Abstract
Background:
Despite the emphasis on exercise to reduce pain and improve function among people with chronic low back pain (cLBP), little is known about the underlying mechanism of the impact of exercise on the neurophysiological and gene transcription alterations that characterize cLBP.
Objectives:
To present a study protocol to examine the feasibility, acceptability, and initial efficacy of Problem-Solving Pain to Enhance Living Well (PROPEL) with the support of nurse consultations and wearable activity-tracking technology on self-management (SM) knowledge, skills, physical activity, and pain and to examine the differential neurophysiological and gene expression profiles in cLBP participants from pre- to post-PROPEL.
Methods:
A pretest and posttest study is employed on 40 adults ages 18–60 years with cLBP who do not have serious complications and/or comorbidities that affect sensorimotor function. Participants will receive video modules focused on SM and biweekly phone consultations to facilitate symptom monitoring and problem-solving while increasing physical activity frequency and duration. Participants will be assessed for outcomes including SM skills, physical activity, and pain every 2 weeks for 12 weeks. We will examine the participants’ differential neurophysiological and gene expression profiles at 12 weeks postintervention and correlate these outcomes with the total duration of physical activity.
Results:
The study began in September 2018. Of the 99 subjects that were screened, 23 were enrolled and 8 completed data collection.
Discussion:
Comparing the neurophysiological and gene expression profiles of people with cLBP exposed to PROPEL could inform the development of interventions that offer personalized physical activity dosage along with general SM support. Web-based programs such as PROPEL have the potential to enhance accessibility of evidence-based interventions that improve functionality and quality of life among people living with cLBP.
Keywords: chronic low back pain, gene expression, neurophysiology, problem-solving, SM
Because of rising healthcare costs as well as the associated problem of opioid abuse, chronic low back pain (cLBP), defined as low back pain lasting for 3 months of the last 6 months (Deyo et al., 2015), is now recognized as one of the most costly public health issues of the 21st century (Shmagel, Krebs, Ensrud, & Foley, 2016). Individuals with cLBP are at risk for functional limitations, including disability (Murray et al., 2015), depression (Ha, Kim, Kim, & Park, 2011), and reduced quality of life (Bailly, Foltz, Rozenberg, Fautrel, & Gossec, 2015).
A precise structural etiology of cLBP is rarely present, yet studies document that a major factor that contributes to the re-fractory nature of cLBP is enhanced peripheral and central pain sensitivity, which can be measured by quantitative sensory testing (QST) using a standardized protocol (Giesecke et al., 2004; Starkweather, Heineman, et al., 2016). Previous work to examine the mechanisms of enhanced pain sensitization in cLBP identified differential gene transcription profiles from whole blood compared to patients with acute resolving pain and healthy controls (Starkweather, Lyon, et al., 2016; Starkweather, Ramesh, et al., 2016). Based on these findings, elucidation of how exercise affects gene transcription profiles of cLBP can provide insight on genetic targets of persistent pain and resolution of pain.
Practice guidelines for cLBP treatment emphasize pain self-management (SM) as first-line standard of care (Qaseem, Wilt, McLean, & Forciea, 2017). A meta-analysis of SM programs for cLBP reported moderate-quality evidence of a moderate effect on pain intensity (Du et al., 2017). Physical activity is an evidence-based component of cLBP treatment and SM (Ontario Ministry of Health and Long-Term Care, 2016). A meta-analysis of walking or exercise for cLBP found that either form of physical activity provides similar outcomes in pain and disability (Vanti et al., 2017), yet dosage effects for cLBP relief are unknown (Lima, Abner, & Sluka, 2017). Guidelines to reduce pain and improve function often refer to a minimum threshold of 150 minutes/week of moderate physical activity (Centers for Disease Control and Prevention, 2008; Geneen et al., 2017). Although individuals with cLBP who only use passive SM strategies, such as analgesics, consume more resources than those using active strategies, there is no evidence on a superior method of physical activity for pain SM (Crawford, Lee, & May, 2014).
To provide a comprehensive SM intervention that incorporates physical activity for cLBP, our team developed the Problem-Solving Pain to Enhance Living Well (PROPEL) SM program that incorporates evidence-based, standard of care methods to target SM knowledge, skills, and confidence to cope with cLBP (see Table 1). An earlier version of PROPEL curriculum without nurse-led support or wearable activity-tracking technology had high satisfaction scores (9 on a 10-point scale) in 12 low back pain participants and showed a large effect size for physical activity (Cohen’s d = 0.916). To gain mechanistic insight into the impact of physical activity on pain phenotype and genetic signatures of cLBP, we will engage cLBP participants in PROPEL, with support of nurse consultations and wearable activity-tracking technology. We aim to (a) examine the feasibility and acceptability of PROPEL with support of nurse consultations and wearable activity-tracking technology; (b) evaluate the initial efficacy of PROPEL on SM knowledge, skills, physical activity, and pain, via a one group, pretest–posttest design; and (c) examine differential neurophysiological and gene expression profiles in cLBP participants from pre- to post-PROPEL and correlate to total duration of physical activity. PROPEL’s feasibility and acceptability will be evaluated via the assessment of enrollment and retention and the completion of and satisfaction with PROPEL. We anticipate that PROPEL will promote pain SM knowledge and skills, as well as increase physical activity. We hypothesize an association (a) between total duration of physical activity and pain severity and (b) between total duration of physical activity and QST measures and gene expression profiles, respectively.
TABLE 1.
Inclusion/Exclusion Criteria
Inclusion criteria
|
Exclusion criteria
|
Note. cLBP = chronic low back pain; EHL = extensor hallucis longus; LBP = low back pain.
Theoretical Framework
As shown in Figure 1, the design of this study was guided by; the Individual and Family SM Theory (IFSMT; Ryan & Sawin, 2009). IFSMT posits that condition-specific factors and individual, family, and environmental factors influence cLBP SM knowledge and beliefs, self-regulation, and social facilitation, which then influence proximal (symptom management) and distal outcomes (quality of life). The pathways in IFSMT have been verified in patients with Type 1 diabetes (Verchota & Sawin, 2016), older adults with polypharmacy (Marek et al., 2013), and women at risk for osteoporosis (Ryan, Maierle, Csuka, Thomson, & Szabo, 2013). Particular to the context of cLBP, context-specific factors such as fear of movement and catastrophizing are known to reduce patients’ engagement in pain SM, particularly movement-based therapies (Poiraudeau et al., 2006). No investigators have examined the way in which fear of movement and/or catastrophizing affect SM behaviors and functional outcomes in cLBP patients. PROPEL targets condition-specific and process factors by integrating education on pain neurophysiology, nurse consultations for personalized goal setting and problem-solving, as well as wearable activity-tracking technology for self-monitoring of physical activity.
FIGURE 1.
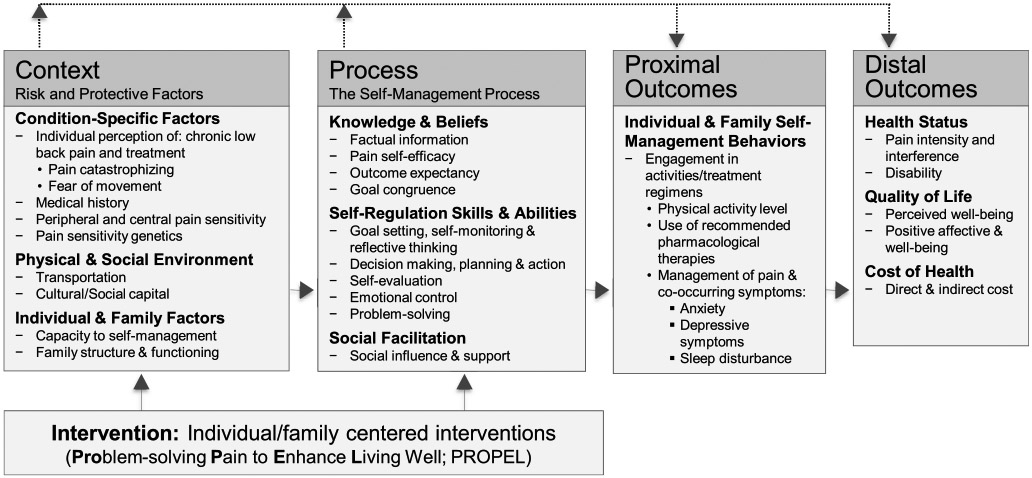
Model of chronic low back pain self-management (cLBP). This illustrates the theoretical framework of this study. This framework views self-management (SM) as “a process by which individuals and families use knowledge and beliefs, self-regulation skills and abilities and social facilitation to achieve health-related outcomes” (Ryan & Sawin, 2009). Interventions aimed at reducing the risk factors of cLBP and/or enhancing SM process factors may enhance promotion of proximal and distal outcomes.
STUDY DESIGN
A longitudinal study design is being used to provide acceptability and feasibility data as well as the initial efficacy of PROPEL among those with cLBP. As shown in Figure 2, participants will be followed for 12 weeks after enrollment, and outcomes will be measured at baseline and 2, 4, 6, 8, 10, and 12 weeks. This study will take approximately 2 years to complete data collection and analysis. The protocol has been approved by the institutional review board.
FIGURE 2.

Study diagram. This illustrates the study schema. The figure represents baseline and follow-up data collections, in addition to PROPEL, which includes 10 video modules, self-monitoring of physical activity via Fitbit, and biweekly nurse consultations. Abbreviations: HIPAA = Health Insurance Portability and Accountability Act; PROPEL = Problem-Solving Pain to Enhance Living Well; RNA = ribonucleic acid. Depending on a participant’s preference, he or she may spend more time reviewing modules and additional resources and practicing the methods that are introduced.
Participants and Recruitment
Forty men and women (ages 18–60 years) will be recruited based on the inclusion/exclusion criteria (Table 1). Participants are being recruited from the general community as well as targeted advertising at two large public university campuses and musculoskeletal clinics in northeastern United States. We will also contact individuals with cLBP enrolled in the Center for Advancement in Managing Pain Registry and receive referrals from clinical collaborators at university-affiliated clinics.
Sample Size
We observed a large effect size (d = 0.916) for physical activity among 12 participants who were exposed to an earlier version of PROPEL. Given that the effect size estimated from a pilot study may be large (Leon, Davis, & Kraemer, 2011), we conservatively estimated the effect size for physical activity as 0.6. Using G*Power, we performed a power analysis with an alpha of .05, a power of .80, and a correlation between the baseline and follow-up of .4, yielding a sample size of 29. Considering a dropout rate of 25%, a sample size of 40 will be large enough to detect within-group differences in physical activity.
The Study Intervention
As shown in Table 2, PROPEL consists of 10 brief video modules that are built upon evidence-based cLBP SM (Qaseem et al., 2017) and provide factual information about pain neurophysiology, instruction on how to reduce pain during physical activity, and methods for strengthening skills in self-regulation and problem-solving. Participants will receive e-mail links for the PROPEL video modules. Each module lasts for 5–10 minutes and provides additional resources for participants to access in order to practice the methods that are introduced. An RN-trained graduate research assistant (GRA) will coordinate biweekly phone consultations with participants to encourage completion of study components and assist with goal setting and problem-solving of SM-related issues. The GRA will strictly follow a script for each phone call, which lasts 20 minutes or less. Fidelity to the intervention scripts and protocol will be monitored regularly by the research team until study completion.
TABLE 2.
Descriptions of PROPEL (Problem-Solving Pain to Enhance Living Well) Modules
| Module topic | Description |
|---|---|
| Chronic low back pain neurophysiology | The pathophysiology of pain perception and musculoskeletal pain sensitivity will be covered in this module using simple terms, definitions of medical terminology, and basic principles of neurophysiology. |
| Catastrophizing | The concept of catastrophizing will be introduced, along with how it affects mood, pain perception, and pain outcomes. Cognitive restructuring exercises, such as recognizing and replacing negative thoughts, identification of catastrophizing triggers and patterns, and self-monitoring, are discussed. |
| Stress reactivity | The concept of stress reactivity will be introduced in relation to pain management and how an individual’s view of stress (as good or bad) can affect their health outcomes. Cognitive and behavioral exercises are introduced with encouragement of goal setting and self-monitoring practices. |
| Fear of movement | Discussion of correct body mechanics and movement, through proper alignment of the spine and techniques to lifting. Guided exercises on proper body mechanisms are provided. |
| Progressive relaxation | Methods to use for progressive muscle relaxation will be discussed, to aid in overall relaxation and peace of mind during painful events. Step-by-step instructions and demonstration of progressive muscle relaxation will be included. |
| Deep breathing | Methods of deep breathing will be shown. Step-by-step instructions on how to use deep breathing for anxiety, stress reduction, and pain will be highlighted. |
| Guided imagery | Methods of using guided imagery will be reviewed. Step-by-step instructions and demonstration will be given. |
| Heat, ice and stretching | Exercises specific to low back pain provided, including yoga poses (standing and seated) and stretching techniques. |
| Strategies for self-activation | Tracking physical and mental health overtime to identify and improve low back pain-associated symptoms. Creates awareness and improvement in confidence in modifying relevant factors, which may affect pain. |
| Problem-solving chronic low back pain management | Instructions for applying all the various concepts into daily life will be discussed with instructions on how to set weekly goals. Examples include incorporating pharmacological and nonpharmacological strategies, such as breathing exercises, activity changes, and coping strategies into daily life. |
Data Collection and Measures
After obtaining Health Insurance Portability and Accountability Act authorization, a trained research staff will perform an interview and a physical examination in a private research lab on the campus to confirm eligibility. Those who fail to meet the criteria receive compensation ($10) for the time spent to make it to the visit. After obtaining informed consent, participants will be asked to complete questionnaires, undergo QST, and venipuncture for collection of a blood specimen. QST is used to measure pain sensitivity and uses standardized stimuli to test both nociceptive and nonnociceptive systems in the periphery and central nervous systems (Rolke, Baron, et al., 2006; Rolke, Magerl, et al., 2006). A standardized QST protocol of administration has been published elsewhere (Starkweather, Heineman, et al., 2016). In brief, seven tests measuring 13 functional sensory pathways can be grouped as follows (Rolke, Baron, et al., 2006): “(1) thermal detection thresholds for the perception of cold, warm, and paradoxical heat sensation; (2) thermal pain thresholds for cold and hot stimuli; (3) mechanical detection thresholds for touch and vibration; and (4) mechanical pain sensitivity including thresholds for pinprick and blunt pressure, stimulus/response-functions for pinprick sensitivity and dynamic mechanical allodynia, and pain summation to repetitive pinprick stimuli (wind-up like pain).” A practice run on the dominant forearm is performed in order to verify the participant’s understanding of the protocol. The medial side of the nondominant forearm is utilized for testing purposes. QST is conducted in the following order: mechanical cutaneous pain, thermal pain, and pressure pain.
Venous blood samples (10 ml) will be collected in one 10-ml PAX Gene tube by an experienced GRA and immediately placed in a biohazard container and transported to the lab where they will be stored in a research-designated −80°C freezer. RNA (ribonucleic acid) degrades rapidly at room temperature due to enzymatic degradation by ribonuclease (RNase) activity. PAX Gene tubes are designed to stabilize intracellular RNA in whole blood samples, resulting in greater total RNA yield for subsequent analysis of gene expression. Samples will be bulk processed for RNA extraction and sequencing. From 30 cLBP participants with complete samples, total RNA extraction will be performed using the PAX Gene (PreAnalytics, Switzerland) total RNA isolation system. From the total RNA yield, mRNA will be reverse transcribed using RNA Quick and RT2 Easy First Strand Kit (Qiagen, Valencia, CA). Quality (defined as a 260/280 ratio of ~2.0) and quantity (100 ng/μl < yield) of total RNA will first be confirmed by biospectrophotometer (Cat. No. 6135000009, Eppendorf Biospectrophotometer Basic, Hauppauge, NY). Following this initial determination, quantitation and quality control of final RNA and cDNA for RNA sequencing will be performed using the 2100 Bioanalyzer (Agilent, Santa Clara, CA). Libraries will be prepared per Illumina standard protocols and sequencing performed using the NovaSeq system to obtain 100-base pair paired-end reads.
Following the baseline visit, participants will be given an activity tracker (Fitbit Flex 2) and given instruction on how to use it for self-monitoring of physical activity. They will then receive follow-up questionnaires every 2 weeks until their 12-week appointment. Participants will be contacted via phone/e-mail up to three times for study-related reminders for each time point. The 12-week visit will follow the same procedures as the baseline visit.
Retention Plan
Participants will be given a $10 gift card to a local store at each time point and a $40 gift card at completion of the study. Participants will be given a Fitbit device to keep. In addition, we will send a weekly e-mail thanking the participant for taking part in the study and provide flexibility in scheduling follow-up visits at times that are convenient to the participant.
Measures
Study measurements will be collected and managed using the REDCap system (Harris et al., 2009). REDCap supports data capture, validated data entry, audit trails for tracking data manipulation, and export procedures. Table 3 outlines the self-reported measurements.
TABLE 3.
Study Measures
| Concept | Measurement | Items |
|---|---|---|
| Demographics and general questionnaire | A questionnaire based on the minimum data set as recommended by the NIH Taskforce on Research Standards for LBP (Deyo et al., 2014) to assess demographics (age, gender, race/ethnicity, education, socio-economic status) and medical history (duration of LBP, comorbidities, medication usage, ongoing treatments). | 40 |
| PROMIS | NINR recommended CDEs including PROMIS fatigue, sleep disturbance + 1 duration question, anxiety, and depressive symptoms (Redeker et al., 2015). | 25 |
| Self-efficacy | The Self-Efficacy for Managing Chronic Illnesses Scale (Lorig, Chastain, Ung, Shoor, & Holman, 1989; Moore et al., 2016) and wording of disease will be replaced with cLBP to measure general self-efficacy for managing cLBP. | 6 |
| Outcome expectancy | Patient-Centered Outcomes Questionnaire (Sanderson et al., 2012) to evaluate outcome expectancy regarding cLBP, relief from pain, and improvement in function. | 3 |
| Catastrophizing and coping strategies | Coping Strategies Questionnaire–Revised (Robinson et al., 1997) to assess 6 cognitive coping responses to pain (i.e., catastrophizing, distraction, praying, ignoring pain, distancing from pain, coping). | 27 |
| Fear of movement | Tampa Scale for Kinesiophobia (Tkachuk & Harris, 2012) to assess fear of movement-related pain in patients with cLBP. | 11 |
| Pain problem-solving | The Pain Solutions Questionnaire (De Vlieger, Van den Bussche, Eccleston, & Crombez, 2006) to evaluate the extent of problem-solving pain management. | 14 |
| Physical activity | A wearable wrist activity tracker given to each participant (Fitbit Flex 2) to measure total daily duration of moderate- to high-intensity physical activity. | N/A |
| Godin Leisure-Time Exercise Questionnaire to assess levels of leisure-time physical activity in patients with cLBP (Godin, 2011; Godin & Shephard, 1985, 1997). | 4 | |
| Pain severity and interference | Brief Pain Inventory–Short Form (Cleeland, 1991) to obtain pain interference score—the mean of the 7 interference items and pain severity score—average pain severity. | 8 |
| Healthcare utilization | Number of visits in the past 6 months to healthcare providers for their cLBP: primary care providers, specialists, mental health visits, complementary/alternative approaches | 6 |
Note. CDEs = common data elements; cLBP = chronic low back pain; LBP = low back pain; NINR = National Institute of Nursing Research; PROMIS = Patient-Reported Outcomes Measurement Information System.
Data Analysis
Data will be exported from the REDCap. Descriptive statistics will be computed to describe the sample. Transformations are anticipated if variables are not normally distributed. With regard to Aim 1, descriptive analysis will be conducted to determine the number of individuals who are screened, the proportion of those who are eligible, and the proportion who provide informed consent. Among those who are enrolled, the frequency of those who complete the 10 video modules will be tallied. We will also examine the completion of biweekly nurse consultations. The minimum of 70% for the completion of study components will be considered adequate to demonstrate feasibility and acceptability of the study protocol (Young, Barnason, & Do, 2015). For Aim 2, we will use repeated-measures analysis of variance to estimate means of SM knowledge and skills, physical activity, and pain severity and interference within groups over time. For Aim 3, we will conduct paired t tests on all QST at baseline and 12 weeks. RNA sequencing data will be transferred to the Bioinformatics Core, which will map the reads to the most recent build of the Ensembl human genome with the short-read aligner TopHat (Trapnell, Pachter, & Salzberg, 2009). The alignments will be merged to form complete alignments to the genome. Differential gene expression by RNA sequencing will be performed using the HTSeq program (Anders & Huber, 2010) against the gene annotation file for the Ensembl human genome build (Love, Huber, & Anders, 2014; Yates et al., 2016). Read counts will be used to measure gene expression. Data normalization and differential expression analysis will be performed with the methods implemented in the R package DESeq2 (Love et al., 2014), which normalizes read counts for sequencing depth and distortion caused by highly differentially expressed genes. A negative binomial model is then used to test the significance of differential expression from pre- to post-PROPEL. The criteria used to select significant genes will include a false discovery rate (FDR) cutoff of less than 0.05 with a more than twofold change in gene expression between pre- to post-PROPEL. Pearson’s and Spearman’s rank correlation will be used to examine the association between total duration of physical activity and (a) pain severity, (b) QST measures, and (c) gene expression profiles. To determine the intervention dose effect, we will estimate the correlations between the tallied intervention dose and physical activity, pain severity and interference, QST measures, and gene expression profiles.
RESULTS
Beginning in September 2018, 99 subjects were screened, 23 were enrolled, 8 completed the data collection process, and 10 are ongoing. The sample included young adults (mean ± SD = 26.3 ± 10.2 years) who were mostly female (68%), White (63.6%), and non-Hispanic (81.8%) and have at least a high school diploma (52.2%).
DISCUSSION
At this time, the most challenging aspect of our study implementation will be retention. Previous retention rates of participants enrolled in longitudinal SM studies report mean retention rates of 75.5% with a range of 70%–81.5% (Young et al., 2015). The research team will implement multiple strategies. The participants will be contacted up to three times via e-mail to send reminders about future appointments. These reminders are built into the daily workings of the research lab. During the consent process, the recruiter will stress the time commitment involved with study participation to improve the potential participant’s comprehension of the study requirements. We will include detailed instructions for watching the modules, using problem-solving technology, as well as the necessity of wearing and charging the Fitbit regularly.
Acknowledgments
This publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number NIH-NINR P20NR016605 (PI Starkweather)–Pilot 3 subaward (PI Kim).
Footnotes
The authors have no conflicts of interest to report.
Ethical Conduct of Research: This protocol has been approved by the Institutional Review Board (IRB protocol #H18-086). All authors complied with institutional ethical standards.
Clinical Trial Registration: The PROPEL protocol is registered at clinicaltrials.gov on August 20, 2018. The registration number is NCT03637998. The first participant was enrolled on September 21, 2018. This is the link to the information on the trial register: https://clinicaltrials.gov/ct2/show/NCT03637998
REFERENCES
- Anders S, & Huber W (2010). Differential expression analysis for sequence count data. Genome Biology, 11, R106. doi: 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly F, Foltz V, Rozenberg S, Fautrel B, & Gossec L (2015). The impact of chronic low back pain is partly related to loss of social role: A qualitative study. Joint, Bone, Spine, 82, 437–441. doi: 10.1016/j.jbspin.2015.02.019 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2008). Physical activity guidelines for Americans. Washington, DC: Author. [Google Scholar]
- Cleeland CS (1991). Pain assessment in cancer. In Osoba D (Ed.), Effect of cancer on quality of life (pp. 293–305). Boca Raton, FL: CRC Press. [Google Scholar]
- Crawford C, Lee C, & May T (2014). Physically oriented therapies for the self-management of chronic pain symptoms. Pain Medicine, 15(Suppl. 1), S54–S65. doi: 10.1111/pme.12410 [DOI] [PubMed] [Google Scholar]
- De Vlieger P, Bussche EV, Eccleston C, & Crombez G (2006). Finding a solution to the problem of pain: Conceptual formulation and the development of the Pain Solutions Questionnaire (PaSol). Pain, 123, 285–293. doi: 10.1016/j.pain.2006.03.005 [DOI] [PubMed] [Google Scholar]
- Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, … Weiner DK (2014). Report of the NIH Task Force on research standards for chronic low back pain. Journal of Pain, 15, 569–585. doi: 10.1016/j.jpain.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S, Hu L, Dong J, Xu G, Chen X, Jin S, … Yin H (2017). Self-management program for chronic low back pain: A systematic review and meta-analysis. Patient Education and Counseling, 100, 37–49. doi: 10.1016/j.pec.2016.07.029 [DOI] [PubMed] [Google Scholar]
- Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, & Smith BH (2017). Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Systematic Review, 4, CD011279. doi: 10.1002/14651858.CD011279.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, & Clauw DJ (2004). Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis and Rheumatology, 50, 613–623. doi: 10.1002/art.20063 [DOI] [PubMed] [Google Scholar]
- Godin G (2011). The Godin-Shephard Leisure-Time Physical Activity Questionnaire. Health and Fitness Journal of Canada, 4, 18–22. [Google Scholar]
- Godin G, & Shephard RJ (1985). A simple method to assess exercise behavior in the community. Canadian Journal of Applied Sport Sciences, 10, 141–146. [PubMed] [Google Scholar]
- Godin G, & Shephard RJ (1997). Godin Leisure-Time Exercise Questionnaire. Medicine & Science in Sports & Exercise, 26 (Suppl. 6), S36–S38. [Google Scholar]
- Ha JY, Kim ES, Kim HJ, & Park SJ (2011). Factors associated with depressive symptoms in patients with chronic low back pain. Annals of Rehabilitation Medicine, 35, 710–718. doi: 10.5535/arm.2011.35.5.710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42, 377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon AC, Davis LL, & Kraemer HC (2011). The role and interpretation of pilot studies in clinical research. Journal of Psychiatric Research, 45, 626–629. doi: 10.1016/j.jpsychires.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima LV, Abner TSS, & Sluka KA (2017). Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. Journal of Physiology, 595, 4141–4150. doi: 10.1113/jp273355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, & Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology, 15, 550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorig K, Chastain RL, Ung E, Shoor S, & Holman HR (1989). Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis and Rheumatism, 32, 37–44. [DOI] [PubMed] [Google Scholar]
- Marek KD, Stetzer F, Ryan PA, Bub LD, Adams SJ, Schlidt A, … O'Brien AM (2013). Nurse care coordination and technology effects on health status of frail older adults via enhanced self-management of medication: Randomized clinical trial to test efficacy. Nursing Research, 62,269–278. doi: 10.1097/NNR.0b013e318298aa55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SM, Schiffman R, Waldrop-Valverde D, Redeker NS, McCloskey DJ, Kim MT, … Grady P (2016). Recommendations of common data elements to advance the science of self-management of chronic conditions. Journal of Nursing Scholarship, 48, 437–447. doi: 10.1111/jnu.12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Barber RM, Foreman KJ, Abbasoglu Ozgoren A, Abd-Allah F, Abera SF, … Vos T (2015). Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological transition. Lancet, 386, 2145–2191. doi: 10.1016/s0140-6736(15)61340x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontario Ministry of Health and Long-Term Care (2016). Low back pain toolkit. Ontario, Canada: Author. [Google Scholar]
- Poiraudeau S, Rannou F, Baron G, Le Henanff A, Coudeyre E, Rozenberg S, … Ravaud P (2006). Fear-avoidance beliefs about back pain in patients with subacute low back pain. Pain, 124, 305–311. doi: 10.1016/j.pain.2006.04.019 [DOI] [PubMed] [Google Scholar]
- Qaseem A, Wilt TJ, McLean RM, & Forciea MA (2017). Non-invasive treatments for acute, subacute, and chronic low back pain: A clinical practice guideline from the American College of Physicians. Annals of Internal Medicine, 166, 514–530. doi: 10.7326/m16-2367 [DOI] [PubMed] [Google Scholar]
- Redeker NS, Anderson R, Bakken S, Corwin E, Docherty S, Dorsey SG, … Grady P (2015). Advancing symptom science through use of common data elements. Journal of Nursing Scholarship, 47, 379–388. doi: 10.1111/jnu.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ME, Riley JL 3rd, Myers CD, Sadler IJ, Kvaal SA, Geisser ME, & Keefe FJ (1997). The Coping Strategies Questionnaire: A large sample, item level factor analysis. Clinical Journal of Pain, 13, 43–49. [DOI] [PubMed] [Google Scholar]
- Rolke R, Baron R, Maier C, Tölle TR, Treede RD, Beyer A, … Wasserka B (2006). Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain, 123, 231–243. [DOI] [PubMed] [Google Scholar]
- Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD (2014). Quantitative sensory testing: a comprehensive protocol for clinical trials. Journal of Pain, 10, 77–88. [DOI] [PubMed] [Google Scholar]
- Ryan P, Maierle D, Csuka ME, Thomson A, & Szabo A (2013). Computer-based intervention to enhance self-management of calcium and vitamin D intake in women. Western Journal of Nursing Research, 35, 986–1010. doi: 10.1177/0193945913483369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P, & Sawin KJ (2009). The individual and family self-management theory: Background and perspectives on context, process, and outcomes. Nursing Outlook, 57, 217–225.e6. doi: 10.1016/j.outlook.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson KB, Roditi D, George SZ, Atchison JW, Banou E, & Robinson ME (2012). Investigating patient expectations and treatment outcome in a chronic low back pain population. Journal of Pain Research, 5, 15–22. doi: 10.2147/jpr.s28636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmagel A, Krebs E, Ensrud K, & Foley R (2016). Illicit substance use in US adults with chronic low back pain. Spine (Phila Pa 1976), 41, 1372–1377. doi: 10.1097/brs.0000000000001702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkweather AR, Heineman A, Storey S, Rubia G, Lyon D, Greenspan J, & Dorsey SG (2016). Methods to measure peripheral and central sensitization using quantitative sensory testing: A focus on individuals with low back pain. Applied Nursing Research, 29, 237–241. doi: 10.1016/j.apnr.2015.03.013 [DOI] [PubMed] [Google Scholar]
- Starkweather AR, Lyon DE, Kinser P, Heineman A, Sturgill JL, Deng X, … Dorsey SG (2016). Comparison of low back pain recovery and persistence: A descriptive study of characteristics at pain onset. Biological Research in Nursing, 18, 401–410. doi: 10.1177/1099800416631819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkweather AR, Ramesh D, Lyon DE, Siangphoe U, Deng X, Sturgill J, … Greenspan J (2016). Acute low back pain: Differential somatosensory function and gene expression compared with healthy no-pain controls. Clinical Journal of Pain, 32, 933–939. doi: 10.1097/ajp.0000000000000347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachuk GA, & Harris CA (2012). Psychometric properties of the Tampa Scale for Kinesiophobia-11 (TSK-11). Journal of Pain, 13, 970–977. doi: 10.1016/j.jpain.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, & Salzberg SL (2009). TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics, 25, 1105–1111. doi: 10.1093/bioinformatics/btp120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanti C, Andreatta S, Borghi S, Guccione AA, Pillastrini P, & Bertozzi L (2017). The effectiveness of walking versus exercise on pain and function in chronic low back pain: A systematic review and meta-analysis of randomized trials. Disability and Rehabilitation, 1–11. doi: 10.1080/09638288.2017.1410730 [DOI] [PubMed] [Google Scholar]
- Verchota G, & Sawin KJ (2016). Testing components of a self-management theory in adolescents with Type 1 diabetes mellitus. Nursing Research, 65, 487–495. doi: 10.1097/NNR.0000000000000180 [DOI] [PubMed] [Google Scholar]
- Yates A, Akanni W, Amode MR, Barrell D, Billis K, Carvalho-Silva D, … Flicek P (2016). Ensembl 2016. Nucleic Acids Research, 44(D1), D710–D716. doi: 10.1093/nar/gkv1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Barnason S, & Do V (2015). Review: Strategies to recruit and retain rural patients participating in self-management behavioral trials. Online Journal of Rural Research & Policy, 10, 1–12. doi: 10.4148/1936-0487.1070 [DOI] [PMC free article] [PubMed] [Google Scholar]


