Abstract
The COVID-19 pandemic has become an unprecedented facilitator of rapid telehealth expansion within rheumatology. Due to demographic shifts and workforce shortages in the future, new models of rheumatology care will be expected to emerge, with a growing footprint of telehealth interventions. Telehealth is already being used to monitor patients with rheumatic diseases and initial studies show good results in terms of safety and disease progression. It is being used as a tool for appointment prioritization and triage, and there is good evidence for using telehealth in rehabilitation, patient education and self-management interventions. Electronic patient-reported outcomes (ePROs) offer a number of long-term benefits and opportunities, and a routine collection of ePROs also facilitates epidemiological research that can inform future healthcare delivery. Telehealth solutions should be developed in close collaboration with all stakeholders, and the option of a telehealth visit must not deprive patients of the possibility to make use of a conventional ‘face-to-face’ visit. Future studies should especially focus on optimal models for rheumatology healthcare delivery to patients living in remote areas who are unable to use or access computer technology, and other patient groups at risk for disparity due to technical inequity and lack of knowledge.
Keywords: health services research, remote care, telehealth, telemedicine
Introduction
Mega trends are macroeconomic and geostrategic forces that are shaping the world. 1 By definition, they are big and influence some of society’s biggest challenges and opportunities.
Demographic shifts and a rise in technology are two megatrends with a major impact on today’s healthcare system. Hence, a report from the United Nations shows that with the current growth in population a tripling of the elderly population is expected by 2050. 2 In addition, the prevalence of rheumatic and musculoskeletal diseases (RMDs) in developed countries has markedly increased by 60% between 1990 and 2010 and is expected to continue rising.3,4 Together with a workforce shortage [i.e. a lack of rheumatologists and other health care professionals (HCPs)], this may have a major negative impact on the quality of rheumatology healthcare delivery in the future.
At the same time, documentation from the International Telecommunication Union (ITU) 5 shows that today 93% of the world population has access to a mobile broadband network. Thus, the technological advancements of the next decade may lead to major shifts in the healthcare system in the form of telehealth solutions.
Telehealth is defined by the World Health Organization (WHO) 6 as ‘the use of telecommunications and virtual technology to deliver health care outside of traditional health-care facilities’.
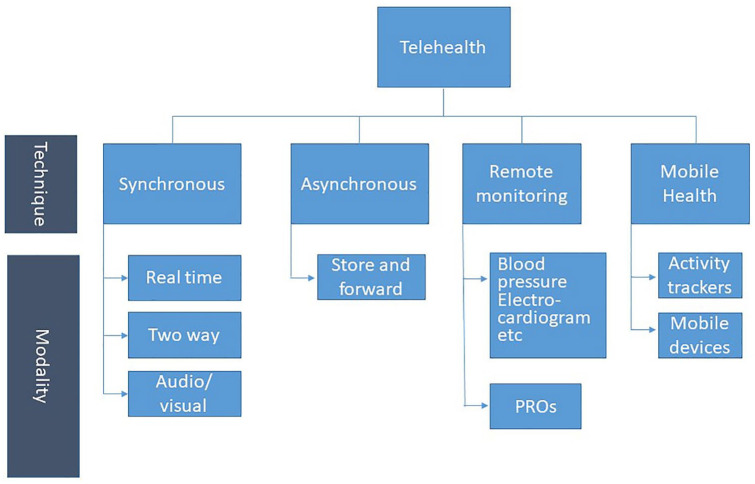
It can be delivered either synchronously (HCP and patient being present at the same time) or asynchronously and be divided into three main types of modalities: Live video and/or audio (synchronous), and the asynchronous modalities: Store and forward (transmission of a recorded health history) and remote patient monitoring (Figure 1). 7
Figure 1.
Different telehealth techniques and mordalities.
In the field of rheumatology, telehealth has been discussed over the last 10–20 years against the background of the demographic change and increasing need for accessible high-quality healthcare. Over the past 2 years, the Coronavirus disease-2019 (COVID-19) pandemic has been breaching the barriers to telehealth faster than anything in history.
A survey among 10 European Alliance of Associations for Rheumatology (EULAR) countries was recently carried out in order to investigate how the first wave of the COVID-19 pandemic influenced decisions of rheumatologists and other HCPs in rheumatology regarding the management of patients with inflammatory RMDs. 8 It was found that 82% of the respondents indicated cancellation or postponement of face-to-face (F2F) visits of new patients due to the pandemic, and 84% of these consultations were replaced by telehealth. Ninety-one percent of follow-up F2F visits were cancelled or postponed, and 96% of them were replaced by a telehealth consultation. 8
Thus, the promise of telehealth is increasing the availability of expertise and access to care, thereby increasing the geographical coverage of health systems. 9 The downside is that with telehealth solutions we place more and more responsibility on the patient’s ability to navigate through the healthcare system, which can increase the risk of disparity due to technical inequity and lack of knowledge.
The aim of this paper is to present some of the current evidence of telehealth within rheumatology and to discuss barriers, facilitators and perspectives for implementation of telehealth interventions into daily clinical practice.
Current evidence
Tele-rheumatology is a relatively new research area with most of the available evidence being reported within the last 20 years. However, in recent years, and especially during the COVID-19 pandemic, the number of publications has markedly grown.
Results from a recent EULAR guideline on remote care show10,11 that the vast majority of studies have been conducted within non-inflammatory RMDs, with osteoarthritis (OA) being the disease where most telehealth interventions have been developed. Within inflammatory RMDs, most interventions have been developed for patients with rheumatoid arthritis (RA).
Interventions
Telehealth in the diagnostic phase
The available evidence covers different phases of the patient pathway. However, only few studies have covered the pre-diagnostic and diagnostic phases,12–14 and these studies have mainly addressed non-inflammatory diseases such as low back pain and fibromyalgia.13,14
Legget et al. published a cohort study in 2001 in which 100 patients with suspected rheumatic diseases were included. Contact between patients, general practitioners (GPs) and rheumatologists took place purely by telephone, while the patients were present in the GP’s office. After an initial anamnesis and discussion of the findings, a video camera was also switched on. The rheumatologist made a tentative diagnosis both after the telephone conversation and after the videotelephony. The patients then had a F2F appointment in the rheumatology outpatient clinic, where the final diagnosis was made. This clinical examination was considered to be the ‘gold standard’. It was found that diagnostic accuracy after the telephone call was 71%. After the video camera was switched on, the accuracy increased to 97%. 13 Similar results were reported in another cohort study where the diagnostic accuracy after a videoconference with a rheumatologist was 79%. 12
These results suggest that telehealth might be useful as a screening tool in the pre-diagnostic phase, either synchronously via teleconsultations or prior to the F2F visit, or asynchronously via electronic patient-reported outcomes (PROs). While telehealth may assist with pre-diagnostic processes, a F2F visit should take place soon after to establish an RMD diagnosis. 15
In a very recent randomized controlled trial (RCT) from Germany, 16 a diagnostic decision support system (DDSS) was compared with diagnosis made by medical students. The DDSS was an artificial intelligence–driven app. Users were asked a varying number of questions depending on the previous answers given. Up to five precise disease suggestions were given together with their probability, triage advice and additional information. 16 It was found that the diagnostic accuracy of medical students was superior to the DDSS, and its usage did not significantly improve students’ diagnostic accuracy. 16 This suggests that the use of the artificial intelligence technology for clinical decision-making may not yet be ready for implementation and widespread use in rheumatology clinics.
Disease monitoring via telehealth
Within rheumatology, the pressure on the healthcare system is mainly driven by disease monitoring and follow-up visits among others due to the treat-to-target strategy in RA and other inflammatory arthrititis (IA). 17 Thus, several studies have investigated the efficacy of monitoring disease activity in IA using a telehealth intervention.15,18–21
Overall, these studies have shown that telehealth is safe to use in disease monitoring among patients with IA with low disease activity or in remission,15,20,21 meaning that overall, no differences were found in disease activity measured by Disease Activity Score (DAS)-28 between patients following a telehealth intervention as compared to usual F2F follow-up.15,20 A recent study investigated the reliability of virtual video consultations during the COVID-19 pandemic. 22 Adult patients with RA, spondyloarthritis (SpA), ankylosing spondylitis (AS) and systemic lupus erythematosus (SLE) attended a virtual video-assisted consultation during the last 2 weeks of lockdown. After 2 weeks, the patients were seen F2F by the same rheumatologist. F2F visits supported the validity of remotely delivered treatment decisions in 89/106 patients (overall sensitivity 84% and specificity 96.7%). However, sensitivity decreased to 55.5% for treatment tapering/cessation and to 36.4% when further tests/examinations were needed. 22
Similarly, in an RCT among 294 Danish RA patients, conventional F2F follow-up by rheumatologists was non-inferior to telehealth follow-up in patients with low disease activity or in remission, measured by the disease activity score using 28 joints (DAS-28) after 1 year of follow-up. 15 Overall, the number of F2F visits decreased fourfold [1.75 (SD, 1.03) visits/year versus 4.15 (SD, 1.0) visits/year]. The study also demonstrated non-inferiority in DAS-28 when the telephone consultations were performed by rheumatology nurses. 15
Telehealth may also be useful as an add-on to F2F visits in early IA, where disease activity is usually moderate to high, in order to ensure tight control, for example when adjusting disease-modifying antirheumatic drug (DMARD) therapy. Hence, Pers et al. 19 investigated the benefits of a smartphone application in RA patients with moderate/high disease activity who were initiated on a DMARD. In this RCT, half of the patients were instructed once a week by email to record some electronic PRO (ePRO) data, including Routine Assessment of Patient Index Data-3 (RAPID-3) and self-assessed DAS-28, via a smartphone application. 19 If the RAPID-3 was >12/30 points, or if insufficient data were entered after 2 months, the patient was contacted by phone and a F2F meeting was scheduled with a rheumatologist. With the other half of the patients, routine F2F checks were done according to the assessment of the rheumatologist in charge. After 6 months, there were no differences between the groups based on DAS-28, RAPID-3 or the health assessment questionnaire (HAQ). In the smartphone application group, the number of F2F visits was significantly lower compared with routine care [0.42 (SD: 0.58) versus 1.93 (SD: 0.55); p < 0.05], while the number of phone calls was significantly higher [2.67 (SD: 2.58) versus 0.41 (SD: 1.19); p < 0.01].
The use of telemedicine can be particularly impactful for patients living in remote areas. An RCT published in 2017 showed that patients with RA (n = 85) from rural areas who had a teleconference every 3 months with a rheumatologist had similar results in DAS-28, Rheumatoid Arthritis Disease Activity Index (RADAI), HAQ, quality of life and patient satisfaction compared with a group that had routine F2F visits in the outpatient clinic. In this study, both clinical examination and teleconference support was provided by a physiotherapist. 20
Rehabilitation, self-management and patient education interventions
Most of the evidence on the use of telehealth in rheumatology comes from rehabilitation and self-management interventions, and there is solid evidence for using telehealth for training and physical activity interventions.23–28
Pedometers and wearable physical activity monitoring devices incorporated in wrist watches and smartphones not only have the potential to increase physical activity through motivation, but may also help to draw conclusions about disease activity in the future. In one study, machine learning of pedometer data was able to determine with high sensitivity (95.7%) and specificity (96.7%) whether or not patients with RA or SpA were experiencing a disease flare. 29 Similar studies also found associations between disease activity (i.e. DAS-28) in patients with RA and other outcomes such as hand grip function (measured using a mobile phone-connected dynamometer) 30 and acceleration when walking. 31
Telehealth is also frequently used as a tool for self-management and goal setting in rehabilitation studies.32–39 These studies have predominantly been conducted in non-inflammatory RMDs such as OA and low back pain. However, one Norwegian RCT with approximately 400 patients with different RMD diagnoses, including IA among others, showed that patients in the tele-rehabilitation arm reported better health-related quality of life (QoL) at discharge compared with the control group. 38
Furthermore, telehealth has also shown promising results as a means to improve adherence to medications40,41 (through the use of reminder text messages and phone calls) and for patient education and counselling.42,43 A very recent study from China explored the effect of web-based patient education among 118 patients with AS. The programme consisted of four online educational sessions, and improvements in QoL and depression were found after 12 weeks. 44
Costs and cost-effectiveness
There is a general lack of studies on cost-effectiveness of telehealth interventions. The overall picture from the published studies are that telehealth interventions are cheaper on-site, however not necessarily cost-effective.
In one study, cost utility and cost-effectiveness of a non-pharmacological F2F treatment programme was compared with a telephone-based treatment programme among 147 patients with generalized OA. 45 This study found the F2F intervention to be most cost-effective; however, the finding did not reach statistical significance. 45 Another study investigated the cost-effectiveness of a blended physiotherapy intervention (e-Exercise) among 209 patients with OA of hip and knee as compared to conventional physiotherapy and found that even though e-Exercise was cheaper, it was not cost-effective. 46 A very recent study investigated the cost-effectiveness of telehealth disease monitoring compared with conventional F2F follow-up offered to 294 patients with RA. 47 This study found that the telehealth intervention seemed to cost less but provided broadly similar health outcomes compared with conventional follow-up. 47
Facilitators and barriers to telehealth interventions
Follow-up appointments by telehealth are generally well received by most patients 48 and are seen as a flexible and less time-consuming model of healthcare than conventional F2F visits in the outpatient clinic. However, both facilitators and barriers to telehealth exist.8,48–59
For telehealth interventions to become successful and fully implemented into daily clinical practice, they must be developed in close collaboration with all relevant stakeholders, including people with RMDs and HCPs, and improvements should be made based on their feedback. 15 Patients should be offered a choice of mode of communication (i.e. phone, video, text/email, F2F) depending on what is right for both the person and situation, remembering that for some patients remote care may never be appropriate. Hence, a recent study has described health literacy (HL) competencies among 895 patients with RA, SpA and gout. 60 Health literacy was measured using all 10 domains from the Health Literacy Questionnaire. 61 It was found that patients were facing most difficulties in the domains: ‘critical appraisal’, ‘navigating the health care system’ and ‘finding good health information’. Hence, individual patient’s HL level and specifically difficulties in these domains should be considered and addressed when deciding whether a patient is a good candidate for telehealth and conducting a telehealth visit.
Despite widespread knowledge regarding the importance of user involvement, a recent systematic literature review has shown that people with RMDs were only involved in approximately 15% of the studies describing mobile health applications for self-management of RMDs. In general, the development process was considered insufficient or not described in most studies. 62
When consultations are delivered remotely, it is important that the quality of personal communication is not compromised. Guidance should be provided to patients in advance so they can best prepare for the encounter, and a clear appointment time should be provided (similar to a F2F appointment) and adhered to as closely as possible, respecting patients’ time.
Greenhalgh et al. 63 developed the non-adoption, abandonment, scale-up, spread, sustainability (NASSS) framework. It can be used for development, but also for evaluation of telehealth interventions. It includes seven domains: (1) the condition, (2) the technology, (3) the value proposition, (4) the adopters, (5) the organization, (6) the wider context and (7) the interaction and adaptation between all of these domains over time. We have adapted the NASSS to the rheumatology field, by adding facilitators and barriers within rheumatology that have been identified in the literature, and have left out field 7 (the interaction and adaption over time), as it is not relevant for the purpose of this review (Table 1).
Table 1.
Facilitators and barriers to telehealth based on the non-adoption, abandonment, scale-up, spread, sustainability (NASSS) framework 63 and adopted to rheumatology.
| NASSSdomain | Content, NASSS domain | Identified facilitators and barriers within rheumatology a | |
|---|---|---|---|
| Facilitator | Barrier | ||
| The condition | What is the complexity of the illness, what are the sociocultural factors and comorbidities? | • Increased accessibility to specialist care, especially for people living in remote areas • Reduced waiting time |
• Lack of physical contact, no body language communication • No possibility of performing tests and clinical examinations which may lead to postponement of treatment decisions |
| The technology | What are the key features of the technology; off-the-shelf and already installed or not yet developed? Is it simple telehealth or complicated, direct or indirect measurements? |
• Simple telehealth, easy to use, high degree of availability Video calls • Possibility of including assistive technology (live captioning, medical interpreter) • Previous experience with telehealth Training of people with RMDs and clinicians |
• Lack of knowledge and confidence with technology Resistance to technology • No suitable equipment No access to Internet Data security concerns |
| The value position, developers | What is the developer’s business case, desirability, efficacy, cost-effectiveness and safety? | • Cost savings for healthcare services | • Reimbursement issues (insurance companies) |
| The adopters | Will there be change of staff roles, and what are the patient expectations? | • Good familiarity with clinicians • Good past treatment experiences • High motivation and engagement • Involvement of family members • High flexibility |
• Lack of training of clinicians Unclear work procedures and expectations among clinicians Lack of privacy |
| The organization | What is the capacity to innovate, readiness for change and who is in charge for implementation? | • Lack of co-ordination and unclear responsibility for implementation | |
RMDs, rheumatic and musculoskeletal diseases.
This list is not exhaustive.
Patient-reported outcome data and self-assessment
The importance of PROs for routine clinical monitoring, clinical trials and observational studies in people with RMDs is increasingly recognized. In the last two decades, rheumatology practice has benefitted from a widespread and growing use of a broad range of PROs (e.g. measures of disease activity, functional status, mental health and QoL) for guiding the treat-to-target approach used to manage rheumatic diseases. 64 The COVID-19 pandemic and abrupt shift to virtual medical care have dramatically accelerated the implementation of PROs in rheumatology in developed countries as an important means of patient evaluation, ensuring adequate control of disease activity and response to antirheumatic treatments in the era of telemedicine. 65 A number of recent studies have shown that PROs, including disease-specific questionnaires for several rheumatic conditions [e.g. rheumatoid arthritis impact of disease (RAID), RADAI, the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), Lupus Impact Tracker (LIT)], are well accepted by patients and perform well with good agreement between remotely delivered and F2F rheumatology consultations when scoring disease activity.22,58,66,67 In a study from Northern Italy, patients with RA, PsA or AS followed by telemedicine via phone had similar general health and visual analogue scale scores for pain compared with patients who had at least one in-person visit during the COVID-19 lockdown. 67 The use of PROs allowed for a 70% reduction in the number of clinical visits. Taken together with other studies showing important benefits of telemedicine for patients (e.g. less travel time, ease of use of the system and shorter waiting periods), 68 these findings demonstrate advantages of the use of PROs for medical professionals and the healthcare system. Recent studies suggest that the American College of Rheumatology (ACR)-recommended RA disease activity and functional status measures [i.e. Clinical Disease Activity Index (CDAI), DAS-28, Simple Disease Activity Index (SDAI), RAPID3, HAQ] can be adapted for use in telehealth settings to support high-quality clinical care. 69 Validated disease-specific PROs for some rheumatic conditions (e.g. Takayasu arteritis) are lacking, identifying areas for future research. 70 However, strategies to improve PRO collection and use in rheumatology care are underway as part of the Rheumatology Informatics System for Effectiveness Learning Collaborative (RISE LC) initiative. 71
The use of digital health applications (DHAs) for self-reporting, self-monitoring and self-management of rheumatic conditions is also on the rise in recent years, with the use of wearable devices and smartphones. 72 A recent study showed that over 70% of rheumatologists and patients with RMDs find DHAs useful in disease management and feel confident with their use, suggesting acceptance of DHAs and its continuous implementation and use in telemedicine. 53 Repetitive collection of ePRO measures in patients with chronic diseases, including rheumatic conditions, in adult and paediatric populations is a key feature of telemonitoring and depends on patients’ adherence to self-assessment and reporting. 73 Other challenges may include a limited ability to perform physical examinations and varying access to technology.68,74
Growing evidence suggests that self-evaluation and self-reporting of PROs can be a feasible and reliable instrument facilitating virtual medical care, particularly in adult populations. Patients’ self-evaluation of the modified HAQ score, tender and swollen joint counts submitted via a smartphone application was highly correlated with the DAS-28 assessment performed by a rheumatologist in a study of 65 patients with RA. 31 However, patient-reported joint swelling is more valuable for flare detection in established RA than for early detection of IA, which may require additional objective measures (e.g. clinical, laboratory and imaging studies). 75
An intensive telemonitoring strategy using a website platform for patients to upload RAID data in a small (n = 41) 12-month RCT ‘Remote Tele-monitoring for Managing Rheumatologic Condition and HEalthcare programs’ (RETE-MARCHE) resulted in faster and higher CDAI remission rates compared with conventional care. 18 PRO-based telehealth strategy for follow-up care in RA patients with low disease activity or in remission was found to be non-inferior to conventional rheumatologist-based follow-up care in achieving tight control of RA disease activity. 15 Web-based self-management programs providing education for improving self-efficacy, problem solving and physical activity guidance have shown improved patient satisfaction, QoL and physical activity in users versus non-users with RA and AS.44,76–78 Wearable physical activity monitors for objective assessment of continuous physical activity showed promising construct validity and reliability in patients with idiopathic inflammatory myopathies, 79 while voice-based teleconsultations have been suggested as a useful means for diagnosing and managing relapses in idiopathic inflammatory myopathies during the pandemic. 80
In 2020, after the COVID-19 pandemic had reached Great Britain, the British Society of Rheumatology (BSR) launched an online PRO measures platform which has shown to be time efficient as PROs can be collected before the consultation (rather than during) and can help to lead its direction. 81 The clinician sends an invite link for the platform prior to the remote consultation and patients can fill out the questionnaire in their own time (the PROs are tailored to their condition). 81 These data can flag up patients who are flaring so they can be called in for a F2F consultation. 22
Overall, the use of PROs in rheumatology is gaining momentum as part of the increasing transition to telemedicine during the COVID-19 pandemic. The growing number of requests for applications from the US National Institute for Health and Rheumatology Research Foundation is intended to support research on telehealth-delivered healthcare for patients with rheumatic conditions, aimed at identifying and testing optimal strategies for assessment, monitoring and management of patients via telehealth, as well as identifying ways to increase access to care for disadvantaged populations. These future studies will be expected to address existing challenges and expand the impact of rheumatology telehealth care delivery.
Conclusion and future perspectives
It is beyond doubt that the COVID-19 pandemic has become an unprecedented facilitator of rapid telehealth expansion in rheumatology. 82 In the future, new models of rheumatology care will be expected to emerge, with a growing footprint of telehealth interventions and a shift to team-based care with prominent roles for advanced practice providers (i.e. clinical nurse specialists, nurse practitioners and physician assistants) and pharmacists as an integral part of the rheumatology HCP team. 24 Not only does this make better use of the skills available, but it is a necessity for the provision of long-term sustainable care given current and predicted future workforce shortages. It is, however, important that HCPs understand the limitations of delivering treatment and care remotely, including, for example, the implications on diagnostic abilities due to limited access to clinical examinations. Therefore, alternatives need to be in place for connecting with patients and caregivers who are facing barriers to remote care, suffer from severe disease, have multiple comorbidities and have low health or digital health literacy. 10 Experiences from the COVID-19 pandemic have shown that it will be crucial that HCPs receive training in telehealth communication, interaction, legacy and clinical assessment. 82 Thus, in the future, telehealth should be incorporated into the existing curricula at universities and other healthcare educational institutions so that HCPs can develop the skills to provide safe and competent telehealth care. 10
However, even though the evidence is rising, there is still a general lack of well-conducted studies on the efficacy and safety of remote care within rheumatology. Furthermore, we also need to focus on the extra burden that patients face in taking part in this transition into a new healthcare system that will place increasing responsibility of treatment and care onto them. Increasing use of telehealth may increase inequity of healthcare, depending on a patient’s geographic location, age, education, socioeconomic status and so on, as potentially limiting factors for access to digital technologies versus routine clinical care. This may potentially create inequality in health, and therefore, a re-design of patient education and self-management strategies is required. Another well-recognized problem is poor adoption and implementation of telehealth interventions, which are not seen through into daily clinical practice. Therefore, implementation of telehealth interventions, as well as societal and health economic consequences, must be covered in future studies.
It is also beyond doubt that telehealth will be part of the solution in future rheumatology care. Hence, based on the current evidence, the following can be concluded for clinical practice:
- Telehealth is already being used to monitor patients with RMDs and initial studies show good results in terms of safety and disease progression.
- Telehealth can be used as a tool for appointment prioritization and triage, although automated, algorithm-based applications are currently too imprecise for routine clinical use.
- There is good evidence for using telehealth in rehabilitation and self-management interventions.
- Electronic patient-reported outcomes (ePROs) offer a number of long-term benefits and opportunities for disease monitoring compared to PROs that are collected via paper questionnaires.
- ePROS such as the RAPID-3 have the potential to determine whether a clinical visit is necessary for patients with RMDs. However, larger studies on this are still lacking.
- A routine collection of ePROS within telehealth also facilitates epidemiological research that informs future healthcare delivery.
- Despite first promising results, the value of smartphone applications in the treatment and monitoring of patients with RMDs is still unclear.
- The option of a telehealth visit must not deprive patients of the possibility to access a conventional ‘face-to-face’ visit.
- Further well-conducted RCTs and cost-effectiveness studies are needed to establish the evidence of telehealth disease monitoring in rheumatology.
- Future studies should especially investigate optimal models for rheumatology healthcare delivery to patients living in remote areas who are unable to use or access computer technology, and other patient groups at risk for disparity due to technical inequity and lack of knowledge.
Footnotes
Author contributions: Annette de Thurah: Project administration; Writing – original draft.
Andréa Marques: Writing – review & editing.
Cynthia S Crowson: Writing – review & editing.
Savia de Souza: Writing – review & editing.
Elena Myasoedova: Writing – original draft.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Annette de Thurah  https://orcid.org/0000-0003-0103-4328
https://orcid.org/0000-0003-0103-4328
Contributor Information
Annette de Thurah, Department of Rheumatology, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99, Aarhus N 8240, Denmark; Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Andrea Marques, Health Sciences Research Unit: Nursing, Higher School of Nursing of Coimbra, Coimbra, Portugal; Rheumatology, Centro Hospitalar e Universitário de Coimbra EPE, Coimbra, Portugal.
Savia de Souza, Centre for Rheumatic Diseases, King’s College London, London, UK.
Cynthia S. Crowson, Department of Qualitative Health Sciences, Mayo Clinic, Rochester, MN, USA Department of Internal Medicine, Division of Rheumatology, Mayo Clinic, Rochester, MN, USA.
Elena Myasoedova, Department of Qualitative Health Sciences, Mayo Clinic, Rochester, MN, USA; Department of Internal Medicine, Division of Rheumatology, Mayo Clinic, Rochester, MN, USA.
References
- 1. Oxford Dictionary. Oxford English Dictionary. Oxford: Oxford University Press, 2015. [Google Scholar]
- 2. United Nations. World population ageing 2019: highlights. New York, 2019, https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf [Google Scholar]
- 3. Briggs AM, Cross MJ, Hoy DG, et al. Musculoskeletal health conditions represent a global threat to healthy aging: a report for the 2015 World Health Organization world report on ageing and health. Gerontologist 2016; 56 Suppl 2: S243–S255. [DOI] [PubMed] [Google Scholar]
- 4. Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis 2019; 78: 1463–1471. [DOI] [PubMed] [Google Scholar]
- 5. International Telecommunication Union (ITU). Measuring digital development. Facts and figures, 2020. Geneva: ITU, 2020. [Google Scholar]
- 6. World Health Organization (WHO). WHO guideline: recommendations on digital interventions for health system strengthening. Geneva: WHO, 2019. [PubMed] [Google Scholar]
- 7. Latifi R. Clinical telemedicine practice: from Ad hoc medicine to modus operandi. In: Lafiti R, Doarn CR, Merrell RC. (eds) Telemedicine, telehealth and telepresence principles, strategies, applications, and new directions. Cham: Springer Nature, 2020, pp. 43–49. [Google Scholar]
- 8. Dejaco C, Alunno A, Bijlsma JW, et al. Influence of COVID-19 pandemic on decisions for the management of people with inflammatory rheumatic and musculoskeletal diseases: a survey among EULAR countries. Ann Rheum Dis 2020. DOI: 10.1136/annrheumdis-2020-218697. [DOI] [PubMed] [Google Scholar]
- 9. Gunasekeran DV, Tham YC, Ting DSW, et al. Digital health during COVID-19: lessons from operationalising new models of care in ophthalmology. Lancet Digit Health 2021; 3: e124–e134. [DOI] [PubMed] [Google Scholar]
- 10. de Thurah AD. C and the EULAR task force on remote care in rheumatic and musculoskeletal diseases. 2021 EULAR Points to consider for remote care in rheumatic and musculoskeletal diseases, 2022. [DOI] [PubMed] [Google Scholar]
- 11. Marques AB, et al. Effectiveness of remote care interventions: a systematic review informing the 2021 EULAR Points to Consider for remote care in rheumatic and musculoskeletal diseases, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nguyen-Oghalai TU, Hunter K, Lyon M. Telerheumatology: the VA experience. South Med J 2018; 111: 359–362. [DOI] [PubMed] [Google Scholar]
- 13. Leggett P, Graham L, Steele K, et al. Telerheumatology – diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract 2001; 51: 746–748. [PMC free article] [PubMed] [Google Scholar]
- 14. Peterson S, Kuntz C, Roush J. Use of a modified treatment-based classification system for subgrouping patients with low back pain: agreement between telerehabilitation and face-to-face assessments. Physiother Theory Pract 2019; 35: 1078–1086. [DOI] [PubMed] [Google Scholar]
- 15. de Thurah A, Stengaard-Pedersen K, Axelsen M, et al. Tele-health followup strategy for tight control of disease activity in rheumatoid arthritis: results of a randomized controlled trial. Arthritis Care Res (Hoboken) 2018; 70: 353–360. [DOI] [PubMed] [Google Scholar]
- 16. Knitza J, Mohn J, Bergmann C, et al. Accuracy, patient-perceived usability, and acceptance of two symptom checkers (Ada and Rheport) in rheumatology: interim results from a randomized controlled crossover trial. Arthritis Res Ther 2021; 23: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016; 75: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salaffi F, Carotti M, Ciapetti A, et al. Effectiveness of a telemonitoring intensive strategy in early rheumatoid arthritis: comparison with the conventional management approach. BMC Musculoskelet Disord 2016; 17: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pers YM, Valsecchi V, Mura T, et al. A randomized prospective open-label controlled trial comparing the performance of a connected monitoring interface versus physical routine monitoring in patients with rheumatoid arthritis. Rheumatology (Oxford) 2021; 60: 1659–1668. [DOI] [PubMed] [Google Scholar]
- 20. Taylor-Gjevre R, Nair B, Bath B, et al. Addressing rural and remote access disparities for patients with inflammatory arthritis through video-conferencing and innovative inter-professional care models. Musculoskeletal Care 2018; 16: 90–95. [DOI] [PubMed] [Google Scholar]
- 21. Wood PR, Caplan L. Outcomes, satisfaction, and costs of a rheumatology telemedicine program: a longitudinal evaluation. J Clin Rheumatol 2019; 25: 41–44. [DOI] [PubMed] [Google Scholar]
- 22. Piga M, Floris A, Congia M, et al. Telemedicine in rheumatology: high specificity and sensitivity of follow-up virtual video consultations during COVID-19 pandemic. Rheumatology (Oxford). Epub ahead of print 5 August 2021. DOI: 10.1093/rheumatology/keab632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bennell KL, Nelligan R, Dobson F, et al. Effectiveness of an internet-delivered exercise and pain-coping skills training intervention for persons with chronic knee pain: a randomized trial. Ann Intern Med 2017; 166: 453–462. [DOI] [PubMed] [Google Scholar]
- 24. Amorim AB, Pappas E, Simic M, et al. Integrating mobile-health, health coaching, and physical activity to reduce the burden of chronic low back pain trial (IMPACT): a pilot randomised controlled trial. BMC Musculoskelet Disord 2019; 20: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Skrepnik N, Spitzer A, Altman R, et al. Assessing the impact of a novel smartphone application compared with standard follow-up on mobility of patients with knee osteoarthritis following treatment with hylan G-F 20: a randomized controlled trial. JMIR Mhealth Uhealth 2017; 5: e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hinman RS, Lawford BJ, Campbell PK, et al. Telephone-delivered exercise advice and behavior change support by physical therapists for people with knee osteoarthritis: protocol for the telecare randomized controlled trial. Phys Ther 2017; 97: 524–536. [DOI] [PubMed] [Google Scholar]
- 27. Kloek CJJ, Bossen D, Spreeuwenberg PM, et al. Effectiveness of a blended physical therapist intervention in people with hip osteoarthritis, knee osteoarthritis, or both: a cluster-randomized controlled trial. Phys Ther 2018; 98: 560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Odole AC, Ojo OD. Is telephysiotherapy an option for improved quality of life in patients with osteoarthritis of the knee. Int J Telemed Appl 2014; 2014: 903816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gossec L, Guyard F, Leroy D, et al. Detection of flares by decrease in physical activity, collected using wearable activity trackers in rheumatoid arthritis or axial spondyloarthritis: an application of machine learning analyses in rheumatology. Arthritis Care Res (Hoboken) 2019; 71: 1336–1343. [DOI] [PubMed] [Google Scholar]
- 30. Espinoza F, Le Blay P, Coulon D, et al. Handgrip strength measured by a dynamometer connected to a smartphone: a new applied health technology solution for the self-assessment of rheumatoid arthritis disease activity. Rheumatology (Oxford) 2016; 55: 897–901. [DOI] [PubMed] [Google Scholar]
- 31. Nishiguchi S, Ito H, Yamada M, et al. Self-assessment tool of disease activity of rheumatoid arthritis by using a smartphone application. Telemed J E Health 2014; 20: 235–240. [DOI] [PubMed] [Google Scholar]
- 32. Cuperus N, Hoogeboom TJ, Kersten CC, et al. Randomized trial of the effectiveness of a non-pharmacological multidisciplinary face-to-face treatment program on daily function compared to a telephone-based treatment program in patients with generalized osteoarthritis. Osteoarthritis Cartilage 2015; 23: 1267–1275. [DOI] [PubMed] [Google Scholar]
- 33. Azma K, RezaSoltani Z, Rezaeimoghaddam F, et al. Efficacy of tele-rehabilitation compared with office-based physical therapy in patients with knee osteoarthritis: a randomized clinical trial. J Telemed Telecare 2018; 24: 560–565. [DOI] [PubMed] [Google Scholar]
- 34. Geraghty AWA, Stanford R, Stuart B, et al. Using an internet intervention to support self-management of low back pain in primary care: findings from a randomised controlled feasibility trial (SupportBack). BMJ Open 2018; 8: e016768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O’Brien KM, Wiggers J, Williams A, et al. Telephone-based weight loss support for patients with knee osteoarthritis: a pragmatic randomised controlled trial. Osteoarthritis Cartilage 2018; 26: 485–494. [DOI] [PubMed] [Google Scholar]
- 36. Friesen LN, Hadjistavropoulos HD, Schneider LH, et al. Examination of an internet-delivered cognitive behavioural pain management course for adults with fibromyalgia: a randomized controlled trial. Pain 2017; 158: 593–604. [DOI] [PubMed] [Google Scholar]
- 37. Ammerlaan J, van Os-Medendorp H, de Boer-Nijhof N, et al. Short term effectiveness and experiences of a peer guided web-based self-management intervention for young adults with juvenile idiopathic arthritis. Pediatr Rheumatol Online J 2017; 15: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berdal G, Bo I, Dager TN, et al. Structured goal planning and supportive telephone follow-up in rheumatology care: results from a pragmatic, stepped-wedge, cluster-randomized trial. Arthritis Care Res (Hoboken) 2018; 70: 1576–1586. [DOI] [PubMed] [Google Scholar]
- 39. Nero H, Ranstam J, Kiadaliri AA, et al. Evaluation of a digital platform for osteoarthritis treatment: study protocol for a randomised clinical study. BMJ Open 2018; 8: e022925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tso LS, Loi D, Mosley DG, et al. Evaluation of a nationwide pharmacist-led phone outreach program to improve osteoporosis management in older women with recently sustained fractures. J Manag Care Spec Pharm 2015; 21: 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Solomon DH, Gleeson T, Iversen M, et al. A blinded randomized controlled trial of motivational interviewing to improve adherence with osteoporosis medications: design of the OPTIMA trial. Osteoporos Int 2010; 21: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kennedy CA, Warmington K, Flewelling C, et al. A prospective comparison of telemedicine versus in-person delivery of an interprofessional education program for adults with inflammatory arthritis. J Telemed Telecare 2017; 23: 197–206. [DOI] [PubMed] [Google Scholar]
- 43. Song Y, Reifsnider E, Zhao S, et al. A randomized controlled trial of the effects of a telehealth educational intervention on medication adherence and disease activity in rheumatoid arthritis patients. J Adv Nurs 2020; 76: 1172–1181. [DOI] [PubMed] [Google Scholar]
- 44. Song Y, Xie X, Chen Y, et al. The effects of WeChat-based educational intervention in patients with ankylosing spondylitis: a randomized controlled trail. Arthritis Res Ther 2021; 23: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cuperus N, van den Hout WB, Hoogeboom TJ, et al. Cost-utility and cost-effectiveness analyses of face-to-face versus telephone-based nonpharmacologic multidisciplinary treatments for patients with generalized osteoarthritis. Arthritis Care Res (Hoboken) 2016; 68: 502–510. [DOI] [PubMed] [Google Scholar]
- 46. Kloek CJJ, van Dongen JM, de Bakker DH, et al. Cost-effectiveness of a blended physiotherapy intervention compared to usual physiotherapy in patients with hip and/or knee osteoarthritis: a cluster randomized controlled trial. BMC Public Health 2018; 18: 1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Skovsgaard CV, Kruse M, Hjollund N, et al. Cost-effectiveness of a telehealth intervention in rheumatoid arthritis: economic evaluation of the Telehealth in RA (TeRA) randomized controlled trial. Scand J Rheumatol. Epub ahead of print 20 January 2022. DOI: 10.1080/03009742.2021.2008604. [DOI] [PubMed] [Google Scholar]
- 48. Knudsen LR, de Thurah A, Lomborg K. Experiences with telehealth followup in patients with rheumatoid arthritis: a qualitative interview study. Arthritis Care Res (Hoboken) 2018; 70: 1366–1372. [DOI] [PubMed] [Google Scholar]
- 49. Almathami HKY, Win KT, Vlahu-Gjorgievska E. Barriers and facilitators that influence telemedicine-based, real-time, online consultation at patients’ homes: systematic literature review. J Med Internet Res 2020; 22: e16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bullock DR, Vehe RK, Zhang L, et al. Telemedicine and other care models in pediatric rheumatology: an exploratory study of parents’ perceptions of barriers to care and care preferences. Pediatr Rheumatol Online J 2017; 15: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferucci ED, Holck P, Day GM, et al. Factors associated with use of telemedicine for follow-up of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2020; 72: 1404–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hinman RS, Nelligan RK, Bennell KL, et al. ‘Sounds a bit crazy, but it was almost more personal’: a qualitative study of patient and clinician experiences of physical therapist-prescribed exercise for knee osteoarthritis via skype. Arthritis Care Res (Hoboken) 2017; 69: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 53. Kernder A, Morf H, Klemm P, et al. Digital rheumatology in the era of COVID-19: results of a national patient and physician survey. RMD Open 2021; 7: e001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lawford BJ, Delany C, Bennell KL, et al. ‘I was really pleasantly surprised’: firsthand experience and shifts in physical therapist perceptions of telephone-delivered exercise therapy for knee osteoarthritis – a qualitative study. Arthritis Care Res (Hoboken) 2019; 71: 545–557. [DOI] [PubMed] [Google Scholar]
- 55. Lawford BJ, Bennell KL, Hinman RS. Consumer perceptions of and willingness to use remotely delivered service models for exercise management of knee and hip osteoarthritis: a cross-sectional survey. Arthritis Care Res (Hoboken) 2017; 69: 667–676. [DOI] [PubMed] [Google Scholar]
- 56. Magnol M, Eleonore B, Claire R, et al. Use of eHealth by patients with rheumatoid arthritis: observational, cross-sectional, multicenter study. J Med Internet Res 2021; 23: e19998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mathijssen EG, Vriezekolk JE, Eijsbouts AM, et al. Support needs for medication use and the suitability of eHealth technologies to address these needs: a focus group study of older patients with rheumatoid arthritis. Patient Prefer Adherence 2018; 12: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Navarro-Millán I, Zinski A, Shurbaji S, et al. Perspectives of rheumatoid arthritis patients on electronic communication and patient-reported outcome data collection: a qualitative study. Arthritis Care Res (Hoboken) 2019; 71: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Opinc A, Łukasik Z, Makowska J. The attitude of Polish rheumatology patients towards telemedicine in the age of the COVID-19 pandemic. Reumatologia 2020; 58: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bakker MM, Putrik P, Rademakers J, et al. Addressing health literacy needs in rheumatology: which patient health literacy profiles need the attention of health professionals? Arthritis Care Res (Hoboken) 2021; 73: 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Osborne RH, Batterham RW, Elsworth GR, et al. The grounded psychometric development and initial validation of the Health Literacy Questionnaire (HLQ). BMC Public Health 2013; 13: 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Najm A, Gossec L, Weill C, et al. Mobile health apps for self-management of rheumatic and musculoskeletal diseases: systematic literature review. JMIR Mhealth Uhealth 2019; 7: e14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Greenhalgh T, Wherton J, Papoutsi C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res 2017; 19: e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Küçükdeveci AA, Elhan AH, Erdogan BD, et al. Use and detailed metric properties of patient-reported outcome measures for rheumatoid arthritis: a systematic review covering two decades. RMD Open 2021; 7: e001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Taylor PC. Adopting PROs in virtual and outpatient management of RA. Nat Rev Rheumatol 2020; 16: 477–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mistry J, Sharif M, Prideaux A, et al. Use of rheumatoid arthritis impact of disease (RAID) in routine care; identification of DAS28 remission and unmet patient-reported outcomes. Rheumatol Adv Pract 2020; 4: rkaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chevallard M, Belloli L, Ughi N, et al. Use of telemedicine during the COVID-19 pandemic in patients with inflammatory arthritis: a retrospective study on feasibility and impact on patient-reported outcomes in a real-life setting. Rheumatol Int 2021; 41: 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bos WH, van Tubergen A, Vonkeman HE. Telemedicine for patients with rheumatic and musculoskeletal diseases during the COVID-19 pandemic; a positive experience in the Netherlands. Rheumatol Int 2021; 41: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. England BR, Barber CEH, Bergman M, et al. Brief report: adaptation of American College of Rheumatology Rheumatoid Arthritis Disease Activity and functional status measures for telehealth visits. Arthritis Care Res (Hoboken) 2021: 73: 1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Misra DP, Rathore U, Patro P, et al. Patient-reported outcome measures in Takayasu arteritis: a systematic review and meta-analysis. Rheumatol Ther 2021; 8: 1073–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Subash M, Liu LH, DeQuattro K, et al. The development of the rheumatology informatics system for effectiveness learning collaborative for improving patient-reported outcome collection and patient-centered communication in adult rheumatology. ACR Open Rheumatol 2021; 3: 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Piga M, Cangemi I, Mathieu A, et al. Telemedicine for patients with rheumatic diseases: systematic review and proposal for research agenda. Semin Arthritis Rheum 2017; 47: 121–128. [DOI] [PubMed] [Google Scholar]
- 73. Wiegel J, Seppen B, van der Leeden M, et al. Adherence to telemonitoring by electronic patient-reported outcome measures in patients with chronic diseases: a systematic review. Int J Environ Res Public Health 2021; 18: 10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Goh YI, Bullock DR, Taylor J, et al. Exploring pediatric tele-rheumatology practices during COVID-19: a survey of the PRCOIN network. Front Pediatr 2021; 9: 642460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rogier C, van Dijk BT, Brouwer E, et al. Realising early recognition of arthritis in times of increased telemedicine: the value of patient-reported swollen joints. Ann Rheum Dis 2021; 80: 668–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shigaki CL, Smarr KL, Siva C, et al. RAHelp: an online intervention for individuals with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013; 65: 1573–1581. [DOI] [PubMed] [Google Scholar]
- 77. Hoving JL, Zoer I, van der Meer M, et al. E-health to improve work functioning in employees with rheumatoid arthritis in rheumatology practice: a feasibility study. Scand J Rheumatol 2014; 43: 481–487. [DOI] [PubMed] [Google Scholar]
- 78. van den Berg MH, Ronday HK, Peeters AJ, et al. Using internet technology to deliver a home-based physical activity intervention for patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum 2006; 55: 935–945. [DOI] [PubMed] [Google Scholar]
- 79. Rockette-Wagner B, Saygin D, Moghadam-Kia S, et al. Reliability, validity and responsiveness of physical activity monitors in patients with inflammatory myopathy. Rheumatology (Oxford) 2021; 60: 5713–5723. [DOI] [PubMed] [Google Scholar]
- 80. Naveen R, Sundaram TG, Agarwal V, et al. Teleconsultation experience with the idiopathic inflammatory myopathies: a prospective observational cohort study during the COVID-19 pandemic. Rheumatol Int 2021; 41: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. British Society of Rheumatology. ePROMs: leading the way in improving patient care, https://www.rheumatology.org.uk/news/details/eproms-leading-the-way-in-improving-patient-care
- 82. Singh JA, Richards JS, Chang E, et al. Management of rheumatic diseases during the COVID-19 pandemic: a national veterans affairs survey of rheumatologists. Arthritis Care Res (Hoboken) 2021; 73: 998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]