5-year survivors†: breast cancer patients with five year postoperative treatment without recurrence; SD‡: standard deviation; ND§: not detected. The limit of detection is defined as 10 pmole/g when 10 mg of proteins are assayed.
Correlation of E2-2,3-Q Derived Adducts With Those of E2-3,4-Q
To examine the relationship between E2-2,3-Q and E2-3,4-Q adducts, we performed simple regression analysis after natural logarithmic transformation of these adducts. Results showed that logged levels of E2-2,3-Q adducts correlated with logged levels of E2-3,4-Q adducts with correlation coefficients at .624 and .336 for Alb and Hb, respectively (P<.001).
Discussion
We acknowledge the limitations of our current study. The relatively small size of the healthy controls, BCP and 5-year survivors hindered observation of both biomarker efficacy as the exploratory endpoint of the different patient group comparison and the breast cancer long term recurrence risk estimation as an individual patient concern. The short duration of the follow-up period before the 20-year analysis prevented us from assessing the biomarker efficacy. The racial diversity of study participants is restricted to ethnicities in Taiwan.
Another limitation of our study is about Student’s t-test that is unreliable when variances differ between underlying populations. The t distribution tends to the normal distribution for large sample sizes and our non-normality of the samples could not be ignored.
Krishnamurthy et al (2012) reported that tamoxifen inhibits estrogen-induced DNA damage and mammary tumorigenesis in the aromatase transgenic mouse model through up-regulation of quinone reductase. Tamoxifen-treated mice showed a significant increase quinone reductase level over the corresponding controls. 19 A novel mechanism of tamoxifen prevention against breast cancer has been proposed where upregulation of NQO1 induced by tamoxifen may inhibit estrogen-induced DNA damage and tumorigenesis in human MCF-10A cells and in transgenic mice.19,20 Additionally, various environmental factors and genetic events may influence the production of estrogen quinones in humans as well.21-23 Cytochrome P450 1A1 and 1B1 mediate metabolic activation of estrogen to generate estrogen catechols and quinones, which are the critical metabolites implicated in the initiation and progression of breast cancer. 24 Several critical enzymes may modulate the formation of estrogen quinone-DNA adducts and oxidative DNA lesions. Genetic predisposition of polymorphisms in these protective enzymes may affect their activities. 25 O-methylation of estrogen catechols mediated by catechol-o-methyl transferase plays a critical role in blocking the oxidation of the estrogen catechols. Other protective enzymes, such as glutathione-S-transferase and NAD(P)H quinone oxidoreductase 1 (NQO1), have key roles in the disposition of estrogen quinones through conjugation with glutathione and quinone reduction pathway, respectively. 26 However, environmental factors, including diet, life style, and occupation, may also modulate gene expression of these enzymes. These influences may reduce or increase levels of formation of reactive metabolites of estrogen. Several studies have demonstrated that the protective agents in the diet modulate the expression of enzymes responsible for estrogen disposition.27,28
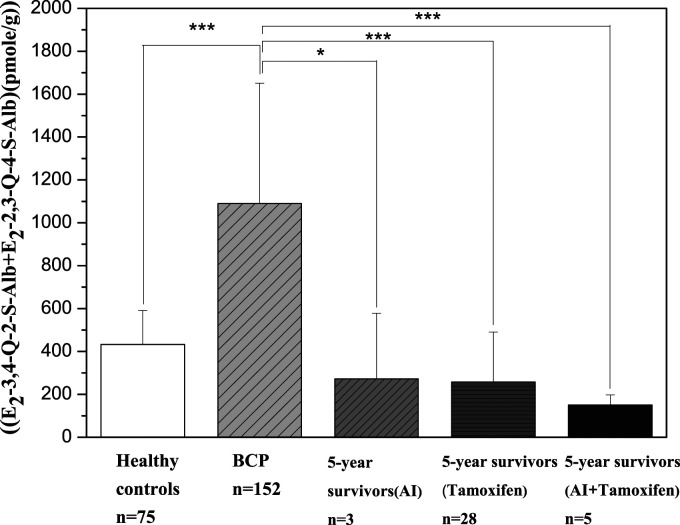
To evaluate the cumulative body burden of estrogen quinones in breast cancer 5-year survivors, levels of estrogen quinone-derived adducts in serum Alb and Hb were measured and compared to those observed in healthy controls and BCP.13,14 Results from our analyses indicated that Alb adducts of estrogen quinones were detected in the breast cancer 5-year survivors, with overall adduct levels (E2-2,3-Q-4-S-Alb plus E2-3,4-Q-2-S-Alb) being dramatically reduced (∼70%) and comparable to those observed in healthy controls Figure 2. Similar observation was detected in Hb adducts of estrogen quinones where overall adduct levels (E2-2,3-Q-4-S-Hb plus E2-3,4-Q-2-S-Hb) were decreased by ∼60% Figure 3. This is the first report of the treatment-related effects, including surgery and hormonal therapy, on the reduction of estrogen quinones in breast cancer survivors Figure 2 and Figure 3. Additionally, we noticed that ratio of E2-3,4-Q-2-S-Alb to E2-2,3-Q-4-S-Alb in breast cancer 5-year survivors was estimated to be .264, whereas .468 and 1.71 was observed for healthy controls and BCP, respectively Table 1. As distinguished by age, similar degree of reduction in estrogen quinone-derived adducts in 5-year survivors was observed except for levels of E2-2,3-Q-4-S-Alb in survivors with age greater than 50 (299 and 402 pmol/g for survivals and BCP, respectively, P>.05). Table 3 and Table 4. This evidence strongly suggests a treatment-related effect on the disposition of estrogen to quinones. Involvement of changes in diet and life style may also contribute to the reduction of estrogen quinones in these breast cancer 5-year survivors.
Figure 2.
Levels of E2-2,3-Q-4-S-Alb and E2-3,4-Q-2-S-Alb in healthy controls, breast cancer patients with less than one year of diagnosis/preoperative treatment (BCP), and breast cancer patients with five-year postoperative treatment without recurrence (5-year survivors). Aromatose inhibitor (AI); Symbols (***) are statistically significantly different from control (P<.001).
Figure 3.
Levels of E2-2,3-Q-4-S-Hb and E2-3,4-Q-2-S-Hb in healthy controls, breast cancer patients with less than one year of diagnosis/preoperative treatment (BCP), and breast cancer patients with five-year postoperative treatment without recurrence (5-year survivors). Symbols (***) are statistically significantly different from control (P<.001).
Table 3.
Levels of estrogen quinone-derived Alb adducts in healthy controls, BCP* and 5-year survivors† as distinguished by age.
| Adducts (pmol/g) mean (SD‡) | Healthy Controls (n=138) | Breast Cancer Patients (n=143) | 5-Year Survivors (n=36) | |
|---|---|---|---|---|
| E2-2,3-Q-4-Alb | <50 | 194 (112) | 396.7 (242.) | 94.8 (27.8)§|| |
| n=102 | n=73 | n=10 | ||
| E2-2,3-Q-4-Alb | >50 | 192 (106) | 402 (275) | 299 (402) |
| n=36 | n=70 | n=26 | ||
| E2-3,4-Q-2-Alb | <50 | 61.7 (41.0) | 725 (321) | 38.3 (15.6) || |
| n=102 | n=73 | n=10 | ||
| E2-3,4-Q-2-Alb | >50 | 62.7 (36.7) | 626 (348) | 61.5 (45.4) || |
| n=36 | n=70 | n=26 |
Note: BCP*: breast cancer patients with less than one year of diagnosis/preoperative treatment; 5-year survivors†: breast cancer patients with five-year postoperative treatment without recurrence; SD‡: standard deviation. Symbols (§) represent values of 5-year survivors are statistically significantly different from healthy controls (P<.05). Symbols (||) represent values of 5-year survivors are statistically significantly different from breast cancer patients (P<.05).
Table 4.
Levels of estrogen quinone-derived Hb adducts in healthy controls, BCP* and 5-year survivors as distinguished by age.
| Adducts (pmol/g) mean (SD‡) | Healthy Controls (n=147) | Breast Cancer Patients (n=143) | 5-Year Survivors (n=32) | |
|---|---|---|---|---|
| E2-2,3-Q-4-Hb | <50 | 82.8 (35.8) n=114 | 488.3 (212.4) n=73 | 209.0 (23.1)§|| n=10 |
| E2-2,3-Q-4-Hb | >50 | 85.0 (50.2) n=33 | 476.9 (211.5) n=70 | 220.0 (47.1)§|| n=22 |
| E2-3,4-Q-2-Hb | <50 | 150.8 (63.0) n=114 | 985.5 (334.7) n=73 | 301.4 (76.3)§|| n=10 |
| E2-3,4-Q-2-Hb | >50 | 150.4 (101.1) n=33 | 936.6 (321.3) n=70 | 397.5 (133.1)§|| n=22 |
Note: BCP*: breast cancer patients with less than one year of diagnosis/preoperative treatment; 5-year survivors†: breast cancer patients with five-year postoperative treatment without recurrence; SD‡: standard deviation. Symbols (§) represent values of 5-year survivors are statistically significantly different from healthy controls (P<.05). Symbols (||) represent values of 5-year survivors are statistically significantly different from breast cancer patients (P<.05).
Overall, our findings add further support to the theme that surgery and hormonal therapy, including AI and Tamoxifen, in cancer patients may dramatically reduce burden of estrogen quinones, DNA damage, and tumorogenesis.19,20 We concluded that treatment-mediated modulation of estrogen disposition and the subsequent decline of burden of estrogen quinones may play roles in the prevention of breast cancer recurrence.
Supplemental Material
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748221084196 for Profiling of Protein Adducts of Estrogen Quinones in 5-Year Survivors of Breast Cancer Without Recurrence by Che Lin, Ding-Ru Chen, Shou-Jen Kuo, Chi-Yen Feng, Dar-Ren Chen, Wei-Chung Hsieh and Po-Hsiung Lin in cancer control
Acknowledgments
This work was supported by the National Science Council, Taiwan, through Grants, NSC97-2314-B-005-001-MY2, NSC98-2314-B-371-004-MY2, and NSC99-2314-B-005-001-MY3. However, the National Science Council, Taiwan, had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication in this research work.
Authors contributions: CL and PHL conceived and designed the study. Ding-Ru Chen, CYF, CL, WCH, and Dar-Ren Chen collected blood samples. Ding-Ru Chen, SYH, PHL, and CL performed instrumental and statistical analyses and interpretation of data. PHL wrote the first draft of the manuscript, which was revised with contributions from WCH, CL, SJK, and Dar-Ren Chen. All authors read and approved the final manuscript. PHL supervised the overall study progress. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ministry of Science and Technology, Taiwan, through Grant, MOST-110-2314-B-005-004 29 . However, the Ministry of Science and Technology, Taiwan, had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication in this research work.
Ethics Statement: This study was approved by the Institutional Review Board Committee of Changhua Christian Hospital (CCH IRB No. 131216). 29 The informed consent was obtained from the patients before blood collection.
Ethics Approval and Consent to Participate: The study protocol was reviewed by the Human Ethics Committee of the Changhua Christian Hospital, Taiwan (No. 131216). Each subject provided informed consent after receiving a detailed explanation of the study.
Availability of Data and Material: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Dar-Ren Chen https://orcid.org/0000-0002-0897-4374
Po-Hsiung Lin https://orcid.org/0000-0003-1905-2778
References
- 1.Cheng SH, Tsou MH, Liu MC, et al. Unique features of breast cancer in Taiwan. Breast Cancer Res Treat. 2000;63(3):213-223. doi: 10.1023/a:1006468514396. [DOI] [PubMed] [Google Scholar]
- 2.Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci USA. 1996;93(18):9776-9781. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martucci CP, Fishman J. P450 enzymes of estrogen metabolism. Pharmacol Ther. 1993;57(2-3):237-257. doi: 10.1016/0163-7258(93)90057-k. [DOI] [PubMed] [Google Scholar]
- 4.Spink DC, Spink BC, Cao JQ, et al. Induction of cytochrome P450 1B1 and catechol estrogen metabolism in ACHN human renal adenocarcinoma cells. J Steroid Biochem Mol Biol. 1997;62(2-3):223-232. doi: 10.1016/s0960-0760(97)00024-1. [DOI] [PubMed] [Google Scholar]
- 5.Butterworth M, Lau SS, Monks TJ. 17β-estradiol metabolism by hamster hepatic microsomes: Comparison of catechol estrogen o-methylation with catechol estrogen oxidation and glutathione conjugation. Chem Res Toxicol. 1996;9(4):793-799. doi: 10.1021/tx9501952. [DOI] [PubMed] [Google Scholar]
- 6.Cao K, Stack DE, Ramanathan R, Gross ML, Rogan EG, Cavalieri EL. Synthesis and structure elucidation of estrogen quinones conjugated with cysteine, N-acetylcysteine, and glutathione. Chem Res Toxicol. 1998;11(8):909-916. doi: 10.1021/tx9702291. [DOI] [PubMed] [Google Scholar]
- 7.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354(3):270-282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 8.Bolton JL, Thatcher GRJ. Potential mechanisms of estrogen quinone carcinogenesis. Chem Res Toxicol. 2008;21(1):93-101. doi: 10.1021/tx700191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parl FF, Egan KM, Li C, Crooke PS. Estrogen exposure, metabolism, and enzyme variants in a model for breast cancer risk prediction. Cancer Inf. 2009;7:CIN.S2262-121. doi: 10.4137/cin.s2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Du F, Ueno NT, Gonzalez-Angulo AM. Breast cancer biomarkers: Utility in clinical practice. Curr Breast Cancer Rep. 2013;5(4):284-292. doi: 10.1007/s12609-013-0125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sestak I, Cuzick J. Markers for the identification of late breast cancer recurrence. Breast Cancer Res. 2015;17(1):10. doi: 10.1186/s13058-015-0516-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh WC, Lin C, Chen DR, et al. Genetic polymorphisms in APE1 Asp148Glu(rs3136820) as a modifier of the background levels of abasic sites in human leukocytes derived from breast cancer patients and controls. Breast Cancer. 2017;24(3):420-426. doi: 10.1007/s12282-016-0719-y. [DOI] [PubMed] [Google Scholar]
- 13.Lin C, Chen DR, Hsieh WC, et al. Investigation of the cumulative body burden of estrogen-3,4-quinone in breast cancer patients and controls using albumin adducts as biomarkers. Toxicol Lett. 2013;218(3):194-199. doi: 10.1016/j.toxlet.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Lin C, Hsieh W-C, Chen D-R, et al. Hemoglobin adducts as biomarkers of estrogen homeostasis: Elevation of estrogenquinones as a risk factor for developing breast cancer in Taiwanese Women. Toxicol Lett. 2014;225(3):386-391. doi: 10.1016/j.toxlet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Skipper PL, Peng X, Soohoo CK, Tannenbaum SR. Protein adducts as biomarkers of human carcinogen exposure. Drug Metabol Rev. 1994;26(1-2):111-124. doi: 10.3109/03602539409029787. [DOI] [PubMed] [Google Scholar]
- 16.Törnqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P. Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J chromatogr. B, Analyt Technol Biomedical Life Sci. 2002;778(1-2):279-308. doi: 10.1016/s1570-0232(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 17.Waidyanatha S, Rappaport SM. Hemoglobin and albumin adducts of naphthalene-1,2-oxide, 1,2-naphthoquinone and 1,4-naphthoquinone in Swiss Webster mice. Chem Biol Interact. 2008;172(2):105-114. doi: 10.1016/j.cbi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Chen DR, Chen ST, Wang TW, et al. Characterization of estrogen quinone-derived protein adducts and their identification in human serum albumin derived from breast cancer patients and healthy controls. Toxicol Lett. 2011;202(3):244-252. doi: 10.1016/j.toxlet.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Krishnamurthy N, Hu Y, Siedlak S, Doughman YQ, Watanabe M, Montano MM. Induction of quinone reductase by tamoxifen or DPN protects against mammary tumorigenesis. Faseb J. 2012;26(10):3993-4002. doi: 10.1096/fj.12-208330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montano MM, Chaplin LJ, Deng H, et al. Protective roles of quinone reductase and tamoxifen against estrogen-induced mammary tumorigenesis. Oncogene. 2007;26(24):3587-3590. doi: 10.1038/sj.onc.1210144. [DOI] [PubMed] [Google Scholar]
- 21.Okobia MN, Bunker CH, Garte SJ, et al. Cytochrome P450 1B1 Val432Leu polymorphism and breast cancer risk in Nigerian women: a case control study. Infect Agents Cancer. 2009;4(suppl 1):S12. doi: 10.1186/1750-9378-4-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin A, Kang D, Choi J-Y, et al. Cytochrome P450 1A1 (CYP1A1) polymorphisms and breast cancer risk in Korean women. Exp Mol Med. 2007;39(3):361-366. doi: 10.1038/emm.2007.40. [DOI] [PubMed] [Google Scholar]
- 23.Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer. Cancer. 2007;109(12 suppl l):2667-2711. doi: 10.1002/cncr.22655. [DOI] [PubMed] [Google Scholar]
- 24.Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78(2):161-170. doi: 10.1016/j.steroids.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Mitrunen K, Hirvonen A. Molecular epidemiology of sporadic breast cancer. Mutat Res Rev Mutat Res. 2003;544(1):9-41. doi: 10.1016/s1383-5742(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 26.Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev. 2000;21(1):40-54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- 27.Zahid M, Gaikwad NW, Ali MF, et al. Prevention of estrogen-DNA adduct formation in MCF-10F cells by resveratrol. Free Radic Biol Med. 2008;45(2):136-145. doi: 10.1016/j.freeradbiomed.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yager JD. Mechanisms of estrogen carcinogenesis: The role of E2/E1-quinone metabolites suggests new approaches to preventive intervention - A review. Steroids. 2015;99(Pt A):56-60. doi: 10.1016/j.steroids.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495-1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748221084196 for Profiling of Protein Adducts of Estrogen Quinones in 5-Year Survivors of Breast Cancer Without Recurrence by Che Lin, Ding-Ru Chen, Shou-Jen Kuo, Chi-Yen Feng, Dar-Ren Chen, Wei-Chung Hsieh and Po-Hsiung Lin in cancer control